Social Robot Interventions for People with Dementia: A Systematic Review on Effects and Quality of Reporting
Abstract
Background:
Using non-pharmacological interventions is a current approach in dementia care to manage responsive behaviors, to maintain functional capacity, and to reduce emotional stress. Novel technologies such as social robot interventions might be useful to engage people with dementia in activities and interactions as well as to improve their cognitive, emotional, and physical status.
Objective:
Assessing the effects and the quality of reporting of social robot interventions for people with dementia.
Methods:
In our systematic review, we included quasi-experimental and experimental studies published in English, French, or German, irrespective of publication year. Searching CINAHL, Cochrane Library, MEDLINE, PsycINFO, and Web of Science Core Collection was supplemented by citation tracking and free web searching. To assess the methodological quality of included studies, we used tools provided by the Joanna Briggs Institute. To assess the reporting of the interventions, we applied CReDECI 2 and TIDieR.
Results:
We identified sixteen studies published between 2012 and 2018, including two to 415 participants with mostly non-defined type of dementia. Eight studies had an experimental design. The predominant robot types were pet robots (i.e., PARO). Most studies addressed behavioral, emotion-related, and functional outcomes with beneficial, non-beneficial, and mixed results. Predominantly, cognitive outcomes were not improved. Overall, studies were of moderate methodological quality.
Conclusion:
Heterogeneous populations, intervention characteristics, and measured outcomes make it difficult to generalize the results with regard to clinical practice. The impact of social robot interventions on behavioral, emotion-related, and functional outcomes should therefore be assessed considering the severity of dementia and intervention characteristics.
INTRODUCTION
Social robots are used to support emotional, cognitive, and physical care of people with dementia in order to maintain their independence and to improve their well-being [1, 2]. There are different types of social robots labeled with non-uniform terminology according to their various functions and appearance [3]. Corresponding to their predominantly intended use, social robots can be classified as pet, assistive, humanoid, and telepresence robots [4]. Pet robots are predominantly intended to enhance social interactions with people affected by dementia and, therefore, to improve their emotional state [4, 5]. The functioning of pet robot interventions is based on animal-assisted therapy’s positive effects on agitation and depressive symptoms [6, 7]. A well-known representative of pet robots is PARO, an animal-shaped robot sounding and moving like a baby seal. It has been developed in Japan [8]. Assistive and humanoid robots are principally deployed to support people with dementia in activities of daily living and to improve their quality of life. Such robotic systems are equipped with various tasks such as social interaction, gesticulation, moving, and recognition of facial expression [9, 10]. The use of telepresence robots mainly intends to provide social connectedness, remote support, care, and medical treatment, e.g., by using a two-way camera system. Telepresence robots are intended to reduce social isolation of people with dementia and, therefore, to enhance their quality of life [11].
Studies on the effectiveness of social robots are characterized by multiple types of robots and heterogeneous study populations including different types and stages of dementia, various intervention formats (e.g., grouped or individualized), and various outcomes (e.g., psychological or physical state, quality of life, and medication dose) [1, 2, 12, 13]. Due to the possible impact of study and intervention characteristics on study results, systematic reviews should, therefore, consider the characteristics of interventions. This is necessary to compare between interventions and their potential effects. A recent systematic review of randomized controlled trials focusing on pet robots aiming to support emotional care shows that behavioral and psychological symptoms such as agitation and depression in people with dementia can be significantly reduced [12]. However, there is a lack of detailed information about the stage of disease in which persons with dementia might benefit from pet robot interventions. Similarly, it is not clear who should apply these interventions and how they should be performed [14]. Therefore, 1) the intervention format (e.g., group or individual format, facilitated or non-facilitated by health professionals), 2) the facilitators’ professional background (e.g., trained or non-trained), and 3) the intervention dose (e.g., duration and frequency) sho-uld be reported [15]. Analyzing the reporting of interventions might fill gaps by specifying intervention effects depending on heterogeneous study samples, intervention details, and outcomes [16, 17].
Furthermore, information about theoretical and methodological underpinnings of social robot interventions are crucial in order to explore the rel-ationship between the intervention and its potential effects as well as to guide the implementation of social robots into clinical practice [18, 19]. The theoretical underpinning and systematic development of social robot interventions should be accompanied by a sound ethical consideration. This is required due to the high vulnerability of this patient group [20, 21]. To clarify to what extent the needs of people with dementia concerning social robot interventions are taken into account, a comprehensive analysis is necessary [19, 21–23]. Thereby, the concerns of formal and informal caregivers of persons with dementia should also be considered. The needs of people with dementia might refer to the social robot’s intended use, appearance, functions, and way of delivery [24]. Considering these needs is indispensable to ensure that social robot interventions have a positive impact on persons with dementia and seem meaningful to them [5, 25]. An ethically responsible implementation of social robot interventions into clinical practice requires to systematically involve persons with dementia in the development of interventions [26].
Up to now, no review investigated the effectiveness of social robot interventions in dementia care in combination with the quality of reporting on the development and evaluation of these interventions. Given the innovative character of social robots in clinical practice, the inclusion of all interventional study designs allows a comprehensive analysis of the available experimental research.
The objectives of this review were to 1) analyze available studies and determine the effectiveness of social robot interventions for people with dementia, 2) to critically appraise the studies, and 3) to assess the quality of reporting.
METHODS
Design
We conducted a systematic review registered in PROSPERO (CRD42019124814, 10 April 2019). The PRISMA statement guided the reporting of this review [27].
Eligibility criteria
We included publications with baseline and post-intervention data (e.g., experimental studies [RCT], quasi-experimental studies [CCT, pre-post trial], qualitative studies, case studies). We were interested in studies aiming to investigate the effects of social robot interventions in people with dementia, published in academic journals in English, French, or German, irrespective of publication year. Concerning the study population, we set no limit concerning the type of dementia and the number of participants. If the sample comprised also people without dementia, separate extraction of data concerning people with dementia should be possible.
We defined social robot interventions as programs integrating the use of a robotic system using the following categorization [4]: pet robot, humanoid robot, and telepresence robot. All types of control groups except for other social robot interventions were included. We considered studies addressing affective, behavioral, cognitive, physical, and psychological outcomes. We therefore excluded outcomes addressing acceptability, usability, and perception of social robots.
Information sources
We performed a comprehensive literature search combining electronic database search and supplementary search methods [28] with database-specific search strategies in MEDLINE via Web of Science, CINAHL, PsycINFO via Ovid, Web of Science Core Collection, and Cochrane Library (November 2018). We searched further studies by forward and backward citation tracking of included studies. For citation tracking, we used Scopus since it might cover the largest number of relevant citations for the purpose of our review [29]. If a reference was not indexed in Scopus, we tracked 1) backward citation manually and 2) forward citation by means of Google Scholar. Additionally, we conducted a free web search via Google Scholar using relevant free text terms in order to identify further studies.
Search
Our search strategy was based on database-specific controlled vocabulary, keywords, and unspecified free search terms. The identification of search terms was based on an orienting search, literature, and the experience of the working group members. To identify relevant search terms, we used MeSH-Browser, COREMINE Medical, and a thesaurus. For unspecified free search terms, we used title, abstract, and keywords search fields, if available. To avoid neglecting publications not yet tagged with controlled vocabulary, we entered controlled vocabulary in these search fields [30]. For free web searching, we used the terms “dementia” and “robot*”. We reviewed and adapted the search strategy by using PRESS [31]. The complete final search strategy is provided in Supplementary Material 1, 2.
Study selection
Two researchers independently screened titles and abstracts of all references as well as full texts of relevant ones for inclusion. We discussed conflicting results within the screening team and, if necessary, with another member of the working group. Additionally, we contacted study authors if we had doubts whether people with dementia were included.
Data collection process
One researcher extracted study characteristics, study results, and the description of interventions. A second researcher checked the extracted data. For the extraction of study characteristics and study results, we developed a standardized, review-specific data extraction sheet. To describe the completeness of intervention reporting, we applied the revised guideline of Criteria for Reporting the Development and Evaluation of Complex Interventions in Healthcare (CReDECI 2) [16] and the Template for Intervention Description and Replication (TIDieR) [17]. By using the CReDECI 2 checklist, we intended to describe the complexity of the given social robot intervention (including training for facilitators, information of staff and/or relatives, etc.). By applying the TIDieR checklist, we delineated the reporting of the explicit social robot session for people with dementia without considering other potential intervention components such as training or information. To meet the CReDECI 2 and TIDIieR criteria, we defined a set of min-imum required information (Supplementary Material 4). Additionally, we assessed the reporting on ethical issues in the included studies. For this purpose, three authors iteratively developed a data extraction sheet including data on ethical approval, informed consent, ongoing consent, and authors’ discussion of ethical issues.
Methodological quality of included studies
To assess the methodological quality of included studies, we used the critical appraisal tools of the Joanna Briggs Institute. Thereby, we determined the extent to which a study addressed the possibility of bias in its design, conduct, and analysis (JBI) [32]. Two researchers appraised the studies independently. They clarified conflicting results with another member of the working group.
Synthesis of study results
We narratively synthesized participants’ aggrega-ted study data based on tabular data extraction. For reasons of clarity, we structured the analysis according to study outcomes. Since the study characteristics indicate the clinical heterogeneity of the interven-tions, we refrained from data pooling. To appropriately report the complexity and heterogeneity of included studies, we graphically summarized effects, critical appraisal, intervention and study characteristics by means of a Harvest plot [33, 34].
RESULTS
Literature search and study selection
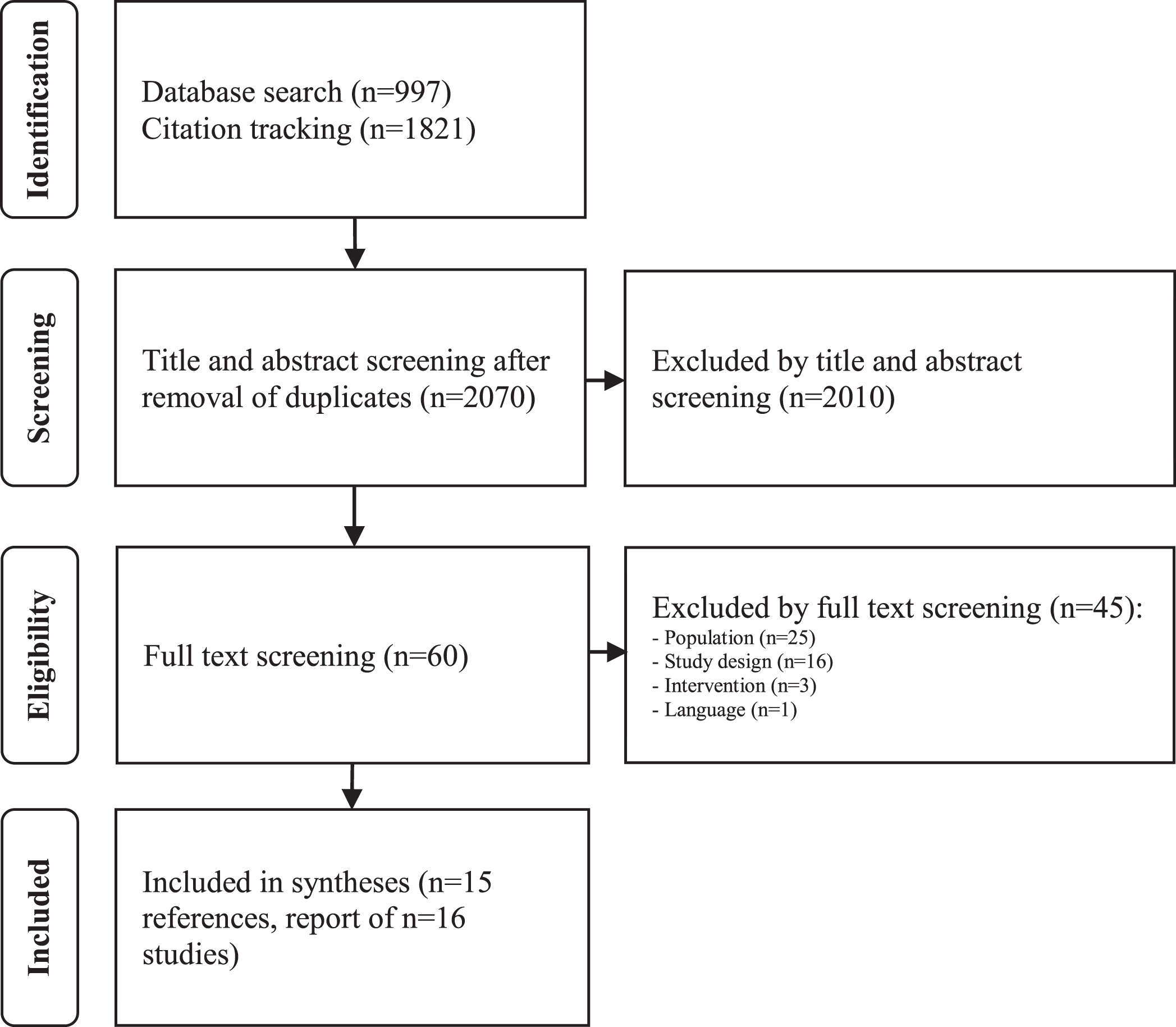
Our comprehensive literature search using dat-abases and citation tracking revealed 2,818 references after removal of duplicates. We screened sixty references by full text, thereby excluding 45. The most frequent reasons for exclusion concerned population (n = 24) and study design (n = 16). Free web searching yielded no further studies to be included. Finally, we included 15 references, all of them identified by database searching. These 15 references report on 16 studies [35]. Figure 1 shows the literature search and study selection process in detail.
Fig. 1
Search and study selection process.
Study and intervention characteristics
Detailed characteristics of the studies are displayed in Supplementary Material 3.
The included studies were published between 2012 and 2018. More than half of them were published since 2016 (n = 9, [36–44]) and were conducted in Australia [37, 39, 44, 45], France [36, 41, 46], Norway [42, 47], Japan [43], the Netherlands [48], New Zealand [38], Spain [35], Sweden [49], and the USA [40].
Eight studies used an experimental design. Four of them were designed as Cluster-RCTs [37, 39, 42, 47], two as RCTs [38, 40], one as individual Crossover-RCT [45], and one as Crossover-Cluster-RCT [35]. Another eight studies were quasi-experimental, four pre-post trials [35, 36, 41, 48] and four pre-post case studies [43, 44, 46, 49]. One pre-post trial used a repeated measures design (ABAB) whereby phase A served as a control group [48]. The setting was mainly long-term care (n = 10) [35, 37, 39, 40, 42, 44, 45, 47–49]. Three studies were conducted in a geriatric hospital [36, 41, 46], each one of them in a day care center [35], a group home [43], and combined in a day care center and at home [38].
For three reasons, the total number of cited studies varies from 16 concerning the following characteristics: 1) Two studies were presented within one reference [35]. These two studies consisted of two study phases with different study characteristics. Therefore, the total number of participants as well as the characteristics of intervention groups and classifications of social robot increased (n = 18). Furthermore, 2) seven studies [35, 36, 41, 43, 44, 46] had no control groups. Three studies comprised two control groups [35, 37, 39]. 3) The remaining studies, except for two [36, 44], examined effects on multiple outcomes.
The number of participants ranged between two and 415. In total, 1,426 persons participated (mean: n = 80; standard deviation: 127). Half of the studies involved 25 or more participants. Ten studies did not report any details about participants’ type of dementia [38–42, 45–49]. In five studies, people with different types of dementia were involved [35–37, 44]. One study included people with Alzheimer’s disease [43]. Participants’ severity of dementia also varied. Eight studies involved people with severe dementia, besides others [35, 42, 45–49]. In five studies, people with mild and/or moderate dementia were included [36, 40, 41, 43, 44]. Three reports did not yield information about participants’ severity of dementia [37–39].
In fourteen studies, the intervention was conducted with a pet robot [35–40, 42, 44, 45–49]. Twelve studies used PARO [35–40, 42, 45–48], one study a pet robot called CuDDler [44], and another study a pet robot called JustoCat [49]. Three studies applied an intervention with a humanoid robot called NAO [35, 41]. One of those studies used PARO and NAO in combination [35]. A telepresence robot called Telenoid was used in one intervention study [43]. Table 1 provides an overview of different types of social robots that were used in the included studies.
Table 1
Description of the different types of social robots used in the included studies
| Type | Description |
| CuDDler | Pet robot, robotic teddy bear, moves its neck, arms, eyelids, limbs and vocally interacts with a growl, developed in Singapore [44]. |
| NAO | Humanoid robot, can use oral language (phrases previously recorded) and move like a human, developed in France [35]. |
| PARO | Pet robot, animal-shaped robot, does not use oral language but sounds and moves like an animal/baby seal, developed in Japan [35]. |
| Telenoid | Telepresence robot, alien-shaped robot, transmits voices, mimics, and head motions using an internet connection, developed in Japan [43]. |
| JustoCat | Pet robot, robotic cat developed based on PARO, purrs, meows, and breaths, developed in Sweden [49]. |
The control interventions varied: nine studies offered usual care [35, 37–39, 40, 42, 45, 47, 48], two studies used a plush toy [37, 39], one study used a dog [35], and another study used reading activity [45]. Six of nine studies described usual care in detail that is displayed in the Supplementary Material [35, 37–40, 45].
With regard to outcomes, the examined effects of social robot interventions were grouped into six domains: 1) behavioral outcomes, i.e., neuropsychiatric symptoms [35, 38, 43, 46], agitation [38, 39, 44, 47, 49], apathy [35, 45, 46], anxiety [39, 40, 45], disturbant behavior [43], and wandering [45]; 2) emotion-related outcomes [36, 41], mood [39, 48], and depressive symptoms [38, 40, 45–47]; 3) well-being [41] and quality of life [35, 42, 43, 45, 49]; 4) functional outcomes such as biochemical indicators for stress and arousal [38, 40], activities of daily living [43, 48], motor activity [37], sleep patterns [37], and weight [46]; 5) medication outcomes, i.e., medication dose [40] and psychotropic [42] or dementia-related medication [38]; 6) cognition [35, 38, 40, 43]. Time of outcome measurement varied between baseline and 24 weeks.
Reporting of the interventions
Assessment by means of CReDECI 2
Table 2 displays the reporting assessment using CReDECI 2. Besides the description of all intervention components of each included study [45] and the contextual characteristics of intervention modelling in two studies [38, 46], no further aspects concerning intervention development were adequately reported. Ten studies were designed as pilot studies [35, 36, 38, 41, 43, 44–46, 49]. Three of the remaining six studies adequately reported the underlying pilot-test and its impact on the definite intervention [37, 39, 48]. No study described the control group intervention and the reason for its selection in detail.
Table 2
Reporting assessment of included studies by means of CReDECI 2 (n = 16)
| Description of . . . | Demange | Moyle | Liang | Moyle | Petersen | Rouaix | Jøranson | Kuwamura | Moyle | Bemelmans | Gustafsson | Jøranson | Valenti | Valenti | Moyle | Sant’Anna |
| et al.,2018 [36] | et al.,2018 [37] | et al.,2017 [38] | et al.,2017 [39] | et al.,2017 [40] | et al.,2017 [41] | et al.,2016 [42] | et al.,2016 [43] | et al.,2016 [44] | et al.,2015 [48] | et al.,2015 [49] | et al.,2015 [47] | Soler et al.,2015 [35]: Nursing home | Soler et al.,2015 [35]: Day care center | et al.,2013 [45] | et al.,2012 [46] | |
| First stage - Development | ||||||||||||||||
| . . . the intervention’s underlying theoretical basis | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| . . . all intervention components | N | N | N | N | N | N | N | N | N | N | N | N | N | N | Y | N |
| . . . any intended interactions between different components | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| . . . the context’s characteristics in intervention modelling | N | N | Y | N | N | N | N | N | N | N | N | N | N | N | N | Y |
| Second stage - Feasibility and piloting | ||||||||||||||||
| . . . the pilot-test and its impact on the definite intervention | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Third stage –Evaluation | ||||||||||||||||
| . . . the control condition (comparator) and reasons for the selection | NA | N | NA | N | N | NA | N | NA | NA | N | NA | N | NA | NA | NA | NA |
| . . . the strategy for delivering the intervention | NA | Y | NA | Y | Y | NA | Y | NA | NA | Y | NA | Y | NA | NA | NA | NA |
| . . . all materials or tools used for the delivery of the intervention | NA | N | NA | N | N | NA | N | NA | NA | N | NA | N | NA | NA | NA | NA |
| . . . fidelity of the delivery process compared to the study protocol | NA | N | NA | N | N | NA | N | NA | NA | N | NA | N | NA | NA | NA | NA |
| . . . a process evaluation and its underlying theoretical basis | NA | Y | NA | Y | N | NA | N | NA | NA | N | NA | N | NA | NA | NA | NA |
| . . . internal facilitators and barriers potentially influencing the delivery of the intervention as revealed by the process evaluation | NA | N | NA | N | N | NA | N | NA | NA | N | NA | N | NA | NA | NA | NA |
| . . . external conditions or factors occurring during the study which might have influenced the delivery of the intervention | NA | N | NA | N | N | NA | N | NA | NA | Y | NA | N | NA | NA | NA | NA |
| . . . costs for the delivery of the intervention | N | Y | N | Y | N | N | N | N | N | N | N | N | N | N | Y | N |
N, no, not reported; NA, not applicable; Y, yes, reported.
Three studies reported costs for the delivery of the intervention [37, 39, 45]. All six studies described the strategy for delivering the intervention [37, 39, 40, 42, 47, 48]. None of the six studies described all materials used for the delivery of the intervention or the fidelity of the delivery process compared to the study protocol. A process evaluation and its underlying theoretical basis was described in two studies [37, 39]. None of those reported internal facilitators and barriers potentially influencing the delivery of the intervention as revealed by the process evaluation. One study described factors which might have influenced the delivery of the intervention [48]. Supplementary Material 4.1 displays the detailed reporting assessment using CReDECI 2.
Assessment by means of TIDieR
Table 3 displays the reporting assessment using TIDieR. All study reports provided the name of the social robot. An underpinning theoretical approach and rationale to develop the robot session and its characteristics was presented in none of the included studies. The procedures and materials needed for the robot session were reported in all studies. Five study reports provided details about the expertise, background, and any specific training given to the person who delivered the robotic session [35, 43–45]. Information about the format of the robot session was given in all studies: individual facilitated sessions in six studies [36, 43, 44, 46, 48, 49], grouped facilitated sessions in five studies [38, 40, 42, 45, 47], individual non-facilitated sessions in three studies [37, 39, 41] as well as the combination of grouped and individual facilitated sessions in two studies [35].
Table 3
Reporting assessment of included studies by means of TIDieR (n = 16)
| Demange | Moyle | Liang | Moyle | Petersen | Rouaix | Jøranson | Kuwamura | Moyle | Bemelmans | Gustafsson | Jøranson | Valenti | Valenti | Moyle | Sant’Anna | |
| et al.,2018 [36] | et al.,2018 [37] | et al.,2017 [38] | et al.,2017 [39] | et al.,2017 [40] | et al.,2017 [41] | et al.,2016 [42] | et al.,2016 [43] | et al.,2016 [44] | et al.,2015 [48] | et al.,2015 [49] | et al.,2015 [47] | Soler et al.,2015 [35]:Nursing home | Soler et al.,2015 [35]:Day care center | et al.,2013 [45] | et al.,2012 [46] | |
| Brief name | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Why | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| What: Materials | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| What: Procedures | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Who provided | N | N | N | N | N | N | N | Y | Y | N | N | N | Y | Y | Y | N |
| How | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Where | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | Y | N | N | N | Y |
| When and how much | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y |
| Tailoring | Y | Y | Y | Y | - | Y | NA | Y | Y | Y | Y | - | Y | Y | NA | - |
| Modifications | - | NA | - | NA | - | NA | NA | - | - | - | - | - | NA | NA | - | - |
| How well (planned) | - | - | - | - | - | Y | - | - | - | - | Y | - | - | - | Y | - |
| How well (actual) | - | - | - | Y | - | Y | Y | - | - | - | - | Y | - | - | - | - |
NA, not applicable because the intervention was not tailored or modified; N, no, not reported; Y, yes, reported; -, unclear whether it was conducted.
The location where the intervention was delivered was not reported in five studies [35, 45, 48, 49]. In eight studies, the intervention was delivered in the patients’ or a separate room [36, 38, 40–42, 44, 46, 47]. Two studies reported intervention delivery wherever the participant was at the time of intervention [37, 39]. In one study, the intervention took place in the public space of a group home [43].
The frequency of the social robot intervention ranged from one to three times a week [35–40, 42–47], consisted of three [41] or ten [48] single sessions or was not reported [49]. The duration of the intervention varied between 15 and 45 minutes or was not reported in two studies [48, 49]. The intervention period varied between one and twelve weeks. In half of the studies the intervention period was ten weeks or longer [35, 37–39, 40, 42, 43, 47].
In three studies it was unclear whether the intervention was tailored or not [40, 46, 47]. All studies, except for two [42, 45], tested tailored interventions. Six interventions were not modified [35, 37, 39, 41, 42]. Other study reports did not provide information concerning modification of interventions. Strategies to improve intervention fidelity were described in three studies [41, 45, 49]. Results about intervention fidelity were mentioned in four studies [39, 41, 42, 47]. Supplementary Material 4.2 displays the detailed reporting assessment using TIDieR.
Assessment of ethical issues
Table 4 displays the reporting assessment of ethical issues in the included studies. In three studies [36, 41, 46], it was judged as unclear whether ethical approval was obtained or not. All studies reported that informed consent was obtained, whereby people with dementia participated in ten studies [35–37, 39–42, 47, 49]. All studies, except for one, involved family members, next-of-kins, informal caregivers, legal representatives, and guardians of people with dementia or health professionals. In one study, solely people with dementia were involved in the process of informed consent [41]. However, detailed information on how informed consent was obtained is missing in all studies. Five studies [36, 37, 39, 44, 48] reported whether ongoing consent was obtained or not. One study [36] provided detailed information on the process of ongoing consent (i.e., how ongoing consent was obtained). None of the studies reported on authors’ discussions of ethical issues. Supplementary Material 4.3 displays the detailed reporting on ethical issues.
Table 4
Reporting assessment on ethical issues in the included studies (n = 16)
| Was ethical approval obtained? | Was informed consent obtained? | Who provided informed consent? | How was informed consent obtained regarding procedures? | Was ongoing consent/assent obtained? | How was ongoing consent/assent obtained regarding procedures? | Were ethical issues discussed by the authors? procedures? | |
| Demange et al., 2018 [36] | ? | Y | L, P | ? | Y | Y | N |
| Moyle et al., 2018 [37] | Y | Y | F, P | ? | Y | ? | N |
| Liang et al., 2018 [38] | Y | Y | F | ? | ? | ? | N |
| Moyle et al., 2017 [39] | Y | Y | F, P | ? | Y | ? | N |
| Petersen et al., 2017 [40] | Y | Y | F, P | ? | ? | ? | N |
| Rouaix et al., 2017 [41] | ? | Y | P | ? | ? | ? | N |
| Jøranson et al., 2016 [42] | Y | Y | F, P | ? | ? | ? | N |
| Kuwamura et al., 2016 [43] | Y | Y | F, H | ? | ? | ? | N |
| Moyle et al., 2016 [44] | Y | Y | L | ? | Y | ? | N |
| Bemelmans et al., 2015 [48] | Y | Y | L | ? | Y | ? | N |
| Gustafsson et al., 2015 [49] | Y | Y | F, P | ? | ? | ? | N |
| Jøranson et al., 2015 [47] | Y | Y | F, P | ? | ? | ? | N |
| Valenti Soler et al., 2015 [35]: | Y | Y | F, L, P | ? | ? | ? | N |
| Nursing home | |||||||
| Valenti Soler et al., 2015 [35]: | Y | Y | F, L, P | ? | ? | ? | N |
| Day care center | |||||||
| Moyle et al., 2013 [45] | Y | Y | F, L | ? | ? | ? | N |
| Sant’Anna et al., 2012 [46] | ? | Y | F | ? | ? | ? | N |
?, no (detailed) information; H, health professional; N, no, not reported; F, family member, next-of-kin, informal caregiver; L, legal representative, guardian; P, person with dementia; Y, yes, reported.
Methodological quality of included studies
Table 5 illustrates the methodological quality of included experimental trials [35, 37–40, 42, 45, 47]. Except for two studies, true randomisation [42, 47] and concealed allocation [35, 40] were reported. Blinding of participants and deliverers of the intervention was judged as not applicable. Outcome assessors were blinded in four studies [35, 37, 39, 45]. Complete follow-up or dropouts were unclear in two studies [38, 40]. Furthermore, it was unknown whether an intention to treat analysis was conducted in these two studies. All studies fulfilled the remaining criteria (Table 5).
Table 5
Critical appraisal using the JBI tool for experimental trials (n = 8)
| Moyle et al., | Liang et al., | Moyle et al., | Petersen et al., | Jøranson et al., | Jøranson et al., | Valenti Soler et al., | Moyle et al., | |
| 2018 [37] | 2017 [38] | 2017 [39] | 2017 [40] | 2016 [42] | 2015 [47] | 2015 [35] | 2013 [45] | |
| True randomisation | Y | Y | Y | Y | U | U | Y | Y |
| Concealed allocation | Y | Y | Y | U | Y | Y | U | Y |
| Groups similar at baseline | Y | Y | Y | Y | Y | Y | Y | Y |
| Participants blinded | NA | NA | NA | NA | NA | NA | NA | NA |
| Deliverers of treatment blinded | NA | NA | NA | NA | NA | NA | NA | NA |
| Outcome assessors blinded | Y | U | Y | U | N | N | Y | Y |
| Groups treated identically | Y | Y | Y | Y | Y | Y | Y | Y |
| Complete follow-up | Y | U | Y | U | Y | Y | Y | Y |
| Intention to treat analysis | Y | U | Y | U | Y | Y | Y | Y |
| Outcome measurement identically for groups | Y | Y | Y | Y | Y | Y | Y | Y |
| Reliable outcome measurement | Y | Y | Y | Y | Y | Y | Y | Y |
| Appropriate statistical analysis | Y | Y | Y | Y | Y | Y | Y | Y |
| Appropriate trial design | Y | Y | Y | Y | Y | Y | Y | Y |
| Overall Y (%) | 85 | 62 | 85 | 54 | 69 | 69 | 77 | 85 |
NA, not applicable; N, no; U, unclear; Y, yes.
Table 6 illustrates the methodological quality of included quasi-experimental trials, i.e., one controlled trial without randomization [48] and three pre-post trials [36, 41, 35]. Completeness of follow-up was unclear in two studies [41, 35]. In one study, it was unclear whether outcome measurement was applied in a reliable way due to lacking details [48]. All studies fulfilled the remaining criteria (Table 6).
Table 6
Critical appraisal using the JBI tool for quasi-experimental trials (n = 4)
| Demange et al., | Rouaix et al., | Bemelmans et al., | Valenti Soler et al., | |
| 2018 [36] | 2017 [41] | 2015 [48] | 2015 [35] | |
| Similar participants | Y | Y | Y | Y |
| Similar treatment | Y | Y | Y | Y |
| Control group | N | N | Y | N |
| Outcome measurement pre and post intervention | Y | Y | Y | Y |
| Complete follow-up | Y | U | Y | U |
| Similar outcome measurement | Y | Y | Y | Y |
| Reliable outcome measurement | Y | Y | U | Y |
| Appropriate statistical analysis | Y | Y | Y | Y |
| Overall Y (%) | 88 | 75 | 88 | 75 |
N, no; U, unclear; Y, yes.
Table 7 displays the methodological quality of included pre-post case studies [43, 44, 46, 49]. Demographics and clinical information of participants were clearly reported in two studies [43, 44]. Outcomes were reported in all studies and the setting was described in three of four studies [43, 44, 49].
Table 7
Critical appraisal using the JBI tool for pre-post case studies (n = 4)
| Kuwamura | Moyle | Gustafsson | Sant’Anna | |
| et al., 2016 [43] | et al., 2016 [44] | et al., 2015 [49] | et al., 2012 [46] | |
| Clear reporting of demographics of participants | Y | Y | N | N |
| Clear reporting of clinical information of participants | Y | Y | N | N |
| Outcomes clearly reported | Y | Y | Y | Y |
| Clear reporting of setting | Y | Y | Y | N |
| Overall Y (%) | 100 | 100 | 50 | 25 |
N, no; Y, yes.
Effects of social robot interventions
Study results vary in terms of social robot intervention, outcome, severity of dementia, setting and intervention format. Figure 2 shows a harvest plot illustrating the benefit and the methodological quality of the included studies at a glance. In our harvest plot, we clustered results of social robot interventions per outcome (no benefit/benefit/mixed results) with information concerning sample characteristics (severity of dementia), intervention format (facilitated/non-facilitated), study design and the methodological quality of each study. In the following sections, we narratively summarize the results on the effects of social robot interventions for people with dementia, separately for each outcome domain and for each robot type according to our harvest plot (Fig. 2). Details on effect size and precision for each outcome are displayed in Supplementary Material 3.
Fig. 2
Harvest plot illustrating the benefit and internal validity of included studies (n = 16) clustered by type of social robot intervention.
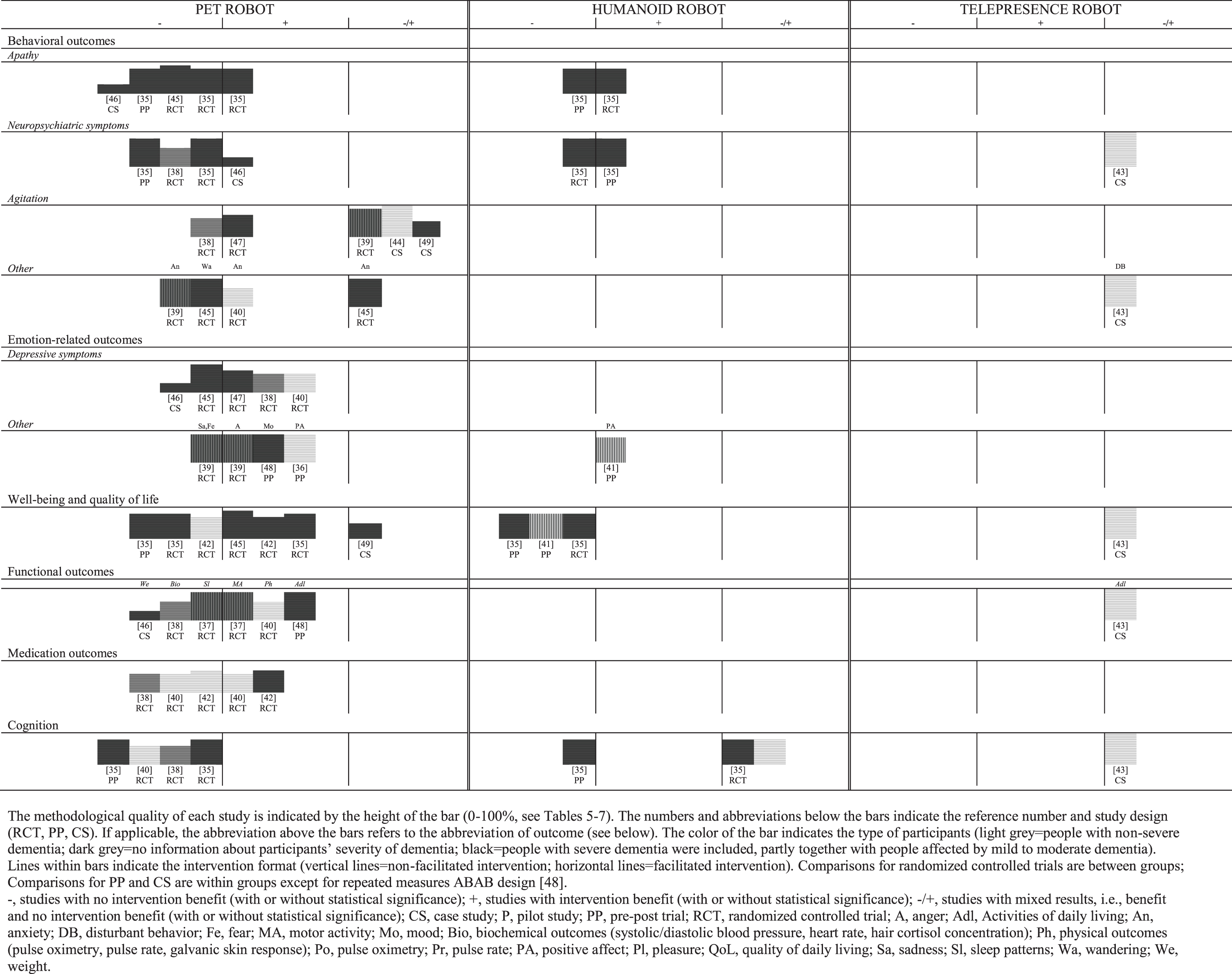
Behavioral outcomes
Pet robot: Facilitated PARO interventions statistically significantly reduced apathy [35], agitation [47] and anxiety [40], compared to usual care in people with mild/moderate/severe dementia [35, 47] and non-severe dementia [40]. A statistically significant improvement of neuropsychiatric symptoms was identified in people with severe dementia within a case study without a control group [46].
However, no benefit from applying facilitated PARO interventions was observed concerning 1) neuropsychiatric symptoms and apathy in people with mild/moderate/severe dementia compared to usual care [35] as well as people with moderate/severe dementia comparing pre- and post-intervention measurement [35], 2) wandering and apathy in people with moderate/severe dementia compared to reading activity [45], and 3) neuropsychiatric symptoms and agitation in people with unclear severity of dementia, compared to usual care [38]. A case study with a facilitated PARO intervention yielded no benefit for people with severe dementia concerning apathy [46].
Mixed results for agitation were observed in two case studies examining a facilitated JustoCat intervention in people with severe dementia [49] as well as in a facilitated CuDDler intervention in people with non-severe dementia [44]. Furthermore, a facilitated PARO intervention for people with moderate/severe yielded contrary results concerning anxiety using 1) the proxy version of an assessment instrument and 2) the version used by people with dementia themselves [45]. A non-facilitated PARO intervention also yielded opposed results concerning agitation in people with unclear severity of dementia using a 1) standardized assessment and 2) video observation [39].
Humanoid robot: A facilitated NAO intervention resulted in statistically significant reductions of 1) apathy in people with mild/moderate/severe dementia compared to usual care [35] and 2) neuropsychiatric symptoms in people with moderate/severe dementia within a pre-post trial [35]. However, contrasting results with no benefit were observed in these studies for neuropsychiatric symptoms in people with mild/moderate/severe dementia, compared to usual care [35], and apathy of people with moderate/severe dementia within the pre-post trial [35].
Telepresence robot: A case study using a telepresence robot yielded mixed results concerning disturbant behavior and neuropsychiatric symptoms in two people with moderate dementia [43].
Emotion-related outcomes
Pet robot: Depressive symptoms were statistically significantly reduced comparing a facilitated PARO intervention with usual care in people with 1) non-severe [40], 2) mild/moderate/severe (follow up measurement twelve weeks post-intervention) [47], and 3) unclear severity of dementia [38]. Anger and pleasure were statistically significantly improved comparing a non-facilitated PARO intervention to plush toy and usual care, respectively [39]. 1) The mood of people with mild/moderate/severe dementia [48] and 2) positive affect in people with non-sev-ere dementia [36] were improved in pre-post trials examining a facilitated PARO intervention. A non-facilitated NAO intervention in a pre-post trial improved the positive affect in people with non-severe dementia [41].
No benefit concerning depressive symptoms were observed for a facilitated PARO intervention for 1) people with moderate/severe dementia, compared to reading activity [45] as well as 2) for people with severe dementia participating in a pre-post trial [46]. No group differences were observed for a non-facilitated PARO intervention concerning anxiety and sadness compared to a plush toy and usual care [39].
Humanoid robot: A classic therapy and non-facilitated NAO intervention yielded a statistically significant improvement of positive affect in people with non-severe dementia [40].
Well-being and quality of life
Pet robot: Three studies yielded beneficial results concerning quality of life of people with 1) moderate/severe dementia [45], 2) severe dementia (sta-tistical significance for twelve weeks follow up but not for post-intervention) [42], and 3) mild/ moderate/severe dementia (study phase 2/2) [35], whereby the facilitated PARO group intervention was compared to usual care [42, 35] and reading activity [45].
No benefit was observed for a facilitated controlled PARO intervention for people with non-severe dementia [42] and mild/moderate/severe dementia (in study phase 1/2) [35], as well as for people with moderate/severe dementia participating in the pre-post trial part of the study [35]. One case study reported mixed results for quality of life [49].
Humanoid robot: No benefit was observed for facilitated [35] and non-facilitated [41] NAO interventions concerning well-being and quality of life in people with mild/moderate/severe dementia.
Telepresence robot: A case study using a telepresence robot yielded mixed results concerning quality of life in two people with moderate dementia [43].
Functional outcomes
Pet robot: One study yielded beneficial results concerning physical and motor activity using a non-facilitated PARO intervention for people with unclear severity of dementia: a statistically significantly reduced step count and/or physical activity were observed for the non-facilitated PARO intervention group compared to the plush toy group and usual care group while daytime. At night, step count and physical activity in the PARO intervention group were reduced, compared to plush toy and usual care, respectively [37]. A facilitated PARO intervention for people with non-severe dementia resulted in benefi-cial and statistically significant group differences, compared to usual care concerning pulse oximetry, pulse rate, and galvanic skin response as indicators for stress and arousal [40]. A positive effect concer-ning activities of daily living in people with mild/moderate/severe dementia was observed within a rep-eated measured trial comparing a facilitated PARO intervention with usual care [44].
No benefit was identified for sleep patterns using a non-facilitated PARO intervention [37] as well as for weight, blood pressure, heart rate, and hair cortisol concentration using facilitated PARO interventions [38, 46].
Telepresence robot: A case study of a telepresence robot yielded mixed results concerning activities of daily living in two people with moderate dementia [43].
Medication outcomes
Pet robot: There was a statistically significant reduction of pain and behavioral medication dose (no details available) for people with non-severe dementia [40]. Psychotropic medication for people with severe dementia [42] was reduced in the facilitated PARO intervention group, compared to usual care. However, 24 weeks follow-up showed no statistical difference between groups of people with severe dementia [42].
No difference between a facilitated PARO intervention and usual care was measured for 1) psycho-tropic medication [42] as well as 2) for sleep and depression medication dose in people with mild and moderate dementia [40], and 3) for dementia-related medication usage in people with dementia of unknown severity [38].
Cognition
Pet robot: No benefit was observed for cognitive outcomes of facilitated PARO interventions for people 1) with mild/moderate/severe [35, 40] and 2) with unclear severity of dementia [38].
Humanoid robot: A facilitated NAO intervention yielded statistically significant cognitive improvement in people with mild/moderate/severe dementia, compared to usual care measured by a standardized assessment. However, there was no benefit measured by the short version of the assessment [35]. Using a facilitated NAO intervention [35], there was also no benefit concerning cognition in people with moderate/severe dementia [35].
Telepresence robot: A case study using a telepresence robot yielded mixed results concerning cognitive outcomes in two people with moderate dementia [43].
DISCUSSION
The results of our review indicate a variety of study designs and clinical heterogeneity of sample populations concerning type and severity of dementia, intervention characteristics such as social robot system, format, duration and frequency as well as outcomes. By analyzing the reporting of included studies, we were able to identify similarities and differences between the development and facilitation of social robot interventions and their components. It is evident that the interventions are heterogeneous, e.g., even for social robot interventions using the same type of robot. It is therefore difficult to perform a synthesis and the generalizability of the results for clinical practice are limited.
Based on our review of eight experimental and eight quasi-experimental studies, pet robot interventions show both benefit and non-benefit for people with dementia concerning all outcomes groups, exc-ept for cognition. There is no evidence that social robot interventions have a positive impact on the cognitive state of people with dementia. Given the evidence on pet robot interventions for people with dementia, improving the cognitive state might not be a desirable outcome [15]. Sample and intervention characteristics such as severity of dementia and facilitation or non-facilitation might explain differences with regard to effectiveness of pet robot interventions on the remaining outcome groups. Therefore, pet robots might not be effective to manage behavioral disorders such as apathy and neuropsychiatric symptoms but might have a beneficial impact on medication and agitation. For emotional and functional outcomes as well as quality of life, the evidence is not clear enough to draw conclusions concerning overall benefit or non-benefit. Such limiting aspects on the available evidence might have been hidden in earlier studies because sample and interventions characteristics were not thoroughly addressed [12].
The sample populations among the identified studies vary in terms of dementia severity or even non-reported severity of dementia. The inclusion of studies with varying sample characteristics also applies to two earlier systematic reviews with study samples of people in various stages of dementia [2, 13]. Another systematic review examining the effects of social robot interventions on older adults included studies with people affected and not affected by cognitive impairment, persons with dementia without providing disease-related details, and some participants with cognitive impairment or dementia [1]. A recently published systematic review and meta-analysis did not address severity of dementia in the sample characteristics [12]. In a systematic review, mixed, differing or unknown sample characteristics lead to clinical and statistical heterogeneity among studies [50]. This might affect the external validity of the review as well as its clinical utility [51]. There is also evidence that the severity of dementia has an impact on the benefit of social robot interventions for study participants [38, 42, 52]. Data about participants’ severity of dementia should therefore be rigorously reported in future studies since detailed information on disease-related characteristics are indispensable. Hence, one option to face cli-nical heterogeneity of study samples in systematic reviews might be the analysis of subgroups to enable the comparison of varying sample populations in terms of disease-related characteristics such as people with mild, moderate, and severe dementia [53].
Studies investigating the effectiveness of huma-noid and telepresence robots for people with dementia seem to be rare. Therefore, evidence is limited. With regard to humanoid robots, we identified three studies determining the effectiveness of NAO for people with dementia, one randomized controlled trial and two pre-post trials [41, 35]. Considering evidence from two studies, there is no benefit concerning quality of life of people with dementia [41, 35]. One study yielded positive results with regard to positive affect of people with dementia [41]. With respect to the cognitive and behavioral state of people with dementia, these studies yielded divergent results [41, 35]. More primary research on humanoid robots and their physical and psychosocial outcomes might be needed.
A recently registered mixed-methods study evaluates the effects on several outcomes such as apathy, burden of care, quality of life, and cognitive state [54]. The results might help to clarify the effect of humanoid robots on such outcomes.
With regard to telepresence robots, we identified one case study including two persons. It yielded mixed results concerning neuropsychiatric symptoms, disturbant behavior, quality of life, activities of daily living, and cognition [43]. The authors did not report details on intervention development and theoretical underpinning of the intended mode of action. Following a recently published integrative review including four studies, telepresence robots have the potential to improve social connectedness of people with dementia and their carers [11]. However, studies determining the effectiveness of telepresence robots are still lacking.
The majority of studies did not report on 1) the professional background and experience of intervention facilitators, 2) whether intervention facilitators received training and if so, 3) which kind of training using which education material. Limited information on education material was already mentioned in an earlier review of PARO interventions [15]. For clinical practice, it is essential to know which persons with which professional background and experience are involved in the intervention and who is training the persons carrying out the intervention [14]. To facilitate the implementation of social robot interventions, information on facilitators and their professional education should therefore be available. Our review provides an overview of social robot interventions as well as background information on their characteristics that might be helpful to facilitate an individualized and disease-specific use of robots for people with dementia in clinical practice.
In the included studies, a wide range of outcomes is investigated. This corresponds to an earlier review on outcome measures [15] indicating a need for the-oretical reasoning concerning the question: How might the intervention be effective? Which impact does the intervention have on people with dementia [55]? The description of theoretical underpinnings (concerning the development and evaluations of intervention) should therefore be accompanied by the development of core outcomes. Core outcomes should be assessed with valid and reliable instruments to ensure comparability among studies and study contexts [56]. Besides the already covered outcomes in the included studies, there might also be a need to explore the effects of social robot interventions on, for instance, pain in people with dementia. This applies to observed or self-reported pain, as suggested by the results of a recently published study using a PARO intervention [57, 58].
Ten of sixteen included studies were classified as pilot studies, given their stage of intervention development according to CReDECI-2 [35, 36, 38, 41, 43–46, 49]. Pilot studies are generally not suitable to determine the effectiveness of an intervention. Rather, they intend to test an intervention and its effect(s) in order to plan the main randomized controlled trial with suffficient sample size and well-determined outcomes [59]. However, the inclusion of pilot studies in our review was essential to draw conclusions on the development stage of social robot interventions [60]. This resulted in the identification of two research sites authoring multiple studies: Australia [37, 39, 44, 45] and France [36, 41, 46]. We can assume that the Australian research group piloted and tested a PARO intervention as indicated by several papers referring to each other [37, 39, 44, 45]. A direct relationship between the studies conducted in France is lacking. The social robot used for intervention is not the same in the three studies [36, 41, 46]. Furthermore, our review is not able to show which pilot studies were tested in a scaled-up trial as long as they were not yet published. Since the majority of studies included can be classified as pilot studies, it might indicate that social robot interventions for people with dementia correspond to an early development stage.
To develop or adapt a social robot intervention, theoretical modelling of its components and its intended mechanisms of action is required [16, 61]. However, none of the studies included in our review reports underlying theoretical assumptions to develop, evaluate, and implement a social robot intervention for people with dementia in a sufficiently comprehensible and transparent manner. Therefore, it remains unclear which mechanisms of action the researchers intended [16]. Without reporting the underlying theoretical assumptions, it remains unclear whether and how people with dementia were involved. Furthermore, it is not clear, whether the needs of people with dementia could have been addressed by the social robot intervention aiming to improve quality of life, well-being, and social engagement of people with dementia [23, 62, 63]. Thus, the overall contribution of social robot interventions to dementia care remains unknown. The implementation might be at risk of failing if the needs of people with dementia are disregarded [19, 20, 64]. Person-centered care is a basic conceptual approach integrating the perspectives of the affected persons [65]. The aim of person-centered care is to ensure and improve the quality of life of persons with dementia [66, 67]. A person-centered approach to the delivery of social robot interventions acknowledges the individuality of people with dementia, the importance of their perspective as well as the significance of their social relationships and interactions [62, 63]. Since an overarching aim of social robot interventions for people with dementia is to promote quality of life, person-centered care might be an expedient approach to developing and implementing individualized and need-driven social robot interventions [4, 63, 68].
Introducing social robots in dementia care settings raises ethical issues with regard to this highly vulnerable population [19, 69, 70]. A person-centered approach might contribute to ensure an ethically responsible dementia care research and practice. This implies to address ethical principles like nonmaleficence, beneficence, personal autonomy, and fairness. The fulfillment of these principles might be threatened in this vulnerable group [21, 71]. It is therefore important to know how people with dementia were involved in the studies, how consent procedures were applied, and how interventions were tailored to the individual needs of people with dementia. However, our assessment of ethical issues revealed an immense lack of detailed information on the procedures of informed and ongoing consent in people with dementia. This also refers to their representatives. Furthermore, there is a lack of information concerning authors’ discussions of ethical issues. Thus, our ethical analysis cannot provide specific implications for an ethically responsible intervention development and implementation as it is often demanded [19–22]. Authors of future studies should transparently and clearly describe their theoretical assumptions when reporting social robot interventions as well as potential ethical conflicts and consent procedures [72]. Researchers should provide information on their dementia-related experience in research and practice. This might help to recognize and understand the necessity of well-guided consent procedures to develop, evaluate, and implement social robot interventions.
The methodological quality of the included studies is limited since most of them were considered as pilot studies. It is therefore probable that sample sizes did not have enough power to result in robust intervention effects [73, 74]. Nevertheless, most of the included studies showed moderate methodological quality, as indicated by fulfilling the majority of items in design-specific JBI tools. This result is supported by an earlier assessment of interventions using technologies for people with dementia [75]. However, for all included randomized controlled studies, blinding of staff and intervention facilitators was judged as not applicable. This increases the risk for bias due to deviations from intended interventions. It also raises concerns whether the addressed outcomes were influenced [76]. Blinding of outcome assessors was conducted in half of the included randomized controlled trials. Thereby, four studies did not report such measures in order to minimize risk of detection bias [38, 40, 42, 47]. Since the blinding of participants and intervention facilitators in social robot interventions seems not applicable, blinding of outcome assessors might be possible. The increasing risk of bias due to non-blinding of participants and intervention facilitators needs to be taken into account when considering the internal validity of studies, i.e., the impact on intervention effectiveness. To minimize the risk for attrition and performance bias, researchers should therefore incorporate additional strategies. One key option might be treating the intervention groups as equal as possible, i.e., increasing the degree of standardization of the social robot intervention [77]. However, standardization should allow social robot interventions tailored to the individual needs of people with dementia.
To assess the degree of standardization, transparent reporting of the development and facilitation of intervention components in primary studies are necessary. For example, information about the background of intervention facilitators and details with regard to tailoring or modification would be helpful. Our review indicates that the majority of included studies does not provide such information in sufficient detail, thereby limiting the assessment of methodological quality.
The strength of this review is a comprehensive search using database and free web searching as well as citation tracking without limitation of publication years to identify potentially all available studies [28]. Furthermore, study selection and critical appraisal by two independent researchers as well as data extraction and assessment of reporting by one researcher (peer-checked by a second researcher) served to ensure high data quality.
This is the first systematic review providing a comprehensive overview of social robot interventions for persons with dementia, including all types of quasi-experimental and experimental studies on all stages of development. Using TIDieR and CReDECI 2 enabled a comprehensive and structured assessment of the reporting of included studies [78, 79]. To assess the studies, two researchers of our team defined minimal requirements ensuring to fulfil the item criteria.
We did not search trial registers and conference proceedings to identify unpublished studies. This raises the risk for overlooking studies and the potential for publication bias. This should be conside-red when interpreting our results. However, we conducted a free web search to minimize the risk of publication bias.
CONCLUSIONS
Our review synthesizes available evidence concerning the effectiveness and quality of reporting of social robot interventions for people with dementia. The implications are relevant for practitioners and scholars developing, evaluating, and reporting interventions intending ethically responsible social robot interventions in dementia care.
Implications for clinical practice
Social robot interventions might not be effect-ive to improve the cognitive state of people with dementia and to manage their behavioral disorders such as apathy and neuropsychiatric symptoms. However, pet robot interventions might have a beneficial impact on agitation and medication. With regard to emotion-related outcomes (e.g., depressive symptoms), functional outcomes, and quality of life, we can draw no conclusions concerning the overall benefit or non-benefit of social robot interventions. However, practitioners are requested to consider the study and intervention characteristics provided in our review. This may be instructive since highly incomplete reporting and the heterogeneity of populations, intervention characteristics, and outcomes hinder generalizability with regard to clinical practice. The-refore, the impact of social robot interventions should be assessed with a focus on the severity of dementia and intervention characteristics. After wei-ghing advantages and disadvantages of social robot interventions, practitioners might facilitate these interventions according to the procedures described.
Implications for the development, evaluation, and reporting of interventions
There is a need for research addressing the effectiveness of social robot interventions using humanoid and telepresence robots since studies are rarely or not available. To develop and evaluate social robot interventions for people with dementia, it is necessary to examine the needs of the target group and to to select interventions and their characteristics. To contribute to person-centered care, it is also required to consider the intended effects and to systematically analyse the available evidence.
Implementing social robots in clinical practice relies on transparent reporting of intervention development and its theoretical underpinnings. Detailed description of intervention characteristics and procedures is essential as well. Study authors should therefore use reporting templates such as CReDECI 2 or TIDieR to describe their intervention rigorously. Moreover, journal editors should ensure mandatory use of reporting guidelines.
Ethical implications
There is a lack of information on ethically sound aspects of developing and implementing social robot interventions in dementia care. The delivery of social robot interventions should be individually tailored to the needs of the person with dementia. Authors should therefore provide transparent information on the involvement of people with dementia as well as on ethical approval and consent procedures. This might contribute to an ethically responsible dementia care research and practice with regard to social robot interventions.
FUNDING
This review is part of an overarching project focusing on dementia and the aging society (AGE-NT) funded by the Swiss State Secretariat for Education, Research and Innovation (SERI). The funder had no influence on the design and the results of this research.
AUTHORS’ DISCLOSURES
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0347r2).
AUTHORS’ CONTRIBUTION
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: All authors
Drafting the work: JH
Revising it critically for important intellectual content: All authors
Final approval of the version to be published: All authors
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200347.
REFERENCES
[1] | Pu L , Moyle W , Jones C , Todorovic M ((2019) ) The effectiveness of social robots for older adults: A systematic review and meta-analysis of randomized controlled studies. Gerontologist 59: , e37–e51. |
[2] | Mordoch E , Osterreicher A , Guse L , Roger K , Thompson G ((2013) ) Use of social commitment robots in the care of elderly people with dementia: A literature review. Maturitas 74: , 14–20. |
[3] | Kachouie R , Sedighadeli S , Khosla R , Chu M-T ((2014) ) Socially assistive robots in elderly care: A mixed-method systematic literature review. Int J Hum Comput 30: , 369–393. |
[4] | Góngora Alonso S , Hamrioui S , La Torre Díez de I , Motta Cruz E , López-Coronado M , Franco M ((2018) ) Social robots for people with aging and dementia: A systematic review of literature. Telemed EHealth 25: , 533–540. |
[5] | Pfadenhauer M , Dukat C ((2015) ) Robot caregiver or robot-supported caregiving?: The performative deployment of the social robot PARO in dementia care. Int J Soc Robot 7: , 393–406. |
[6] | Hu M , Zhang P , Leng M , Li C , Chen L ((2018) ) Animal-assisted intervention for individuals with cognitive impairment: A meta-analysis of randomized controlled trials and quasi-randomized controlled trials. Psychiatry Res 260: , 418–427. |
[7] | Robinson H , MacDonald BA , Kerse N , Broadbent E ((2013) ) The psychosocial effects of a companion robot: A randomized controlled trial. JAMDA 14: , 661–667. |
[8] | Shibata T ((2012) ) Therapeutic seal robot as biofeedback medical device: Qualitative and quantitative evaluations of robot therapy in dementia care. Proc IEEE 100: , 2527–2538. |
[9] | Feil-Seifer D , Mataric MJ ((2005) ) Defining socially assistive robotics. International Conference on Rehabilitation Robotics, pp. 465–468. |
[10] | Papadopoulos I , Koulouglioti C , Lazzarino R , Ali S ((2020) ) Enablers and barriers to the implementation of socially assistive humanoid robots in health and social care: A systematic review. BMJ Open 10: , e033096. |
[11] | Moyle W , Arnautovska U , Ownsworth T , Jones C ((2017) ) Potential of telepresence robots to enhance social connectedness in older adults with dementia: An integrative review of feasibility. Int Psychogeriatr 29: , 1951–1964. |
[12] | Leng M , Liu P , Zhang P , Hu M , Zhou H , Li G , Yin H , Chen L ((2018) ) Pet robot intervention for people with dementia: A systematic review and meta-analysis of randomized controlled trials. Psychiatry Res 271: , 516–525. |
[13] | Bemelmans R , Gelderblom GJ , Jonker P , Witte de L ((2012) ) Socially assistive robots in elderly care: A systematic review into effects and effectiveness. JAMDA 13: , 114–120. |
[14] | Vernooij-Dassen M , Moniz-Cook E ((2014) ) Raising the standard of applied dementia care research: Addressing the implementation error. Aging Ment Health 18: , 809–814. |
[15] | Kang HS , Makimoto K , Konno R , Koh IS ((2020) ) Review of outcome measures in PARO robot intervention studies for dementia care. Geriatr Nurs 41: , 207–214. |
[16] | Möhler R , Köpke S , Meyer G ((2015) ) Criteria for reporting the development and evaluation of complex interventions in healthcare: Revised guideline (CReDECI 2). Trials 16: , 204. |
[17] | Hoffmann TC , Glasziou P , Boutron I , Milne R , Perera R , Moher D , Altman DG , Barbour V , MacDonald H , Johnston M , Lamb SE , Dixon-Woods M , McCulloch P , Wyatt JC , Chan A-W , Michie S ((2014) ) Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 348: , g1687. |
[18] | Scoglio AA , Reilly ED , Gorman JA , Drebing CE ((2019) ) Use of social robots in mental health and well-being research: Systematic review. J Med Internet Res 21: , e13322. |
[19] | Hung L , Liu C , Woldum E , Au-Yeung A , Berndt A , Wallsworth C , Horne N , Gregorio M , Mann J , Chaudhury H ((2019) ) The benefits of and barriers to using a social robot PARO in care settings: A scoping review. BMC Geriatr 19: , 232. |
[20] | Ienca M , Lipps M , Wangmo T , Jotterand F , Elger B , Kressig RW ((2018) ) Health professionals’ and researchers’ views on Intelligent Assistive Technology for psychogeriatric care. Gerontechnology 17: , 139–150. |
[21] | Körtner T ((2016) ) Ethical challenges in the use of social service robots for elderly people. Z Gerontol Geriatr 49: , 303–307. |
[22] | Diaz-Orueta U , Hopper L , Konstantinidis E ((2020) ) Shaping technologies for older adults with and without dementia: Reflections on ethics and preferences. Health Informatics J 26: , 3215–3230. |
[23] | Robillard JM , Goldman IP , Prescott TJ , Michaud F ((2020) ) Addressing the ethics of telepresence applications through end-user engagement. J Alzheimers Dis 76: , 457–460. |
[24] | Hung L , Gregorio M , Mann J , Wallsworth C , Horne N , Berndt A , Liu C , Woldum E , Au-Yeung A , Chaudhury H ((2019) ) Exploring the perceptions of people with dementia about the social robot PARO in a hospital setting. Dementia (London). doi: 10.1177/1471301219894141 |
[25] | Ziegler S , Bleses HM ((2017) ) [Deutung von Deutungen in und zu Begegnungssituationen von Personen mit Demenz und Robotern]. In: [Theoretische Einsichten. Im Kontext empirischer Arbeit], Burzan N, Hitzler R, eds., Springer Fachmedien: Wiesbaden, pp. 125–146. |
[26] | Hirt J , Burgstaller M , Zeller A , Beer T ((2019) ) Needs of people with dementia and their informal caregivers concerning assistive technologies: A scoping review. Pflege 32: , 295–304. |
[27] | Liberati A , Altman DG , Tetzlaff J , Mulrow CD , Gøtzsche PC , Ioannidis JPA , Clarke M , Devereaux PJ , Kleijnen J , Moher D ((2009) ) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339: , b2700. |
[28] | Cooper C , Booth A , Varley-Campbell J , Britten N , Garside R ((2018) ) Defining the process to literature searching in systematic reviews: A literature review of guidance and supporting studies. BMC Med Res Methodol 18: , 85. |
[29] | Li J , Burnham JF , Lemley T , Britton RM ((2010) ) Citation analysis: Comparison of Web of Science®, Scopus™, SciFinder®, and Google Scholar. J Med Libr Assoc 7: , 196–217. |
[30] | Rodriguez RW ((2016) ) Comparison of indexing times among articles from medical, nursing, and pharmacy journals. Am J Health Syst Pharm 73: , 569–575. |
[31] | McGowan J , Sampson M , Salzwedel DM , Cogo E , Foerster V , Lefebvre C ((2016) ) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75: , 40–46. |
[32] | Critical appraisal tools, https://joannabriggs.org/ebp/critical_appraisal_tools, Accessed March 21, 2020. |
[33] | Ogilvie D , Fayter D , Petticrew M , Sowden A , Thomas S , Whitehead M , Worthy G ((2008) ) The harvest plot: A method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol 8: , 1–8. |
[34] | Higgins JPT , López-López JA , Becker BJ , Davies SR , Dawson S , Grimshaw JM , McGuinness LA , Moore THM , Rehfuess EA , Thomas J , Caldwell DM ((2019) ) Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health 4: , e000858. |
[35] | Valenti Soler M , Aguera-Ortiz L , Olazaran Rodriguez J , Mendoza Rebolledo C , Perez Munoz A , Rodriguez Perez I , Osa Ruiz E , Barrios Sanchez A , Herrero Cano V , Carrasco Chillon L , Felipe Ruiz S , Lopez Alvarez J , Leon Salas B , Canas Plaza JM , Martin Rico F , Abella Dago G , Martinez Martin P ((2015) ) Social robots in advanced dementia. Front Aging Neurosci 7: , 133. |
[36] | Demange M , Lenoir H , Pino M , Cantegreil-Kallen I , Rigaud AS , Cristancho-Lacroix V ((2018) ) Improving well-being in patients with major neurodegenerative disorders: Differential efficacy of brief social robot-based intervention for 3 neuropsychiatric profiles. Clin Interv Aging 13: , 1303–1311. |
[37] | Moyle W , Jones C , Murfield J , Thalib L , Beattie E , Shum D , O’Dwyer S , Mervin MC , Draper B ((2018) ) Effect of a robotic seal on the motor activity and sleep patterns of older people with dementia, as measured by wearable technology: A cluster-randomised controlled trial. Maturitas 110: , 10–17. |
[38] | Liang A , Piroth I , Robinson H , MacDonald B , Fisher M , Nater UM , Skoluda N , Broadbent E ((2017) ) A pilot randomized trial of a companion robot for people with dementia living in the community. JAMDA 18: , 871–878. |
[39] | Moyle W , Jones CJ , Murfield JE , Thalib L , Beattie ER , Shum DK , O’Dwyer ST , Mervin MC , Draper BM ((2017) ) Use of a robotic seal as a therapeutic tool to improve dementia symptoms: A cluster-randomized controlled trial. JAMDA 18: , 766–773. |
[40] | Petersen S , Houston S , Qin H , Tague C , Studley J ((2017) ) The utilization of robotic pets in dementia care. J Alzheimers Dis 55: , 569–574. |
[41] | Rouaix N , Retru-Chavastel L , Rigaud A-S , Monnet C , Lenoir H , Pino M ((2017) ) Affective and engagement issues in the conception and assessment of a robot-assisted psychomotor therapy for persons with dementia. Front Psychol 8: , 950. |
[42] | Jøranson N , Pedersen I , Rokstad AMM , Ihlebæk C ((2016) ) Change in quality of life in older people with dementia participating in Paro-activity. J Adv Nurs 72: , 3020–3033. |
[43] | Kuwamura K , Nishio S , Sato S ((2016) ) Can we talk through a robot as if face-to-face? Long-term fieldwork using teleoperated robot for seniors with Alzheimer’s disease. Front Psychol 7: , 1066. |
[44] | Moyle W , Jones C , Sung B , Bramble M , O’Dwyer S , Blumenstein M , Estivill-Castro V ((2016) ) What effect does an animal robot called CuDDler have on the engagement and emotional response of older people with dementia? A pilot feasibility study. Int J Soc Robot 8: , 145–156. |
[45] | Moyle W , Cooke M , Beattie E , Jones C , Klein B , Cook G , Gray C ((2013) ) Exploring the effect of companion robots on emotional expression in older adults with dementia: A pilot randomized controlled trial. J Gerontol Nurs 39: , 46–53. |
[46] | Sant’Anna M , Morat B , Rigaud AS ((2012) ) Adaptabilité du robot Paro dans la prise en charge de la maladie d’Alzheimer sévére de patients institutionnalisés. NPG Neurol Psychiatr Gériatr 12: , 43–48. |
[47] | Jøranson N , Pedersen I , Rokstad AMM , Ihlebæk C ((2015) ) Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: A cluster-randomized controlled trial. JAMDA 16: , 867–873. |
[48] | Bemelmans R , Gelderblom GJ , Jonker P , Witte de L ((2015) ) Effectiveness of robot paro in intramural psychogeriatric care: A multicenter quasi-experimental study. JAMDA 16: , 946–950. |
[49] | Gustafsson C , Svanberg C , Mullersdorf M ((2015) ) Using a robotic cat in dementia care: A pilot study. J Gerontol Nurs 41: , 46–56. |
[50] | Thompson SG ((1995) ) Why sources of heterogenity in meta-analysis should be investigated. In Systematic reviews, Chalmers I, Altman DG, eds., BMJ Publishing Group, London, pp. 48–63. |
[51] | West SL , Gartlehner G , Mansfield AJ , Poole C , Tant E , Lenfestey N , Lux LJ , Amoozegar J , Morton SC , Carey TC , Viswanathan M , Lohr KN ((2010) ) Comparative Effectiveness Review Methods: Clinical Heterogeneity, Agency for Healthcare Research and Quality, 10-EHC070-EF, Rockville. |
[52] | Jones C , Moyle W , Murfield J , Draper B , Shum D , Beattie E , Thalib L ((2018) ) Does cognitive impairment and agitation in dementia influence intervention effectiveness? findings from a cluster-randomized-controlled trial with the therapeutic robot, PARO. JAMDA 19: , 623–626. |
[53] | Richardson M , Garner P , Donegan S ((2019) ) Interpretation of subgroup analyses in systematic reviews: A tutorial. Clin Epidemiol Glob Health 7: , 192–198. |
[54] | Schüssler S , Zuschnegg J , Paletta L , Fellner M , Lodron G , Steiner J , Pansy-Resch S , Lammer L , Prodromou D , Brunsch S , Holter M , Carnevale L , Russegger S ((2020) ) The effects of a humanoid socially assistive robot versus tablet training on psychosocial and physical outcomes of persons with dementia: Protocol for a mixed methods study. JMIR Res Protoc 9: , e14927. |
[55] | Higgins JPT , Green S ((2011) ) Guide to the contents of a Cochrane protocol and review. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, Higgins JPT, Green S, eds.,Version 5.1.0,Wiley-Blackwell, Chichester, pp. 51–79. |
[56] | Prinsen CAC , Vohra S , Rose MR , Boers M , Tugwell P , Clarke M , Williamson PR , Terwee CB ((2016) ) How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” - a practical guideline. Trials 17: , 449. |
[57] | Pu L , Moyle W , Jones C ((2019) ) How people with dementia perceive a therapeutic robot called PARO in relation to their pain and mood: A qualitative study. J Clin Nurs 29: , 437–446. |
[58] | Pu L , Moyle W , Jones C , Todorovic M ((2020) ) The effect of using PARO for people living with dementia and chronic pain: A pilot randomized controlled trial. JAMDA 21: , 1079–1085. |
[59] | Leon AC , Davis LL , Kraemer HC ((2011) ) The role and interpretation of pilot studies in clinical research. J Psychiatr Res 45: , 626–629. |
[60] | Mühlhauser I , Lenz M , Meyer G ((2011) ) [Entwicklung, Bewertung und Synthese von komplexen Interventionen - eine methodische Herausforderung]. Z Evid Fortbild Qual Gesundhwes 105: , 751–761. |
[61] | Rohwer A , Booth A , Pfadenhauer L , Gerhardus A , Moz-ygemba K , Oortwijn W , Tummers M , Wilt van der GJ , Rehfuess E ((2016) ) Guidance on the use of logic models in health technology assessments of complex interventions,http://www.integrate-hta.eu/wp-content/uploads/2016/02/Guidance-on-the-use-of-logic-models-in-health-technology-assessments-of-complex-interventions.pdf ,Last updated 2016, Accessed October 30, 2019. |
[62] | National Institute for Health and Care Excellence ((2018) ) Dementia: Assessment, management and support for people living with dementia and their carers, https://www.nice.org.uk/guidance/ng97 ,Last updated 2018, Accessed March 4, 2020. |
[63] | Manthorpe J , Samsi K ((2016) ) Person-centered dementia care: Current perspectives. Clin Interv Aging 11: , 1733–1740. |
[64] | Ienca M , Fabrice J , Elger B , Caon M , Pappagallo AS , Kressig RW , Wangmo T ((2017) ) Intelligent assistive technology for Alzheimer’s disease and other dementias: A systematic review. J Alzheimers Dis 56: , 1301–1340. |
[65] | Blake D , Berry K , Brown LJE ((2020) ) A systematic review of the impact of person-centred care interventions on the behaviour of staff working in dementia care. J Adv Nurs 76: , 426–444. |
[66] | Kitwood T ((1997) ) Dementia reconsidered: The person comes first, Rethinking Ageing, Open University Press, Maidenhead. |
[67] | Chenoweth L , Stein-Parbury J , Lapkin S , Wang A , Liu Z , Williams A ((2019) ) Effects of person-centered care at the organisational-level for people with dementia. A systematic review. PLoS One 14: , e0212686. |
[68] | Sugihara T , Fujinami T , Phaal R , Ikawa Y ((2015) ) A technology roadmap of assistive technologies for dementia care in Japan. Dementia 14: , 80–103. |
[69] | Ienca M , Jotterand F , Vica C , Elger B ((2016) ) Social and assistive robotics in dementia care: Ethical recommendations for research and practice. Int J Soc Robotics 8: , 565–573. |
[70] | Sharkey A , Sharkey N ((2012) ) Granny and the robots: Ethical issues in robot care for the elderly. Ethics Inf Technol 14: , 27–40. |
[71] | Beauchamp TL , Childress JF ((2013) ) Principles of Biomedical Ethics, 7th Edition, Oxford University Press, New York. |
[72] | Manzeschke A , Weber K , Rother E , Fangerau H ((2013) ) Ethical questions in the area of age appropriate assisting systems. Results of the study, https://www.researchgate.net/publication/304743219_Ethical_questions_in_the_area_of_age_appropriate_assisting_systems, Accessed November 6, 2020. |
[73] | Kim J , Seo BS ((2013) ) How to calculate sample size and why. Clin Orthop Surg 5: , 235–242. |
[74] | Altman DG , Bland JM ((1995) ) Statistics notes: Absence of evidence is not evidence of absence. BMJ 311: , 485. |
[75] | Brims L , Oliver K ((2019) ) Effectiveness of assistive technology in improving the safety of people with dementia: A systematic review and meta-analysis. Aging Ment Health 23: , 942–951. |
[76] | Higgins JPT , Savović J , Page MJ , Elbers RG , Sterne JAC ((2019) ) Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions: Version 6, Higgins JPT, Thomas J, eds., 2nd Edition, Wiley Online Library: Hoboken, pp. 205–228. |
[77] | Karanicolas PJ , Farrokhyar F , Bhandari M ((2010) ) Blinding: Who, what, when, why, how? Can J Surg 53: , 345–348. |
[78] | Booth A , Moore G , Flemming K , Garside R , Rollins N , Tuncalp Ö , Noyes J ((2019) ) Taking account of context in systematic reviews and guidelines considering a complexity perspective. BMJ Glob Health 4: , e000840. |
[79] | Altman DG , Simera I ((2014) ) Using reporting guidelines effectively to ensure good reporting of health research. In: Guidelines for reporting health research: A user’s manual, Moher D, Altman DG, Schulz KF, Simera I,Wager E, eds., John Wiley & Sons, Chichester, pp. 32–40. |




