Sensory Cueing of Autobiographical Memories in Normal Aging and Alzheimer’s Disease: A Comparison Between Visual, Auditory, and Olfactory Information
Abstract
Background:
Alzheimer’s disease (AD) is a chronic, neurodegenerative disease resulting in a progressive decline of autobiographical memories (AMs) which favors the development of psycho-behavioral disorders. One of the most popular psychosocial interventions in dementia care, Reminiscence Therapy, commonly uses sensory cueing to stimulate AMs retrieval. However, few empirical studies have investigated the impact of sensory stimulation on AMs retrieval in AD.
Objective:
Our goal was to determine the most relevant cue for AMs retrieval in patients with early to mild AD when comparing odors, sounds and pictures.
Methods:
Sixty AD patients, 60 healthy older adults (OA), and 60 healthy young adults (YA) participated in our study. Participants were presented with either 4 odors, 4 sounds, or 4 pictures. For each stimulus, they were asked to retrieve a personal memory, to rate it across 3 dimensions (emotionality, vividness, rarity) and then to date it.
Results:
Overall, results showed no clear dominance of one sensory modality over the others in evoking higher-quality AMs. However, they show that using pictures is the better way to stimulate AD patients’ AM, as it helps to retrieve a higher number of memories that are also less frequently retrieved, followed by odors. By contrast, auditory cueing with environmental sounds presented no true advantage.
Conclusion:
Our data should help dementia care professionals to increase the efficiency of Reminiscence Therapy using sensory elicitors. Other clinical implications and future directions are also discussed.
INTRODUCTION
Alzheimer’s disease (AD) is a chronic, neurodegenerative disease resulting in a progressive decline of cognitive functions that reduces the patient’s abil-ity to perform everyday life activities. The behavioral and functional impairments that characterize this form of dementia constitute one of the major causes of disability worldwide, significantly impacting pati-ents’ and their families’ quality of life. As the effe-ctiveness of pharmacological treatments are limited, non-pharmacological interventions have gained atte-ntion in recent years, targeting cognitive, functional, behavioral, and affective aspects of dementia.
One cognitive hallmark of AD is memory decline affecting multiple memory systems, including those involved in autobiographical memory (AM). AM has been defined as personal events from one’s life [1], which underlies the sense of self and helps to regulate current and future behaviors [2–4]. Autobiographical information is organized at many levels of abstraction, from sensory details to conceptual lifetime periods [5]. Importantly, AMs can be re-constructed and re-experienced along with their sensory details. This allows individuals to experience a congruent sense of self, corresponding to the phenomenological feeling of being someone, a continuous physical presence experiencing the world [6–8].
AM retrieval mechanisms change across the lifespan. Studies in normal aging have shown that later in life, memories lose in quality, i.e., they are less specific and vivid and more like abstracted summaries of extended and repeated events (e.g., [9]; for a related general theory, see [10]). These changes are amplified in pathological aging. Several studies have shown an even more important decline in specificity (i.e., the richness in episodic details) of autobiographical events recalled by AD patients: a phenomenon known as the overgenerality of autobiographical memory, first identified by Williams and Broadbent [11] in parasuicidal patients and later considered as one of the main characteristics of AM in AD [12–18]. Because of unspecific AMs coupled with an amnestic syndrome affecting both anterograde and retrograde memory, AD patients have limited access to memories that shape their self-knowledge, self-consciousness, and self-image, resulting in a diminished sense of identity [16, 19–21] which, in turn, favors the development of psycho-behavioral and affective disorders [19, 21]. It is therefore of great interest to find a way to compensate the autobiographical memory changes in normal and pathological aging. Helping AD patients enhance their AM quality can have particularly important therapeutic implications.
In line with the encoding specificity principle [22], according to which matching the encoding contexts of information at recall assists in the retrieval of episodic memories, sensory cueing can favor autobiographical event retrieval. Reminiscence Therapy (i.e., a non-pharmacological approach to dementia care, based on evocation and discussion with another person or a group about past activities, events and experiences, using a variety of supporting materials; [23]), for example, is often implemented using specific sensory elicitors (music, pictures, objects, etc.) (see for example [24] for a meta-analysis). Few studies, however, have investigated the impact of each form of sensory stimulation on AM retrieval in normal and pathological cognition.
Some evidence suggests that, in normal cognition, odors could be a better cue to retrieve AMs, compared with other types of stimuli, but the results from the literature are not always consistent. In 1995, Herz and Cupchik [25] showed that odor-evoked memories were always experienced as more emotional than memories elicited by cues presented in any other sensory modality (see [26–30] for similar results but see also [31–33] for different results). Several studies also indicated that olfactory-evoked autobiographical information is ontogenetically older than memories evoked by visual, auditory, or verbal information [30, 32–34] and that old memories elicited by odors may also be more likely rare memories (i.e., infrequently relived compared to other episodic events [32, 35]). Lastly, Chu and Downes [34] showed that memories originally cued by a verbal label referring to an odor were more vivid when they were later retrieved with a congruent odor (matching the original verbal cue), compared with an incongruent one. However, several other authors failed to observe any differences in vividness of retrieved autobiographical events across cue modalities (i.e., words, pictures, or odors) [30, 32, 33, 36, 37].
An important amount of data suggest that the visual system is also central for AM [38]. This is in line with the so-called “visual dominance hypothesis”. According to this hypothesis, there is a sensory modality hierarchy, where vision dominates the other senses because visual information tends to be more reliable than other sources of information, and the central nervous system integrates information in a statistically optimal fashion [39, 40]. For example, the “imageability” of a verbal cue was found to influence the age, specificity, and vividness of AMs [41–44]. There is also support for the notion that visual dominance could influence AM retrieval [45]. On the other hand, a strong literature relating the potency of auditory cues to evoke emotional AM also exists. Music in particular has been widely reported to be an effective stimulus in eliciting AMs. In healthy individuals, music-evoked AM have been found to be richer in details and more vivid than those elicited by other kinds of cues [46–49]. Similar findings have been reported in AD [50]. However, when it comes to compare simple environmental sounds versus other sensory stimuli, the outcomes are different. Willander and colleagues [33], for example, showed that visual information contributes to retrieval outcome more than auditory one. Consistently, Knez and colleagues [51] reported that visual stimuli, compared with auditory and olfactory ones, cue memories involving both higher cognitive and emotional components of the self (see [52] for a multicomponent conceptualization of the self).
The effect of odors on AM retrieval in AD has recently been a key research topic for Glachet and colleagues [53–56]. Results from this line of research showed various advantages in exposing AD patients to odors before retrieving AMs, when comparing to the absence of sensory exposition. Patients with AD reported a higher number of memories, linked with a higher arousal and a best subjective reliving after odor exposure. These memories were also systematically more emotional and specific, and this was observed over an extensive lifespan period going from childhood memories to future thinking (i.e., the capacity to imagine or simulate events that might occur in one’s personal future; [57]). However, to the best of our knowledge, only one of these studies has compared the efficacity of different kind of sensory stimuli, including odors, in evoking AMs. El Haj and colleagues [58] compared odor-evoked and music-evoked AMs recalled by AD participants and found that memories retrieved after odor and music exposure in AD participants had higher specificity, emotional experience, mental time travel, and shorter retrieval time than in the control condition (no cue condition). In parallel, Ally, Gold, and Budson [59] demonstrated a picture superiority effect using a recognition paradigm where participants were tested on memory for words versus pictures. Compared to old healthy adults, this picture superiority effect was markedly greater in the AD group, suggesting that visual stimuli may be advantageous to AD patients. However, no study has compared the benefit of different sensory stimuli in AD patients’ AMs retrieval performance.
In the present study, our goal was to determine the most relevant cue for AM retrieval in patients with early to mild AD, comparing odors, sounds and pictures. In line with the work of Glachet and colleagues (i.e., [53–56]), we suggest that odors may actually be the more appropriate way to stimulate AM in AD patients. At first glance, this idea might be surprising given the well-documented presence of olfactory impairments in these patients as early as the pre-clinical stages of the disease [60–62]. However, such deficits are observed with tasks requiring an explicit semantic processing of the odorous stimuli (e.g., choosing the label that best fits the perception; see for example [63]). Yet, it has been suggested that the best way to measure odor effects may be implicit, especially when emotional effects are expected (e.g., [64, 65]). It is of great interest to investigate implicit processing of odors since an implicit pre-semantic episodic memory for odors could be preserved in AD.
Here, we used a simple and ecological procedure that can be easily replicated during Reminiscence Therapy sessions (inspired from [36]). Participants were presented with either 4 odors, 4 sounds, or 4 pictures. For each stimulus, they were asked to retrieve a personal memory, to rate it across 3 dimensions—emotionality, vividness (i.e., the clarity and detail of visual imagery in a memory), and rarity—and then to date it. We recruited AD patients, as well as older participants without cognitive impairment, and healthy young subjects, to distinguish effects pertaining to normal versus pathological aging. Compared with the other sensory stimuli, we expected odors to be the most efficient cues to retrieve AMs, in all groups. Namely, we predicted that odors would evoke a greater number of AMs, owing to their less effortful retrieval [58], and that these memories would be judged as more emotional, older and rarer than memories evoked by sounds or pictures, in all groups of participants. We also expected to observe an overall decrease in the intensity of these effects in patients with AD relative to healthy individuals and in healthy elderly participants relative to young ones (due to age-related autobiographical memory changes and general lowering of olfactory function).
METHODS
Participants
A total of 180, right-handed, native French-speaking participants were included in the study: 60 patients with diagnosed AD (39 women; mean age = 80.9±6.1 years), 60 healthy older adults recruited from the community (OA, 45 women; mean age = 80.1±6.2 years), and 60 healthy young adults (YA, 38 women; mean age = 22.2±2.9 years).
YAs were recruited by advertisements posted on a French internet database of volunteers willing to participate in psychology or neuroscience research. OAs lived in the community and were recruited by advertisements and notices distributed around senior citizen organizations in the Paris area. Patients with AD were recruited from a local memory center and were at the early to mild stage of the disease with a Mini-Mental State Examination (MMSE) score ranging from 15 to 24 [66]. All participants reported a normal sense of smell, had normal or corrected-to-normal vision and hearing, and were naive to the aim of the experiment. They provided written informed consent according to institutional guidelines of the local research ethics committee and in compliance with the Declaration of Helsinki. The whole procedure was approved by the local ethics committee (Comité de Protection des Personnes Ile-de-France-X, protocol 02(2) 2015 –ALCOM n° 2014-A01141-46).
All participants underwent structured interviews and neuropsychological testing to assess cognitive functioning. A full description of the participant groups is presented in Table 1. The diagnosis of probable or possible AD was determined by a neurologist following the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Associations (NINCDS/ADRDA) [67]. AD patients were excluded if they were deemed unable to understand task instructions. For controls, the following exclusion criteria were applied: history of neurological disorders, traumatic brain injury with loss of consciousness, or significant history of psychological or psychiatric disorders.
Table 1
Means and SDs of demographics, general neuropsychological efficiency and depression scores in (a) total sample, (b) YA, (c) OA, and (d) AD patients. All comparisons between subgroups were non-significant
| Young adults (YA) | Older adults (OA) | AD patients | Differences between YA and OA | Differences between OA and AD patients | |||
| t | p | t | p | ||||
| N (F:M) | 60 (38:22) | 60 (45:15) | 60 (39:21) | –a | n.s. | –a | n.s. |
| Age (y) | 22.2 (2.9) | 80.1 (6.2) | 80.9 (6.2) | –64.8 | < 0.000 | –0.6 | n.s. |
| Level of education (y) | 13.5 (1.9) | 11.3 (3.9) | 10.2 (4.5) | 3.8 | < 0.000 | 1.5 | n.s. |
| General cognitive efficiency MMSE (30) b | 28.3 (1.4) | 27.6 (1.7) | 21.7 (3.7) | 2.4 | < 0.05 | 11.2 | < 0.000 |
| Frontal efficiency FAB (18) b | 17.0 (0.9) | 15.9 (1.9) | 12.9 (1.7) | 3.8 | 0.001 | 6.8 | < 0.000 |
| Episodic memory 5-word test (10) b | 9.9 (0.1) | 9.4 (0.9) | 6.4 (2.2) | 4.3 | < 0.000 | 9.1 | < 0.000 |
| Attention & working memory | |||||||
| Forward digit span | 6.3 (1.1) | 5.5 (1.3) | 5.2 (1.1) | 3.4 | < 0.001 | 1.3 | n.s. |
| Backward digit span | 4.9 (1.2) | 4.0 (1.3) | 3.6 (0.9) | 3.9 | < 0.001 | 1.6 | 0.09 |
| Depression GDS (cut-off < 7/15) | –c | 1.9 (1.8) | 2.2 (1.8) | –c | –c | –0.9 | n.s. |
Two-tailed t-tests for independent samples were used. SD, standard deviation; n.s., not significant; AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination; FAB, Frontal Assessment Battery; GDS, Geriatric Depression Scale. aGender distribution across groups was tested by using a two-sided Fisher’s exact test for Count Data. bMaximum possible score. cNot applicable. Young adults were screened for current major depression by using the Mini International Psychiatric Interview 5.0.0 (French version, [74]).
The neuropsychological evaluation consisted in exploring global cognition with the MMSE [68], frontal lobe and executive functions with the Frontal Assessment Battery [69], productive language with a verbal fluency task [70], episodic memory with the 5-word test [71], and attention and working memory with the forward and backward digit spans [72]. Regarding the psychiatric evaluation, older participants (healthy controls and AD patients) completed the 15-item Geriatric Depression Scale [73] and those who scored 7 or more were excluded from the study. The Mini International Psychiatric Interview 5.0.0 (French version, [74]) was administered to young participants to screen for current major depression. All healthy participants performed within the normal range on all neuropsychological screening tests (i.e., scores within 1.65 SDs of the group means, which are provided in the test norms). All healthy older participants had an MMSE score of 26 or higher and none of them expressed any memory complaints. All were paid 10 euros for their participation.
Materials
Based on previous preliminary testing, twelve sensory stimuli (4 odors, 4 sounds, 4 pictures) were selected for this experiment (see Table 2). Overall, our stimuli were rated as mildly to highly representing the real items, mildly pleasant, concrete, and evocative across the different sensory forms. Please refer to the Supplementary Material for more details.
Table 2
Stimuli selection. Selecting different items for olfactory modality allowed us to minimize the differences in stimuli’s properties across modalities. Please refer to the Supplementary Material for further information about our stimuli’s selection process
| Odors | Sounds | Pictures |
| Laundry | Sound of cutting bread | A picture of typical French bread |
| Apple | Sound of apple crunching | A picture of an apple |
| Fresh-cut grass | Sound of wood crackling | A picture of a wood fire |
| Coffee bottle opening | Sound of wine | A picture of a wine bottle |
Olfactory stimuli were 4 fragrances provided by International Flavors & Fragrances Inc. (Apple, Coffee, Fresh-cut grass, and Laundry) presented to the participants on sniffing sticks (provided by Burghart Messtechnik, Germany). Auditory stimuli were 4 recording samples of ecological noises: “Crackling fire”, “Wine bottle opening”, “Bread cutting”, and “Apple crunching”. Each audio file lasted 10 s. Visual stimuli were 640×480 pixel color pictures of a wood fire, a bottle of wine, bread, and an apple shown on a black background. A 15.6-inch Toshiba computer screen with a set of audio speakers were used to present visual and auditory stimuli.
Design
The study was conducted in a single session. Each participant was tested individually in a quiet distraction-free room. After providing written informed consent, participants underwent structured interviews and neuropsychological assessment. They were then asked to sequentially assess their own visual, auditory and sensory aptitudes on a 6-points Likert scale ranging from 0 = “Very poor [vision/ sense of hearing/sense of smell]” to 5 = “Perfect [vision/hearing/sense of smell]”. The specific questions posed were: “How good or bad is your [vision/olfaction/hearing] as compared with other persons of your own age?” [30].
We used a three-way between-subjects design. The experimental conditions were: olfactory stimulation, auditory stimulation, and visual stimulation. Participants were assigned to one of three experimental groups on the basis of their highest score stemmed from their self-evaluated sensory functions. Next, we created nine independent groups of 20 participants: 3 subgroups of AD patients (AD-Odors, AD-Sounds, AD-Pictures), 3 subgroups of Older Adults (OA-Odors, OA-Sounds, OA-Pictures), and 3 subgroups of Young Adults (YA-Odors, YA-Sounds, YA-Pictures). OA and AD patients were matched between subgroups for age, gender distribution, years of education and level of depression (see details in Table 1).
All participants were cued four times, once with each of the four stimuli belonging to the same sensory group. Stimulus order presentation was counterbalanced between participants. The cues were introduced sequentially to the participants by giving them the verbal label of the presented stimulus (the experimenter said: “Here you have the picture/the sound of/the odor of”). We included verbal labels for every stimulus to facilitate object label retrieval across groups and control for the semantics linked to each stimulus.
Participants were asked to come up with a specific personal memory concerning a particular person, place or event for each item presented [36].
On each condition, subjects were allowed to “process” the stimulus (i.e., sniff the odors, listen to the sound, or look at the picture) either until they could produce an autobiographical event or until they were sure that no memory was forthcoming. In cases where an autobiographical event was recalled, participants were asked to report it by giving “as much detail as possible”.
Participants were then asked to report if their memory was emotionally charged (i.e., if they felt an emotion associated with their memory). If they answered affirmatively, they were asked to report emotional valence by choosing between the labels “Positive” or “Negative”, before rating emotion intensity on an 8-points Likert scale (0 = “Not intense at all”, 7 = “Extremely intense”). Participants were then asked to rate the vividness of their memory on another 8-points Likert scale (0 = “Not vivid at all”, 7 = “Extremely vivid”). They were then asked to date their autobiographical episode by giving either the year it occurred or their age at the time of the event. Finally, they were asked to rate the frequency with which each memory was evoked (i.e., its rarity). Using a 5-alternative nominal scale, participants were asked to select the best response between: “All the time”, “Frequently”, “Once in a while”, “Rarely”, and “Never”.
In cases where a stimulus evoked more than one specific autobiographical event, all the memories were collected and scored, one after the other.
Finally, participants were asked to assess the following stimuli properties: “Stimulus-reality” matc-hing (i.e., to what extent the stimulus evoked the real item on a 10-points Likert scale; 1 = “Not at all”, 9 = “Extremely”); Pleasantness, on an 11-poi-nts Likert scale (-5 = “Extremely unpleasant”, 5 = “Extremely pleasant”); Intensity (for olfactory and auditory stimuli only), on a 6-points Likert scale (0 = “Not intense at all”, 5 = “Very intense”). A printed version of each measurement scale was systematically provided by experimenters. Once the participant had completed the ratings, the experimenter introduced the next stimulus.
After the procedure was completed (i.e., once all four stimuli belonging to the same sensory group were presented), subjects were fully debriefed. The procedure was identical across all sensory conditions.
Statistical analysis
Demographics and neuropsychological data
In order to examine group differences in the total sample (N = 180), we applied a two-sided Fisher’s exact test for Count Data for categorical variables and Analyses of Variance (ANOVAs) for continuous variables, respectively. The tested factors were gender distribution, age, level of education, general cognitive efficiency (MMSE score), other more specific neuropsychological measures and level of depression. Following the ANOVA, we performed planned comparisons by using bilateral Student t-tests when main effects or interactions were observed (significance level≤0.05). The same analyses were performed to compare the three independent experimental groups, for each experimental condition.
Variables of interest
Our variables of interest were overall percentage of memories (i.e., the number of evoked memories over the total number of cued memories for each participant), percentage of emotional memories, emotional valence, emotional intensity, vividness, age and rarity. Participants’ responses for the rarity assessment were coded on a 4-point scale. We attributed 0 points to the response “All the time”; 1 point to “Frequently”; 2 points to “Once in a while”; 3 points to “Rarely”; and 4 points to “Never”. The memory age analysis was computed only for the OA and AD groups. We clustered participants’ responses in 4 categories corresponding to the same time periods covering the entire lifespan: (i) 0–18 years old; (ii) 19–30 years old; (iii) > 30 years old except for the last 5 years; (iv) last 5 years (based on Piolino et al., 2003 [75]). Since the total number of memories/emotional memories, emotional valence, and age were categorical variables, two-tailed Fisher’s exact tests were used in order to analyze the different contingency tables resulting from the crossing of two factors: Type of sensory stimulation (Olfactory/Auditory/Visual) and Group (AD/OA/YA). Residuals analysis were then performed in order to identify those specific contingency tables’ cells making the greatest contribution to the omnibus test result (i.e., the Fisher’s exact test): the larger the residual, the greater the contribution of the cell to the magnitude of the resulting test value [76]. Statistically significant differences between observed and expected adjusted residuals (i.e., the values that should be observed in the absence of statistical difference between conditions) were thus reported to further our understanding of the results. Independent measures ANOVAs with Type of sensory stimulation (Olfactory/Auditory/Visual) and Group (AD/OA/YA) as between-subjects factors were conducted on the other AM characteristics (i.e., emotional intensity, vividness, and rarity). We then compared the same characteristics across the 3 experimental conditions, i.e., the Type of sensory stimulation, separately for AD, OA, and YA. Tukey’s HSD tests were used to perform post-hoc analyses.
Lastly, we performed some control analyses on our stimuli. We carried out an ANOVA with Type of sensory stimulation (Olfactory/Auditory/Visual) and Group (AD/OA/YA) as between-subjects factors on the following properties: Representativeness (i.e., how accurately the sensory stimulus represents the real item), Pleasantness and Intensity (uniquely for Odors and Sounds). Results for these analyses are reported in the Supplementary Material.
For all tests, significance was set at p≤0.05, and p-values between 0.051 and 0.099 were considered trends. Partial Eta-squared (η2p) and Cramer’s V (V) are reported for parametric and non-parametric ANOVAs, respectively, as effect size indexes. As suggested by Cohen [77], we considered effect sizes as being small when η2p < 0.06, medium when 0.06≤η2 p < 0.14, and marked when η2 pthinsp;≥0.14. For significant comparisons, Cohen’s d was used to determine effect size with d < 0.3 corresponding to a small effect, 0.3 < d < 0.8 to a medium effect and d > 0.8 to a large effect [77].
RESULTS
Descriptive statistics for all three groups are listed in Table 1.
Overall percentage of memories
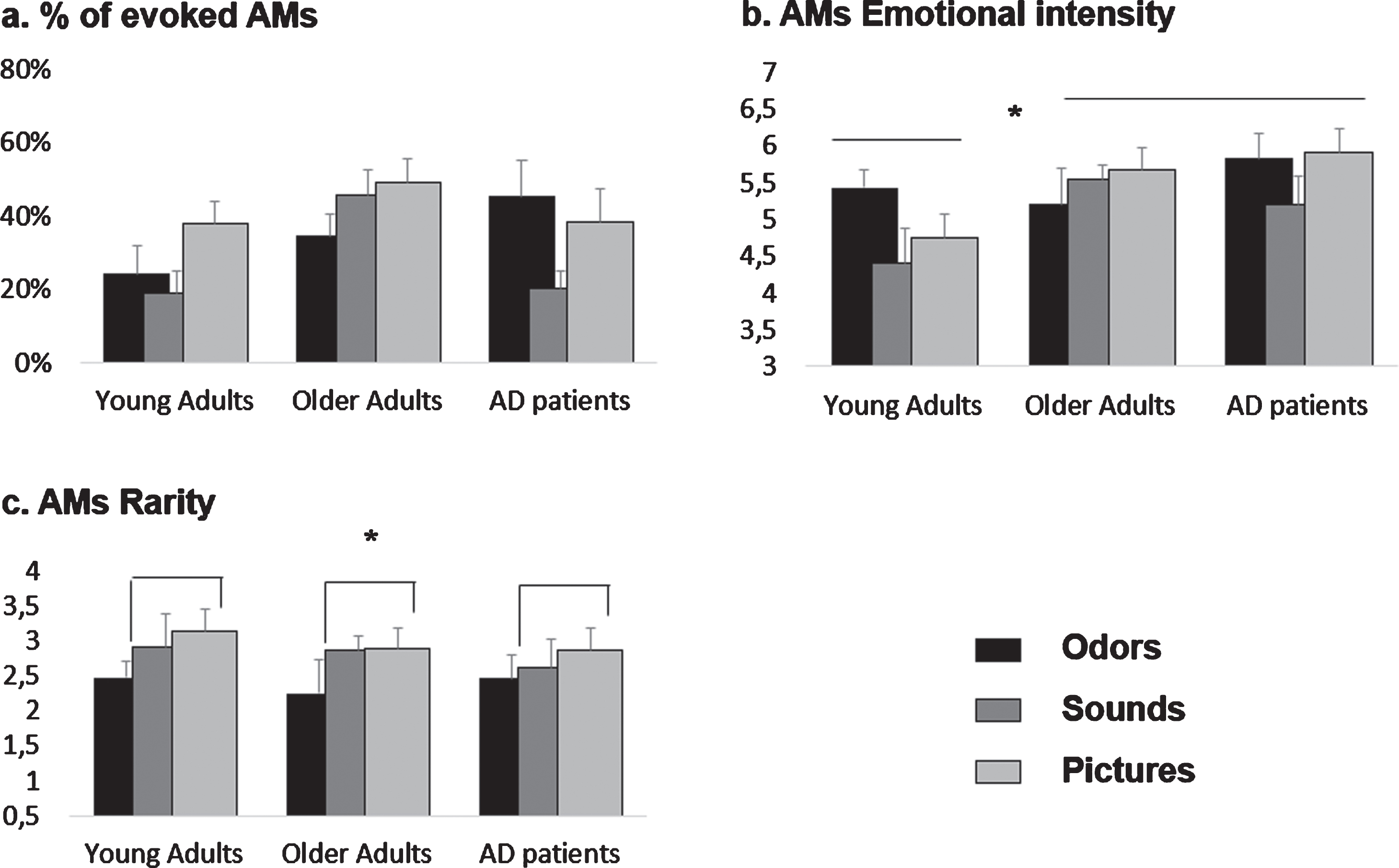
Overall, OAs and YAs produced respectively more (44%, z = 3.66; p < 0.05) and fewer (26%, z = –3.77; p < 0.05) memories than expected, while Pictures and Sounds evoked significantly more (42%, z = 2.68; p < 0.05) and fewer (29%, z = –2.51; p < 0.05) memories than expected, respectively. The percentage of memories evoked by the subgroups OA-Pictures and OA-Sounds was significantly higher than expected (50%, z = 2.91 and 47%, z = 2.22, respectively; p < 0.05). YA-Odors, YA-Sounds, and AD-Sounds recalled significantly fewer memories than expected (22%, z = –2.05; 18%, z = –3.48 and 20%, z = –2.56, respectively; p < 0.05).
Within the AD group, a Fisher’s exact test indicated a significant association between Type of sensory stimulation and the presence of an evoked memory (p = 0.008, V = 0.19). This was driven by the subgroup AD-Sounds evoking significantly fewer memories then expected (19%, z = –3.01; p < 0.05). No association was observed in the OA group (p = 0.23, V = 0.10), while in the YA group (p = 0.05, V = 0.14), the subgroup YA-Pictures evoked significantly more memories than expected (33%, z = 2.23; p < 0.05) (Fig. 1a).
Fig. 1
Effects of olfactory, auditory and visual cueing on AM characteristics for young adults (YA), older adults (OA), and AD patients. Means and standard errors of the mean are reported. a) %of evoked AMs. These data were analyzed categorically by computing Fisher’s exact test. Significant effects are described in Table 3. b) AMs emotional intensity. Both AD patients and OA rated their memories as more emotional than YA, (c) AMs rarity. Overall, pictures-evoked memories were significantly rarer than odors-evoked ones.
Memories’ emotional characteristics
Percentage of emotional memories
All groups evoked a similar percentage of emotional memories over the total amount of evoked memories: 81%for YA, 78%for OA, and 82%for AD. Odors, Sounds, and Pictures evoked 27%, 28%, and 43%of the total number of evoked memories, respectively. We observed no significant association between this variable and Group or Type of sensory stimulation (Fisher’s Exact Test, all p > 0.10). There was also no interaction between these two factors (p > 0.10, V = 0.21).
No associations were found separately for the AD and YA groups (all p > 0.1). The subgroup OA-Odors recalled significantly fewer emotional memories than expected (19%, z = –2.4; p < 0.05).
Memories’ emotional valence
Overall, only 7%of all emotional memories evoked by our participants was judged as “Negative” (14%for YAs, 6%for OAs, and 4%for AD patients). No significant associations nor interactions were observed between Group and Type of sensory stimulation over the memories’ emotional valence (Positive versus Negative) (Fisher’s Exact Test, all ps > 0.20).
No association between Type of sensory stimulation and memories’ emotional valence was observed separately for each of our groups (all ps > 0.20).
Memories’ emotional intensity
The ANOVA conducted on positive memories’ emotional intensity with Group and Type of sensory stimulation as between-subjects factors revealed a main effect of Group (F (2,85) = 3.91; p = 0.02; η2p = 0.08). Tukey post-hoc comparisons showed that both AD patients (mean = 5.67±0.9) and OAs (mean = 5.59±1.1) rated their memories as more emotional than YAs (mean = 4.82±1.1, respectively p = 0.01, d = 0.85 and p = 0.02, d = 0.07). No differences were found between AD patients and OAs (p > 0.1). No effect of Type of sensory stimulation, nor interaction between Type of sensory stimulation and Group were found for this variable (all ps > 0.1).
There was no effect of the Type of sensory stimulation as a between-subjects factor for any of the groups, when analyzed separately (all ps > 0.1) (Fig. 1b).
Memories’ vividness
The ANOVA conducted on memories’ vividness with Group and Type of sensory stimulation as between-subjects factor revealed no effects of Group, Type of sensory stimulation, nor an interaction between these two factors (all ps > 0.1).
No effect of the Type of sensory stimulation as between-subjects factor was observed for any of the groups when analyzed separately (all ps > 0.1).
Memories’ rarity
The ANOVA conducted on memories’ rarity with Group and Type of sensory stimulation as between-subjects factors revealed a main effect of Type of sensory stimulation (F (2,88) = 2.92; p = 0.05; η2p = 0.06). Overall, Pictures evoked memories that were significantly rarer (mean = 2.96±1.08) than Odors (mean = 2.39±1.01, p = 0.03, d = 0.54). No other differences were observed (all ps > 0.1). No effect of Group, nor interaction between Type of sensory stimulation and Group were found for this variable (all ps > 0.1).
No effect of the Type of sensory stimulation as a between-subjects factor on memories’ rarity was found for any of the groups when analyzed separately (all ps > 0.1) (Fig. 1c).
Memories’ age
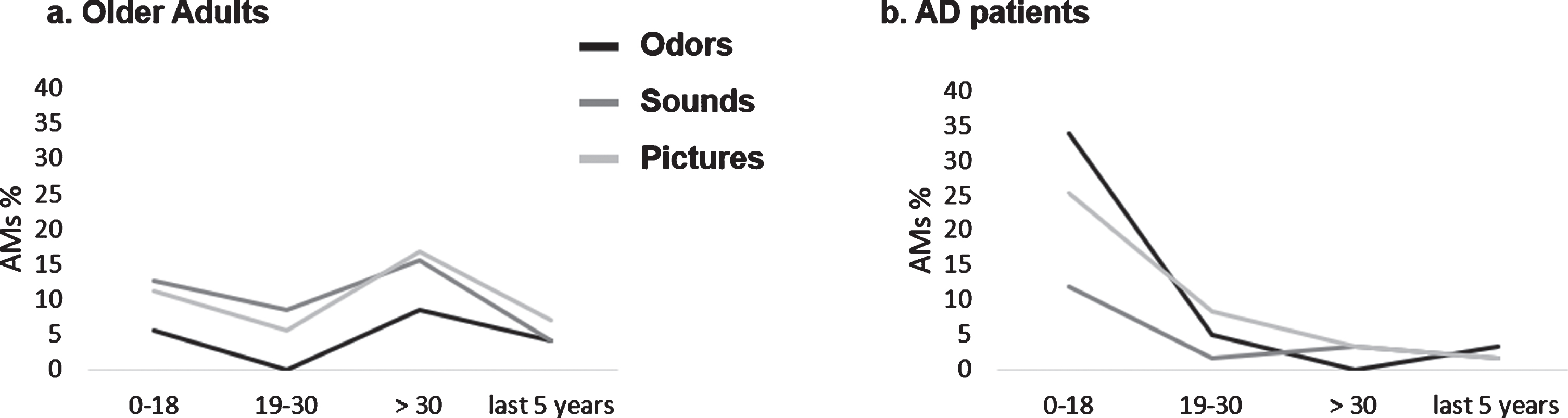
We observed a significant association between Group (OA/AD) and memories’ age (Fisher’s Exact Test, p < 0.000, V = 0.39). Two key periods explained this result: overall, AD patients evoked more memories than expected for the period “0–18” (70%, z = 5.36; p < 0.05) and fewer memories than expected for the period “ > 30 years except for the last 5 years” (9%, z = –4.79; p < 0.05), while this pattern was reversed—even though in a more balanced fashion—in OAs (33%related to the period “0–18”, z = –5.36; p < 0.05 and 38%related to the period “ > 30 years except for the last 5”, z = 4.79). No significant association between Type of sensory stimulation and memories’ age was observed (p = 0.16, V = 0.14). However, we did observe an interaction between Type of sensory stimulation and Group (Fisher’s Exact Test, p < 0.000, V = 0.26). This was explained by:
– OA (Odors and Sounds) and AD (Odors and Pictures) evoking respectively fewer and more memories than expected for the period “0–18” (all absolute values of z > 2.30; p < 0.05)
– OA (Odors and Pictures) and AD (Odors and Pictures), evoking respectively more and fewer memories than expected for the recent period “ > 30 years except for the last 5” (all absolute values of z > 2.20; all ps < 0.05). See Fig. 2.
No association between Type of sensory stimulation and memories’ age was observed for any of the groups when analyzed separately (all ps > 0.1) (Fig. 2).
Fig. 2
Graphic representation of AMs age distribution as a function of the type of sensory cueing for (a) older adults (OA) and (b) AD patients. Mean percentages are report.
DISCUSSION
The aim of this study was to identify which cues (odors, sounds, or pictures) would best stimulate AM retrieval in patients with early to mild AD. We hypothesized that the best stimulators would be odors. However, our data did not support this view. Odor-evoked AMs were not more emotional, older, and rarer than those evoked by sounds or pictures across the populations tested. Globally, pictures were the most efficient way of stimulating AM in all our groups, as they helped to retrieve a bigger, and potentially rarer, number of memories. Our data thus bring support to “the visual dominance hypothesis”. Lastly, our results showed that the effectiveness of non-visual sensory cues in retrieving AMs is submitted to changes in normal and pathological aging: compared to YA, olfactory stimulation seems to gain in relevance for both OA and AD patients while auditory stimulation seems to gain relevance for OA but not AD patients. As a result, we defend the view that pictures are the most efficient way of stimulating AM in AD patients, followed by odors.
Memories’ quantitative retrieval performance
Firstly, our results showed that OAs are most likely to elicit AMs, followed by AD patients and then YAs. This is consistent with the well-known memory deficits affecting retrieval mechanisms in AD pathology [78]. Visual cueing was the condition leading to the best memories’ quantitative retrieval performance across our three groups of participants. The analyses computed separately for each group indicated that this was especially true for YA. Auditory cueing, by contrast, was the condition leading to the worst quantitative retrieval performances in AD patients. Olfactory cueing elicited a mild benefit across our groups of older adults (with or without AD) (see Table 3 for a summary).
Table 3
Schematic representation of the beneficial effects of sensory cueing on AM quantitative retrieval performance, as a function of group and type of stimulation
| Visual stimulation | Auditory stimulation | Olfactory stimulation | |
| Young adults | ++ | - | - |
| Older adults | + | + | ± |
| AD patients | ± | - - | ± |
Plus and minus signs (“+”, “-“) indicate a gain and a disruption in retrieval performance, respectively, compared with the expected values. The plus-minus sign (“±”) indicates expected performance. As two Fisher’s exact tests were computed (i.e., with and without Group as a factor, see Methods), a single sign indicates a significant effect in at least one analysis whereas a double sign indicates a significant effect in both analyses
These patterns lead to several interpretations: first, our data globally fit the “Visual dominance hypothesis” [39] and especially the proposal of Willander and colleagues [33] suggesting that visual information contributes more to retrieval than auditory information. Interestingly, our cross-generational approach allows us to make some assumptions about the evolution of the mechanisms underpinning sensory cueing and its effects on AM retrieval in normal and pathological aging.
Visual dominance is observed in YAs, consistently with previous reports [42, 44]. However, it seems like in aging, information coming from other sensory systems gains relevance. Interestingly, some have suggested that the underlying mechanism for this visual cue-dominance is related to attentional processing of sensory information. For example, experiments have suggested that visual dominance is the result of more attention being directed toward visual information compared to information pertaining to other sensory systems (e.g., [79, 80]. It is therefore possible that the age-related changes in attentional resources ([81], but see [82] for a more recent review) lead to an overall decrease of visual dominance with age, resulting in a more homogeneous distribution of attentional resources between sensory modalities.
It should be noted, however, that auditory stimuli clearly disrupted AD patients’ quantitative retrieval performance. This may be related to hearing loss and its well-established association with dementia (see [62] for a review). There is evidence showing an association between hearing impairment and the rate of decline in whole brain volume [83]. Regional loss of right temporal lobe volume in patients with dementia suggests increased loss in areas important for auditory processing. Other proposed mechanisms include the effects of social isolation [84] and increased cognitive effort secondary to hearing impairment [85]. Interestingly, olfactory stimuli did not lead to the same outcomes, despite the well-documented presence of olfactory impairments in AD patients discussed above [62]. This is in line with data suggesting that all the olfactory functions are not equally affected in AD: while patients are mostly impaired in those relying upon higher sensory functions (i.e., odors’ identification and recognition; see [62] for a recent review), our data suggest that odor detection still allows implicit processes to take place and favor AMs retrieval. Our data therefore confirm the relevance to use olfactory stimulation with these patients.
Memories’ emotional characteristics
All groups evoked a similar percentage of emotional memories over the total amount of evoked memories, converging with the view that emotion is a strongly associated, cross-generational component of AM ([65] but see also [87] or [88] for a review). Moreover, independently of groups or types of stimulation, almost all the retrieved AMs were emotionally charged in a positive way. This is in line with the view that we more often recall positive memories [1] because we are generally motivated to see ourselves in a positive manner [89, 90]. This finding also replicates Knez and colleagues’ results [51] in young adults, showing that positive affective states were more involved in autobiographical memory than negative affective states, independently of the sensory cueing modality.
However, interestingly, Group, but not Type, of sensory stimulation, modulated AM emotional intensity. Our older participants (with and without AD) indeed evoked significantly more emotionally intense AMs than YAs. A potential explanation can be grounded in the theoretical framework of Socioemotional Selectivity Theory (SST) [91, 92], a lifespan theory of motivation. According to SST, people have a sense of their time left in life, which shifts attention to emotionally meaningful aspects of life over time. OAs’ time perception appears to be limited, so positive emotional experience becomes the preeminent motivation, leading individuals to tune attentional, cognitive, and social investments to maximize emotional feelings and positive affect. However, according to SST, older participants were also supposed to show a greater proportion of positive memories compared to young participants, an effect that we did not report in our study. The absence of such effect in our data is likely due to the overwhelming proportion of “positive” memories, across groups (i.e., 93%) that limits the power of the frequency’s distribution analysis.
Why olfactory cueing had no impact on AM emotional characteristics is harder to explain. As noted above, the emotional advantage of the odor-evoked AMs is still a quietly controversial topic. Previous findings have suggested that odor-evoked AMs are more emotional than those evoked by other modalities (e.g., [36, 37]). However, some studies failed to replicate these findings in young participants [31, 33], while Willander & Larsson [32] failed to show the effect in healthy older adults. They reported that “rather, [ . . . ] memory representations evoked by visual information were experienced with a stronger emotional connotation than were memories cued by verbal and olfactory information.” ([32], p. 243). More recently, El Haj and colleagues [58] showed a richer emotional experience for AMs evoked after odor exposure than in the control condition (i.e., no sensory cueing) in AD patients but not in the healthy older adults. In sum, the current literature focusing on whether olfactory cueing leads to more emotional AMs is far from being consensual. This is probably linked to a lack of consistency in methodological approaches and more data are needed to shed new light on this topic. Future research should consider using an objective index of emotional states in order to assess differences in the emotional arousal triggered by different forms of sensory-cued AMs (e.g., galvanic skin response recordings, a noninvasive technique that has been used in AD studies) [93].
Memories’ other phenomenological features
Group or type of sensory cueing had no impact on AM vividness, consistent with previous findings reporting no differences in vividness of retrieved autobiographical events across cue-modalities [30, 32, 33, 36, 37]. In contrast, AM rarity was modulated here as a function of sensory cues. More specifically, picture-cued AMs were overall rarer than olfactory-cued ones, independently of the group.
Compared with other phenomenological features, research in AM “rarity” (a memory “thought of and talked about less often”), has generated proportionally fewer data. To the best of our knowledge, only two studies have investigated this issue, and both showed that odor-evoked memories were rarer than memories evoked by words or pictures [32, 35]. It has been proposed that cue specificity may underlie this effect. Odor cues are more specific than verbal or visual cues, and would therefore match fewer, and potentially rarer, representations than more generic cues such as words or pictures [94]. However, there is no compelling connection between “generic cue” and “frequently cued event” since, despite a more frequent exposure to items’ verbal or visual forms, we are not required to be constantly engaged in a memory recollection process. Plus, some objects are more often present in our environment under their olfactory, rather than visual or verbal, form. We suggest that sensory-cued AM rarity may rather be mediated by cues’ imageability. This would be in line with the idea that visual imagery plays a central role in autobiographical memory [95], that is: the richer the visual representation of an item, the greater the chance it will evoke rare events related to it.
The age distribution analysis mainly showed that most AMs evoked by AD patients referred to periods during childhood and adolescence, whereas OAs showed a more balanced pattern. This can be explained by the fact that AD patients (differently than OAs) show a pattern of retrograde amnesia that obeys Ribot’s Law [96] (i.e., old memories are better preserved than recent ones [97, 98]; see also [99] for a more recent study comparing different recollection paradigms). However, no association between Type of sensory stimulation and memories’ age was observed separately for each of our groups, suggesting that the type of sensory cueing did not modify the original patterns related to AM distribution in OAs and AD patients. Rather, we show that olfactory and visual cues actually strengthened the existing patterns in both groups. These findings contradict those of Willander & Larsson [30, 32], showing that AMs triggered by olfactory information were older than memories associated with visual information in OAs. However, as stated above, they also showed that semantic knowledge of an odor’s name significantly affected the age distribution of memories such that the memory peak for odors in childhood is reduced. In our study, we chose to provide participants with the verbal label for every stimulus. As stated in our methods section, we did so to attenuate differences in retrieval difficulty across the sensory groups. Also, in both studies mentioned above, participants were stimulated 20 times with 20 different stimuli, leading to a much greater number of generated AMs (ranging from a mean of 7.58 to 10.79 for each participant across conditions in [30] versus 1.11 to 1.67 in our study). This allowed for tighter clustering of participants’ responses within shorter time periods (e.g., 9 years) and, consequently, a deeper analysis of the sensory cueing effects. Altogether, these methodological choices may have prevented us from observing the age distribution of odor-cue modulation of AMs.
AD group’s specificities: Clinical implications and future directions
Globally, our data show that pictures appear to be the most efficient way of stimulating AM in AD patients, as they helped to retrieve a bigger, and potentially rarer, number of memories. Interestingly, however, since auditory non-musical stimuli were the least effective cues to stimulate AM evocation in these patients, we suggest that the second most relevant sensory cues could be precisely odors. This advantage of olfactory stimulation in AD is particularly interesting, considering that the literature has predominantly focused on the well-known olfactory deficits caused by AD histopathology (see [62] for a review). Our data are in line with results from Glachet and colleagues [53–56], who provided strong evidence of the beneficial effects of odors on AD patients’ AM when compared to absence of stimulation. It must be noted that a key difference between our methods and Glachet’s ones is that these authors do not use odors as explicit memory cues. They exposed participants to odors before asking them to evoke memories. Thus, participants could evoke memories that were not related to the presented odors. The consistency of their findings clearly strengthens the idea that the best way to measure odor effects in AD patients is implicit, especially when emotional effects are expected, as previously suggested [64, 65].
An important clinical implication stemming from these considerations is that AM’s rehabilitation program can be tailored to suit differences and preferences between individuals regarding sensory stimulation (e.g., some patients may prefer or benefit more from visual cues, whereas others may be more oriented towards olfactory ones). Another possibility consists in targeting a multisensorial cueing approach for AM rehabilitation in AD: combining visual and olfactory cueing should allow to maximize the beneficial effects.
By contrast, simple auditory stimulation not involving music seems to have no real impact or even to reduce AD patients’ memory retrieval skills. Future investigations should shed some light on this point and verify, for example, whether hearing loss in AD actually plays a role and to what extent.
AMs’ Vividness was not impacted by group. In other words, AD patients did not report lower vividness than other groups, contrary to what previous data in the literature suggest [100]. This indicates that sensory stimulation has globally compensate this decline in phenomenological quality of AD patients’ AMs. One may have expected, however, that visual stimulation (because it helps visual imagery; see for example [101]) or odors (because they activate the emotional network; [27, 30, 102]) would have particularly enhanced vividness in AD patients. We did not report such a result as vividness was not impacted by sensory cues either. It would be of clinical interest to further investigate which properties of sensory stimulation is efficient to improve vividness in AD.
Lastly, our findings globally suggest that sensory cueing of AMs in general tends to elicit strong and positive-toned affect in AD patients, which have therapeutic implications beyond episodic memory rehabilitation.
Limitations
The present study has several limitations. First, we did not analyze memories’ content objectively. The whole experimental session was recorded for each participant. We started to transcribe the 180 recorded sessions but need time and funding to finish the work. Once done, more objective data related to memories’ richness and details will be processed and presented. Here, we were interested in reporting participants’ point of view regarding sensory stimulation benefits in AM retrieval. This can be done by collecting memories’ subjective experience (see for example [33, 51, 103, 104]). The use of a subjective approach namely allows to focus on patients’ psychological experience, which is particularly relevant in a clinical framework. We provide useful data for dementia care professionals who conduct Reminiscence Therapy using sensory elicitors.
Still, one may wonder about the ability of some of the patients, with sometimes significant cognitive deficits, to use a measurement scale and to evaluate the phenomenological properties of stimuli that refer to abstract notions. As previously stated, printed versions of measurement scales were always provided by experimenters who were all trained neuropsychologists.
It would also have been interesting to objectively test participants’ sense of smell. As this was not materially possible (i.e., olfactory testing instruments are expensive and time-consuming, particularly when considering our three-way between-subjects design), we decided to ask the participants to self-rate their sensory capacities. In any case, experimenters were instructed to abort the session if a participant was not able to perceive our stimuli.
Lastly, our stimuli selection was purposely limited. Each participant was indeed cued only 4 times, mainly to limit the duration of the experimental task and, consequently, the risk of causing early fatigability in AD participants. Reducing the duration of the experiment is a real challenge when testing pathological populations. As an example, in their three-way within-subjects design, El Haj and colleagues [58] planned three experimental sessions (with a 3- to 5-day interval between sessions) in order to cue each participant 6 times (i.e., 2 times per session). Asking participants to attend more than one experimental session (like Kirk and colleagues [13] also did in their study) is logistically complicated for AD subjects and their caregivers and predicts a high number of withdrawals. That is the reason why we privileged a design requiring a unique session. Minimizing the duration of testing was also the reason why we did not collect further control variables like, for example, participants’ vividness of mental imagery [105] which has been found to influence odor detection, as well as vividness and emotion of evoked mental representations [106, 107].
CONCLUSION
Through a rigorous experimental design, our study suggests that visual and olfactory stimuli are the most relevant cues for stimulating autobiographical memory in mild to moderate AD, while auditory cueing seems to present no real advantage. This should be considered by dementia care professionals who conduct Reminiscence Therapy using sensory elicitors.
ACKNOWLEDGMENTS
The authors thank Arnaud Montet, Consumer Science Global Director, and all the International Flavors & Fragrances (Inc.) perfumers and chemical engineers for providing the stimuli as well as providing their fundamental expertise in managing the olfactory material. We also thank Jérôme Blin, M.D., Stéphanie Marchand, M.D., Claire Gautherot and Oriane Breysse, neuropsychologists, and the staff at the private geriatric hospital “Les Magnolias”, as well as Severine Rose, psychologist, and the staff at the day care centre for people living with dementia “Mémoire Plus” for their kind and helpful assistance.
This study was funded by the French “Agence Nationale de la Recherche” (ANR) (“AlCom” project ANR-13-JSH2-0001-01).
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0841r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200841.
REFERENCES
[1] | Conway AM , Pleydell-Pearce C ((2000) ) The construction of autobiographical memories in the Self-Memory System. Psychol Rev 108: , 83–95. |
[2] | Conway MA ((2005) ) Memory and the self. J Mem Lang 53: , 594–628. |
[3] | Pillemer DB ((2003) ) Directive functions of autobiographical memory: The guiding power of the specific episode. Memory 11: , 193–202. |
[4] | Rathbone CJ , Moulin CJA , Conway MA ((2008) ) Self-centered memories: The reminiscence bump and the self. Mem Cogn 36: , 1403–1414. |
[5] | Williams HL , Conway MA , Cohen G ((2008) ) Autobiographical memory. Mem Real World 3: , 21–90. |
[6] | Fivush R , Habermas T , Waters TEA , Zaman W ((2011) ) The making of autobiographical memory: Intersections of culture, narratives and identity. Int J Psychol 46: , 321–345. |
[7] | Boyer P , Wertsch JV ((2009) ) Memory in mind and culture, Cambridge University Press. |
[8] | Prebble SC , Addis DR , Tippett LJ ((2014) ) Autobiographical memory and sense of self. Psychol Bull 139: , 815–840. |
[9] | Piolino P , Desgranges B , Benali K , Eustache F ((2002) ) Episodic and semantic remote autobiographical memory in ageing. Memory 10: , 239–257. |
[10] | Park DC , Reuter-Lorenz P ((2009) ) The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol 60: , 173–196. |
[11] | Williams JM , Broadbent K ((1986) ) Autobiographical memory in suicide attempters. J Abnorm Psychol 95: , 144. |
[12] | Irish M , Hornberger M , Lah S , Miller L , Pengas G , Nestor PJ , Hodges JR , Piguet O ((2011) ) Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia 49: , 2694–2702. |
[13] | Kirk M , Berntsen D ((2018) ) A short cut to the past: Cueing via concrete objects improves autobiographical memory retrieval in Alzheimer’s disease patients. Neuropsychologia 110: , 113–122. |
[14] | Leyhe T , Müller S , Milian M , Eschweiler GW , Saur R ((2009) ) Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 47: , 2464–2469. |
[15] | Ivanoiu A , Cooper JM , Shanks MF , Venneri A ((2006) ) Patterns of impairment in autobiographical memory in the degenerative dementias constrain models of memory. Neuropsychologia 44: , 1936–1955. |
[16] | EL Haj M , Antoine P , Nandrino J-L , Kapogiannis D ((2015) ) Autobiographical memory decline in Alzheimer’s disease, a theoretical and clinical overview. Ageing Res Rev 23: , 183–192. |
[17] | Barnabe A , Whitehead V , Pilon R , Arsenault-Lapierre G , Chertkow H ((2012) ) Autobiographical memory in mild cognitive impairment and Alzheimer’s disease: A comparison between the Levine and Kopelman interview methodologies. Hippocampus 22: , 1809–1825. |
[18] | Moses A , Culpin V , Lowe C , McWilliam C ((2004) ) Overgenerality of autobiographical memory in Alzheimer’s disease. Br J Clin Psychol 43: , 377–386. |
[19] | Addis DR , Tippett LJ ((2004) ) Memory of myself: Autobiographical memory and identity in Alzheimer’s disease. Memory 12: , 56–74. |
[20] | Morris RG , Mograbi DC ((2013) ) Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex 49: , 1553–1565. |
[21] | Piolino P ((2008) ) A la recherche du self: Théorie et pratique de la mémoire autobiographique dans la maladie d’Alzheimer. Encephale 34: , S77–S88. |
[22] | Tulving E , Thomson DM ((1973) ) Encoding specificity and retrieval processes in episodic memory. Psychol Rev 80: , 352–373. |
[23] | Cotelli M , Manenti R , Zanetti O ((2012) ) Reminiscence therapy in dementia: A review. Maturitas 72: , 203–205. |
[24] | Mileski M , Topinka JB , Brooks M , Lonidier C , Linker K , Veen Vander K ((2018) ) Sensory and memory stimulation as a means to care for individuals with dementia in long-term care facilities. Clin Interv Aging 13: , 967–974. |
[25] | Herz RS , Cupchik GC ((1995) ) The emotional distinctiveness of odor-evoked memories. Chem Senses 20: , 517–528. |
[26] | Herz RS ((1998) ) Are odors the best cues to memory? A cross-modal comparison of associative memory stimuli. Ann N Y Acad Sci 855: , 670–674. |
[27] | Royet JP , Zald D , Versace R , Costes N , Lavenne F , Koenig O , Gervais R ((2000) ) Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: A positron emission tomography study. J Neurosci 20: , 7752–7759. |
[28] | Herz RS , Eliassen J , Beland S , Souza T ((2004) ) Neuroimaging evidence for the emotional potency of odor-evoked memory. Neuropsychologia 42: , 371–378. |
[29] | Bonfigli L , Kodilja R , Zanuttini L ((2002) ) Verbal versus olfactory cues: Affect in elicited memories. Percept Mot Skills 94: , 9–20. |
[30] | Willander J , Larsson M ((2007) ) Olfaction and emotion: The case of autobiographical memory. Mem Cognit 35: , 1659–1663. |
[31] | Toffolo MBJ , Smeets MAM , van den Hout MA ((2012) ) Proust revisited: Odours as triggers of aversive memories. Cogn Emot 26: , 83–92. |
[32] | Willander J , Larsson M ((2006) ) Smell your way back to childhood: Autobiographical odor memory. Psychon Bull Rev 13: , 240–244. |
[33] | Willander J , Sikström S , Karlsson K ((2015) ) Multimodal retrieval of autobiographical memories: Sensory information contributes differently to the recollection of events. Front Psychol 6: , 1681. |
[34] | Chu S , Downes JJ ((2002) ) Proust nose best: Odors are better cues of autobiographical memory. Mem Cognit 30: , 511–518. |
[35] | Rubin DC , Groth E , Goldsmith DJ ((1984) ) Olfactory cuing of autobiographical memory. Am J Psychol 97: , 493–507. |
[36] | Herz RS ((2004) ) A naturalistic analysis of autobiographical memories triggered by olfactory visual and auditory stimuli. Chem Senses 29: , 217–224. |
[37] | Herz R , Schooler J ((2009) ) A naturalistic study of autobiographical and visual evoked by olfactory memories the Proustian cues: Testing the Proustian hypothesis. Am J Psychol 115: , 21–32. |
[38] | Greenberg DL , Rubin DC ((2003) ) The neuropsychology of autobiographical memory. Cortex 39: , 687–728. |
[39] | Schmid C , Büchel C , Rose M ((2011) ) The neural basis of visual dominance in the context of audio-visual object processing. Neuroimage 55: , 304–311. |
[40] | Witten IB , Knudsen EI ((2005) ) Why seeing is believing: Merging auditory and visual worlds. Neuron 48: , 489–496. |
[41] | Greenberg DL , Knowlton BJ ((2014) ) The role of visual imagery in autobiographical memory. Mem Cogn 42: , 922–934. |
[42] | Rasmussen KW , Berntsen D ((2014) ) “I can see clearly now”: The effect of cue imageability on mental time travel. Mem Cogn 42: , 1063–1075. |
[43] | Rubin DC , Schulkind MD ((2011) ) Properties of word cues for autobiographical memory. Psychol Rep 81: , 47–50. |
[44] | Williams JMG , Healy HG , Ellis NC ((1999) ) The effect of imageability and predicability of cues in autobiographical memory. Q J Exp Psychol 52A: , 555–579. |
[45] | Karlsson K , Sikström S , Willander J ((2013) ) The semantic representation of event information depends on the cue modality: An instance of meaning-based retrieval. PLoS One 8: , e73378. |
[46] | Baird A , Brancatisano O , Gelding R , Thompson WF ((2018) ) Characterization of music and photograph evoked autobiographical memories in people with Alzheimer’s disease. J Alzheimers Dis 66: , 693–706. |
[47] | Belfi AM , Karlan B , Tranel D ((2016) ) Music evokes vivid autobiographical memories. Memory 24: , 979–989. |
[48] | Schulkind MD , Woldorf GM ((2005) ) Emotional organization of autobiographical memory. Mem Cognit 33: , 1025–1035. |
[49] | Ford JH , Rubin DC , Giovanello KS ((2014) ) Effects of task instruction on autobiographical memory specificity in young and older adults. Memory 22: , 722–736. |
[50] | El Haj M , Postal V , Allain P ((2012) ) Music enhances autobiographical memory in mild Alzheimer’s disease. Educ Gerontol 38: , 30–41. |
[51] | Knez I , Ljunglöf L , Arshamian A , Willander J ((2017) ) Self-grounding visual, auditory and olfactory autobiographical memories. Conscious Cogn 52: , 1–8. |
[52] | Klein SB , Gangi CE ((2010) ) The multiplicity of self: Neuropsychological evidence and its implications for the self as a construct in psychological research. Ann N Y Acad Sci 1191: , 1–15. |
[53] | Glachet O , El Haj M ((2019) ) Emotional and phenomenological properties of odor-evoked autobiographical memories in Alzheimer’s disease. Brain Sci 9: , 135. |
[54] | Glachet O , El Haj M ((2020) ) Effects of olfactory stimulation on past and future thinking in Alzheimer’s disease. Chem Senses 45: , 313–320. |
[55] | Glachet O , Moustafa AA , Gallouj K , El Haj M ((2019) ) Smell your memories: Positive effect of odor exposure on recent and remote autobiographical memories in Alzheimer’s disease. J Clin Exp Neuropsychol 41: , 555–564. |
[56] | Glachet O , Gandolphe MC , Gallouj K , Antoine P , El Haj M ((2018) ) Effects of olfactory stimulation on autobiographical memory in Alzheimer’s disease. Geriatr Psychol Neuropsychiatr Vieil 16: , 311–320. |
[57] | Schacter DL , Benoit RG , Szpunar KK ((2017) ) Episodic future thinking: Mechanisms and functions. Curr Opin Behav Sci 17: , 41–50. |
[58] | EL Haj M , Gandolphe MC , Gallouj K , Kapogiannis D , Antoine P ((2017) ) From nose to memory: The involuntary nature of odor-evoked autobiographical memories in Alzheimer’s disease. Chem Senses 43: , 27–34. |
[59] | Ally BA , Gold CA , Budson AE ((2009) ) The picture superiority effect in patients with Alzheimer’s disease and mild cognitive impairment. Neuropsychologia 47: , 595–598. |
[60] | Devanand DP , Lee S , Manly J , Andrews H , Schupf N , Doty RL , Stern Y , Zahodne LB , Louis ED , Mayeux R ((2015) ) Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84: , 182–189. |
[61] | Velayudhan L ((2015) ) Smell identification function and Alzheimer’s disease: A selective review. Curr Opin Psychiatry 28: , 173–179. |
[62] | Murphy C ((2019) ) Olfactory and other sensory Impairments in Alzheimer’s disease. Nat Rev Neurol 15: , 11–24. |
[63] | Royet JP , Croisile B , Williamson-Vasta R , Hibert O , Serclerat D , Guerin J ((2001) ) Rating of different olfactory judgements in Alzheimer’s disease. Chem Senses 26: , 409–417. |
[64] | Degel J , Piper D , Köster EP ((2001) ) Implicit learning and implicit memory for odors: The influence of odor identification and retention time. Chem Senses 26: , 267–280. |
[65] | Köster EP ((2005) ) Does olfactory memory depend on remembering odors? Chem Senses 30: (Suppl 1), 236–237. |
[66] | Feldman HH , Woodward M ((2005) ) The staging and assessment of moderate to severe Alzheimer disease. Neurology 65: , S10–S17. |
[67] | McKhann G , Knopman DS , Chertkow H , Hymann B , Jack CR , Kawas C , Klunk W , Koroshetz W , Manly J , Mayeux R , Mohs R , Morris J , Rossor M , Scheltens P , Carrillo M , Weintrub S , Phelphs C ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[68] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[69] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB. Neurology 55: , 1621–1626. |
[70] | Cardebat D , Doyon B , Puel M , Goulet P , Joanette Y ((1990) ) Evocation lexicale formelle et sémantique chez des sujets normaux. Performances et dynamiques de production en fonction du sexe, de l’âge et du niveau d’étude. Acta Neurol Belg 90: , 207–217. |
[71] | Dubois B , Touchon J , Portet F , Ousset P-J , Vellas B , Michel B ((2002) ) “Les 5 mots”, épreuve simple et sensible pour le diagnostic de la maladie d’Alzheimer. Press Med 31: , 1696–9. |
[72] | Wechsler D ((1997) ) WAIS-III administration and scoring manual. |
[73] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1983) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[74] | Lecrubier Y , Sheehan D , Hergueta T , Weiller E ((1998) ) The mini international neuropsychiatric interview. Eur Psychiatry 13: , 198s. |
[75] | Piolino P , Desgranges B , Belliard S , Matuszewski V , Lalevée C , De La Sayette VD , Eustache F ((2003) ) Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain 126: , 2203–2219. |
[76] | Sharpe D ((2015) ) Your chi-square test is statistically significant: Now what? Pract Assess Res Eval 20: , 1–10. |
[77] | Cohen J ((1988) ) Statistical power analysis for the behavioural sciences. Lawrence Earlbaum Assoc, Hillside, NJ. |
[78] | Grober E , Buschke H , Korey SR ((1987) ) Genuine memory deficits in dementia. Dev Neuropsychol 3: , 13–36. |
[79] | Posner MI , Nissen MJ , Klein RM ((1976) ) Visual dominance: An information-processing account of its origins and significance. Psychol Rev 83: , 157–171. |
[80] | Sinnett S , Spence C , Soto-Faraco S ((2007) ) Visual dominance and attention: The Colavita effect revisited. Percept Psychophys 69: , 673–686. |
[81] | Craik FIM , Byrd M ((1982) ) Aging and cognitive deficits: The role of attentional resources. In Aging and Cognitive Processes, Springer, pp. 191–211. |
[82] | Buckner RL ((2004) ) Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 44: , 195–208. |
[83] | Lin FR , Ferrucci L , An Y , Goh JO , Doshi J , Metter EJ , Davatzikos C , Kraut MA , Resnick SM ((2014) ) Association of hearing impairment with brain volume changes in older adults. Neuroimage 90: , 84–92. |
[84] | Weinstein BE , Ventry IM ((1982) ) Hearing impairment and social isolation in the elderly. J Speech Lang Hear Res 25: , 593–599. |
[85] | Lin FR , Albert MS ((2014) ) Hearing loss and dementia-who is listening? Aging Ment Health 18: , 671–673. |
[86] | Holland AC , Kensinger EA ((2010) ) Emotion and autobiographical memory. Phys Life Rev 7: , 88–131. |
[87] | Fivush R ((2011) ) The development of autobiographical memory. Annu Rev Psychol 62: , 559–582. |
[88] | Gilboa A , Rosenbaum RS , Mendelsohn A ((2018) ) Autobiographical memory: From experiences to brain representations. Neuropsychologia 110: , 1–6. |
[89] | Demblon J , D’Argembeau A ((2017) ) Contribution of past and future self-defining event networks to personal identity. Memory 25: , 656–665. |
[90] | Schwartz SJ , Luyckx K , Vignoles VL (2011) Handbook of Identity Theory and Research. Springer Science+Business Media. |
[91] | Carstensen LL , Isaacowitz DM , Charles ST , Prakash RS , De Leon AA , Patterson B , Schirda BL , Janssen AL ((1999) ) Taking time seriously. Am Psychol 54: , 165–181. |
[92] | Löckenhoff CE , Carstensen LL ((2004) ) Socioemotional selectivity theory, aging, and health: The increasingly delicate balance between regulating emotions and making tough choices. J Pers 72: , 1395–1424. |
[93] | Irish M , Cunningham CJ , Walsh JB , Coakley D , Lawlor BA , Robertson IH , Coen RF ((2006) ) Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dement Geriatr Cogn Disord 22: , 108–120. |
[94] | Larsson M , Willander J , Karlsson K , Arshamian A ((2014) ) Olfactory LOVER: Behavioral and neural correlates of autobiographical odor memory. Front Psychol 5: , 312. |
[95] | Rubin DC , Greenberg DL ((1998) ) Visual memory-deficit amnesia: A distinct amnesic presentation. Proc Natl Acad Sci U S A 95: , 5413–5416. |
[96] | Ribot T (1882) Diseases of memory: An essay in the positive psychology, Appleton D, ed. |
[97] | Greene JDW , Hodges JR , Baddeley AD ((1995) ) Autobiographical memory and executive function in early dementia of Alzheimer type. Neuropsychologia 33: , 1647–1670. |
[98] | Kopelman MD ((1992) ) The” new” and the” old”: Components of the anterograde and retrograde memory loss in Korsakoff and Alzheimer patients. In Neuropsychology of Memory, Squire LR, Butters N, eds. Guilford Press, New York, pp. 130–146. |
[99] | Kirk M , Berntsen D ((2018) ) The life span distribution of autobiographical memory in Alzheimer’s disease. Neuropsychology 32: , 906–919. |
[100] | El Haj M , Kapogiannis D , Antoine P ((2016) ) Phenomenological reliving and visual imagery during autobiographical recall in Alzheimer’s disease. J Alzheimers Dis 52: , 421–431. |
[101] | Dijkstra N , Bosch SE , van Gerven MAJ ((2017) ) Vividness of visual imagery depends on the neural overlap with perception in visual areas. J Neurosci 37: , 1367–1373. |
[102] | Bywaters M , Andrade J , Turpin G ((2004) ) Determinants of the vividness of visual imagery: The effects of delayed recall, stimulus affect and individual differences. Memory 12: , 479–488. |
[103] | Goddard L , Pring L , Felmingham N ((2005) ) The effects of cue modality on the quality of personal memories retrieved. Memory 13: , 79–86. |
[104] | Herz RS , Cupchik GC ((1992) ) An experimental characterization of odor-evoked memories in humans. Chem Senses 17: , 519–528. |
[105] | Andrade J , May J , Deeprose C , Baugh SJ , Ganis G ((2014) ) Assessing vividness of mental imagery: The plymouth sensory imagery questionnaire. Br J Psychol 105: , 547–563. |
[106] | Djordjevic J , Zatorre RJ , Petrides M , Jones-Gotman M ((2004) ) The mind’s nose: Effects of odor and visual imagery on odor detection. Psychol Sci 15: , 143–148. |
[107] | Bensafi M , Rouby C ((2007) ) Individual differences in odor imaging ability reflect differences in olfactory and emotional perception. Chem Senses 32: , 237–244. |




