Primary Prevention of Dementia: An Ethical Review
Abstract
Dementia poses important medical and societal challenges, and of all health risks people face in life, dementia is one of the most feared. Recent research indicates that up to about 40% of all cases of dementia might be preventable. A series of environmental, social, and medical risk-factors have been identified that should be targeted from midlife onwards when people are still cognitively healthy. At first glance, this seems not merely advisable, but even imperative. However, these new developments trigger a series of new ethical questions and concerns which have hardly been addressed to date. Pro-active ethical reflection, however, is crucial to ensure that the interests and well-being of those affected, ultimately all of us, are adequately respected. This is the goal of the current contribution. Against the background of a concrete case in primary dementia prevention, it provides a systematic overview of the current ethical literature and sketches an ethical research agenda. First, possible benefits of increased well-being must be balanced with the burdens of being engaged in particularly long-term interventions for which it is unclear whether they will ever pay out on a personal level. Second, while knowledge about one’s options to maintain brain health might empower people, it might also undermine autonomy, put high social pressure on people, medicalize healthy adults, and stigmatize those who still develop dementia. Third, while synergistic effects might occur, the ideals of dementia prevention might also conflict with other health and non-health related values people hold in life.
INTRODUCTION
Dementia poses a significant societal challenge, as the number of people affected steadily increases. According to the current estimates, over 50 million people are living with dementia worldwide. It isexpected that this number will increase to 152 million by 2050. The current annual costs for dementia are estimated at 1 billion US dollars and these costs are expected to double in the next 10 years [1]. People with dementia experience significant suffering and decline in their daily life functioning and quality of life [2]. Of all health risks people face in life, dementia is one of the most feared [3]. Dementia is particularly feared because of its potential impact on people’s autonomy, on their mental and cognitive capacities, and because of personality changes that have been attributed to it. Because of this double degeneration, affecting physical but also cognitive and mental aspects, people’s caregivers experience very significant burdens and detrimental effects as well [4–6]. In addition to being a personal and familial problem, dementia poses a significant societal challenge as the number of people affected steadily increases. According to the current estimates, over 50 million people are living with dementia worldwide. It is expected that this number will increase to 152 million by 2050. The current annual costs for dementia are estimated at 1 billion US dollars and these costs are expected to double in the next 10 years [7].
Current research heavily invests to better understand dementia, its underlying brain processes, and to develop biomarkers and genetic risk factors, with the ultimate aim to develop means to prevent, slow down, stop or cure degeneration in affected individuals as well as those considered at-risk [7, 8]. Next to this biomedical research, recent findings indicate that up to about 40% of all cases of dementia cases might be due to modifiable risk factors and hence be preventable by behavioral change and public health measures [9, 10]. To that aim, a series of environmental, social, and medical risk-factors that play a role already during midlife have been identified that should be targeted from early in life onwards by corresponding interventions [11]. As a consequence, research is increasingly shifting towards the prevention of dementia by targeting modifiable risk factors early in life, when people are still cognitively healthy. Conceivable measures are public policies aiming at better childhood education, smoking cessation, healthy diets, management of depression, diabetes, hypertension, prevention of hearing-loss, as well as social and cognitive engagement. These have also been addressed by the Journal of Alzheimer’s Disease’s special issue on dementia prevention edited by the International Research Network on Dementia Prevention (IRNDP) [12, 13].
Important public and population health gains appear achievable if universal prevention is successful: less people developing the condition and more people staying autonomous and self-reliant until old(er) ages and consequently less burden being put on (informal) caregivers and less expenditure on health care funding. Against this background, early dementia prevention by means of lifestyle changes and medical risk management appears to be not merely advisable, but even imperative. However, regardless of the specific interventions invoked, such preventive measures are likely to have two central features: 1) they will target large groups of the population rather than selected individuals such as patients or individuals at-risk; and 2) they will aim at relatively young populations such as people during midlife or even younger. These new foci trigger a series of new ethical questions and concerns that may have an impact on how we design such health initiatives. For example, what might it mean to the personal identity of young and healthy people if they are advised to care about preventing a disease that is still far away and for which health benefits, if any, will become visible only decades from now? But also, how will preventive campaigns targeting dementia relate to other health campaigns that also focus on lifestyle and behavior? It is of great importance to identify ethical questions like these in order to ensure that the interests and well-being of those affected, ultimately all of us, are adequately respected. Now is the time to do so, as primary prevention interventions are upcoming, early pilots are being implemented and (inter)national roll-outs are on the research and public health agenda’s (e.g., [14–17]. Profound ethical reflection that accompanies these efforts can contribute to the further development of upcoming interventions in ways that are not only scientifically effective but also desirable from an ethical and social point of view. However, while biomedical screening and biomarker-based early diagnosis have immediately attracted profound ethical attention [18–22], to date systematic ethical reflection and guidance regarding this kind of primary dementia prevention seems to be largely lacking. The current contribution wants to ignite this debate. To this end, we first present a recent concrete case of primary dementia prevention in the Netherlands, we then provide a systematic overview of the current literature and finally sketch an ethical agenda of primary dementia prevention.
THE CASE
We want to start this discussion by presenting a case of early dementia prevention covering lifestyle changes and general medical care. The recent Dutch public health campaign “We zijn zelf het medicijn” [‘We are the medicine ourselves’] ran from March 2018 to February 2019 in the Province of Limburg in the Netherlands and was supported by an accompanying mobile app called “MijnBreincoach” [‘MyBraincoach’] [23]. Although not being evidence-based—yet—, these practices are innovative examples in the context of upcoming early dementia prevention.
The public health campaign was based on the in-creasing consensus about the public relevance of dementia prevention, such as the UK Blackfriars consensus [24], the G8 Dementia Summit of December 11, 2013 [25], and the recommendation of the Lancet Commission on Dementia Prevention, Intervention and Care to be “ambitious about dementia prevention” [10]. Recommendations were based on the well-validated LIBRA (LIfestyle for BRAin Health)-index [11, 13, 26], which had been developed previously as part of the European In-MINDD project [27]. LIBRA consists of twelve modifiable dementia risk-factors which can be targeted by lifestyle interventions and prevention strategies in primary care: physical inactivity, smoking, alcohol use, cognitive activity, healthy diet, depression, hypertension, obesity, diabetes, hypercholesterolemia, coronary heart disease, and renal disease. The campaign targeted people aged 40–75 years and aimed at increasing their level of awareness on the relationship between the various lifestyle and medical factors and dementia. Secondary goals were to increase people’s motivation to change behavior by engaging in relevant lifestyle changes and seeking effective risk management and treatment. The public was targeted with the notion that lifestyle has an impact on brain health and dementia through mass media, social media, public displays of posters and leaflets, public lectures and newspaper articles and local TV programs. Posters put questions like “Does learning Spanish diminish the chance of dementia?”, “Does having a dog reduce the chance of dementia”, or “Does visiting a museum diminish the chance of dementia?” These materials had the intention to grab attention and direct people to the campaign website, where further information about lifestyle factors related to dementia, local events and a link to the eHealth platform could be found.
The eHealth platform included the app ‘MijnBreincoach’, which was developed to give people insight into their personal ‘room-for-improvement’ and individual target behaviors (based on the LIBRA-index). Registered users could choose a risk factor to work on for the next two weeks. They received a daily notification that contained a different content every day (e.g., a behavior challenge, fact, tip, quiz-question). An example of a behavioral challenge could be as follows: ‘Try to eat fatty fish on two occasions in the coming week. Do you accept this challenge?’ A tip for those who do accept the challenge then provides a recipe for a mackerel salad. The app was downloaded 13,000 times since the start of the campaign.
Already during the development phase, not only scientific questions arose, but also ethical issues came to the fore. Concerns about the possible implications of the campaign slogan were exemplary in this regard. Given that the campaign aimed at changing individual behaviors, it was first considered to call it “I am the medicine myself’. However, this emphasis on ‘I’ could also trigger undue responsibilization and ‘blaming the victim’, as if one is personally responsible for either or not developing dementia. However, that would be a highly undesirable situation. In this sense, a recent open letter signed by 67 health professionals from the Netherlands [28] explicitly stated that public health measures on dementia prevention should not lead to a situation in which people with dementia get the impression ‘that it was their own fault’ they contracted the condition. Unfortunately, until today, it is still rather unclear how one can get the one (prevention) without risking the other (blaming patients). Considering this danger and aiming at a reasonable balance, the campaign leaders chose for the plural terminology in ‘we are the medicine ourselves’ (emphasis added) in order to stress the role of social determinants of health operating at the wider societal and political level, next to individual risk behavior. That slogan and the campaign material were also tested within the Alzheimer Centre Limburg’s client panel consisting of various stakeholders in the domain (including people with dementia and their caregivers). They unanimously agreed to move forward with the campaign, using this slogan.
OVERVIEW OF THE ETHICAL LITERATURE
Obviously, within dementia research and clinical dementia care, ethical discussions are common. However, by today ethical discourses seem to focus on other issues than primary prevention in general populations and via lifestyle changes. Prominent issues concern the clinical care for people with dementia, particularly when they are severely affected. This covers questions on how to uphold care that respects people’s dignity, the problem of mental incapacity in following up the person’s wishes, but also issues regarding end-of-life care and euthanasia [29–33]. Ethical discussions have also emerged on the concept of ‘prodromal dementia’ or ‘asymptomatic dementia’, arguing that genetic and other biomarker-based screening of cognitively healthy people is ethically questionable, as is the disclosure of scientific research findings to participants [18, 20, 34, 35]. Finally, ethical research has targeted people with mild cognitive impairment (MCI) and early symptoms of dementia. Discussions cover disclosure of early diagnosis, pointing toward the problem of the medicalization of typical aging, the current therapeutic gap, the danger of increasing despair, the possibly widespread off-label medication usage, the often only moderate to low predictive value of initial findings, and finally the delicate balance of the right to know with the wish not to know [19, 21, 36].
In general, biological and biomarker-based res-earch and efforts that include individual testing have rather easily triggered ethical attention. In contrast, approaches that focus on social, psychological and personal aspects or that target society at large seem to be hardly discussed. A scooping review with the terms ‘prevention’ AND ‘dementia’ AND ‘ethic*’ in the title or abstract of articles in the database PubMed conducted on 4th December 2019 confirms this impression.
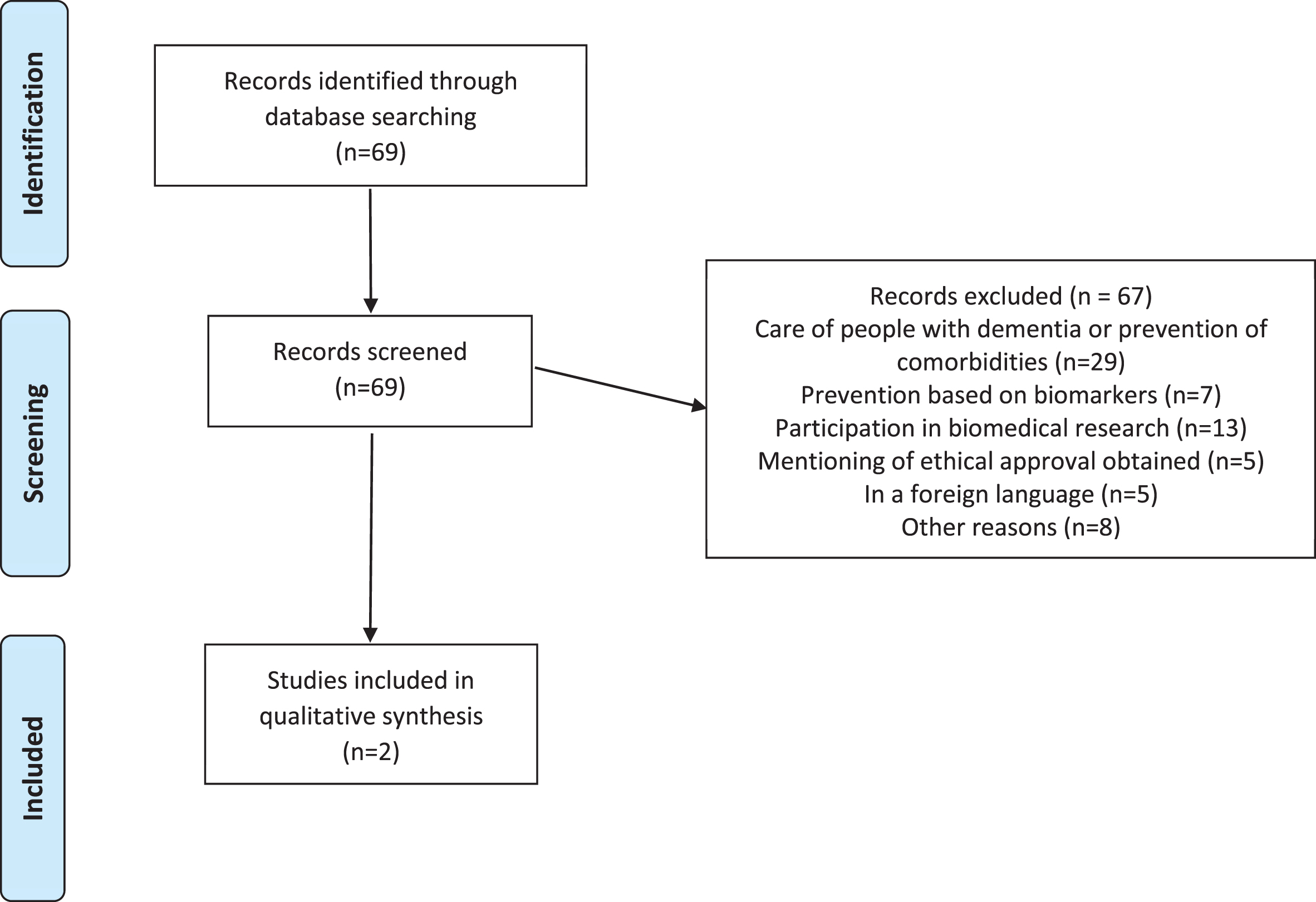
It returned a set of 69 initial hits, from which, however, most articles had to be excluded because they did not cover ethical issues in primary dementia prevention aiming at social and psychological factors. Selection was based on abstracts. 29 articles discussed the care of people with dementia or the prevention of any comorbidities in dementia, 7 articles discussed prevention in the context of biomarkers and early testing, and 13 articles discussed the involvement of people with dementia in biomedical research. Another 5 articles only mentioned “ethical approval” without discussing ethical issues themselves, 5 articles were written in a language other than English, German, or Dutch and finally, 8 articles were excluded for other reason (see Fig. 1). So in the end, only two articles [37, 38] discussed ethical issues in the context of the primary prevention of dementia in general populations and independent of personal risk-status.
Fig. 1
Review of the ethics of primary prevention of dementia.
Lawless M, Augoustinos M, and LeCouteur A (2017)
Lawless et al. [37] studied the content of publicly available websites on health communication and promotion with special emphasis on the prevention of dementia as a potential public health priority. Their main interest was to understand how neutral or normatively steering the presented information actually was. The initial concern was that such public material could be used to not merely increase public awareness about the preventability of dementia, but also to put increasing one-sided emphasis on personal responsibility to actually engage in certain kinds of behavior. This seems desirable from the perspective of behavioral change. However, it might imply or lead to a “repressive ethic of risk management and social control” (p. 1540), particularly if presented solely as an individual responsibility rather than a social or public issue, or as a definite health priority rather than an option people are free to engage in. In the end, this is largely what the authors argue to indeed have found in expressions used on these websites, for example when portraying the potential causes of dementia and the possibilities to contribute to or engage in relevant lifestyle changes. It is mainly individuals who are addressed and who get reminded directly of their personal possibilities to ensure healthy aging and to uphold cognitive abilities. Lawless et al. point out why such an attitude is problematic. They argue that, first of all, prevention of dementia is not that straightforward and that the predictive validity of lifestyle risks is low. Therefore, a one-sided focus on individual responsibilities to change behavior could lead to a situation in which collective and public efforts that enable or facilitate healthier lifestyles could get diminished for the sake of individualized approaches. In addition, stigmatization of those already living with the disease might increase, because apparently they had behaved irresponsibly while still being young and healthy. So in line with our own discussion on the campaign ‘We are the medicine ourselves’; Lawless et al. support the idea that it is not findings and ambitions alone that are important, but that the way they get presented in public spaces, the language invoked to shape these presentations, and ultimately the freedom to follow recommendations have a huge impact on their social and ethical implications.
Whitehouse (2019)
In the second paper, Whitehouse [38] critically discusses public health aspects of dementia. He is particularly concerned about the narrow focus of actual and possible dementia prevention campaigns. According to this author, prevention of dementia should not be seen independently from the prevention of other medical conditions, and issues regarding sustaining quality of life in aging population should not be considered independently from the quality of life of other groups in society, like for example young families. Upcoming primary dementia prevention efforts, however, run the danger to become single-issue campaigns, focusing on one specific final goal and one part of the population only. Looking from an overall perspective, however, he is concerned that such campaigns might contribute to a situation where different groups in society get more and more isolated from each other and start to compete for just their piece of cake from limited public resources. This is not merely an unfavorable situation, but more seriously, potential synergistic effects are overlooked or even undone if for each social group or each medical condition separate research projects and prevention campaigns are being set-up. In this sense, he discusses a series of examples that could be very conducive for a goal-directed dementia prevention campaign, but just as well in several other regards. For example, to arrange for safe public green spaces can support that elderly people continue to walk and move regularly, but will likewise increase the well-being of children and young families. Invoking the example of art and music, Whitehouse asks almost rhetorically: “How much evidence do we need that people - with dementia - are stimulated by such activities just as the rest of us?” (p. 103, emphasis added). Instead of disease- or group-specific prevention campaigns, Whitehouse pleads for general supportive social infrastructures, because they are likely to have similar health effects, but do not risk to pull people apart or let them compete with one another for public resources.
Similar to Lawless et al., Whitehouse raises concerns that a focus on brain-health or brain-fitness in the context of dementia prevention might implicate a focus on single individuals with specific conditions. To avoid this, he advocates that targeting quality of life more generally and for everybody contributes to dementia prevention while also increasing health of the population in general. Generally supportive social infrastructures might trigger healthy behaviors more effectively than interventions aimed at specific subgroups of individuals, because actual behavior changes are much harder to achieve individually than in societies that facilitate healthy living conditions for all, so their argument. Renewed attention to the significance of social arrangements appears as the first outcome of the emerging ethical debate on primary dementia prevention.
SKETCHING AN ETHICAL RESEARCH AGENDA
Early diagnosis of dementia based on biomarker testing as well as attempts to identify individuals at risk via biomedical screenings have immediately attracted critical ethical attention. Concerns have been uttered regarding, among others, low predictive validity, a wide therapeutic gap, the potential to increase stigmatization, and an ever-higher emphasis on individual rather than social responsibility [19–21]. Even though ethical reflection is still sparse, our review indicates that public health approaches to dementia prevention and interventions that target social, psychological, behavioral, and medical factors in larger populations, might raise similar ethical questions and concerns. This indicates that the current ‘bio-exceptionalism’ in the ethical debate is unfounded. That is, it is inappropriate that the ethical debate almost exclusively focuses on biomedical developments, but largely ignores wider social and public measures with similar main aims. Therefore, independent of their disciplinary origin, all attempts to prevent dementia deserve ethical attention. Put differently, to date, it seems unclear how the ambitious aims of maximum dementia prevention could be achieved [9, 10], while ‘a repressive ethics of social control’ [37], or an exaggerated individual responsibilization and narrowing down on issues relevant for old age [38] is avoided. Therefore, scientific work on primary dementia prevention and brain-health will benefit from ethical reflection in corresponding add-on or sub-studies as they recently have been advocated for in other highly debated areas of neuro-intervention [39, 40]. As a start, an ethical research agenda of primary dementia prevention is to be set.
First, a failure to apply and translate upcoming insights on dementia prevention might also be a significant moral failure, because without installing what might be helpful, we allow the potentially avoidable harm and suffering of people with dementia and their families. That is, as soon as social, psychological, and behavioral, or medical risk-factors allow for the development of goal-directed preventive interventions, we must consider also the downsides of not making use of this upcoming knowledge. An early review from the US Agency of Healthcare Research and Quality and the National Institute on Aging indeed concluded that “the overall quality of evidence was low” and “the current literature does not provide adequate evidence to make recommendations for interventions” [41] (our emphasis). This position to only accept new findings on causal factors for dementia and to rely solely on the outcome of large scale randomized controlled trials (RCTs) to recommend public health interventions, however, has also come under pressure. It forces one almost by definition to forgo certain kinds of knowledge and as a consequence the potential benefits resulting from it. Regarding primary dementia prevention, such studies, however, are hardly feasible in their most pure form as they required “to study 10.000 subjects over 40 years randomly assigned to groups of low and high saturated fat in the diet, head injury, and high or low levels of mental activity, physical activity, inactivity, as well as smoking and non-smoking” [42]. A methodologically rigorous position on medical and lifestyle risk factors for dementia runs the danger that upcoming knowledge with some, but not a definite, degree of certainty, gets silenced and that a blind eye is turned on possible, albeit not guaranteed, benefits of preventive interventions. More recent reports indeed tend to take these considerations into account, to depart from an over harshly RCT-centrism and to acknowledge the value of conditional evidence, for example longitudinal or observational studies [43, 44]. In this sense, efforts are also undertaken to consider qualitative and quantitative evidence, to combine different studies with various strengths and weaknesses and to achieve a good promise of evidence by triangulation [11, 45]. This basically epistemic question on what counts as knowledge, becomes also of crucial ethical importance as it steers the debate on which scientific findings justify the implementation of preventive interventions [46] and allow for corresponding health gains.
A second issue concerns the relationship of primary dementia prevention with other health-oriented prevention in a given society [47]. Particularly when it comes to implementation or up-scaling, tensions might arise between what is required from a science point of view and what is desirable or feasible in real-world circumstances, which are often characterized by an already complex prevention landscape. On the one hand, adding primary dementia prevention advice might allow for particular synergy effects. In this sense, Whitehouse [38] pointed out how safe public spaces or music classes are beneficial for various purposes and groups of the population, among which brain-health in middle-aged and elderly people. In addition, much lifestyle advice in primary dementia prevention seems to conform to that regarding other health issues like the prevention of cardiovascular disease: “What is good for your heart is also good for your brain”. This holds for example for the advice to move regularly and to eat low saturated fat. So many ways to prevent or delay onset of dementia is well in line with other health recommendations. In this way, people who are hard to motivate to engage for reasons of cardiovascular health might change their mind if they could positively influence their brain-health by the same means and measures as well and potentially avoid a condition desperately feared by many.
This situation, however, can also raise questions about apt priorities. As discussed before, subsuming dementia prevention advice among general public health campaigns might avoid a series of negative side effects. However, it could also dilute the message of the preventability of dementia and undermine collateral positive effects for other health conditions (“what’s good for your brain is good for your heart”). Moreover, different health advice is not necessarily in line with each other and tensions can arise between the advice given by two different health prevention initiatives. Most strikingly, recent advice to stay home and distance socially to prevent infection with COVID-19 does not mesh with emphatic pleads for social, cognitive, and physical activity to reduce risks for common non-communicable diseases including dementia. By today, a plethora of health campaigns exists providing people with abundant and sometimes contradictory advice on what to do and leave for a healthy living. Overall, this can result in a situation where people abandon health advice altogether and achieve even worse outcomes [48]. In addition, certain health advice challenges other values people hold in life. For example, the wish for a sustainable lifestyle, where one does not support overfishing of the ocean can be in conflict with the advice to eat fatty fish twice a week. In this sense, a healthy diet is not necessarily an environmentally sustainable diet, requesting people to make decisions between health and non-health related sets of values [49].
Against this background, primary dementia prevention can never be solely an issue of identifying causal mechanisms and defining effective interventions. It will always raise wider ethical and social questions about which health and non-health topics to prioritize when, and why. Also, it will require researchers and campaigners alike to justify the position of potential benefits of dementia prevention in relation to other values in life and to various way to stay healthy.
Finally, primary dementia prevention is special in the way it concerns individuals. It aims at comparatively young people who are still brain-healthy and requests a rather extensive adaption of a certain lifestyle for a long period; that is, for decades rather than for months or years. Therefore, people will not see results, if any, for a very long time, even if they closely live up to the advice of primary prevention dementia. In a positive sense, this might contribute to growing awareness of dementia overall and it might give people a correspondingly long-term sense of control and an enduring feeling of empowerment to fight a serious condition. However, this particularly long-term engagement and a focus on goals very late in life might also foster a change in people’s personal identity. That is, rather young and healthy people might develop a proto-dementia identity and come to perceive themselves as the could-be-dementia-patient of the future. This might unduly medicalize the lives of large parts of society and mental health consumers are created long before there is an actual mental health need and in situations where such a need may never develop [50]. Along with such potential proto-identities, the long-term perspective and the—intended—invisibility of successes might also enforce iatrogenic effects of despair and hopelessness. This might result exactly because no positive impact is seen at all and insecurity remains even in the face of great dedication. Such feelings, however, could reduce people’s well-being on the short-term, and given the devastating effects of enduring mental stress be counterproductive in the long-term. Still another point concerns the question who is likely and able to incorporate lifestyle advice in their daily lives and who is not. In so far as advice better fits some social classes than others or if certain groups of people are more likely, or maybe also more economically pressured to set other priorities in life, large scale prevention campaigns run the danger to further increase health disparities and social injustices. This might hold even more so, in case intervention tools or (e-)health platforms are getting commercialized and accessible only upon payment. While this might create a financially sustainable basis for further explorations, it will increase selective uptake and draw differences between those who can and cannot afford participation [47]. In this sense, the ethical and social implications of primary dementia prevention advice are not to be set solely in a theoretical way. Much will depend on how interventions actually get implemented, how they are integrated with other health and non-health related values, and on how people actually perceive and deal with public dementia prevention advice in their personal and daily lives. However, to date this ‘social life’ of dementia prevention is hardly known. The best way to explore it and get a grip on the ethical and social implications of primary dementia prevention is to carry out detailed empirical ethical research and to conduct stakeholder analyses of all groups of individuals potentially affected. That is, covering not only people with dementia and their caregivers, but in particular also those who are rather young, brain-healthy and typically busy with other matters in life. Such pro-active ethical research is needed to inform ongoing scientific research and upcoming policy-making to ensure that the mentioned benefits can be achieved while people are also protected from foreseeable harm and counterproductive effects.
CONCLUSION
Early prevention of dementia has gained increasing scientific interest over the past decade and more and more emphasis is put on the potential to intervene in the general population, from midlife ages onwards and with lifestyle and medical treatments. With an increasing frequency, local public health campaigns that aim to increase awareness on the preventability of dementia are being set-up and health-apps get released that intend to support individuals to change their behavior. While the main aim to reduce the incidence of dementia is hardly controversial, the route toward that end can evoke a series of ethical questions and concerns. The current article has sketched these questions and pointed out several urgent dilemmas that concern the implementation of dementia prevention in a real-world setting. It has also pointed toward problems that occur when these findings are withheld from general populations. While explicit ethical literature to this avail is very sparse at the moment, some trends are emerging. First of all, it will be important to balance the possible benefit of increased well-being and the possibility that more people will become able to lead healthier lives when growing old, with the burdens of being engaged for decades in interventions while benefits only pay out in the long run, if ever. Second, gaining knowledge about one’s personal options to engage in brain health might not only empower people and provide them with an internal locus of control and sense of self-efficacy; it might also undermine autonomy and put high social pressure on people to engage in certain science-supported behaviors and not decide in favor of other lifestyles or priorities in life. In addition, stigmatization of those who still come to develop dementia is also a danger that might increase when more emphasis is put on prevention. Third, there are also issues of justice and how dementia prevention efforts might change general public health investments, potentially worsening the situation of some groups of the population or at least contributing to a society in which different groups and different public health aims are played off against each other. At the same time, good risk management might also lead to a situation where dementia prevention supports synergy effects with other public health and non-health aims because of shared goals. How such synergy effects can be achieved while competition between prevention aims can be avoided will be one of the challenges of the time to come.
In any case, while currently early dementia prevention is winning ground, it is of great importance to think through its ethical and social implications and ensure that research, campaigns and interventions are set up in a way that are also desirable from an ethical point of view as well as socially sustainable. We hope that our contribution will be the start of this debate on what it means to do ethically good primary dementia prevention and how to achieve that in real world settings.
ACKNOWLEDGMENTS
The study has been carried out without external funding.
Authors’ disclosures available online https://www.j-alz.com/manuscript-disclosures/20-1104r1.
REFERENCES
[1] | Alzheimer’s Disease International 2019 World Alzheimer Report 2019: Attitudes to dementia. Alzheimer’s Disease International, London. |
[2] | Berr C , Wancata J , Ritchie K ((2005) ) Prevalence of dementia in the elderly in Europe. Eur Neuropsychopharmacol 15: , 463–471. |
[3] | Roberts JS , Connell CM ((2000) ) Illness representations among first-degree relatives of people with Alzheimer disease. Alzheimer Dis Assoc Disord 14: , 129–136. |
[4] | de Vugt ME , Verhey FRJ ((2013) ) The impact of early dementia diagnosis and intervention on informal caregivers. Prog Neurobiol 110: , 54–62. |
[5] | McCurry SM When a family member has dementia: Steps to becoming a resilient caregiver Praeger, Westport, CO. |
[6] | Oken BS , Fonareva I , Wahbeh H ((2011) ) Stress-related cognitive dysfunction in dementia caregivers. J Geriatr Psychiatry Neurol 24: , 191–198. |
[7] | Alzheimer’s Disease International 2018 World Alzheimer Report 2018. The state of the art of dementia research. Alzheimer’s Disease International, London. |
[8] | Makin S ((2018) ) The amyloid hypothesis on trial. Nature 559: , S4–S7. |
[9] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchi K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[10] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[11] | Deckers K , van Boxtel MPJ , Schiepers OJG , de Vugt M , Muñoz Sánchez JL , Anstey KJ , Brayne C , Dartigues J-F , Engedal K , Kivipelto M , Ritchie K , Starr JM , Yaffe K , Irving K , Verhey FRJ , Köhler S ((2015) ) Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. J Geriatr Psychiatry Neurol 30: , 234–246. |
[12] | Anstey KJ , Peters R ((2019) ) Dementia, risk, risk reduction, and translation into practice: An international research network for dementia prevention (IRNDP) special issue. J Alzheimers Dis 70: , s1–s3. |
[13] | Deckers K , Nooyens A , van Boxtel M , Verhey FRJ , Verschuren M , Köhler S ((2019) ) Gender and educational differences in the association between lifestyle and cognitive decline over 10 years: The Doetinchem cohort study. J Alzheimers Dis 70: , s31–s41. |
[14] | Centres for Disease Control and Prevention, Accelerating Risk Reduction and Promoting Brain Health, https://www.cdc.gov/aging/healthybrain/issue-maps/risk-reduction.html. |
[15] | National Health Services, NHS Health Check Programme Introducing the Dementia Component Increasing Awareness & Signposting E-learning resource for practitioners, http://www.healthcheck.nhs.uk/increasing-dementia-awareness-training-resource/#1, |
[16] | Prodemos, Prevention of dementia using mobile phone applications, trail registration http://www.isrctn.com/ISRCTN15986016. |
[17] | Public Health England, Health matters: Midlife approaches to reduce dementia risk, https://www.gov.uk/government/publications/health-matters-midlife-approaches-to-reduce-dementia-risk/health-matters-midlife-approaches-to-reduce-dementia-risk. |
[18] | Bunnik EM , Richard E , Milne R , Schermer MHN ((2018) ) On the personal utility of Alzheimer’s disease-related biomarker testing in the research context. J Med Ethics 44: , 830–834. |
[19] | Mattsson N , Brax D , Zetterberg H ((2010) ) To know or not to know: Ethical issues related to early diagnosis of Alzheimer’s disease. Int J Alzheimers Dis 2010: , 841941. |
[20] | Stites SD ((2018) ) Cognitively healthy individuals want to know their risk for Alzheimer’s Disease: What should we do? J Alzheimers Dis 62: , 499–502. |
[21] | Vanderschaeghe G , Dierickx K , Vandenberghe R ((2018) ) Review of the ethical issues of a biomarker-based diagnoses in the early stage of Alzheimer’s disease. J Bioeth Inq 15: , 219–230. |
[22] | Wright CF , Hall A , Matthews FE , Brayne C ((2009) ) Biomarkers, dementia, and public health. Ann N Y Acad Sci 1180: , 11–19. |
[23] | Heger I , Deckers K , van Boxtel M , de Vugt M , Hajema K , Verhey F , Köhler S ((2019) ) Dementia awareness and risk perception in middle-aged and older individuals: Baseline results of the MijnBreincoach survey on the association between lifestyle and brain health.. BMC Public Health 19: , 678. |
[24] | Lincoln P , Fenton K , Alessi C , Prince M , Brayne C , Wortmann M , Patel K , Deanfield J , Mwatsama M ((2014) ) The Blackfriars Consensus on brain health and dementia. Lancet 383: , 1805–1806. |
[25] | UK Government, G8 dementia summit: Global action against demention; Discussion 2: Preventing and delaying dementia https://www.gov.uk/government/publications/g8-dementia-summit-global-action-against-dementia/g8-dementia-summit-global-action-against-dementia-11-december-2013#discussion-2–preventing-and-delaying-dementia. |
[26] | Deckers K , Cadar D , van Boxtel MPJ , Verhey FRJ , Steptoe A , Köhler S ((2019) ) Modifiable risk factors explain socioeconomic inequalities in dementia risk: Evidence from a population-based prospective cohort study. J Alzheimers Dis 71: , 549–557. |
[27] | O’Donnell CA , Browne S , Pierce M , McConnachie A , Deckers K , van Boxtel MPJ , Manera V , Köhler S , Redmond M , Verhey FRJ , van den Akker M , Power K , Irving K , In MT ((2015) ) Reducing dementia risk by targeting modifiable risk factors in mid-life: Study protocol for the Innovative Midlife Intervention for Dementia Deterrence (In-MINDD) randomised controlled feasibility trial. Pilot Feasibility Stud 1: , 40. |
[28] | van’t Land K, Olde Rikkert M, van Winkelhof M, Richard E, Gerjoke W, Scheltens P, Lahuis B, Biessels G-J, Scherder E, Wenselaar R, & 67 care professionals (21st October2019) in NRC Handelsblad. |
[29] | Alzheimer Europe (2014), Ethical dilemmas faced by carers and people with dementia, Alzheimer Europe Luxembourg. |
[30] | Gauthier S , Leuzy A , Racine E , Rosa-Neto P ((2013) ) Diagnosis and management of Alzheimer’s disease: Past, present and future ethical issues. Prog Neurobiol 110: , 102–113. |
[31] | Howe EG ((2006) ) Ethical issues in diagnosing and treating Alzheimer disease. Psychiatry (Edgmont) 3: , 43–53. |
[32] | Johnson RA , Karlawish J ((2015) ) A review of ethical issues in dementia. Int Psychogeriatr 27: , 1635–1647. |
[33] | Lavazza A , Reichlin M ((2018) ) Of meatballs, autonomy, and human dignity: Neuroethics and the boundaries of decision making among persons with dementia. AJOB Neurosci 9: , 88–95. |
[34] | Bemelmans SASA , Tromp K , Bunnik EM , Milne RJ , Badger S , Brayne C , Schermer MH , Richard E ((2016) ) Psychological, behavioral and social effects of disclosing Alzheimer’s disease biomarkers to research participants: A systematic review. Alzheimers Res Ther 8: , 46. |
[35] | Milne R , Bunnik E , Tromp K , Bemelmans S , Badger S , Gove D , Maman M , Schermer M , Truyen L , Brayne C , Richard E ((2017) ) Ethical issues in the development of readiness cohorts in Alzheimer’s disease research. J Prev Alzheimers Dis 4: , 125–131. |
[36] | Lowenthal J , Chandros S , Pearson SD ((2012) ) The ethics of early evidence - preparing for a possible breakthrough in Alzheimer’s disease. N Engl J Med 367: , 488–490. |
[37] | Lawless M , Augoustinos M , LeCouteur A ((2017) ) “Your Brain Matters”: Issues of risk and responsibility in online dementia prevention information. Qual Health Res 28: , 1539–1551. |
[38] | Whitehouse PJ ((2019) ) Ethical issues in early diagnosis and prevention of Alzheimer disease. Dialogues Clin Neurosci 21: , 101–108. |
[39] | Nuttin B , Wu H , Mayberg H , Hariz M , Gabriëls L , Galert T , Merkel R , Kubu C , Vilela-Filho O , Matthews K , Taira T , Lozano AM , Schechtmann G , Doshi P , Broggi G , Régis J , Alkhani A , Sun B , Eljamel S , Schulder M , Kaplitt M , Eskandar E , Rezai A , Krauss JK , Hilven P , Schuurman R , Ruiz P , Chang JW , Cosyns P , Lipsman N , Voges J , Cosgrove R , Li Y , Schlaepfer T ((2014) ) Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry 85: , 1003. |
[40] | Park RJ , Singh I , Pike AC , Tan JOA ((2017) ) Deep brain stimulation in anorexia nervosa: Hope for the hopeless or exploitation of the vulnerable? The Oxford Neuroethics Gold Standard Framework. Front Psychiatry 8: , 44. |
[41] | Plassman BL , Williams JW Jr. Burke JR , Holsinger T , Benjamin S ((2010) ) Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 153: , 182–193. |
[42] | Friedland RP , Nandi S ((2013) ) A modest proposal for a longitudinal study of dementia prevention (with apologies to Jonathan Swift, 1729) J Alzheimers Dis 33: , 313–315. |
[43] | National Academies of Sciences E, Medicine, (2017) Preventing Cognitive Decline and Dementia: A Way Forward, Downey A, Stroud C, Landis S, Leshner AI, eds. National Academies Press, Washington (DC). |
[44] | World Health Organization (2019) Risk reduction of cognitive decline and dementia: WHO guidelines, World Health Organization. |
[45] | Glymour MM , Whitmer RA ((2019) ) Using cross-cultural studies to improve evidence on dementia prevention: Lessons from the special issue sponsored by the International Research Network on Dementia Prevention (IRNDP). Int J Alzheimers Dis 70: , S5–S10. |
[46] | Fricker M ((2007) ), Epistemic injustice: Power and the ethics of knowing, Oxford University Press, Oxford, UK. |
[47] | Leadbeater BJ , Dishion T , Sandler I , Bradshaw CP , Dodge K , Gottfredson D , Graham PW , Lindstrom Johnson S , Maldonado-Molina MM , Mauricio AM , Smith EP ((2018) ) Ethical challenges in promoting the implementation of preventive interventions: Report of the SPR Task Force. Prev Sci 19: , 853–865. |
[48] | Nagler RH ((2014) ) Adverse outcomes associated with media exposure to contradictory nutrition messages. J Health Commun 19: , 24–40. |
[49] | Macdiarmid JI ((2013) ) Is a healthy diet an environmentally sustainable diet? Proc Nutr Soc 72: , 13–20. |
[50] | Verweij M ((1999) ) Medicalization as a moral problem for preventive medicine. Bioethics 13: , 89–113. |




