Brain γ-Tocopherol Levels Are Associated with Presynaptic Protein Levels in Elderly Human Midfrontal Cortex
Abstract
Background:
Higher vitamin E intake has been widely related to lower risks of cognitive decline and dementia. Animal models suggest that this relationship might be (partially) explained by the protection of vitamin E against presynaptic protein oxidation.
Objective:
In this cross-sectional study, we aimed to examine the associations between brain tocopherols and presynaptic protein levels in elderly humans.
Methods:
We examined associations of α- and γ-tocopherol brain levels with presynaptic protein levels in 113 deceased participants (age 88.5±6.0 years, 45 (40%) female) from the prospective Memory and Aging project. Three distinct presynaptic proteins, a SNARE protein composite, a synaptotagmin synaptophysin composite and the protein-protein interaction between synaptosomal-associated protein 25 (SNAP-25), and syntaxin were measured in two cortical brain regions. Linear regression models assessed associations of brain tocopherols with presynaptic protein levels.
Results:
Higher brain γ-tocopherol levels were associated with higher levels of the SNARE protein composite, complexin-I, complexin-II, the synaptotagmin synaptophysin composite, and septin-5 in the midfrontal cortex (B(SE) = 0.272 to 0.412 (0.084 to 0.091), p < 0.001 to 0.003). When additionally adjusted for global Alzheimer’s disease pathology, cerebral infarcts, and Lewy body disease pathology, these associations remained largely similar. No associations were found between α-tocopherol and presynaptic protein levels.
Conclusion:
In this cross-sectional study, we found higher brain γ-tocopherol levels were associated with presynaptic protein levels in the midfrontal cortex. These results are consistent with a proposed role of vitamin E to maintain presynaptic protein levels.
INTRODUCTION
Lower intake and blood levels of several vitamin E isoforms are reported to be associated with increased risks of cognitive decline and dementia [1–4]. In a previous study, we found that higher vitamin E brain levels were associated with lower Alzheimer’s disease (AD) neuropathology; lower brain γ-tocopherol levels were associated with higher amyloid load and neurofibrillary tangle severity, while higher α-tocopherol in combination with low γ-tocopherol levels were associated with higher amyloid load [5]. These findings support a protective role of vitamin E in AD and specifically an important role for γ-tocopherol. The underlying mechanisms for this relationship, however, remain unclear.
Synapses play a central role in brain function. Previous studies showed that synaptic density and function are highly related to normal neurological function and cognition [6–11]. Synaptic function, often measured as presynaptic protein levels, is therefore considered an indicator of cognitive brain reserve [12]. Consistent with this role for presynaptic proteins as measure of cognitive reserve, we previously reported that presynaptic protein levels are related to a clinical diagnosis of dementia independent of global AD neuropathology and cerebral infarcts [13]. Synapses are thus key for cognitive function; however, they are highly susceptible to oxidative stress that induces presynaptic protein loss and synaptic dysfunction [14–16].
α-Tocopherol is well known for having antioxidant properties that prevent free radicals from oxidizing polyunsaturated lipids of cell membranes, lipid bodies, and lipoproteins [17, 18]. In addition, γ-tocopherol may exert anti-inflammatory effects [19]. Previous animal studies showed that α-tocopherol may counteract oxidative stress processes and help to maintain presynaptic protein levels [20, 21]. We therefore hypothesized that one potential biological mechanism underlying the relation of tocopherols with cognitive health is preservation of cognitive reserve by sustaining higher levels of presynaptic proteins. In this study, we aimed to examine the associations between brain tocopherols and presynaptic proteins in elderly human brains.
METHODS
Study sample
This study sample is comprised of 115 autopsied cases of participants of the Rush Memory and Aging Project (MAP) who were analyzed for both brain tocopherols and presynaptic proteins. The Rush MAP is an ongoing clinical-neuropathological epidemiologic study of persons living in Chicago continuous care retirement communities and subsidized housing that began in 1997 [22]. Volunteers are free of known dementia at enrollment and agreed to annual clinical evaluations and to brain autopsy at death. Written informed consent was obtained from all study participants and the study was approved by the Institutional Review Board of Rush University Medical Center.
Brain neuropathology
Brains were examined by a board-certified neuropathologist blinded to clinical data. Brain autopsies for the study sample were performed on average, 6.5 hours after death in a standard fashion as previously described [22]. Brains were processed and stored at the Rush Alzheimer’s Disease Center (RADC) laboratory. Slabs from one cerebral hemisphere were placed in a –80°C freezer. This tissue was used for the tocopherol and presynaptic protein quantification as described below. The contralateral hemisphere was fixed in 4% paraformaldehyde and stored in 20% glycerol and 2% dimethylsulfoxide. After fixation, tissue was dissected, processed, embedded, cut and stained from multiple standard regions for neuropathologic evaluation of AD and other dementia related pathologies including infarcts and Lewy bodies [23]. The density of neuritic plaques, diffuse plaques, and neurofibrillary tangles was assessed using Bielschowsky silver stain 6 micron sections for visualization and a graticule to count total number of each measure in a 1 mm2 area of highest density. Counts for each characteristic were completed in the entorhinal cortex, hippocampus, midtemporal, inferior parietal, and midfrontal cortex and then converted to standardized scores. The standardized scores were then averaged across the five regions to obtain a summary score for each measure. A measure of global AD pathology was obtained by averaging summary scores for diffuse plaques, neuritic plaques, and neurofibrillary tangles [24, 25]. Macroscopic and microscopic infarcts (acute, subacute, and chronic measures separately) were converted to a dichotomous variable indicating absence or presence (1 or more) of any chronic infarcts [26]. Lewy bodies were graded on a four-point scale (0-not present, 1-nigral predominant, 2-limbic type, 3-neocortical type), as previously described [27].
Brain tocopherol analyses
Frozen brain tissue from two cortical brain regions (inferior temporal cortex and midfrontal cortex) were thawed and analyzed for tocopherol concentrations using high performance liquid chromatography coupled to electrochemical detection as previously described [28, 29]. Extraction losses were corrected for recoveries of the internal standard, δ-tocopherol. For data-analyses, tocopherol levels are expressed as picomoles (pmol) per mg protein. We eliminated from the analyses two cases with extreme values (α-tocopherol >10.000 pmol/mg; γ-tocopherol >900 pmol/mg).
Vitamin E intake
Vitamin E dietary and supplement intake was assessed using a semiquantitative food frequency questionnaire (FFQ) validated in a sample of older Chicago residents [30]. Daily intake of vitamin E was obtained by multiplying the vitamin E content of each food item (from the Harvard nutrient database) by reported frequency of intake, and summing over all food items. All nutrients were calorie adjusted by the regression-residual method. Supplement use and dosage was calculated from vitamin E (α-tocopherol) and multivitamin supplements. Dietary and supplement intake were averaged over all valid FFQs available for each participant.
Presynaptic protein measurements
Monoclonal antibodies were used to quantify immunoreactivity for eight presynaptic proteins (synaptophysin, synaptotagmin, septin 5, syntaxin, synaptosomal associated protein 25 (SNAP-25), vesicle-associated membrane protein (VAMP), complexin-I, complexin-II) in two cortical brain regions (inferior temporal cortex and midfrontal cortex). Frozen samples of gray matter were assayed with an enzyme-linked immunosorbent assay (ELISA). The protein concentration needed to acquire equal fixed optical density value across all samples was identified from dilution curve fitting. The total homogenate protein concentration was inversely related to the present quantity of target antigen. Protein-protein interactions between syntaxin/SNAP-25 and SNAP-25/syntaxin were measured using a high-throughput immunoprecipitation strategy implemented with a heterologous capture ELISA [31]. Purified antibody directed against one of the targets was fixed on the ELISA plate, serially diluted brain homogenate samples were incubated on the ELISA plate, subsequently a second antibody was added to detect the protein-binding partner of the initially captured target. Primary antibodies were produced in house. The following quality control actions were performed. Titer tests were performed on tissue culture supernatants to ensure potency of detection antibodies. Purified antibodies for the protein-protein interaction assays were used at an optimized, fixed protein concentration. Duplicate serial dilution curves were carried out for all sample assays on each plate, and linearity was evaluated from the results. Samples were run twice on separate days, with run-to-run correlations required to exceed r = 0.8. In each run multiple replicates of a reference sample were included; the within-run coefficient of variation was required to be <10%.
Statistical analyses
Mann Whitney-U tests were used to compare levels of tocopherols in the inferior temporal and midfrontal brain regions. We studied levels of α- and γ-tocopherol, presynaptic proteins and protein-protein interactions in two different brain regions (inferior temporal cortex and midfrontal cortex). Because tocopherol, presynaptic protein data and vitamin E intake were not normally distributed, all values were log transformed and subsequently standardized to z-scores. Presynaptic protein data were additionally inverted to make higher values correspond to higher protein concentrations. Some presynaptic proteins were highly correlated, serve related functions and were therefore summarized in three composite scores; 1) soluble N-ethylmaleimide-sensitive factor protein attachment protein receptor (SNARE) proteins composite (syntaxin, VAMP, SNAP-25, Spearman correlation coefficients range: 0.74–0.87, p < 0.001), 2) Syntaxin/SNAP-25 protein-protein interaction composite (syntaxin/SNAP-25 and SNAP-25/syntaxin, Spearman correlation coefficients range: 0.58–0.59, p < 0.001), 3) Synaptophysin synaptotagmin vesicular protein composite (Synaptophysin and synaptotagmin, Spearman correlation coefficients range 0.83–0.86, p < 0.001). Composite scores for each brain region separately were calculated averaging z-scores of the individual presynaptic proteins or protein-protein interactions. Spearman’s correlations were used to assess correlations between dietary and total (dietary + supplement) intake of vitamin E and brain tocopherol levels. Linear regression models were used to examine the associations of vitamin E intake and brain tocopherol levels in separate models (continuous determinants) with presynaptic proteins and protein-protein interactions (outcome) by region. The basic model was adjusted for age at death, sex, years of education, APOE ɛ4 genotype carriers and the time interval from death to autopsy (hours). In a second model we additionally adjusted for global AD pathology, cerebral infarcts and Lewy body disease pathology. Models that examined associations of the tocopherols with the syntaxin/SNAP-25 interaction composite were additionally adjusted for the distinct levels of syntaxin and SNAP-25. All analyses were performed in R (3.4.2 (2017-09-28). Results are reported at a threshold of p < 0.05. In addition, we report the results that surpass correction for multiple testing using the Bonferroni correction after which p = 0.05/48 = 0.001 is considered significant.
RESULTS
Sample characteristics
Descriptives of the study sample are shown in Table 1. Mean±SD age was 88.5±6.0 years and the majority of our sample was female (60%). The mean±SD years of education was 14.9±2.6 and 30 (26%) participants were carriers of at least one APOE ɛ4 allele. The mean±SD time till autopsy was 6.5±3.2 h. Levels of α- and γ-tocopherol did not differ between inferior temporal and midfrontal brain regions.
Table 1
Descriptives of 113 Memory and Aging Project participants
| General | |
| Age at death, mean y±SD | 88.5±6.0 |
| Female, n (%) | 45 (40%) |
| Education, mean y±SD | 14.9±2.6 |
| APOE ɛ4, n (%) with at least one allele | 30 (27%) |
| Clinical AD diagnosis, n (%) | 38 (34%) |
| Neuropathology | |
| Postmortem autopsy, mean h±SD | 6.5±3.2 |
| Global AD pathology, mean±SD | 0.6±0.5 |
| Presence of cerebral infarctions, n (%) | 60 (53%) |
| Presence of Lewy body pathology, n (%) | 19 (17%) |
| Vitamin E intake (mg/d) | |
| Dietary vitamin E intake, median (IQR) | 5.6 (4.9, 6.6) |
| Total vitamin E intake, median (IQR) | 28.7 (12.8, 146.4) |
| Brain vitamin E levels (pmoles/mg) | |
| α-tocopherol in the inferior temporal cortex, median (IQR) | 172.9 (49.6, 327.0) |
| α-tocopherol in the midfrontal cortex, median (IQR) | 231.8 (93.8, 325.0) |
| γ-tocopherol in the inferior temporal cortex, median (IQR) | 56.7 (34.4, 113.8) |
| γ-tocopherol in the midfrontal cortex, median (IQR) | 57.8 (36.4, 84.4) |
APOE, apolipoprotein E; IQR, interquartile range; SD, standard deviation. Vitamin E intake levels were calorie adjusted, Lewy body pathology was assessed on a 4-point scale (0–3) as explained in the text, in this table we used a dichotomous variable indicating any presence (score 1–3) of Lewy body pathology.
Intake of vitamin E
Total (dietary + supplement) intake of vitamin E was moderately correlated with brain α-tocopherol levels in the inferior temporal cortex (r = 0.25, p = 0.01) but not in the midfrontal cortex (r = 0.17, p = 0.07) or with γ-tocopherol brain levels (r = –0.01, p = 0.90, inferior temporal cortex, r = –0.06, p = 0.52, midfrontal cortex). Dietary intake alone was not correlated with brain α- or γ-tocopherol levels (r(range) = –0.09 to 0.04).
Associations between brain tocopherols and presynaptic proteins
Linear regression analysis adjusted for sex, age, education, APOE ɛ4 genotype, and the postmortem time interval were used to examine the associations of brain tocopherols with presynaptic proteins by region (Fig. 1 and Table 2). In the midfrontal cortex, higher γ-tocopherol brain levels were associated with higher levels of the SNARE protein composite, the synaptotagmin synaptophysin composite, complexin-I, complexin-II, and septin-5. Only the association with the synaptotagmin synaptophysin composite did not survive Bonferroni correction. When the linear regression models were additionally adjusted for global AD pathology, cerebral infarcts, and Lewy body pathology, the effect sizes of associations were not materially changed, but only the association with complexin-II surpassed the more stringent Bonferroni corrected threshold for significance (p < 0.001) (Table 2). Dietary and/or supplement intake of vitamin E was not associated with presynaptic protein levels (data not shown).
Fig. 1
Linear regression models corrected for sex, age, education, postmortem interval, and APOE ɛ4 genotype. Dashed lines represent p = 0.05. a) SNARE proteins, synaptotagmin + synaptophysin, and SNAP-25/syntaxin are calculated composites as described in text. b) SNAP-25/syntaxin is additionally adjusted for region specific SNAP-25 and syntaxin concentrations in both models.
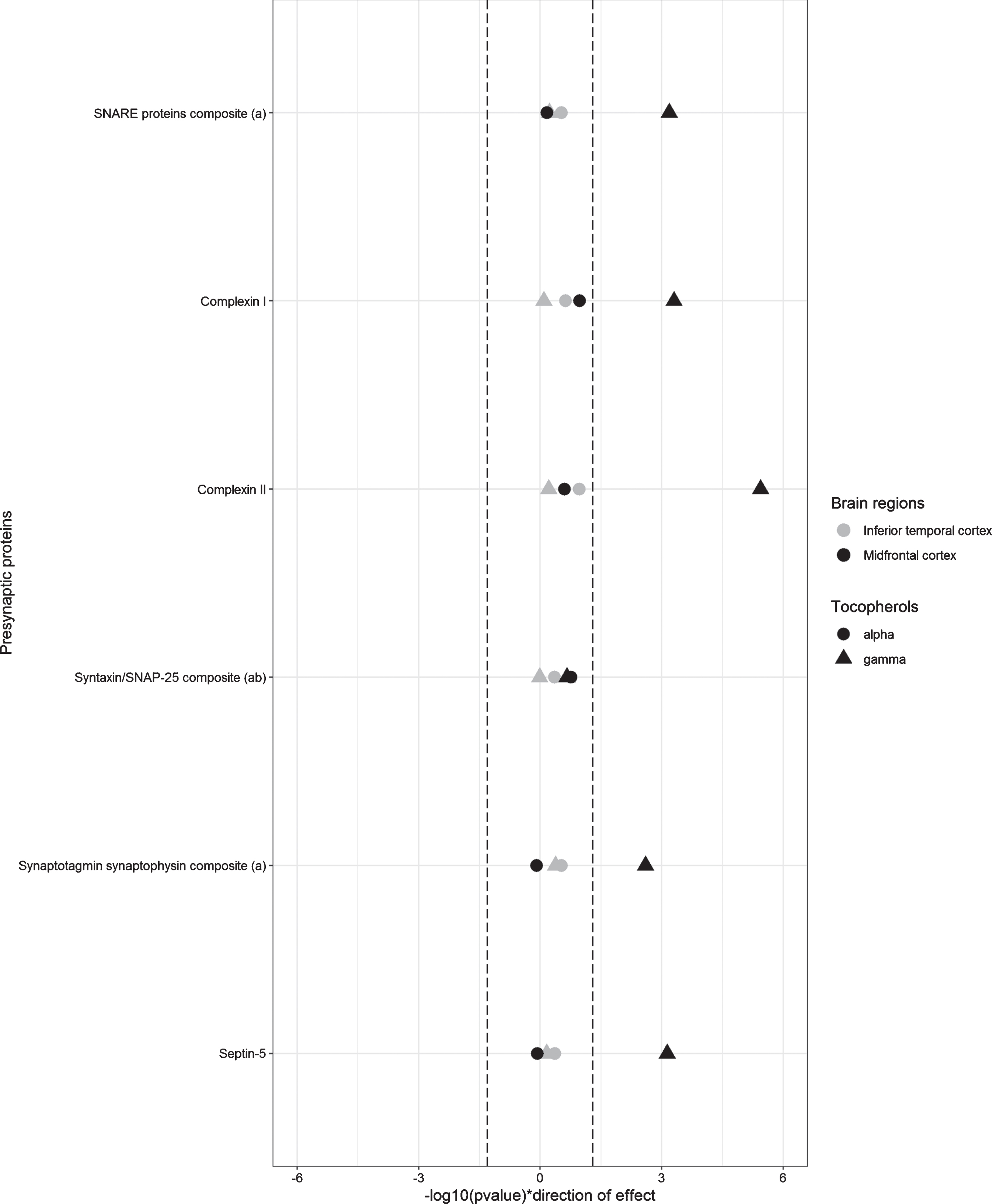
Table 2
Association of brain levels of α and γ-tocopherol with presynaptic protein concentrations
| Determinant | α-tocopherol | γ-tocopherol | |||||||
| Outcome | Brain region | Basic model | Basic model + AD pathology + infarcts + | Basic model | Basic model + AD pathology + infarcts + | ||||
| Lewy Body pathology | Lewy Body pathology | ||||||||
| B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p | ||
| SNARE proteins compositea | IT | 0.098 (0.093) | 0.297 | 0.119 (0.096) | 0.215 | 0.051 (0.095) | 0.595 | 0.065 (0.101) | 0.522 |
| SNARE proteins compositea | MF | 0.038 (0.092) | 0.684 | 0.030 (0.093) | 0.749 | 0.302 (0.086) | <0.001* | 0.267 (0.088) | 0.003 |
| Complexin-I | IT | 0.117 (0.098) | 0.235 | 0.141 (0.101) | 0.166 | 0.023 (0.100) | 0.817 | 0.083 (0.107) | 0.439 |
| Complexin-I | MF | 0.159 (0.097) | 0.105 | 0.154 (0.100) | 0.129 | 0.327 (0.091) | <0.001* | 0.313 (0.095) | 0.001 |
| Complexin-II | IT | 0.158 (0.098) | 0.108 | 0.154 (0.099) | 0.121 | 0.050 (0.100) | 0.617 | 0.088 (0.104) | 0.399 |
| Complexin-II | MF | 0.109 (0.094) | 0.250 | 0.103 (0.093) | 0.271 | 0.412 (0.084) | <0.001* | 0.369 (0.085) | <0.001* |
| Syntaxin/SNAP-25 compositeab | IT | 0.070 (0.091) | 0.440 | 0.082 (0.094) | 0.387 | –0.004 (0.090) | 0.964 | –0.027 (0.096) | 0.779 |
| Syntaxin/SNAP-25 compositeab | MF | 0.121 (0.088) | 0.172 | 0.132 (0.090) | 0.146 | 0.118 (0.095) | 0.217 | 0.128 (0.097) | 0.187 |
| Synaptotagmin synaptophysin composite a | IT | 0.100 (0.096) | 0.300 | 0.103 (0.098) | 0.292 | 0.078 (0.097) | 0.422 | 0.075 (0.103) | 0.466 |
| Synaptotagmin synaptophysin composite a | MF | –0.022 (0.094) | 0.816 | –0.031 (0.094) | 0.741 | 0.272 (0.088) | 0.003 | 0.233 (0.090) | 0.011 |
| Septin-5 | IT | 0.077 (0.098) | 0.434 | 0.091 (0.099) | 0.358 | 0.038 (0.099) | 0.700 | 0.053 (0.104) | 0.612 |
| Septin-5 | MF | –0.018(0.096) | 0.850 | –0.034 (0.095) | 0.723 | 0.311(0.089) | <0.001* | 0.260 (0.090) | 0.005 |
IT, inferior temporal, MF, midfrontal, SE, standard error. Basic model adjusted for age at death (y), sex, education (y), APOE ɛ4 (any ɛ4 versus none) and postmortem autopsy time (h). aSNARE proteins, synaptotagmin + synaptophysin, and SNAP-25/syntaxin are calculated composites as described in text. bSNAP-25/syntaxin is additionally adjusted for region specific SNAP-25 and syntaxin concentrations in both models. *Significant after Bonferroni correction p < 1.0×10–3.
DISCUSSION
The main finding of this study is that higher brain γ-tocopherol levels relate to higher levels of presynaptic proteins in the midfrontal cortex. To our knowledge, this cross-sectional study is the first study to address the relation between tocopherols and presynaptic proteins in human brain. As such, we can only speculate on the causality or directionality of effects. Our findings might however, indicate that γ-tocopherol supports synaptic function.
The finding that higher brain tocopherol levels are associated with higher presynaptic protein levels confirms earlier findings from mice studies [20, 21]. Kaneai et al. found that vitamin E deficient mice express lower levels of SNARE proteins [20]. Moreover, oxidative stress reduced levels of presynaptic proteins, but this effect could be partly counteracted with vitamin E supplementation. With these findings, one could hypothesize that tocopherols inhibit the oxidation of presynaptic proteins under oxidative stress. This hypothesis is further supported by two studies describing the potential antioxidant properties of vitamin E in the brain [32, 33].
We found that γ-tocopherol levels were associated with individual presynaptic protein levels in the midfrontal cortex, but not in the inferior temporal cortex. The current knowledge on regional differences in tocopherols and presynaptic protein function is, however limited, thus, we can only speculate on the interpretation of these findings. Previous studies have highlighted the midfrontal cortex as highly active area and suitable to study synaptic abnormalities [34–36]. This could perhaps explain why the associations we found between γ-tocopherol and presynaptic protein levels were limited to the midfrontal cortex. Larger studies are however needed to further examine regional differences in tocopherols and presynaptic protein function.
The associations between brain tocopherols and presynaptic protein levels in this study, were independent of AD pathology, cerebral infarcts, and Lewy body disease pathology. In previous work, we showed that presynaptic protein levels contributed importantly to cognitive function independent of pathology [13]. Together with the results from this study, this gives support to a model of tocopherols that create an antioxidant environment that protects against oxidation of presynaptic proteins, helps to maintain cognitive reserve, and subsequently might prevent cognitive decline and dementia. In previous work, however, we also observed associations between vitamin E and AD brain pathology [5]. Together this might indicate that vitamin E, and in particular γ-tocopherol might have multiple pathways to protect the brain against neurodegenerative processes both via direct effects on pathology and on synapses.
As an essential nutrient, vitamin E must be obtained from food and cross the blood-brain barrier to reach different areas of the brain [37, 38]. In the brain, we found associations between γ-tocopherol levels and presynaptic proteins. We did not observe direct associations between dietary and/or (α-tocopherol) supplement intake and presynaptic protein levels. Moreover, total (diet + supplement) vitamin E intake was only correlated with α-tocopherol brain levels. This could perhaps be explained by the transport and regulation of vitamin E in humans. While the majority of our dietary vitamin E intake is γ-tocopherol, α-tocopherol is considered the most biologically active form of vitamin E, and comprises the main isoform of vitamin E and multivitamin supplements. Due to the high affinity of vitamin E transporters for α-tocopherol, 90% of the vitamin E in plasma as well as in the brain samples of this study is α-tocopherol [5, 38]. This suggests that our dietary intake of γ-tocopherol only reaches the brain in very low concentrations. Perhaps this explains why the associations between γ-tocopherol and presynaptic proteins in the brain were not seen using the dietary intake measure in this study. Previous studies showed that γ-tocopherol is quickly metabolized into 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman (γ-CEHC) which has similar anti-oxidative and anti-inflammatory properties as γ-tocopherol. [39, 40] It might therefore be of interest to include γ-CEHC measurements as marker for γ-tocopherol function in future investigations. Another explanation could be that the sample size of this study is too small to study the potentially subtle association between dietary intake and presynaptic proteins that could also affected by multiple metabolic factors and recall bias of dietary intake. The additional measurement of γ-tocopherol and γ-CEHC in blood in future studies might help to further assess the complex relationship between dietary intake of γ-tocopherol and presynaptic proteins.
Limitations of this study include the cross-sectional design, and uncertainty concerning the temporal relationship between tocopherols and presynaptic protein levels. The direction of effects cannot be determined. Our data, however, support a model where nutritional vitamin E intake contributes to increasing presynaptic protein levels, rather than a brain (disease) effect associated with reduced brain tocopherols or vitamin E intake. First, we found moderate associations of brain α-tocopherol levels with total vitamin E intake, supporting a direct relation between brain tocopherols and nutritional intake. Second, our findings were independent of pathological AD, cerebral infarcts, or Lewy body disease pathology, indicating that disease effects are not (directly) related to our findings. Lastly, our findings are consistent with previous animal studies that showed similar effects in vitamin E supplemented mice. Longitudinal animal studies are, however, needed to further define the exact cascade of events. Currently, only a few studies measured brain levels of tocopherols; measured concentrations of α-tocopherol and γ-tocopherol levels varied considerably among studies [41, 42]. Inconsistencies in findings could be explained by differences in population or measurement methods. For example, our study sample was younger and included more males, compared to the Georgian Centenarian Study [42]. Although all studies measured tocopherols using HPLC, differences in internal standards or other (pre-)analytical factors could contribute to conflicting findings. Future research should therefore aim to measure tocopherols in more brain regions and to compare different analytical methods. This study demonstrated associations between γ-tocopherol levels, but not α-tocopherol levels and presynaptic protein levels. Contrary to α-tocopherol, γ-tocopherol has important anti-inflammatory properties, future studies might benefit from including indicators of neuroinflammation [19]. Among the strengths of our present study is that our cohort is very well defined, offered information on the most important potential confounders, and detailed neuropathological measures that were obtained after a relatively short postmortem time interval. In addition, we had information on both dietary and supplement intake of vitamin E as brain tocopherol levels. The sample size of our cohort, with over 100 brain cases, is relatively large in comparison to the limited other autopsy studies in nutrition research. Besides, as a population-based cohort, our results might be extrapolated to a larger population. We had many presynaptic protein levels measured in two cortical brain regions, which raises issues of multiple testing. However, we summarized highly correlated presynaptic proteins in composite scores to limit these risks and have additionally used a significance threshold that corrects for multiple testing.
In conclusion, we found associations for higher γ-tocopherol levels with higher levels of presynaptic protein levels in the midfrontal cortex. These results support an important role for γ-tocopherol to maintain presynaptic protein levels and thereby potentially reduce risks of cognitive decline.
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging (R01AG031553, R01AG054476, and R01AG17917). F.d.L. is appointed to the NUDAD project, which is funded by NWO-FCB (project number 057-14-004). F.d.L. was supported by the Alzheimer Nederland Fellowship Grant No. WE. 15-2018-03.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0166r1).
REFERENCES
[1] | Morris MC , Evans DA , Tangney CC , Bienias JL , Wilson RS , Aggarwal NT , Scherr PA ((2005) ) Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr 81: , 508–514. |
[2] | Morris MC , Evans DA , Bienias JL , Tangney CC , Wilson RS ((2002) ) Vitamin E and cognitive decline in older persons. Arch Neurol 59: , 1125–1132. |
[3] | Mangialasche F , Solomon A , Kareholt I , Hooshmand B , Cecchetti R , Fratiglioni L , Soininen H , Laatikainen T , Mecocci P , Kivipelto M ((2013) ) Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp Gerontol 48: , 1428–1435. |
[4] | Joseph JA , Shukitt-Hale B , Denisova NA , Prior RL , Cao G , Martin A , Taglialatela G , Bickford PC ((1998) ) Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci 18: , 8047–8055. |
[5] | Morris MC , Schneider JA , Li H , Tangney CC , Nag S , Bennett DA , Honer WG , Barnes LL ((2015) ) Brain tocopherols related to Alzheimer’s disease neuropathology in humans. Alzheimers Dement 11: , 32–39. |
[6] | Tobin MK , Musaraca K , Disouky A , Shetti A , Bheri A , Honer WG , Kim N , Dawe RJ , Bennett DA , Arfanakis K , Lazarov O ((2019) ) Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24: , 974–982.e3. |
[7] | Washbourne P , Thompson PM , Carta M , Costa ET , Mathews JR , Lopez-Bendito G , Molnar Z , Becher MW , Valenzuela CF , Partridge LD , Wilson MC ((2002) ) Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5: , 19–26. |
[8] | Glynn D , Bortnick RA , Morton AJ ((2003) ) Complexin II is essential for normal neurological function in mice. Hum Mol Genet 12: , 2431–2448. |
[9] | Drew CJ , Kyd RJ , Morton AJ ((2007) ) Complexin 1 knockout mice exhibit marked deficits in social behaviours but appear to be cognitively normal. Hum Mol Genet 16: , 2288–2305. |
[10] | Schoch S , Deak F , Konigstorfer A , Mozhayeva M , Sara Y , Sudhof TC , Kavalali ET ((2001) ) SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 294: , 1117–1122. |
[11] | Schmitt U , Tanimoto N , Seeliger M , Schaeffel F , Leube RE ((2009) ) Detection of behavioral alterations and learning deficits in mice lacking synaptophysin. Neuroscience 162: , 234–243. |
[12] | Stern Y ((2009) ) Cognitive reserve. Neuropsychologia 47: , 2015–2028. |
[13] | Honer WG , Barr AM , Sawada K , Thornton AE , Morris MC , Leurgans SE , Schneider JA , Bennett DA ((2012) ) Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry 2: , e114. |
[14] | Urano S , Asai Y , Makabe S , Matsuo M , Izumiyama N , Ohtsubo K , Endo T ((1997) ) Oxidative injury of synapse and alteration of antioxidative defense systems in rats, and its prevention by vitamin E. Eur J Biochem 245: , 64–70. |
[15] | Scheff SW , Ansari MA , Mufson EJ ((2016) ) Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol Aging 42: , 1–12. |
[16] | Abidin I , Aydin-Abidin S , Bodur A , Ince I , Alver A ((2018) ) Brain-derived neurotropic factor (BDNF) heterozygous mice are more susceptible to synaptic protein loss in cerebral cortex during high fat diet. Arch Physiol Biochem 124: , 442–447. |
[17] | Traber MG , Atkinson J ((2007) ) Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43: , 4–15. |
[18] | Zhang X , Feng M , Liu F , Qin L , Qu R , Li D , Wang Z ((2014) ) Subacute oral toxicity of BDE-15, CDE-15, and HODE-15 in ICR male mice: Assessing effects on hepatic oxidative stress and metals status and ascertaining the protective role of vitamin E. Environ Sci Pollut Res Int 21: , 1924–1935. |
[19] | Hensley K , Benaksas EJ , Bolli R , Comp P , Grammas P , Hamdheydari L , Mou S , Pye QN , Stoddard MF , Wallis G , Williamson KS , West M , Wechter WJ , Floyd RA ((2004) ) New perspectives on vitamin E: Gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med 36: , 1–15. |
[20] | Kaneai N , Arai M , Takatsu H , Fukui K , Urano S ((2012) ) Vitamin E inhibits oxidative stress-induced denaturation of nerve terminal proteins involved in neurotransmission. J Alzheimers Dis 28: , 183–189. |
[21] | Kaneai N , Fukui K , Koike T , Urano S ((2013) ) Vitamin E prevents hyperoxia-induced loss of soluble n-ethylmaleimide-sensitive fusion protein attachment protein receptor proteins in the rat neuronal cytoplasm. Biol Pharm Bull 36: , 1500–1502. |
[22] | Bennett DA , Schneider JA , Buchman AS , Barnes LL , Boyle PA , Wilson RS ((2012) ) Overview and findings from the rush memory and aging project. Curr Alzheimer Res 9: , 646–663. |
[23] | Bennett DA , Wilson RS , Boyle PA , Buchman AS , Schneider JA ((2012) ) Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 72: , 599–609. |
[24] | Felsky D , Roostaei T , Nho K , Risacher SL , Bradshaw EM , Petyuk V , Schneider JA , Saykin A , Bennett DA , De Jager PL ((2019) ) Neuropathological correlates and genetic architecture of microglial activation in elderly human brain. Nat Commun 10: , 409. |
[25] | Bennett DA , Buchman AS , Boyle PA , Barnes LL , Wilson RS , Schneider JA ((2018) ) Religious orders study and rush memory and aging project. J Alzheimers Dis 64: , S161–S189. |
[26] | Arvanitakis Z , Leurgans SE , Barnes LL , Bennett DA , Schneider JA ((2011) ) Microinfarct pathology, dementia, and cognitive systems. Stroke 42: , 722–727. |
[27] | Schneider JA , Arvanitakis Z , Yu L , Boyle PA , Leurgans SE , Bennett DA ((2012) ) Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 135: , 3005–3014. |
[28] | Hensley K , Barnes LL , Christov A , Tangney C , Honer WG , Schneider JA , Bennett DA , Morris MC ((2011) ) Analysis of postmortem ventricular cerebrospinal fluid from patients with and without dementia indicates association of vitamin E with neuritic plaques and specific measures of cognitive performance. J Alzheimers Dis 24: , 767–774. |
[29] | Williamson KS , Gabbita SP , Mou S , West M , Pye QN , Markesbery WR , Cooney RV , Grammas P , Reimann-Philipp U , Floyd RA , Hensley K ((2002) ) The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide 6: , 221–227. |
[30] | Morris MC ((2003) ) Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 158: , 1213–1217. |
[31] | Barakauskas VE , Beasley CL , Barr AM , Ypsilanti AR , Li HY , Thornton AE , Wong H , Rosokilja G , Mann JJ , Mancevski B , Jakovski Z , Davceva N , Ilievski B , Dwork AJ , Falkai P , Honer WG ((2010) ) A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology 35: , 1226–1238. |
[32] | Alzoubi KH , Khabour OF , Salah HA , Hasan Z ((2013) ) Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: The role of oxidative stress. Physiol Behav 119: , 72–78. |
[33] | Tome AR , Feng D , Freitas RM ((2010) ) The effects of alpha-tocopherol on hippocampal oxidative stress prior to in pilocarpine-induced seizures. Neurochem Res 35: , 580–587. |
[34] | Bell KF , Bennett DA , Cuello AC ((2007) ) Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci 27: , 10810–10817. |
[35] | DeKosky ST , Scheff SW ((1990) ) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol 27: , 457–464. |
[36] | Honer WG , Ramos-Miguel A , Alamri J , Sawada K , Barr AM , Schneider JA , Bennett DA ((2019) ) The synaptic pathology of cognitive life. Dialogues Clin Neurosci 21: , 271–279. |
[37] | Mardones P , Rigotti A ((2004) ) Cellular mechanisms of vitamin E uptake: Relevance in alpha-tocopherol metabolism and potential implications for disease. J Nutr Biochem 15: , 252–260. |
[38] | Lee P , Ulatowski LM ((2019) ) Vitamin E: Mechanism of transport and regulation in the CNS. IUBMB Life 71: , 424–429. |
[39] | Jiang Q , Christen S , Shigenaga MK , Ames BN ((2001) ) γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 74: , 714–722. |
[40] | Wiser J , Alexis NE , Jiang Q , Wu W , Robinette C , Roubey R , Peden DB ((2008) ) In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med 45: , 40–49. |
[41] | Craft NE , Haitema TB , Garnett KM , Fitch KA , Dorey CK ((2004) ) Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging 8: , 156–162. |
[42] | Tanprasertsuk J , Mohn ES , Matthan NR , Lichtenstein AH , Barger K , Vishwanathan R , Johnson MA , Poon LW , Johnson EJ ((2019) ) Serum carotenoids, tocopherols, total n-3 polyunsaturated fatty acids, and n-6/n-3 polyunsaturated fatty acid ratio reflect brain concentrations in a cohort of centenarians. J Gerontol A Biol Sci Med Sci 74: , 306–314. |




