Death Rate Due to COVID-19 in Alzheimer’s Disease and Frontotemporal Dementia
Abstract
We aimed to evaluate the frequency and mortality of COVID-19 in patients with Alzheimer’s disease (AD) and frontotemporal dementia (FTD). We conducted an observational case series. We enrolled 204 patients, 15.2% of whom were diagnosed with COVID-19, and 41.9% of patients with the infection died. Patients with AD were older than patients with FTD (80.36±8.77 versus 72.00±8.35 years old) and had a higher prevalence of arterial hypertension (55.8% versus 26.3%). COVID-19 occurred in 7.3% of patients living at home, but 72.0% of those living at care homes. Living in care facilities and diagnosis of AD were independently associated with a higher probability of death. We found that living in care homes is the most relevant factor for an increased risk of COVID-19 infection and death, with AD patients exhibiting a higher risk than those with FTD.
INTRODUCTION
According to recent studies, the presence of dementia is a major risk factor for Coronavirus Disease-19 (COVID-19) severity [1]. At the same time, the risk of exposure to the infection seems to be higher in patients with dementia [2]. However, to our knowledge, previous studies have not evaluated the influence of the specific diseases causing dementia in the frequency and impact of COVID-19. Patients with different neurodegenerative disorders exhibit distinct clinical features and clinical course that may modify either the risk or the severity of COVID-19. On the one hand, patients with frontotemporal dementia (FTD) show behavioral disorders and may have more difficulties to follow the preventative measures [3]. On the other hand, the APOE ɛ4 genotype, a risk factor for Alzheimer’s disease (AD), could be associated with an increased COVID-19 severity, according to a recent and preliminary investigation [4].
In this study, we aimed to evaluate the frequency and mortality of COVID-19 in a case series of patients with AD and FTD.
METHODS
Study design
This is an observational case series study evaluating the frequency and mortality of COVID-19 among patients with a previous diagnosis of AD and FTD. All patients were under follow-up in a consultation of behavioral neurology of a tertiary hospital in Madrid, Spain. All patients were contacted by phone from May 15 to June 12, 2020 by the neurologist responsible for the care of these patients [5]. All patients with a previous appointment in the specified period and meeting diagnosis of AD and FTD were included in the study. A semi-structured interview was conducted to the principal caregivers and included the following items: diagnosis of COVID-19 by reverse transcription polymerase chain reaction (RT-PCR) or serological tests; symptoms of COVID-19; the need for hospital admission (at any level of hospitalization, except the transient assistance in the emergency room) and death; and place of residence. Data and previous dementia diagnosis were obtained, and the information available from the hospital’s electronic database was also used to confirm the information reported by patients and caregivers. The diagnosis of the cause of dementia was performed according to the clinical diagnostic criteria [6, 7], with support of 18F-FDG-PET and/or cerebrospinal fluid biomarkers in all cases of FTD and non-amnestic AD, and when considered necessary by the clinician in amnestic AD (e.g., differential diagnosis with other entities, early onset, or doubts about clinical progression in early stages before the development of dementia). Patients were classified according to the clinical stage of dementia using the Clinical Dementia Rating into very mild, mild, moderate, and severe dementia [8]. At the time of the closure of the study, the total number of patients with a diagnosis of COVID-19 in the Region of Madrid was 70,998, with 14,972 deaths.
Only patients that had a previously scheduled appointment in our department within the period comprising from May 11 to June 19, 2020 were included in the study. The hospital’s Ethics Committee approved the research protocol, and patients or caregivers gave oral informed consent.
Statistical analysis
Statistical analysis was performed using SPSS Statistics version 20.0. Descriptive data are shown as frequency (percentage) or mean. Chi-squared test or Fisher test was used to compare qualitative data between groups. A two-sample t-test was used to compare means after the normal distribution of the variables was checked according to the Kolmogorov-Smirnov test. A binary logistic regression analysis (enter method) was performed to identify clinical and demographic characteristics independently associated with COVID-19 infection and death. Significant variables in univariate analysis were introduced in the regression model. A two-tailed p-value <0.05 was considered statistically significant.
RESULTS
Sample description
We included 204 patients, 147 (72.1%) with AD and 57 (27.9%) with FTD. The mean age was 78.02±9.42 years old, and 119 (58.3%) were females. According to the clinical stage, 34 (16.7%) patients were classified as very mild, 57 (27.9%) mild, 58 (28.4%) moderate, and 55 (27.0%) as severe dementia. Patients with AD debuted as amnestic AD in 117 (79.5%) cases, logopenic aphasia in 26 (17.6%), and posterior cortical atrophy in 4 (2.7%). Regarding FTD, the phenotype at onset was the behavioral variant in 19 (33.3%) cases, non-fluent primary progressive aphasia in 26 (45.6%), and semantic dementia in 12 (21.0%).
Frequency and mortality of COVID-19
Thirty-one patients (15.2%) were diagnosed with COVID-19. According to the diagnosis, 22 (15.0%) of patients with AD and 9 (15.8%) with FTD (χ2 = 0.022, p = 0.883) presented COVID-19. Eight patients with AD (36.4%) and 2 (22.2%) with FTD (χ2 = 0.585, p = 0.677) were transferred to the hospital.
One hundred and seventy-nine (87.7%) patients lived at home and 25 (12.3%) in care homes. The frequency of COVID-19 was 13 (7.3%) in patients living at home, and 18 (72.0%) in care homes (χ2 = 71.33 p < 0.001).
Total deaths amount to 14. Of those, 13 patients died of COVID-19; that is 6.9% of all patients, and 41.9% of COVID-19 positive patients. The majority of deaths (12, 92.3%) in COVID-19 positive patients were due to respiratory complications and one case passed away due to an intracranial hemorrhage two weeks after the onset of symptoms. In the 204 patients included in the study, death was associated with older age (83.92±6.76 versus 77.59±9.48, t = 2.77, p = 0.015) and with an advanced clinical dementia stage (χ2 = 8.58, p = 0.035). Furthermore, living at care homes was identified as a significant factor associated with death. Ten out of 25 (40%) patients living in care homes died versus 4 out of 179 (2.2%) patients living at home; χ2 = 48.94, p < 0.001) (Table 1).
Table 1
Main characteristics and results of the sample. Comparison between AD and FTD
| Whole sample | AD | FTD | p | |
| (n = 204) | (n = 147) | (n = 57) | ||
| Age | 78.02±9.42 | 80.36±8.77 | 72.00±8.35 | <0.001 |
| Females | 119 (58.3%) | 87 (59.2%) | 32 (56.1%) | 0.692 |
| Clinical stage | ||||
| Very mild | 34 (16.7%) | 22 (15.0%) | 12 (21.1%) | 0.433 |
| Mild | 57 (27.9%) | 43 (29.3%) | 14 (24.6%) | |
| Moderate | 58 (28.4%) | 45 (30.6%) | 13 (22.8%) | |
| Advanced | 55 (27.0%) | 37 (25.2%) | 18 (31.6%) | |
| Living at home | 179 (87.7%) | 132 (89.8%) | 47 (82.5%) | 0.151 |
| Arterial hypertension | 97 (47.5%) | 82 (55.8%) | 15 (26.3%) | <0.001 |
| COVID-19 | 31 (15.2%) | 22 (15.0%) | 9 (15.8%) | 0.883 |
| Death | 14 (6.9%) | 13 (8.8%) | 1 (1.8%) | 0.072 |
In patients with COVID-19, death was also associated with older age (84.00±7.03 versus 75.00±10.00, t = 2.77, p = 0.009). Similarly, in patients with COVID-19, living at care homes was associated with death. Ten out of 18 patients (55.6%) living at care homes died versus 3 out of 13 (23.1%) living at home (χ2 = 3.70, p = 0.071).
We did not find statistically significant differences in the frequency of infection between patients with AD (22, 15.1%) and FTD (8, 14.8%) (χ2 = 0.002, p = 0.964). However, the diagnosis of AD was associated with death although it did not reach the statistical significance in the whole sample. Thirteen out of 147 (8.8%) cases with AD versus 1 out of 57 (1.8%) patients with FTD died (χ2 = 3.22, p = 0.119). Conversely, in patients with COVID-19 infection, 12 out of 22 (54.5%) patients with AD died, in comparison to 1 out of 9 (7.7%) of patients with FTD (χ2 = 4.94, p = 0.045).
Main characteristics of patients with COVID-19 infection are shown as Supplementary Material.
Logistic regression analysis
Place of residence (care homes) was independently associated with COVID-19 (R2 = 0.369, correct classification rate of 90.2%, p < 0.001). Furthermore, living in care homes, and diagnosis of AD were independently associated with a higher probability of death (R2 = 0.445, correct classification rate of 94.6%, p < 0.001). Age, arterial hypertension, and dementia stages were not independent predictors when these factors were introduced in the logistic regression model associated with place of living.
DISCUSSION
In this study, we aimed to evaluate COVID-19 infection in patients with AD and FTD. Our study confirms that care homes are high-risk settings for outbreaks of COVID-19, as has been recently suggested in a study showing a rapid spread of COVID-19 in a nursing home [9]. In this regard, in our study, living in care homes was independently associated with a higher rate of infection and death. The frequency of COVID-19 in patients living in care homes was 72%, which is clearly higher than patients living at home. Although cases officially diagnosed with COVID-19 in our region are around 1%, a recent epidemiological study suggested a prevalence of 11.3% (95% confidence interval 9.8–13.0%) [10], which is slightly lower than the prevalence of COVID-19 in our whole sample. The referred report [10] used serological tests to identify COVID-19 patients, which may also diagnose asymptomatic patients. Therefore, the frequency of COVID-19 in patients living at home in our study (7.3%) could be interpreted as comparable. This suggests that dementia itself may not be a risk factor for COVID-19 infection, but rather the place of residence. Although patients with AD and FTD may have increased difficulties to follow public health recommendations and protective measures, the mandatory lockdown decreed in our country and the precautions taken by their caregivers may have reduced this theoretical higher risk of infection. The high risk of death by COVID-19 in care homes is probably related to the fact that living in such organizations increases the likelihood of contracting the infection. However, some additional issues could also explain the higher mortality in patients living in care home. For instance, a rapid dissemination of the infection in care homes would potentially overwhelm medical assistance. And furthermore, repeat and constant exposure to circulating virus occurring in enclosed environments increases the viral load, which has, on its part, been associated with more severe COVID-19 and worse clinical outcomes [11, 12].
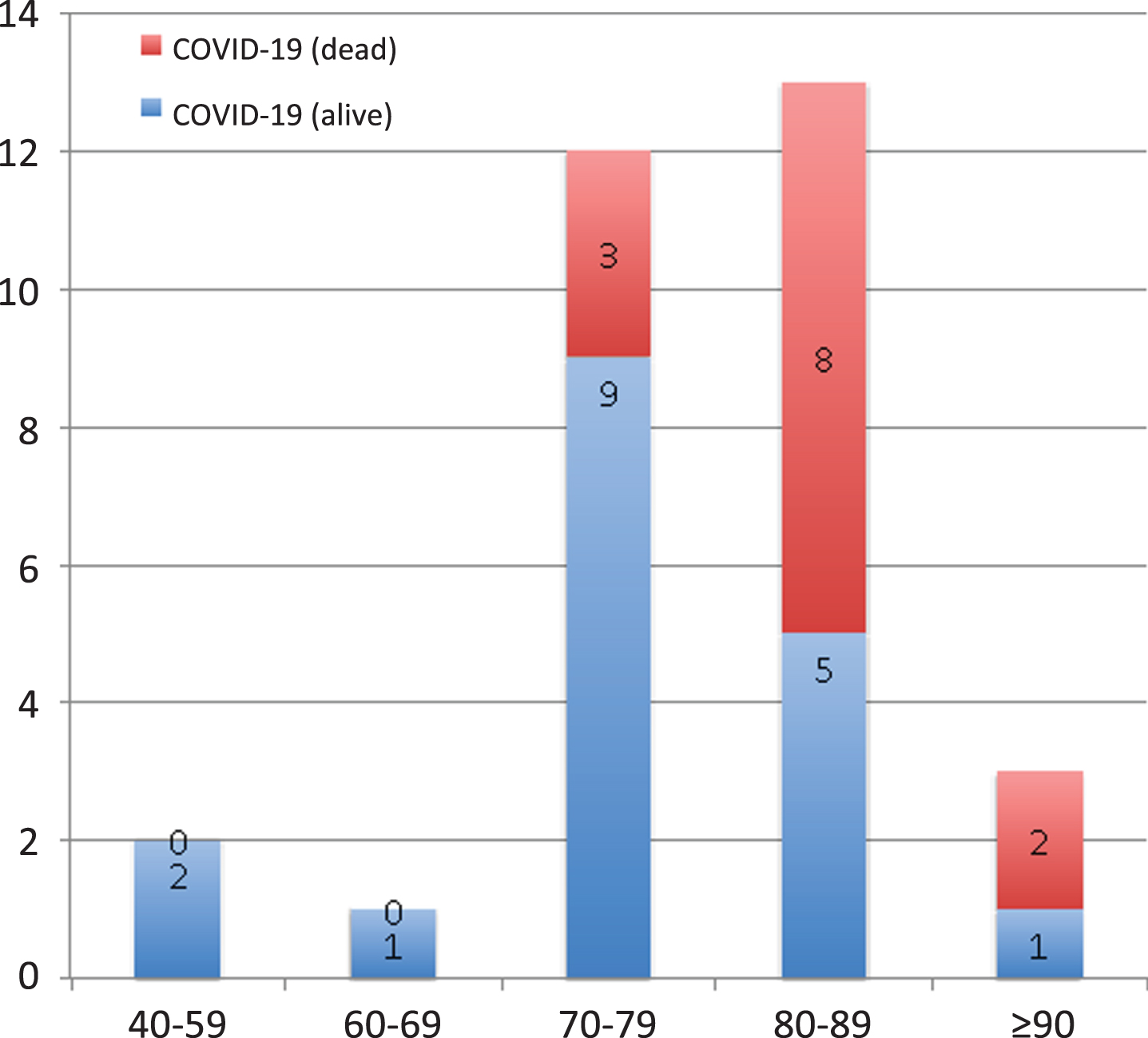
One of the most interesting aspects of our study is the inclusion of a large cohort of patients with FTD, including the behavioral variant, non-fluent progressive aphasia, and semantic dementia. To our knowledge, this is the first report of COVID-19 in patients with FTD and primary progressive aphasia. These patients did not show a higher frequency of COVID-19 in comparison to AD. Intriguingly, patients with FTD showed a lower risk of mortality associated with COVID-19. There were no differences in the stage of dementia or the percentage of admissions to the hospital between patients with AD or FTD. Thus, the lower risk of death in FTD patients with COVID-19 could be hypothetically explained due to the effect of age, which has been consistently associated with higher severity and was higher in patients with AD. In this regard, Fig. 1 shows the impact of age in mortality in our cohort. In addition, the impact of the specific neurodegenerative disorder due to certain genetic factors like APOE ɛ4, or comorbidities like arterial hypertension, which is more frequent in AD than FTD, could also be potential reasons for the difference observed. Regression analysis confirmed that living at care homes and AD were independently associated with death due to COVID-19, while other factors such as age or clinical stage were less significant. Nevertheless, considering that the number of cases included in our study with FTD and COVID-19 is limited, further research is necessary to confirm these findings regarding a potential lower severity of COVID-19 in patients with FTD. According to the current evidence, age and arterial hypertension are probably the best candidates to explain the differences in the severity of COVID-19 between AD and FTD. Unfortunately, we did not have the APOE genotyping in our cohort, which may have been useful to confirm the potential role of genetic factors in COVID-19 severity [4].
Fig. 1
Frequency (absolute) of patients with COVID-19 and outcome considering the different age ranges.
In conclusion, our study found that living in care homes is the most relevant factor associated with the risk of COVID-19 infection and death in patients with dementia. Patients with AD showed a higher risk of death in COVID-19 than patients with FTD. Differences in age and rate of arterial hypertension, two well-known risk factors for severity of COVID-19, may be influencing factors. Our findings warrant further studies to understand clinical and genetic characteristics associated with greater COVID-19 severity in patients with dementia. Identifying patients more susceptible to the effects of COVID-19 is crucial to adopt future strategies for the long-term care of these patients.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0940r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200940.
REFERENCES
[1] | Atkins JL , Masoli JAH , Delgado J , Pilling LC , Kuo CL , Kuchel GA , Melzer D ((2020) ) Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community cohort. J Gerontol A Biol Sci Med Sci, doi:10.1093/gerona/glaa183 |
[2] | Brown EE , Kumar S , Rajji TK , Pollock BG , Mulsant BH ((2020) ) Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry 28: , 712–721. |
[3] | Suzuki M , Hotta M , Nagase N , Yamamoto Y , Hirakawa N , Satake Y , Nagata Y , Suehiro T , Kanemoto H , Yoshiyama K , Mori E , Hashimoto M , Ikeda M ((2020) ) The behavioral pattern of patients with frontotemporal dementia during the COVID-19 pandemic. Int Psychogeriatr, doi:10.1017/S104161022000109X |
[4] | Kuo CL , Pilling LC , Atkins JL , Masoli JAH , Delgado J , Kuchel GA , Melzer D ((2020) ) ApoE e4e4 Genotype and Mortality With COVID-19 in UK Biobank. J Gerontol A Biol Sci Med Sci 75: , 1801–1803. |
[5] | Matías-Guiu J , Porta-Etessam J , Lopez-Valdes E , García-Morales I , Guerrero-Solá A , Matías-Guiu JA ((2020) ) Management of neurological care during the COVID-19 pandemic. Neurologia 35: , 233–237. |
[6] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[7] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EG , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini ML , Rosen H , Prioleau-Lathan CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[8] | Morris J ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[9] | McMichael TM , Currie DW , Clark S , Pogosjans S , Kay M , Schwartz NG , Lewis J , Baer J , Baer A , Kawakami V , Lukoff MD , Ferro J , Brostrom-Smith C , Rea TD , Sayre MR , Riedo FX , Russell D , Hiatt B , Montgomery P , Rao AK , Chow EJ , Tobolowsky F , Hughes MJ , Bardossy AC , Oakley LP , Jacobs JR , Stone ND , Reddy SC , Jernigan JA , Honein MA , Clark TA , Duchin JS , Public Health-Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team ((2020) ) Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med 382: , 2005–2011. |
[10] | https://www.mscbs.gob.es/ciudadanos/ene-covid/docs/ES_TUDIO_ENE-COVID19_SEGUNDA_RONDA_INFORME_PRELIMINAR.pdf. Accessed 14 June 2020. |
[11] | Chen D , Hu C , Su F , Song Q , Wang Z ((2020) ) Exposure to SARS-CoV-2 in a high transmission setting increases the risk of severe COVID-19 compared with exposure to a low transmission setting? J Travel Med 27: , taaa094. |
[12] | Little P , Read RC , Amlot R , Chdborn T , Rice C , Bostock J , Yardley L ((2020) ) Reducing risks from coronavirus transmission in the home-the role of viral load. BMJ 639: , m1728. |




