Mortality in Dementia from 1996 to 2015: A National Registry-Based Cohort Study
Abstract
Background:
It remains unclear whether the increased focus on improving healthcare and providing appropriate care for people with dementia has affected mortality.
Objective:
To assess survival and to conduct a time trend analysis of annual mortality rate ratios (MRR) of dementia based on healthcare data from an entire national population.
Methods:
We assessed survival and annual MRR in all residents of Denmark ≥65 years from 1996–2015 using longitudinal registry data on dementia status and demographics. For comparison, mortality and survival were calculated for acute ischemic heart disease (IHD) and cancer.
Results:
The population comprised 1,999,366 people (17,541,315 person years). There were 165,716 people (529,629 person years) registered with dementia, 131,321 of whom died. From 1996–2015, the age-adjusted MRR for dementia declined (women: 2.76 to 2.05; men: 3.10 to 1.99) at a similar rate to elderly people without dementia. The sex-, age-, and calendar-year-adjusted MRR was 2.91 (95%CI: 2.90–2.93) for people with dementia. MRR declined significantly more for acute IHD and cancer. In people with dementia, the five-year survival for most age-groups was at a similar level or lower as that for acute IHD and cancer.
Conclusion:
Although mortality rates declined over the 20-year period, MRR stayed higher for people with dementia, while the MRR gap, compared with elderly people without dementia, remained unchanged. For the comparison, during the same period, the MRR gap narrowed between people with and without acute IHD and cancer. Consequently, initiatives for improving health and decreasing mortality in dementia are still highly relevant.
INTRODUCTION
During the last two decades, there has been in-creased focus on improving health in the rapidly increasing elderly population [1, 2]. These initiatives are important for maintaining good quality of life for the individual, for increased life expectancy, and for limiting societal economic costs. With age as the predominant risk factor for dementia, prevalence of dementia worldwide is expected to increase considerably from 46.8 million in 2015 to 131.5 million by 2040 [1].
Even though mortality is markedly increased in dementia [3], it may not be perceived of as a fatal disease in the same way as acute ischemic heart disease (IHD) and cancer are, for example.
Currently, no cure or disease-modifying treatments for dementia are available [4]. The existing antidementia drugs are only symptomatic, with documented efficacy on Alzheimer’s disease, Lewy body dementia, and Parkinson’s disease with dementia. Dementia research has nonetheless seen a variety of other improvements occur in recent decades, including the acquisition of greater knowledge about the underlying pathophysiological changes, leading to better and more precise diagnostics and, thus, also improvements in the evaluation and management of dementia. Consequently, these changes have been accompanied by increased awareness, a change in the perception of dementia disorders as brain disorders, and greater knowledge about the need for psychosocial support, not to mention improved treatment of comorbidities. As in other high income countries, the most frequent causes of death in Denmark are heart disease and cancer [5, 6]. In the general population, these are perceived as life-threatening, where as a dementia disorder may not be perceived as a fatal condition. In contrast to dementia disorders, the treatment of acute IHD and cancer, including identification of risk factors, early detection, and novel disease modifying interventions, has improved markedly in the last 20 years, leading to a significant decline in mortality. Hence, it is of interest to examine whether improvements in the management of dementia disorders have been accompanied by a decline in mortality in people with dementia and then to put results into a context, comparing with changes in mortality in people with acute IHD and cancer.
Evidence for the time trend of mortality with dementia is scarce [7–11, 21]. Monitoring time trends is challenging as the date of dementia diagnosis must be linked to the date of death at an individual level. Of the six studies investigating changes in mortality in dementia over time, four found a declining trend in mortality rates in dementia [8, 10, 11, 21]. Nevertheless, these results were based on small cohorts, covered only a few years, or included limited observation points in time. National Danish registries are uniquely poised to provide data enabling exploration of time trends of mortality in an entire population over decades [12].
We hypothesized that mortality was higher in people with dementia compared with the general population without dementia. Furthermore, we also sug-gested that the mortality in people with dementia had declined more rapidly than in the general population due to a higher awareness of providing appropriate care. The aim of this study was to assess time trends in all-cause mortality in people with dementia compared to the general elderly population using healthcare data from the entire population of Denmark from 1996–2015. In addition, we aimed to compare all-cause mortality in dementia with all-cause mortality in people with acute IHD and cancer. Finally, our goal was to study the current survival in people with dementia compared to both the general elderly population and people with acute IHD or cancer.
METHODS
Study setting
This cohort study was based on data from national Danish registries. Since 1968, the Danish Civil Registration System has provided all permanent residents with a unique 10-digit personal identification number that includes vital information on an individual level, such as date of birth and death [13]. This number enables individual linkage on demographic and healthcare data for the entire population [13].
Data sources
Danish Civil Registration System data was merged with data from three national registries. The first two, the Danish National Patient Registry and the Psychiatric Central Research Register, began registering data on all hospital admissions in 1977 and 1969, respectively. Since 1995, they have also collected data on contacts to emergency departments and hospital-based outpatient clinics [12]. Date of admission, date of discharge, and primary and optional secondary diagnoses have been registered according to the International Classification of Diseases (ICD), 10th Revision at every patient contact since 1994 [12]. Before then, ICD 8th Revision was in use [12]. The third registry, the Danish National Prescription Registry, has collected data on filled prescription medication since 1995 that includes date of dispensing, dose, strength, and the anatomical therapeutic chemical code [14].
Study period
The observation period was defined as January 1, 1996 to December 31, 2015. For the survival analyses calculating cumulative incidences, we restricted the study period to January 1, 2000 to December 31, 2015 as we were unable to adjust for calendar period in the cumulative incidences and because 1996–2000 had a higher all-cause mortality and incidence of dementia than the subsequent period.
Study population
All Danish residents age ≥65 years as of January 1, 1996 were included in the assessment of mortality. During follow-up, people were included the day they turned 65 and then censored if they died, left the country permanently, were lost to follow-up, or at the end of the study period. When assessing mortality in dementia, individuals registered with dementia age <65 years were excluded due to our focus on late onset dementia, while individuals not registered with dementia served as the reference group. For comparison, mortality and survival were calculated for people age ≥65 years with acute IHD and cancer using the same methodology. Thus, the analysis assessing an-nual mortality rate ratios (MRRs) excluded individuals diagnosed with acute IHD and cancer age <65 years.
The methodology was adjusted in three ways to calculate cumulative incidences of death. First, the study period began January 1, 2000. Second, only in-cidence cases were assessed, which meant that people with dementia, acute IHD, and cancer diagnosed before January 1, 2000 were excluded from their res-pective analyses. Lastly, we constructed a group to represent the general population (see Supplementary Material). Apart from these adjustments, the same criteria for inclusion, exclusion, and censoring were used when assessing MRRs and cumulative incidence of death.
Definition of dementia, acute IHD, and cancer
Dementia was defined as either a registered dem-entia diagnosis (primary or secondary) in the Danish National Patient Registry or the Psychiatric Central Research Register (ICD–8 codes: 290.9–11, 290.18–19, 293.09–19; ICD–10 codes: F00.0–9, F01.0–9, F02.0, F03.9, G30.0–9, G31.8–9) or having filled a prescription for an antidementia drug (ATC-codes N06DA02 (donepezil), N06DA03 (rivastigmine), N06DA04 (galantamine), and N06DX01 (memantine)). Onset date was defined as the first contact with a registered dementia diagnosis, or a prescription filled for an antidementia drug, whichever came first. Denmark has universal tax-funded healthcare and less than one percent of hospital beds are in private hospitals [15]. When people experience symptoms of dementia, they usually make an appointment with their general practitioner, who performs a preliminary examination. If the general practitioner suspects de-mentia, the person is referred to a hospital memory clinic, which performs diagnostic evaluation. If sym-ptoms are obvious and additional workup safely de-emed unnecessary, the general practitioner may assign the diagnosis without referring the patient to the hospital. Another factor is that some people, though a highly limited number, are seen in a private healthcare setting. In an effort to include the last two groups mentioned, we used data on prescriptions filled for antidementia drugs.
The definition of acute IHD and cancer were based on diagnosis codes as defined in the Charlson Comorbidity Index (acute IHD, ICD–8 code: 410, ICD10-codes: I21–23; cancer: ICD–8 codes: 140–189, 195–199, 200–207, 275.59, ICD–10 codes: C00–85, C88, C90–96) [16].
Statistics
Mortality
Time trends in mortality in people with dementia from 1996 to 2015 were assessed by MRRs analyzed using Poisson regression analysis, which is equivalent to a Cox regression [17]. In our first model, the exposure was dementia and the outcome was death. All analyses were stratified by sex, as mortality differs among women and men. We tested whether there was a difference in the time trend of the mortality rates in people with dementia compared to the elderly population without dementia and chose the reference to be the mortality rate for women and men without dementia in 1996 (reference value was 1.00). Our second and third Poisson regression models were used to calculate MRR trends for individuals registered with acute IHD and cancer, respectively, compared to people without either disease using the same methods as described for dementia. Furthermore, we examined differences in change in mortality rates between people with dementia, acute IHD, cancer, and the total elderly population by assigning the reference value of 1.00 to all four groups in 1996. The assumption of piecewise constant intensity in the age groups was verified, as should be the case before applying a Poisson regression analysis [18].
Cumulative incidences
We assessed cumulative incidence rates of death in people registered with dementia, acute IHD, cancer, and in the general elderly population age ≥65 years from 2000–2015 using Kaplan-Meier analyses to calculate survival probabilities for women and men in strata of 5-year age groups (65–69, 70–74, 75–79, 80–84, 85–89, 90+). The Kaplan-Meier methods cannot take changes due to calendar year properly into account. In order to try and limit the effect of changes related to calendar year, we chose to exclude the years 1996–1999 because mortality was highest in this period and our previous study showed a marked increase in incidence of dementia those years as well [19]. This left us with the time period from 2000–2015 when assessing survival. To prevent any identification of individual cases, the two oldest age groups were combined into one group of people aged ≥85 for the Kaplan-Meier curves, thus maintaining anonymity. In figures, the maximum follow-up time was also limited to ten years to preserve anonymity.
Table 1
Distribution of number of deaths, person years, mortality rates, and mortality rate ratios in people with and without dementia who died from 1996–2015 for women and men, for age-standardized 5-year age groups, and age- and sex-adjusted five-year calendar periods
| Variable | Dementia | No dementia | MRRa (95%CI) | ||||
| n | PY | MR | n | PY | MR | ||
| Women | 81,855 | 353,211 | 231.7 | 405,048 | 9,529,139 | 42.5 | 2.77 (2.75 2.80)* |
| Men | 49,466 | 176,418 | 280.4 | 369,622 | 7,415,073 | 49.8 | 3.13 (3.10 3.16)* |
| Age groups | |||||||
| 65–69 | 1767 | 17,127 | 103.2 | 92,770 | 5,390,626 | 17.2 | 5.54 (5.28 5.80)* |
| 70–74 | 7289 | 54,893 | 132.8 | 115,363 | 4,222,806 | 27.3 | 4.81 (4.70 4.93)* |
| 75–79 | 17,564 | 102,533 | 171.3 | 140,657 | 3,230,175 | 43.5 | 3.99 (3.92 4.05)* |
| 80–84 | 31,426 | 140,918 | 223.0 | 152,891 | 2,220,656 | 68.8 | 3.34 (3.30 3.38)* |
| 85–90 | 38,638 | 132,920 | 290.7 | 139,527 | 1,259,591 | 110.8 | 2.72 (2.69 2.75)* |
| 90+ | 34,637 | 81,239 | 426.4 | 133,462 | 620,357 | 215.1 | 2.08 (2.06 2.11)* |
| Time periods | |||||||
| 1996–2000 | 21,909 | 79,664 | 275.0 | 215,235 | 3,908,805 | 55.1 | 2.91 (2.87 2.95)* |
| 2001–2005 | 32,003 | 123,854 | 258.4 | 197,639 | 3,928,311 | 50.3 | 2.89 (2.86 2.93)* |
| 2006–2010 | 38,276 | 157,020 | 243.8 | 184,273 | 4,214,293 | 43.7 | 2.85 (2.82 2.88)* |
| 2011–2015 | 39,133 | 169,092 | 231.4 | 177,523 | 4,892,802 | 36.3 | 2.95 (2.91 2.98)* |
Mortality rates are listed in 1000 person years. MRR, mortality rate ratio; CI, confidence interval; n, number of deaths; PY, person years, MR, mortality rates; a, adjusted/stratified for age, calendar year, and sex; *p < 0.00001.
A p-value was considered significant at 0.05 for all analyses, which were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Approvals and data availability
This study was approved by the Danish Data Protection Agency (ID no.: 2007-58-0015/30-0667), Statistics Denmark, and the Danish Health and Med-icines Authority (ID no.: 6-8011-907/1). As this study used anonymized data, Danish law does not require ethics committee approval or informed patient consent.
This study was based on data from nationwide public registries and, according to Danish law, sharing such datasets is not allowed. To gain access to Danish registry data, individual research projects must seek approval from the Danish Data Protection Agency. Hence, further data sharing of this project is not possible.
RESULTS
Between 1996 and 2015, there were 1,999,366 people (women: 1,079,276; men: 920,090) age ≥65 years living in Denmark. They were observed over 17,541,315 person years (women: 9,912,480; men: 7,628,835), resulting in an average of 8.77 years at risk per person. There were 165,716 people living- with a dementia diagnosis (women: 103,683; men: 62,033), observed over 529,629 person years (wo-men: 353,211; men: 176,418). A total of 15,300 people with dementia was solely identified using prescription data.
In the same period, there were 144,380 people living with an acute IHD diagnosis (women: 65,622; men: 78,758), observed over 665,640 person years (women: 290,760; men: 374,880), and 400,610 people living with a cancer (women: 195,483; men: 205,127), observed over 1,565,110 person years (women: 841,299; men: 723,811).
The number of people with dementia who died was 131,321 (women: 81,855; men: 49,466). In the acute IHD group, the number of deaths was 109,154 (women: 51,172; men: 57,982), and in the cancer group, it was 283,143 (women: 137,834; men: 145,309).
Table 1 presents mortality rates and MRRs stratified by sex, age, and 5-year calendar periods for people with dementia compared with the general population without dementia. Age- and calendar-year-adjusted MRRs were higher for both women and men with dementia. MRRs in dementia decreased with age from 5.54 (95%CI: 5.28–5.80) in people age 65–69 years to 2.08 (2.06–2.11) in people age ≥90 years. Sex- and age-adjusted MRRs were similar in 1996–2000, 2001–2005, 2006–2010, and 2011–2015.
Figure 1 presents the MRR time trends for women and men with and without dementia, acute IHD, and cancer. MRRs were significantly higher in dementia compared with people without dementia throughout the 20-year period. Similar trends were observed for acute IHD and cancer. Adjusted for age, sex, and calendar year, the MRR was 2.91 (95%CI: 2.90–2.93) in people with dementia compared with the general elderly population without dementia.
Fig. 1
Time trend of all-cause mortality for women and men. Graphs showing age-adjusted mortality rate ratios for women and men with dementia, acute ischemic heart disease (IHD), and cancer compared to people without. The reference value was defined as 1.00 in 1996 for women and men without dementia, acute IHD, and cancer. Error bars represent 95%confidence interval.
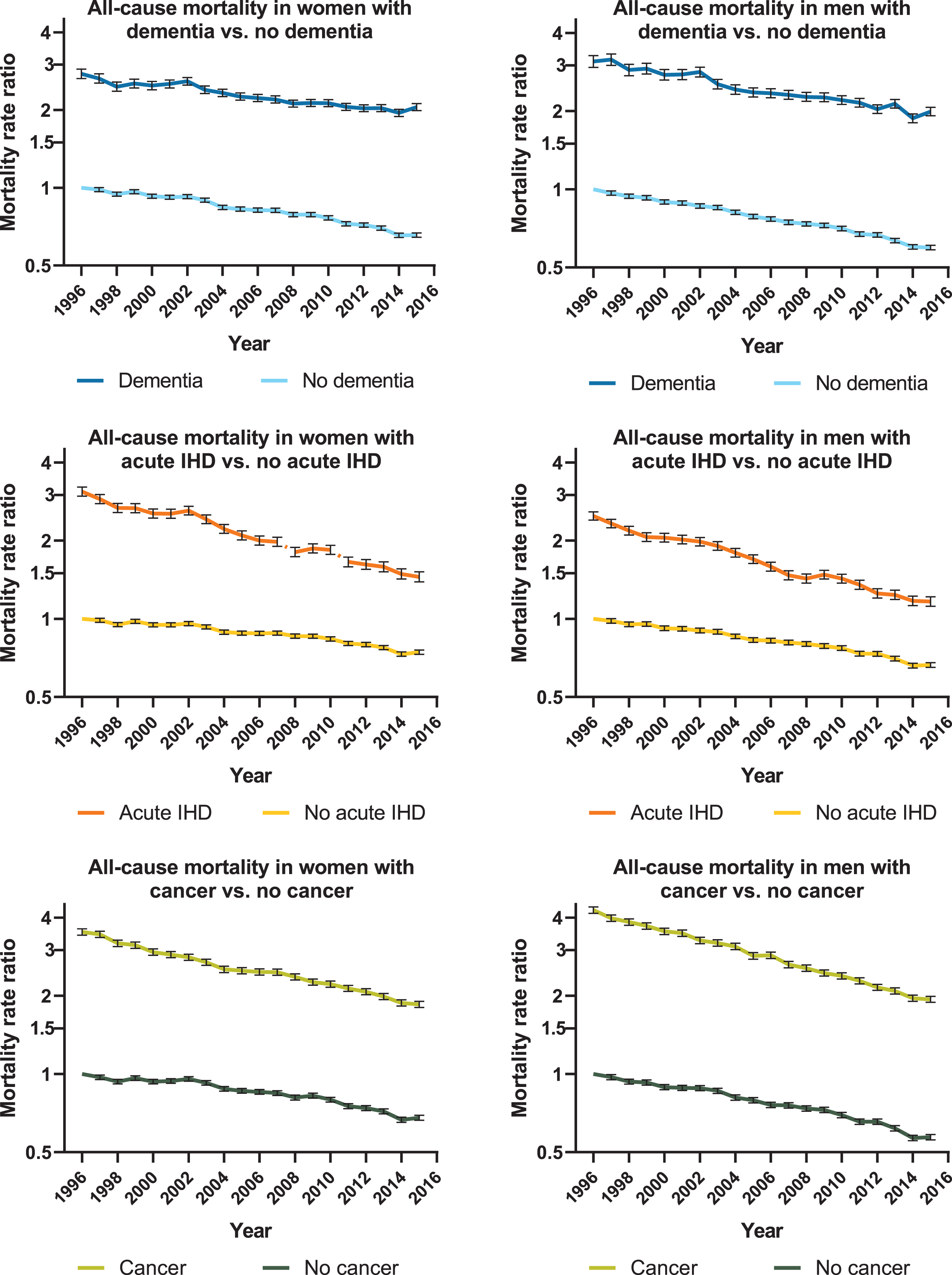
Figure 2 presents MRR time trends using the reference value of 1.00 in 1996 for dementia, acute IHD, cancer, and the total elderly population. For both women and men with dementia, the MRRs de-clined at the same speed as in the general population, just as a similar decline was observed in MRRs for both groups when comparing each year from 1997–2015 to the index year 1996. The MRRs, in contrast, declined significantly faster in acute IHD and cancer compared with the general elderly population.
Fig. 2
Time trend of all-cause mortality for women and men. Age-adjusted mortality rate ratios for women and men with dementia, acute ischemic heart disease (IHD), cancer, and the general elderly population. The reference value was defined as 1.00 in 1996. A) All-cause mortality in women. B) All-cause mortality in men.
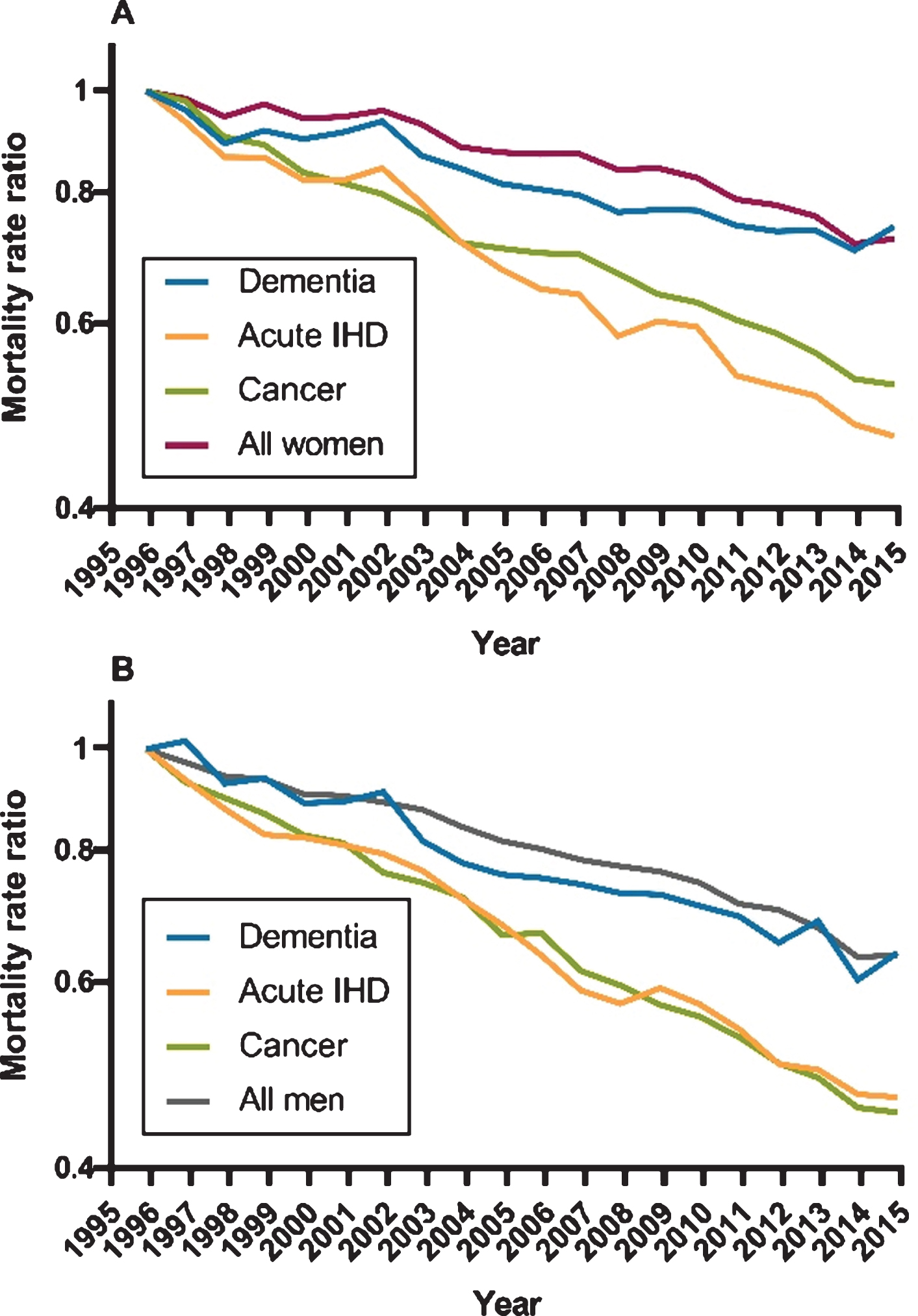
Figure 3 shows Kaplan-Meier curves for women and men with dementia, acute IHD, cancer, and the general elderly population. A steeper decline in survival was observed in the first months after onset of acute IHD and cancer compared with dementia.
Fig. 3
Survival for women and men. Age-stratified Kaplan-Meier survival curves for women and men with dementia, acute ischemic heart disease (IHD), cancer, and the general elderly population, 2000–2015.
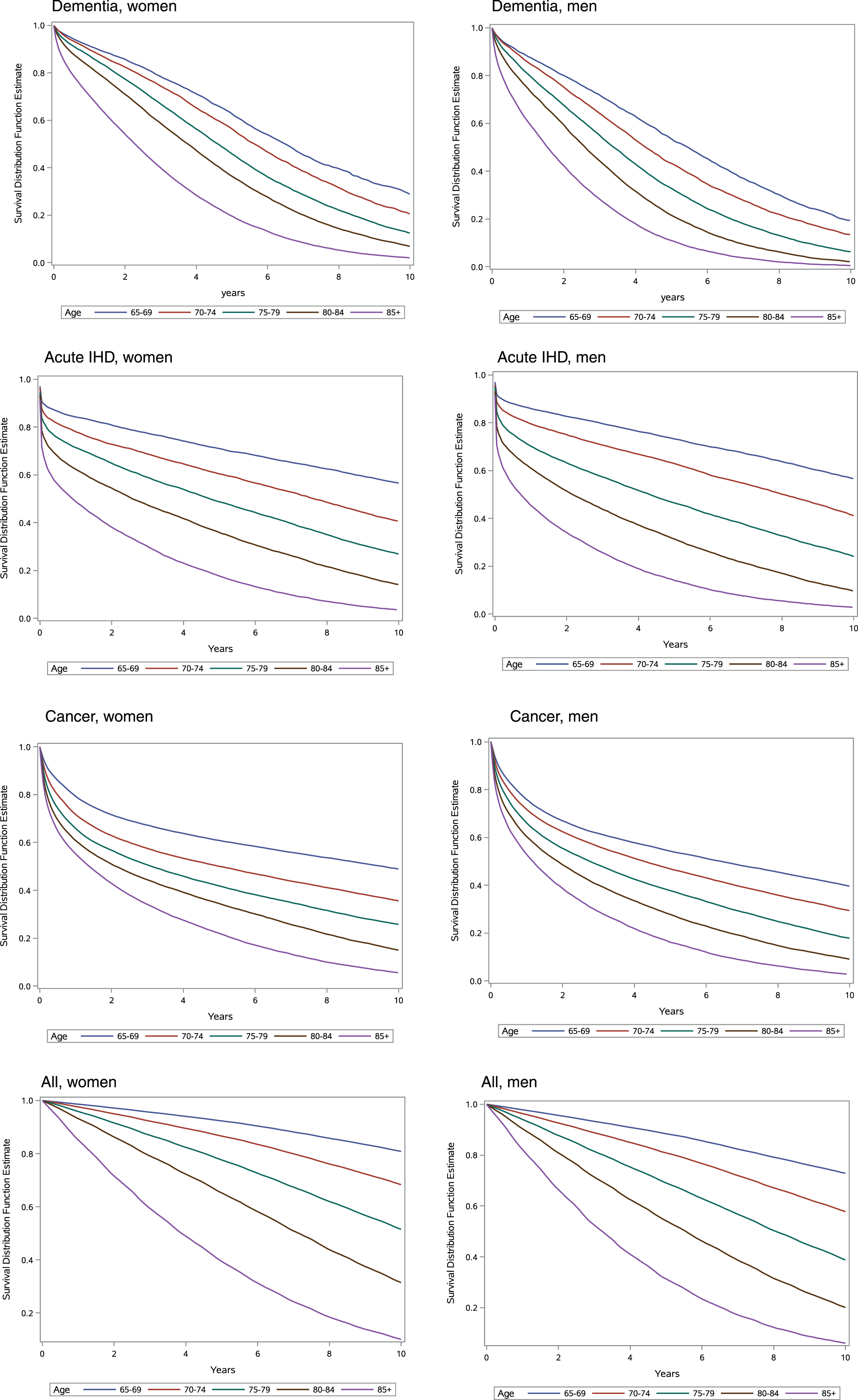
Figure 4 presents 1- and 5-year probabilities of survival in the same groups. In women and men with dementia, the 1-year survival probabilities were lower compared with the whole elderly population in all age groups. However, they were higher in women and men with dementia compared with acute IHD and cancer. The 5-year probability of survival was lower for the dementia, acute IHD, and cancer groups compared with the general elderly population in both sexes for all age strata. In women, the 5-year survival probability was lower in dementia than in acute IHD in women age <80 years, whereas in age ≥80 years, they were comparable. Women with dementia age <85 years had a higher 5-year probability of survival compared with cancer, whereas it was lower in women with dementia age ≥85 years.
Fig. 4
1-year and 5-year survival for women and men. A) Women, 1-year survival probabilities. B) Men, 1-year survival probabilities. C) Women, 5-year survival probabilities. D) Men, 5-year survival probabilities. Survival probabilities presented as 1-year and 5-year for women and men with dementia, acute ischemic heart disease (IHD), cancer, and the general elderly population from 2000–2015. Error bars represent standard errors.
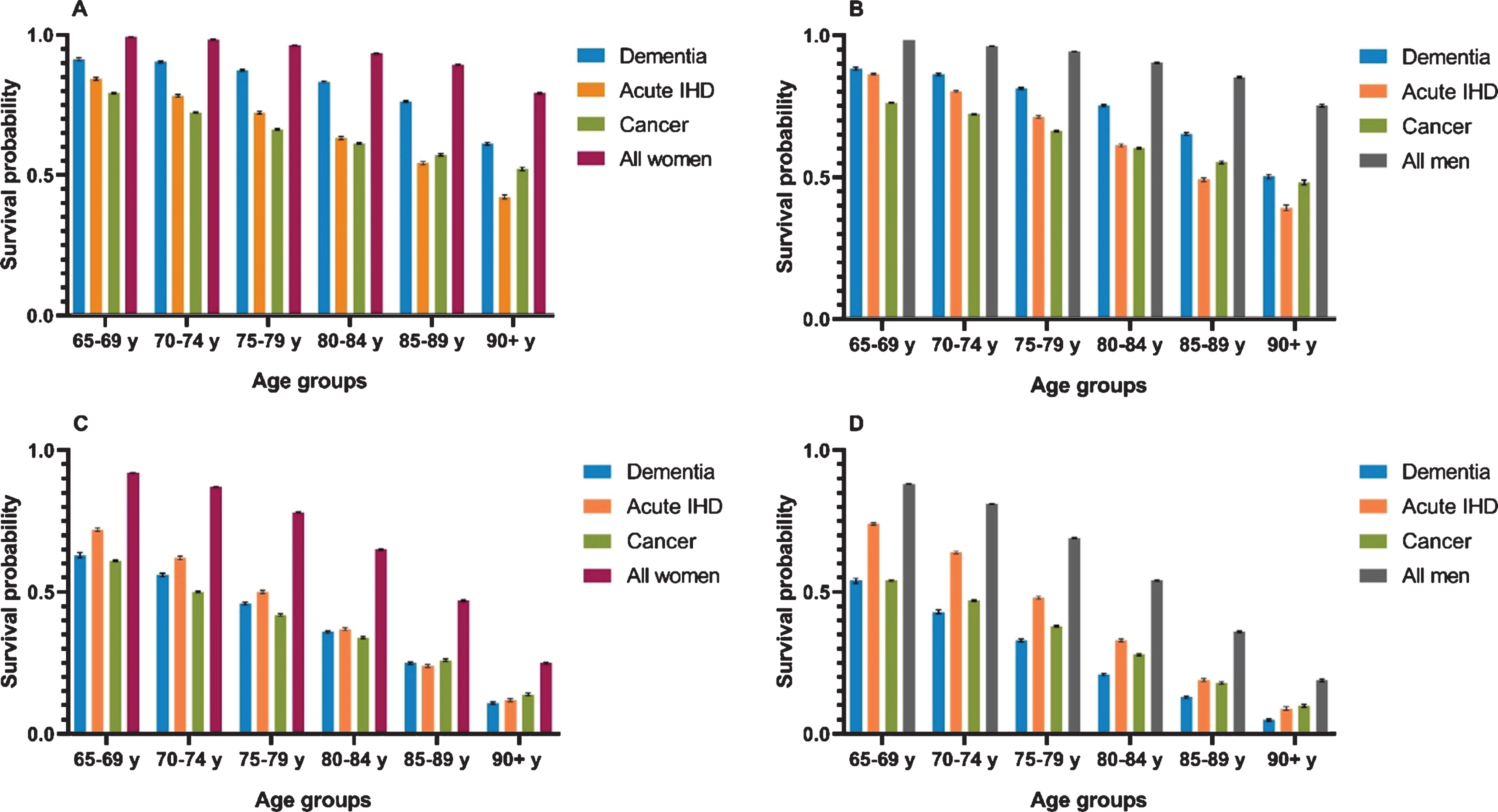
When comparing 5-year survival probabilities in men, they were substantially lower in dementia than in acute IHD. Comparing men with dementia to those with cancer, 5-year survival probabilities were similar in the age group 65–69 years, but in all older age groups, the probability of survival was lower for men with dementia than for men with cancer.
Lastly, we calculated the 50%fractiles of the survival curves, i.e., how many years have passed before half of the population in each subgroup has died (Supplementary Tables 1, 2). In women and men with dementia ≤75 years, the 50%fractiles were comparable to or lower than for those with acute IHD, cancer, and the general elderly population.
DISCUSSION
To our knowledge, this is the first study of time trends in mortality for dementia in an entire national population. In accordance with previous findings, our study confirmed that all-cause mortality rates in dementia were higher than in people without dementia [3]. Although MRRs in dementia declined over the 20-year period, it occurred at the same rate as in those without dementia, leaving the excess mortality in people with dementia unchanged. In contrast, the excess mortality from acute IHD and cancer was significantly reduced during the same period.
Our results highlight the fact that dementia is associated with increased mortality at all ages. Even th-ough the MRRs were higher in the youngest of the elderly when comparing people with and without dementia, a dementia diagnosis was associated with significantly higher MRRs in women and men in all age groups. Furthermore, the 5-year probability of survival after a dementia diagnosis was largely comparable to the 5-year probability of survival after acute IHD or a cancer diagnosis. We believe this age-related difference in MRRs is associated to the fact that the risk of mortality increases with age, which means that it is generally high in people age >90 years. The risk of mortality is also affected by comorbidities, which are more frequent in the most elderly people. Thus, when comparing the people in the youngest dementia groups with the general elderly population in the same age group, the latter will have fewer comorbidities and are at a lower risk of dying. However, in the oldest age groups mortality rates are generally higher and the frequency of comorbidities are similar in people with and without dementia. Thus, in the youngest there is a greater difference in MRR between people with and without dementia because the background mortality is low and, compared to their peers, people with dementia have more comorbidities.
We found that, over a 20-year period, the age- and sex-adjusted MRR was about 2.9 times higher in people with dementia compared with people without dementia. In their meta-analysis, Dewey and Saz estimated a similarly increased mortality risk for people with dementia (odds ratio: 2.63, 95%CI: 2.17–3.21) [20]. However, only few studies have investigated time trends of mortality. Our findings of declining mortality are in line with findings from four population-based studies, one from Sweden, two from the Netherlands, and one from Japan [8, 10, 11, 21]. In contrast, two other studies found a tendency of increasing mortality in dementia. A German study based on health claims registry data from 2006–2007 to 2009–2010 found that mortality with dementia tended to be lower in the first period, although this was only significant in women [9]. This study observed mortality in dementia over a relatively short period. Similarly, a U.S. study based on data from a population-based longitudinal survey observed that the 2-year mortality was higher in individuals with moderate and severe cognitive impairment in 2002–2004 compared with 1993–1995 [7]. However, these results were not significant. To conclude, there is limited evidence of an increase in mortality in dementia, and our study results, based on data from an entire national elderly population over a 20-year period, add weight to the scientific evidence that mortality in dementia is declining.
From 1996–2015, the mortality rates declined more in men compared with women in the groups with dementia, cancer, and the general elderly population. For men and women with acute IHD, the mortality rates declined at a similar rate. Interestingly, life expectancy is higher in women than in men. From 2000–2015, this sex gap in Denmark narrowed by nearly one year [22]. Thus, we believe that the steeper decline in mortality rates seen in most groups of men may be explained by an increased life expectancy in men in general.
Most dementias are progressive, fatal brain diseases, which means that dementia alone contributes to an increased risk of death [23]. It is also well-established that increased mortality in dementia is related to the level of severity and subtype of dementia [24–28]. We did not investigate the potential contributors of other possible causes for the excess mortality in people with dementia found in our study. Previous studies suggest that increased mortality in dementia may also be related to polypharmacy [23, 29], antipsychotics [30], frailty [29, 31], co-occurring chronic conditions [24], and functional impairment [32]. Furthermore, it is possible that insufficient treatment of other health conditions, unhealthy lifestyle, and compromised access to healthcare due to a dem-entia diagnosis may contribute to the excess mortality.
We observed a decline in mortality rates in elde-rly people with and without dementia. There are several possible explanations for this finding. First, the health of the entire elderly population with and without dementia has generally improved. Second, increased awareness about dementia and improved dementia care during our study period may have also contributed to the decline in mortality. Third, an artificial decline in mortality rates of dementia unrelated to improved health may be due to earlier age at diagnosis. If there had been a shift towards earlier diagnosis while overall life expectancy remained unchanged, this would, somewhat misleadingly, re-sult in increased survival after dementia diagnosis. Even though, our previous study on declining incidence of dementia in Denmark showed that median age at diagnosis did not change significantly from 1996 to 2015 [19], it is still possible that diagnoses towards the end of the study were registered at an earlier stage of the disease process.
The mortality gap between people with acute IHD or cancer and the general elderly population was reduced during the same 20-year period. The period covered by the present study saw the development of better and more efficacious treatments, just as the diseases we examined were and are a priority in the Danish healthcare sector, with full government support [33, 34]. For example, the treatment and organization of healthcare for acute IHD improved. From 1997–1998 to 2009–2010, the practice of revascularization (percutaneous coronary intervention) after onset of acute myocardial infarction increased from 2.5%to 38.2%, while the use of statins and clopidogrel also increased even more markedly [34].
Although multiple disease-modifying drugs aga-inst Alzheimer’s disease are in the pipeline, no new medications have been approved since 2003, and numerous attempts to find more effective drugs have failed [35]. Nonetheless, several actions can be taken to possibly reduce mortality in dementia, for instance, better treatment of comorbidities, a reduction in pol-ypharmacy and inappropriate medications, and im-proved access to healthcare to reduce functional impairment and frailty in people with dementia.
This study also assessed survival after first diagnoses. For 1- and 5-year survival probabilities, we observed that survival was higher in the general elderly population, as compared to people with dem-entia, acute IHD, or cancer. Nonetheless, 1-year sur-vival for women and men with dementia was significantly higher than for those with acute IHD and cancer. We believe this reflects the difference in the disease development and time course of the three conditions examined. Acute IHD has the highest mortality on the day of onset and closely after, which is why the general standard for reporting mortality is 30-day survival [36]. Our definition of cancer covers a broad range of cancer diseases with very large differences in disease progression patterns. The reason why the 1-year survival in cancer was lower than for dementia is related to the fact that some cancer types are fatal very early in the course of the disease, e.g., pancreas, liver, brain, lung, and esophagus cancer [37].
In terms of the 5-year survival probabilities, we observed different patterns for women and men when comparing dementia with cancer and acute IHD. In general, the 5-year survival probability for the three disease groups ranged from 0.72–0.11 in women and 0.74–0.04 in men, according to age groups. In women age <80 years, the 5-year survival was lowest in cancer, higher in dementia, and highest in acute IHD, whereas in women age ≥80 years, the three diseases are largely similar.
Overall, being diagnosed with dementia was more dangerous in men than a diagnosis of acute IHD and cancer. We believe that one of the reasons why survival in men with acute IHD was relatively high may be related to our selection of the group. From 1997–2010, the median age of first acute myocardial infarction in men was 64–66 years and 73–75 years in women [34]. The fact that we excluded people with a previous history of acute IHD prior to the age of 65 may have led to selection bias, possibly favoring men who were healthier than the average male with acute IHD.
This study, which is based on routine healthcare data from the secondary sector, has limitations. Hence, for dementia, one of the limitations is that we do not have access to data on, e.g., cognitive status or physical performance status, that may affect mortality in dementia. Studies have shown that mortality differs according to the various subtypes of dementia [24–26]. We would be interested in performing additional analyses investigating subtypes but, even though the dementia syndrome diagnosis has high validity in Danish national registries, the validity of dementia subtype diagnoses is limited [38]. Our definition of cancer is heterogeneous. As mortality varies with cancer subtypes [33], the time trend of mortality may have changed differently for the various subtypes during our study period. Furthermore, the steep decrease in cancer mortality may partly be mediated by earlier time of diagnosis due to screening regimes for some cancer subtypes. In addition, not everyone with dementia is registered in our secondary healthcare registries. Our previous study showed that there were 36,129 people age ≥65 years registered with dementia in Denmark in 2015 [19]. Nevertheless, extrapolations from population-based studies estimate the prevalence of dementia in Denmark to be around 87,000 [39]. Thus, a considerable number of people with dementia are either undiagnosed or diagnosed in primary care only. We hypothesize that possibly unregistered individuals with dementia may represent those with the mildest and the most severe symptoms. The inability to include these groups may have influenced our results. However, having the entire Danish elderly population as our reference group we believe the contamination of the comparison group is limited. Thus, the underreporting of dementia in the registries may only lead to a limited underestimate of the association between dementia and excess mortality.
Among the strengths of our study is the use of data from an entire large, unselected national cohort with minimal dropout, no missing data, and a large sample size. Second, our data is continuous in terms of time, enabling us to assess time trends at yearly intervals. This strengthens our time trend analysis as compared to only comparing two or a few points in time.
The validity of dementia syndrome diagnoses in the Danish registries used in this study has been assessed and found to be correct in 85.8%of cases [38].
We believe our results are generalizable to high-income Western countries with similar improvements in health in the elderly population over the last 20 years and with equal access to healthcare.
Conclusion
This study demonstrated that dementia is associated with a considerably increased mortality in women and men of all age groups. The mortality rates decl-ined for dementia over the 20-year period under study. However, contrary to our hypothesis, we found that the mortality gap between people with dementia and the general elderly population was not reduced, de-spite a stronger focus on appropriate care for people with dementia during the study period. In the future, the discovery of more efficacious disease-modifying treatments may hopefully contribute to lower mortality. In the meantime, initiatives for improving health and decreasing mortality in people with dementia are still highly relevant and can include improving the treatment of comorbidities and the organization of support and healthcare services for people with dementia.
ACKNOWLEDGMENTS
The authors would like to thank the Danish Ministry of Health for supporting the Danish Dementia Research Centre. Additionally, the authors thank Kasper Jørgensen, Danish Dementia Research Centre, for his thorough review of this paper.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0823r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-200823.
REFERENCES
[1] | Prince M , Wimo A , Guerchet M , Ali G , Wu Y , Prina M ((2015) ) World Alzheimer Report 2015 The Global Impact of Dementia. Alzheimer’s Disease International, London. |
[2] | Golinowska S , Groot W , Baji P , Pavlova M ((2016) ) Health promotion targeting older people. BMC Health Serv Res 16: , 4–6. |
[3] | Guehne U , Riedel-Heller S , Angermeyer MC ((2005) ) Mortality in dementia: A systematic review. Neuroepidemiology 25: , 153–162. |
[4] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Jiska P , Phd C-M , Cooper C , Fox N , Gitlin LN , Howard R ((2017) ) The Lancet International Commission on Dementia Prevention and Care. Lancet 390: , 2673–2734. |
[5] | https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. The webpage was accessed on September 25, 2020. |
[6] | Sundhedsdatastyrelsen ((2019) ) Dødsårsagsregisteret 2018 Tal og analyse. |
[7] | Langa KM , Larson EB , Karlawish JH , Cutler DM , Kabeto MU , Kim SY , Rosen AB ((2008) ) Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4: , 134–144. |
[8] | Qiu C , Von Strauss E , Bäckman L , Winblad B , Fratiglionis L ((2013) ) Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology 80: , 1888–1894. |
[9] | Doblhammer G , Fink A , Zylla S , Willekens F ((2015) ) Compression or expansion of dementia in Germany? An observational study of shortterm trends in incidence and death rates of dementia between 2006/07 and 2009/10 based on German health insurance data. Alzheimers Res Ther 7: , 1–11. |
[10] | Ohara T , Hata J , Yoshida D , Mukai N , Nagata M , Iwaki T , Kitazono T , Kanba S , Kiyohara Y , Ninomiya T ((2017) ) Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 88: , 1925–1932. |
[11] | Schrijvers EMC , Verhaaren BFJ , Koudstaal PJ , Hofman A , Ikram MA , Breteler MMB ((2012) ) Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78: , 1456–1463. |
[12] | Schmidt M , Schmidt SAJ , Sandegaard JL , Ehrenstein V , Pedersen L , Sørensen HT ((2015) ) The Danish National patient registry: A review of content, data quality, and research potential. Clin Epidemiol 7: , 449–490. |
[13] | Pedersen CB ((2011) ) The Danish Civil Registration System. Scand J Public Health 39: , 22–25. |
[14] | Pottegård A , Schmidt SAJ , Wallach-Kildemoes H , Sørensen HT , Hallas J , Schmidt M ((2017) ) Data resource profile: The Danish National Prescription Registry. Int J Epidemiol 46: , 798–798f. |
[15] | Schmidt M , Schmidt SAJ , Adelborg K , Sundbøll J , Laugesen K , Ehrenstein V , Sørensen HT ((2019) ) The Danish health care system and epidemiological research: From health care contacts to database records. Clin Epidemiol 11: , 563–591. |
[16] | Thygesen SK , Christiansen CF , Christensen S , Lash TL , Sørensen HT ((2011) ) The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 11: , 83. |
[17] | Iacobelli S , Carstensen B ((2013) ) Multiple time scales in multi-state models. Stat Med 32: , 5315–5327. |
[18] | Andersen PK , Keiding N ((2002) ) Multi-state models for event history analysis. Stat Methods Med Res 11: , 91–115. |
[19] | Taudorf L , Nørgaard A , Islamoska S , Jørgensen K , Laursen TM , Waldemar G ((2019) ) Declining incidence of dementia: A national registry-based study over 20 years. Alzheimers Dement 15: , 1383–1391. |
[20] | Dewey ME , Saz P ((2001) ) Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int J Geriatr Psychiatry 16: , 751–761. |
[21] | van de Vorst IE , Vaartjes I , Bots ML , Koek HL ((2018) ) Decline in mortality in patients with dementia: Results from a nationwide cohort of 44 258 patients in the Netherlands during 2000 to 2008. Int J Geriatr Psychiatry 33: , 1620–1626. |
[22] | OECD/European Observatory on Health Systems and Policies ((2019) ) Denmark: Country Health Profile 2019, State of Health in the EU. |
[23] | Schubert CC , Boustani M , Callahan CM , Perkins AJ , Carney CP , Fox C , Unverzagt F , Hui S , Hendrie HC ((2006) ) Comorbidity profile of dementia patients in primary care: Are they sicker? J Am Geriatr Soc 54: , 104–109. |
[24] | Snowden MB , Steinman LE , Bryant LL , Cherrier MM , Greenlund KJ , Leith KH , Levy C , Logsdon RG , Copeland C , Vogel M , Anderson LA , Atkins DC , Bell JF , Fitzpatrick AL ((2017) ) Dementia and co-occurring chronic conditions: A systematic literature review to identify what is known and where are the gaps in the evidence? Int J Geriatr Psychiatry 32: , 357–371. |
[25] | Brodaty H , Seeher K , Gibson L ((2012) ) Dementia time to death: A systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatrics 24: , 1034–1045. |
[26] | Mueller C , Soysal P , Rongve A , Isik AT , Thompson T , Maggi S , Smith L , Basso C , Stewart R , Ballard C , O’Brien JT , Aarsland D , Stubbs B , Veronese N ((2019) ) Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: A meta-analysis of longitudinal studies. Ageing Res Rev 50: , 72–80. |
[27] | Strand BH , Knapskog AB , Persson K , Edwin TH , Amland R , Mjørud M , Bjertness E , Engedal K , Selbæk G ((2018) ) Survival and years of life lost in various aetiologies of dementia, mild cognitive impairment (MCI) and subjective cognitive decline (SCD) in Norway. PLoS One 13: , 1–14. |
[28] | Andersen K , Lolk A , Martinussen T , Kragh-Sørensen P ((2010) ) Very mild to severe dementia and mortality: A 14-year follow-up - The Odense study. Dement Geriatr Cogn Disord 29: , 61–67. |
[29] | Porter B , Arthur A , Savva GM ((2019) ) How do potentially inappropriate medications and polypharmacy affect mortality in frail and non-frail cognitively impaired older adults? A cohort study. BMJ Open 9: , 1–11. |
[30] | Schneider-Thoma J , Efthimiou O , Huhn M , Krause M , Reichelt L , Röder H , Davis JM , Salanti G , Leucht S ((2018) ) Second-generation antipsychotic drugs and short-term mortality: A systematic review and meta-analysis of placebo-controlled randomised controlled trials. Lancet Psychiatry 5: , 653–663. |
[31] | Sampson EL , Leurent B , Blanchard MR , Jones L , King M ((2013) ) Survival of people with dementia after unplanned acute hospital admission: A prospective cohort study. Int J Geriatr Psychiatry 28: , 1015–1022. |
[32] | Connors MH , Ames D , Boundy K , Clarnette R , Kurrle S , Mander A , Ward J , Woodward M , Brodaty H ((2016) ) Predictors of mortality in dementia: The PRIME Study. J Alzheimers Dis 52: , 967–974. |
[33] | Allemani C , Matsuda T , Di Carlo V , Harewood R , Matz M , Niksić M , Bonaventure A , Valkov M , Johnson CJ , Estéve J , Ogunbiyi OJ , Azevedo E Silva G , Chen WQ , Eser S , Engholm G , Stiller CA , Monnereau A , Woods RR , Visser O , Lim GH , Aitken J , Weir HK , Coleman MP; CONCORD Working Group ((2018) ) Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391: , 1023–1075. |
[34] | Gjesing A , Gislason GH , Køber L , Gustav Smith J , Christensen SB , Gustafsson F , Olsen AMS , Torp-Pedersen C , Andersson C ((2014) ) Nationwide trends in development of heart failure and mortality after first-time myocardial infarction 1997–2010: A Danish cohort study. Eur J Intern Med 25: , 731–738. |
[35] | Cummings J , Lee G , Ritter A , Sabbagh M , Zhong K ((2019) ) Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y) 5: , 272–293. |
[36] | Norum J , Hansen TM , Hovland A , Balteskard L , Haug B , Olsen F , Trovik T ((2017) ) Calculating the 30-day survival rate in acute myocardial infarction: Should we use the treatment chain or the hospital catchment model? Heart Int 12: , e24–e30. |
[37] | John S , Broggio J ((2019) ) Cancer survival in England: Adult, stage at diagnosis and childhood - patients followed up to 2018. |
[38] | Phung TKT , Andersen BBB , Høgh P , Kessing LV , Mortensen PBB , Waldemar G ((2007) ) Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 24: , 220–228. |
[39] | Jørgensen K , Waldemar G ((2014) ) Prævalens af demens i Danmark. Ugeskr Læger 177: , 1041–1044. |




