Endostatin as a Mediator Between Endothelial Function and Cognitive Performance in Those at Risk for Vascular Cognitive Impairment
Abstract
Background:
Patients with coronary artery disease have an increased risk for developing vascular cognitive impairment. Endothelial function is often diminished and has been associated with lower cognitive performance in these patients. The link between endothelial function and cognition in coronary artery disease is not fully understood. Angiogenesis may play a role in mediating the association between endothelial function and cognition since angiogenic processes rely heavily on the endothelium.
Objective:
The aim of this study was to determine if markers of angiogenesis mediate the relationship between endothelial function and cognition in coronary artery disease patients.
Methods:
In 50 participants with coronary artery disease, endothelial function was assessed using peripheral arterial tonometry. Vascular endothelial growth factor (pro-angiogenic) and endostatin (anti-angiogenic) were measured in peripheral serum samples. Cognition was assessed using the Montreal Cognitive Assessment. A mediation analysis, using a bias corrected inferential bootstrapping method with 10,000 permutations, was used to determine if vascular endothelial growth factor or endostatin mediated an association between peripheral arterial tonometry measures and cognitive performance on the Montreal Cognitive Assessment.
Results:
Endostatin, but not vascular endothelial growth factor, mediated a relationship between endothelial function and cognitive performance when controlling for total years of education, body mass index, coronary artery bypass graft, stent, diabetes, and diuretic use. This analysis was also significant when delayed recall was substituted for the overall score on the Montreal Cognitive Assessment.
Conclusion:
These results suggest that endostatin mediates an association between endothelial function and cognitive performance in coronary artery disease.
INTRODUCTION
Coronary artery disease (CAD), a major cause of cardiovascular disease worldwide [1], is strongly associated with vascular cognitive impairment (VCI); prospective studies indicate that those with CAD have a 45% greater risk of developing cognitive deficits [2]. Many risk factors for CAD overlap with those for cognitive impairment: hypertension, hyperlipidemia, increased body mass index (BMI), smoking, and hyperglycemia [3]. Therefore, the CAD population is an at-risk group for developing VCI, and cognitive impairment in CAD can also lead to functional deficits and mortality [4, 5]. Cognitive performance in CAD patients is associated with the cerebrovascular alterations in patients with VCI, including white matter hyperintensities and signs of cerebral hypoperfusion [6–10].
Sufficient tissue perfusion depends highly on the vascular endothelium, which is a monolayer of cells within the vasculature that is responsible for regulating vascular tone and vascular homeostasis. Both CAD and VCI are associated with endothelial dysfunction, which is characterized by impaired nitric oxide mediated vascular tone [11–14]. Further, endothelial dysfunction is linked to cerebrovascular damage and cognitive deficits [13, 15, 16], which provides rationale for a mechanistic association between CAD and VCI. Importantly, impaired endothelial function plays a critical role in the impairment of angiogenic processes that results in microvascular rarefaction, which has been linked to tissue hypoperfusion [17, 18]. It has been demonstrated that impaired Wnt signaling (an important glycoprotein responsible for regulating several cellular processes) can lead to endothelial dysfunction, which then results in impaired angiogenesis and altered expression of angiogenesis markers [19]. This provides rationale that endothelial dysfunction precedes impaired angiogenesis. While impairment in angiogenic processes has been associated with poorer cognitive performance in mild cognitive impairment and Alzheimer’s disease [20, 21], most of the evidence to suggest the role of angiogenesis in VCI has been established through animal models [22, 23]. Nonetheless, impairments in angiogenesis may be critical in VCI given the vascular etiology of these diseases. Additionally, more studies are required to establish a link between endothelial dysfunction, angiogenesis, and cognition in at risk populations for VCI to further our understanding of the underlying mechanisms that may contribute to cognitive decline.
Since it has been suggested that peripheral markers of endothelial function and angiogenesis reflect cerebrovascular alterations and cognitive performance, this study aimed to assess the link between peripheral endothelial function, markers of angiogenesis, and overall cognitive performance in a CAD population. It is hypothesized that angiogenesis will mediate the relationship between endothelial function and cognition given that impaired angiogenesis is a likely result of poorer endothelial function and a contributor to brain hypoperfusion, which is highly associated with cognitive decline. Furthermore, studies have indicated that angiogenic promoters and inhibitors are differentially affected in CAD and each respond differently to aerobic exercise [24, 25], an intervention known to have a positive impact on cognition in CAD patients [26, 27]. Therefore, this study aimed to investigate both pro- and anti-angiogenic markers to determine how each signal mediates the relationship between endothelial function and cognition. Additionally, altered angiogenesis markers have been shown to increase apoptotic signaling and decrease endothelial cell proliferation in diabetes [28]. Therefore, this study will also investigate endothelial function as a mediator between levels of angiogenesis markers and cognitive performance.
METHODS
Participants
This study was approved by the research ethics boards at Sunnybrook Health Sciences Centre and the Toronto Rehabilitation Institute at the University Health Network, where participants were recruited before entry into a cardiac rehabilitation program. All participants provided written informed consent before enrolling into the study. All participants had CAD, which was defined as having one or more of the following: myocardial infarction, angiographic evidence showing ≥50% blockage in at least one major coronary artery, percutaneous coronary intervention, or coronary artery bypass graft surgery (CABG). Participants were excluded based on previously diagnosed neurodegenerative illness including all-cause dementia, active cancer, surgery planned within 12 months, schizophrenia, bipolar affective disorder, and substance use disorder. Participants with standardized Mini-Mental Status Examination of 24 or less were excluded because significant cognitive impairment would preclude participants from participating in the cardiac rehabilitation program independently and from completing the cognitive testing.
Demographic and clinical characteristics, as well as a detailed medical history for eligible participants who provided written informed consent, were collected from patient interviews. Cardiac diagnoses, concomitant medications, vascular risk factors, and anthropometrics were obtained from patient charts at the Toronto Rehabilitation Institute. BMI was calculated per standard definition [kg/m2].
Endothelial function
Peripheral arterial tonometry (PAT) (EndoPAT; Itamar Medical, Israel) of the index digits was used to assess endothelial function. Participants fasted for 12 h before measurements were taken in a dimly lit temperature-controlled room. Participants were placed in a supine position and the EndoPAT probes were placed on the index digits of both hands. The digital pulse amplitude was measured until it was stable, then for an additional 5 min (pre-occlusion). Using a blood pressure cuff on the non-dominant arm, the brachial artery was occluded by increasing the cuff pressure to 60 mm Hg above systolic blood pressure for 5 min (the absolute pressure was no less than 200 mm Hg and no more than 300 mm Hg). The pressure in the cuff was then released and the digital pulse amplitude was recorded for an additional 5 min (post-occlusion) during hyperemia. The responsiveness of the microvasculature to hyperemia is indicative of endothelial function and is expressed using the reactive hyperemia index (RHI). The RHI is calculated as the post- to pre-occlusion ratio of the digital pulse amplitude in the occluded arm divided by the same ratio in the non-occluded arm, corrected for baseline vascular tone. Increased RHI is indicative of better endothelial function. The non-occluded arm acts as an internal control for non-endothelial dependent alterations (i.e., systemic changes) in the digital pulse amplitude.
The Endo-PAT2000 is approved for assessing endothelial function by USA Food and Drug Administration [29, 30]. This device has an 82% sensitivity and 77% specificity for diagnosing coronary artery endothelial dysfunction when compared to the gold standard of assessing endothelial function, the intracoronary acetylcholine challenge method [30]. The RHI obtained from PAT has been shown to significantly correlate with the cerebral endothelial function measured using cerebrovascular reactivity to 7% CO2 [31]. PAT has been significantly correlated with other measures of peripheral endothelial function such as flow mediated dilation (FMD) [32]. However, PAT has the advantage of feasibility and cost-effectiveness, whereas FMD requires a costly ultrasound device and a trained technician to operate the device. Additionally, PAT has the advantage of an internal control for correction of systemic changes that FMD cannot control for.
Angiogenesis markers
Fasting blood samples were drawn from the median cubital vein using venipuncture and stored in a serum separator tube. After clotting for 30 min, samples were centrifuged at 1000×g for 15 min and serum was extracted and stored at –80°C for batch analysis. The Human Endostatin Quantikine® ELISA Kit (DNST0) from R&D Systems (Minneapolis, MN, USA) was used to measure the serum endostatin concentration. Samples were diluted using a 50-fold dilution factor (20μL of serum +980μL of diluent) as per the manufacturer’s requirements. The Human VEGF Quantikine® Kit (DVE00) from R & D Systems was used to measure serum vascular endothelial growth factor (VEGF) concentrations. For both kits, samples were analyzed in duplicates or triplicates using a standard microplate reader (BioTek Synergy Neo2 microplate reader; Winooski, VT, USA) at 450 nm corrected to 540 nm. The standard manufacturer’s protocol was followed for both assays.
Cognitive testing
The Montreal Cognitive Assessment (MoCA) was administered as an assessment of global cognition. The MoCA is recommended by the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network to assess global cognition for the investigation of VCI [33]. Each section of the MoCA measures a different cognitive domain and the scores are summed to get a score of global cognition out of 30. The MoCA tests for short-term memory, visuospatial abilities, executive function, attention, concentration, working memory, language, and orientation to time and space. Cognitive testing was administered by a trained researcher at a standardized time of 09 : 30 and participants refrained from consuming caffeine-containing products for at least 4 h prior to testing.
Statistics
Continuous variables were summarized as means and standard deviations (SD). Categorical variables were expressed as percentages. Participant characteristics were compared using Pearson’s correlation for continuous variables and independent sample t-test for categorical variables. Significance for Pearson’s correlations and t-tests were set a p < 0.05.
Reliability estimates for serum VEGF and endostatin concentrations were calculated using the coefficient of variation (CV%) between replicates and an average CV% was obtained. A CV% equal or lower to the CV% published by the manufacturer was considered appropriate. RHI, angiogenesis, and MoCA data were assessed using a frequency plot and data ware transformed for normality as required. Mediation effects between RHI, angiogenesis markers, and MoCA scores were tested using the PROCESS macro (version 3.4) for SPSS. We hypothesized an indirect effect of RHI on MoCA scores that is mediated by concentrations of the angiogenesis markers (calculated as the product of the linear regression coefficients a×b) [34]. A bias corrected inferential bootstrapping method with 10,000 permutations was used to obtain a 95% confidence interval (CI) for the indirect effect, which offers superior control of type I error compared to other methods such as the Soble test and Barron and Kenny test [35]. A significant indirect effect is one where the 95% bootstrap CI does not cross 0. The model controlled for total years of education, which has been well documented as an independent predictor of cognitive performance [36, 37]. Post-hoc models controlled for potential confounders that were identified in univariate analyses. To test for possible reciprocal effects between endothelial function and angiogenesis markers, the indirect effect of angiogenesis markers on MoCA scores mediated by RHI was tested using the same methods described above.
RESULTS
Participant characteristics
Table 1 summarizes the characteristics for the 50 participants who were assessed for endothelial function, endostatin and VEGF serum levels, and who completed the MoCA. The mean BMI was in the overweight range of >25 kg/m2. The mean RHI (1.8) indicated normal endothelial function (greater than 1.67). The RHI values ranged from 0.86–3.92 (Table 1) and 43% of participants had an RHI below 1.67. Both serum endostatin and VEGF concentrations were comparable to the levels found in other studies on CAD [38, 39]. The mean MoCA scores (25.8) indicated that this population was on average slightly below the cutoff for cognitive impairment (<26). The MoCA scores ranged from 19–30 (Table 1), and 40% of participants had a score below 26. The CV% for endostatin was 4.2% (manufacturer CV% = 5.9%), and the CV% for VEGF was 4.0% (manufacturer CV% v= 4.5%).
Table 1
Participant characteristics and their correlation to the analysis variables
| Characteristic 1 | Mean (SD) or n (%) | RHI r, p or t, p | Endostatin r, p or t, p | VEGF r, p or t, p | MoCA r, p or t, p |
| Sociodemographic | |||||
| Age | 64 (6.6) | 0.139, 0.277 | 0.238, 0.096 | –0.024, 0.868 | –0.242, 0.090 |
| Sex, male | 43 (86%) | –1.006, 0.320 | 0.043, 0.966 | 1.296, 0.201 | 1.999, 0.051 |
| Ethnicity, Caucasian | 43 (86%) | –0.657, 0.514 | –0.376, 0.709 | 0.676, 0.503 | –1.507, 0.138 |
| Marital status, married | 36 (72%) | –0.423, 0.674 | 1.254, 0.261 | –0.546, 0.587 | 0.143, 0.887 |
| Years of education | 16 (3) | –0.112, 0.383 | –0.195, 0.174 | 0.132, 0.361 | 0.337, 0.019* |
| Smoking history, smoked | 30 (60%) | 2.040, 0.047* | –1.722, 0.092 | –0.319, 0.751 | 0.232, 0.817 |
| Body composition | |||||
| Body mass index, kg/m2 | 29 (5.5) | –0.098, 0.443 | 0.333, 0.018* | 0.062, 0.667 | –0.135, 0.348 |
| Waist circumference, cm | 99.6 (14.4) | –0.059, 0.645 | 0.264, 0.063 | 0.099, 0.494 | –0.016, 0.914 |
| Cardiac history | |||||
| Myocardial infarction | 26 (52%) | 0.199, 0.843 | 1.325, 0.191 | –0.928, 0.358 | 0.398, 0.692 |
| Coronary artery bypass graft | 12 (24%) | 0.531, 0.598 | –2.617, 0.012* | 0.411, 0.683 | –0.598, 0.553 |
| Stent | 35 (70%) | –0.775, 0.442 | 2.054, 0.045* | –1.329, 0.190 | –1.697, 0.096 |
| Comorbidities | |||||
| Hypertension | 46 (92%) | 0.636, 0.528 | 0.043, 0.966 | 1.235, 0.223 | 0.313, 0.756 |
| Diabetes | 11 (22%) | 1.655, 0.105 | –2.522, 0.015* | –0.159, 0.875 | 0.657, 0.514 |
| Depression | 9 (18%) | 1.006, 0.319 | 0.826, 0.413 | –1.399, 0.168 | 0.345, 0.731 |
| Cardiopulmonary fitness | |||||
| VO2peak, ml/kg/min | 19.9 (5.8) | 0.108, 0.403 | –0.337, 0.018* | 0.183, 0.209 | 0.362, 0.011* |
| Medication | |||||
| B-adrenergic receptor blockers | 40 (80%) | –0.522, 0.604 | 1.396, 0.169 | –0.444, 0.659 | –0.181, 0.857 |
| Calcium channel blockers | 7 (14%) | 1.300, 0.200 | 0.320, 0.750 | 1.171, 0.247 | –1.872, 0.067 |
| Diuretics | 7 (14%) | –0.689, 0.516 | –0.318, 0.752 | –0.271, 0.787 | 2.519, 0.015* |
| Angiotensin-converting enzyme inhibitors | 30 (60%) | –0.586, 0.561 | 0.694, 0.419 | –0.822, 0.415 | 0.244, 0.808 |
| Platelet inhibitors | 49 (98%) | 0.075, 0.942 | –0.504, 0.616 | –1.135, 0.262 | –0.683, 0.498 |
| Analysis variables2 | |||||
| Reactive hyperemia index | 1.8 (0.5) [0.86–3.28] | - | –0.297, 0.038* | –0.066, 0.654 | –0.025, 0.861 |
| Endostatin, ng/ml | 157.2 (33.2) | –0.297, 0.038* | - | 0.060, 0.678 | –0.352, 0.012* |
| VEGF, pg/mL | 276.2 (152.8) | –0.066, 0.654 | 0.060, 0.678 | - | –0.197, 0.170 |
| MoCA | 25.8 (2.7) [19–30] | –0.025, 0.861 | –0.352, 0.012* | –0.197, 0.170 | - |
1Continuous variables were expressed as means±SD and Pearson’s correlation (r) was used to assess their relationship to the mediators and outcome variable. Categorical variables are expressed as n (%) and an unpaired t-test was used to assess their relationship to analysis variables. Significant (*) analyses are those where p < 0.05. t = test statistic for the t-test. 2Values in square brackets represent the range of values for reactive hyperemia index (RHI) and the Montreal Cognitive Assessment (MoCA). VEGF = vascular endothelial growth factor.
Mediation effect of angiogenesis markers between endothelial function and cognitive scores
In a mediation model controlling for total years of education, endostatin levels mediated a significant indirect association between RHI and MoCA scores (0.66, 95% CI [0.11, 1.135]; Fig. 1). The indirect association remained significant in post-hoc analyses controlling for CABG, stent, diabetes, BMI, or diuretic use (Table 2). The indirect association was not significant when controlling for VO2peak (Table 2). An indirect association of RHI on MoCA scores mediated by VEGF concentrations was not significant (0.07, 95% CI [–0.73, 0.65]). Finally, there was no significant interaction effect between RHI and endostatin concentrations on MoCA scores (F = 0.99, p = 0.33).
Fig. 1
A mediation pathway between endothelial function (RHI), endostatin (log transformed), and cognitive performance (MoCA scores) in a CAD population. Total years of education was used a priori as a covariate. a = regression coefficient between RHI and LogEndostatin (±standard error), b = regression coefficient between LogEndostatin and MoCA (±standard error), a×b = indirect effect, c’ = c–a×b where c is the regression coefficient between RHI and MoCA, CI = 95% bias corrected bootstrap (10,000 permutations) confidence interval for the indirect effect. Significant path coefficient at p < 0.05, significant indirect effect when the CI does not cross 0.
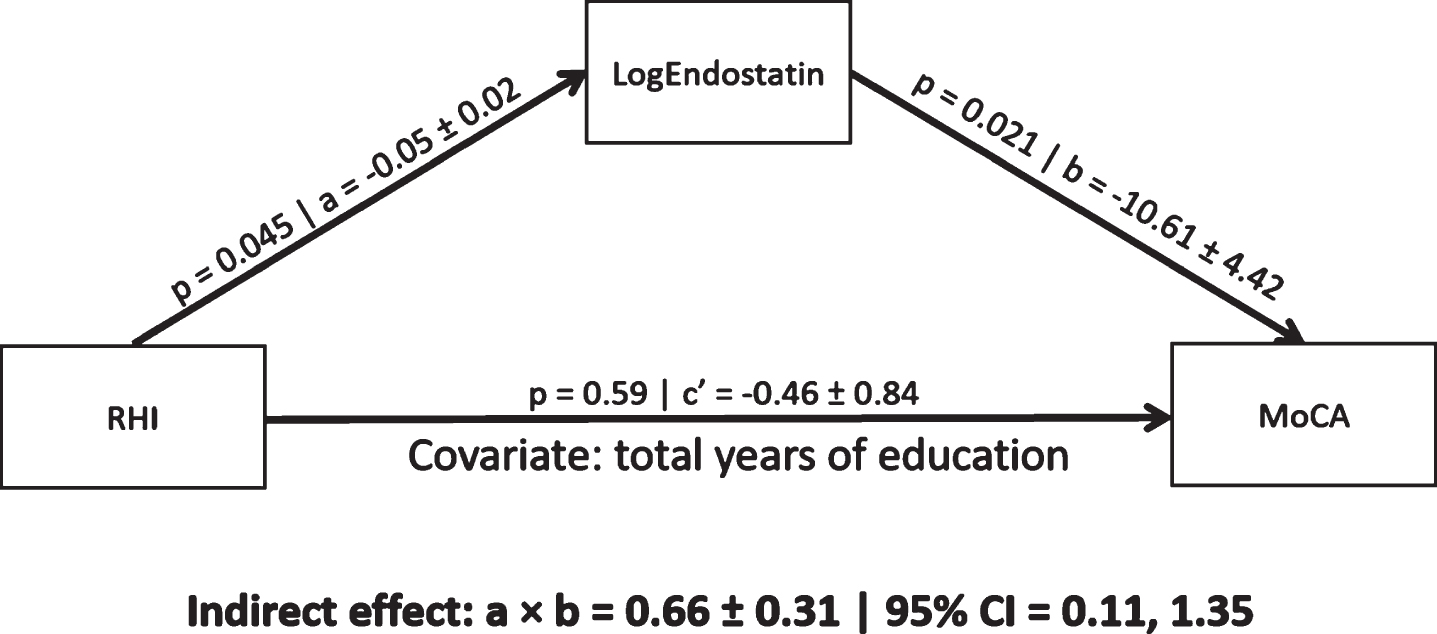
Table 2
Mediation results between RHI, LogEndostatin, and MoCA with different covariates
| Covariate1 | a, p | b, p | a×b [95% CI] | |
| CABG | –0.05, 0.05* | –15.5, 0.001* | 0.79 | [0.16, 1.73]* |
| Stent | –0.05, 0.06 | –11.4, 0.02* | 0.57 | [0.06, 1.32]* |
| Diabetes | –0.04, 0.12 | –12.88, 0.008* | 0.54 | [0.02, 1.29]* |
| BMI | –0.05, 0.06 | –12.6, 0.01* | 0.61 | [0.04, 1.40]* |
| Diuretics | –0.06, 0.04* | –11.63, 0.008* | 0.69 | [0.18, 1.60]* |
| VO2peak | –0.05, 0.06 | –9.9, 0.04* | 0.49 | [–0.03, 1.16] |
1Each covariate was run separately. A significant p is < 0.05 and a significant 95% CI does not cross 0.
Mediation effect of endothelial function between angiogenesis markers and cognitive scores
RHI did not mediate an association between serum endostatin levels and MoCA scores (1.21 95% CI [–1.88, 4.12]). Additionally, RHI did not mediate an association between serum VEGF levels and MoCA scores (0.02, 95% CI [–0.12, 1.29]).
Mediation effects of angiogenesis markers between endothelial function and sub-sections of the MoCA
Additional mediation analyses demonstrated that endostatin concentrations mediated an indirect association between RHI and delayed recall scores on the MoCA (0.59, 95% CI [0.19, 1.22]; Table 3) when controlling for total years of education. The indirect association with delayed recall remained significant when controlling for CABG (0.56, 95% CI [0.12, 1.26]), stent (0.49, 95% CI [0.10, 1.09]), diabetes (0.45, 95% CI [0.01, 1.12]), BMI (0.53, 95% CI [0.09, 1.18]), diuretic use (0.57, 95% CI [0.20, 1.3]), and VO2peak (0.45, 95% CI [0.08, 1.00]). The indirect association was not significant between RHI and sub-sections of the MoCA when VEGF was used as the mediating variable (data not shown).
Table 3
Mediation analysis between RHI, LogEndostatin, and sub-sections of the MoCA
| Sub -section1 | Average score | a, p | b, p | a×b [95% CI] | |
| Visuospatial | 4.62/5 | –0.05, 0.04* | –0.26, 0.80 | 0.01 | [–0.12, 0.14] |
| Naming | 2.86/3 | –0.05, 0.04* | –1.26, 0.07 | 0.07 | [–0.02, 0.26] |
| Attention | 5.66/6 | –0.05, 0.04* | –0.38, 0.77 | 0.02 | [–0.12, 0.15] |
| Language | 2.16/3 | –0.05, 0.04* | –1.20, 0.44 | 0.07 | [–0.11, 0.23] |
| Abstraction | 1.80/2 | –0.05, 0.04* | –0.44, 0.61 | 0.02 | [–0.08, 0.14] |
| Delayed recall | 2.68/5 | –0.05, 0.04* | –10.58, 0.0002* | 0.59 | [0.19, 1.22]* |
| Orientation | 5.9/6 | –0.05, 0.04* | –5.00, 0.36 | 0.28 | [–0.39, 1.32] |
1 Total years of education was chosen as a covariate a priori. Average scores are out of the highest possible score achievable for the given sub-section. Significant p < 0.05 and a significant 95% CI does not cross 0.
DISCUSSION
The contributions of vascular diseases to cognitive deterioration has emerged as an important avenue of investigation to understand, prevent, slow, and reverse declines in cognitive function. Diminished endothelial function has been associated with the degree of cognitive impairment and cerebrovascular pathologies in Alzheimer’s disease and mild cognitive impairment [20, 40]. In elderly populations with cardiovascular diseases, endothelial function has been associated with white matter hyperintensity volume, which has been associated with impaired cognitive processes [15]. Additionally, greater endothelial dysfunction correlated with higher white matter hyperintensity volume, greater prevalence of silent infarcts, and lower total cerebral brain volume in individuals with vascular dementia-type brain aging [41]. In addition to its role in regulating vascular tone, the endothelium also plays a major role in angiogenesis. Nonetheless, no studies have investigated angiogenesis as a potential contributor to the mechanistic link between endothelial function and cognition in CAD. The current study shows that decreases in endothelial function in CAD may contribute to decreased cognitive performance through increased concentrations of the anti-angiogenic factor endostatin.
Specifically, endostatin mediated the relationship between endothelial function and delayed recall. This may indicate that delayed recall is one of the first cognitive function to be affected by cardiovascular damage. One study demonstrated that patients with CAD were most impaired in verbal memory compared to controls [42]. Further, neuroimaging studies have shown that white matter hyperintensities and deteriorations in microstructural integrity in the frontal-subcortical white matter tracks affected verbal memory tasks [43, 44]. In those with mild VCI, alterations in the frontal-subcortical white-matter were associated with worse performance on the MoCA; interestingly, they also performed worse on the delayed recall task compared to controls [45]. There is also promising evidence to indicate that verbal memory can be improved in CAD patients, which suggests that early intervention may be able to delay the progression of cognitive decline. In one study, CAD patients showed improvements in verbal memory after completing a 1-year cardiac rehabilitation program that involved regular exercise sessions [26], which is known to improve endothelial function and alter markers of angiogenesis. Therefore, endothelial function and angiogenesis may be good targets for improving cognitive deficits, such as impaired verbal memory, in patients with CAD.
The current study also found that VEGF did not act as a mediator between endothelial function and cognitive test performance while endostatin did mediate the indirect association. One may expect to see an inverse association between VEGF and endostatin, and potentially opposite results for the mediation, given the antagonistic functions of VEGF and endostatin. However, endostatin mainly acts as a VEGF antagonist by binding VEGF receptors, not by altering levels of VEGF [46]. Furthermore, the current finding may indicate that changes in endothelial function in this population of CAD patients led to specific alterations in markers of angiogenesis. One study showed that there was no difference in plasma VEGF between CAD patients and controls [24]. While one study disagrees [47], it has also been shown that VEGF levels do not correlate with endothelial function in CAD populations [38]. Thereby, increases in VEGF might be facilitated in a non-endothelial dependent manner in CAD. In contrast, increased serum endostatin levels are found in several cardiovascular associated diseases including CAD, heart failure, diabetes, and hypertension [48]. Specific to CAD, serum endostatin has been shown to be significantly elevated in CAD versus healthy controls, and greater levels of serum endostatin in CAD patients is significantly associated with poorer collateralization, increased disease severity, and a greater risk for future cardiovascular events [39, 49–52]. Additionally, impairment in endothelial function is significantly associated with increased serum endostatin levels in those with cardiovascular risk factors, such as hypertension [53]. Therefore, endostatin may play a more pronounced role, compared to VEGF, in impaired angiogenic processes as a result of endothelial dysfunction in CAD. Additionally, there may be a molecular mechanism that helps to explain the connection between endothelial dysfunction and endostatin concentrations in CAD. Cathepsin L is one endothelial derived protease that is responsible for the cleavage of endostatin from collagen XVIII [54]. Increased cathepsin L has been linked to endothelial dysfunction and anti-angiogenesis [55, 56]. Interestingly, it has been documented that those with cardiovascular disease, particularly atherosclerotic plaques, can have increased levels of cathepsin L and endostatin [54, 57]. Therefore, endothelial dysfunction may be linked to endostatin levels via over expression of cathepsin L in CAD. This may help to explain why changes in endothelial function were related specifically to endostatin concentrations in this CAD population.
This study has potential limitations. First, only a small sample was available to conduct the mediation analysis. Therefore, these results may not reflect a true expression of the indirect association between EndoPAT and MoCA scores through peripheral endostatin levels. However, this study employed a bias corrected bootstrapping method, which is robust to variations from normality in distribution and offers greater control of type I error compared to the Sobel test and Baron and Kenny method [35, 58]. Nonetheless, future studies should replicate these analyses using a larger sample size. Additionally, the cross-sectional nature of this study limits any causal inferences and it does not provide insight into the longitudinal association between endothelial function, angiogenic markers, and cognitive performance in CAD [59]. Therefore, this study cannot demonstrate the role that angiogenic markers play in mediation as the disease progresses or over the course of a treatment strategy, such as exercise therapy. For this reason, future studies should replicate these analyses using longitudinal data. Furthermore, there was no significant direct relationship between RHI and MoCA scores (the c’ path). While the traditional approach to mediation proposed by Baron and Kenny necessitate a significant c’ path [35], more recent research suggests that this requirement is overly restrictive and unnecessary for mediation analyses [60–62]. In the same context, the c’ path showed an insignificant negative association between RHI and MoCA. Since c’=c –ab, the c’ path may very well be a negative association if the indirect path (ab) is larger than the total effect (c path). Further work, especially using longitudinal data, should be done to clarify the direct association between RHI and MoCA. Another potential limitation is the larger representation of males in this population (86%). While this is typical of the cardiac rehabilitation population [13], it precluded analysis of sex differences in the mediating effect of angiogenesis markers. Though sex was not associated with the MoCA in our univariate analysis, it has been shown to independently predict MoCA scores [63] and should be addressed in future studies with larger samples of females. Additionally, EndoPAT and serum endostatin are indirect measures of cerebral endothelial function and cerebral levels of angiogenic markers, respectively. Therefore, it is possible that EndoPAT does not reflect cerebral endothelial function and serum endostatin does not reflect cerebral levels of endostatin. However, it has been shown in populations with cardiovascular risk factors (hypertension and/or diabetes) that PAT correlates with cerebral endothelial function measured via cerebrovascular reactivity to CO2 [31, 64]. Further, decreased peripheral endothelial function has been associated with an increased risk of cerebrovascular events [65], which may indicate that peripheral markers of endothelial function give insights into cerebrovascular health. As for serum endostatin, postmortem analyses of Alzheimer’s disease brains with evidence of cerebrovascular pathology showed increased deposits of cerebral endostatin compared to healthy control brains [66]. Additionally, a rabbit model of cerebral ischemia showed that there were increases in cerebrovascular endostatin levels after induced ischemia [67]. Furthermore, peripheral injection of endostatin in mice results in reduction of choroidal vascularization [68]. Although these are animal models, they demonstrate that endostatin may play a role in cerebrovascular pathologies of cognitive impairment and that peripheral endostatin can alter cerebral angiogenesis. Lastly, the sub-sections of the MoCA may not be sensitive enough to detect minor changes in specific cognitive domains that would be expected in a CAD population who is at risk of cognitive decline. The total score (global cognition) for the MoCA has a sensitivity of 96.3% for detecting mild cognitive impairment when patients scored below 26 [69]. While there are no studies exploring the sensitivity of each domain on the MoCA in CAD, delayed recall was the second most sensitive sub-section (visuospatial was first) for detection of impairment in a Parkinson’s disease population [70]. Future studies should utilize more domain-specific neuropsychological tests to investigate the mediation effect. Two appropriate tests might include Trails B for executive function and processing speed and the California Verbal Learning Test for delayed recall, both of which assess domains that might be affected in patients with mild VCI and CAD [13, 26, 27, 71–74].
In conclusion, peripheral endostatin levels may mediate a relationship between endothelial function and cognitive performance in a population at risk of developing VCI. This mediation effect may indicate the involvement of a specific anti-angiogenic process because a similar mediation effect was not detected using a pro-angiogenic marker. Lastly, endostatin may particularly mediate a relationship between endothelial function and memory performance. Since exercise therapy has had cognitive benefits in those at risk of VCI due to CAD, but has not shown positive results for some [27], further studies might examine whether endostatin changes in response to exercise might explain variability in cognitive benefits.
ACKNOWLEDGMENTS
Thank you to the Canadian Institute of Health Research (LanctotMOP-114913) and the Ontario Mental Health Foundation for research grant funding. Thank you to the Canadian Institute of Health Research (Canadian Graduate Scholarship –Master’s) and the Heart and Stroke Foundation (Queen Elizabeth II Graduate Scholarship in Science and Technology) for graduate funding. The above institutions were not involved in study management, the collection, analysis, and interpretation of data, or the preparation, review, or approval of the manuscript.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0058r1).
REFERENCES
[1] | Wong ND ((2014) ) Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol 11: , 276–289. |
[2] | Deckers K , Schievink SHJ , Rodriquez MMF , van Oostenbrugge RJ , van Boxtel MPJ , Verhey FRJ , Kohler S ((2017) ) Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS One 12: , e0184244. |
[3] | Gorelick PB , Scuteri A , Black SE , Decarli C , Greenberg SM , Iadecola C , Launer LJ , Laurent S , Lopez OL , Nyenhuis D , Petersen RC , Schneider JA , Tzourio C , Arnett DK , Bennett DA , Chui HC , Higashida RT , Lindquist R , Nilsson PM , Roman GC , Sellke FW , Seshadri S , American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia ((2011) ) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: , 2672–2713. |
[4] | Selnes OA , Grega MA , Borowicz LM , Royall RM , McKhann GM , Baumgartner WA ((2003) ) Cognitive changes with coronary artery disease: A prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg 75: , 1377–1386. |
[5] | Tilvis RS , Kähönen-Väre MH , Jolkkonen J , Valvanne J , Pitkala KH , Strandberg TE ((2004) ) Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol 59A: , 268–274. |
[6] | Santiago C , Herrmann N , Swardfager W , Saleem M , Oh PI , Black SE , Bradley J , Lanctot KL ((2018) ) Subcortical hyperintensities in the cholinergic system are associated with improvements in executive function in older adults with coronary artery disease undergoing cardiac rehabilitation. Int J Geriatr Psychiatry 33: , 279–287. |
[7] | Santiago C , Herrmann N , Swardfager W , Saleem M , Oh PI , Black SE , Lanctot KL ((2015) ) White matter microstructural integrity is associated with executive function and processing speed in older adults with coronary artery disease. Am J Geriatr Psychiatry 23: , 754–763. |
[8] | Rajagopalan B , Raine AEG , Cooper R , Ledingham JGG ((1984) ) Changes in cerebral blood flow in patients with severe congestive cardiac failure before and after captopril treatment. Am J Med 76: (5B), 86–90. |
[9] | Jefferson AL , Poppas A , Paul RH , Cohen RA ((2007) ) Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging 28: , 477–483. |
[10] | de la Torre JC ((2012) ) Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012: , 367516. |
[11] | Fujiyoshi K , Yamaoka-Tojo M , Minami Y , Kutsuna T , Obara S , Kakizaki R , Nemoto T , Hashimoto T , Namba S , Shimohama T , Tojo T , Ako J ((2018) ) Endothelial dysfunction is associated with cognitive impairment of elderly cardiovascular disease patients. Int Heart J 59: , 1034–1040. |
[12] | Widmer RJ , Lerman A ((2014) ) Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014: , 291–308. |
[13] | Saleem M , Herrmann N , Dinoff A , Mazereeuw G , Oh PI , Goldstein BI , Kiss A , Shammi P , Lanctot KL ((2019) ) Association between endothelial function and cognitive performance in patients with coronary artery disease during cardiac rehabilitation. Psychosom Med 81: , 184–191. |
[14] | Adamski MG , Sternak M , Mohaissen T , Kaczor D , Wieronska JM , Malinowska M , Czaban I , Byk K , Lyngso KS , Przyborowski K , Hansen PBL , Wilczynski G , Chlopicki S ((2018) ) Vascular cognitive impairment linked to brain endothelium inflammation in early stages of heart failure in mice. J Am Heart Assoc 7: , e007694. |
[15] | Hoth KF , Tate DF , Poppas A , Forman DE , Gunstad J , Moser DJ , Paul RH , Jefferson AL , Haley AP , Cohen RA ((2007) ) Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke 38: , 308–312. |
[16] | Johnson NF , Gold BT , Brown CA , Anggelis EF , Bailey AL , Clasey JL , Powell DK ((2017) ) Endothelial function is associated with white matter microstructure and executive function in older adults. Front Aging Neurosci 9: , 255. |
[17] | Toth P , Tarantini S , Csiszar A , Ungvari Z ((2017) ) Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: , H1–H20. |
[18] | Ungvari Z , Tarantini S , Kiss T , Wren JD , Giles CB , Griffin CT , Murfee WL , Pacher P , Csiszar A ((2018) ) Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15: , 555–565. |
[19] | Durak-Kozica M , Paszek E , Stepien EL ((2019) ) Role of the Wnt signalling pathway in the development of endothelial disorders in response to hyperglycaemia. Expert Rev Mol Med 21: , e7. |
[20] | Dede DS , Yavuz B , Yavuz BB , Cankurtaran M , Halil M , Ulger Z , Cankurtaran ES , Aytemir K , Kabakci G , Ariogul S ((2007) ) Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? J Am Geriatr Soc 55: , 1613–1617. |
[21] | Huang L , Jia J , Liu R ((2013) ) Decreased serum levels of the angiogenic factors VEGF and TGF-beta1 in Alzheimer’s disease and amnestic mild cognitive impairment. Neurosci Lett 550: , 60–63. |
[22] | Zhang R , Kadar T , Sirimanne E , MacGibbon A , Guan J ((2012) ) Age-related memory decline is associated with vascular and microglial degeneration in aged rats. Behav Brain Res 235: , 210–217. |
[23] | Wang J , Fu X , Jiang C , Yu L , Wang M , Han W , Liu L , Wang J ((2014) ) Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia. Behav Brain Res 265: , 171–180. |
[24] | Alber HF , Frick M , Dulak J , Dorler J , Zwick RH , Dichtl W , Pachinger O , Weidinger F ((2005) ) Vascular endothelial growth factor (VEGF) plasma concentrations in coronary artery disease. Heart 91: , 365–366. |
[25] | Danzig V. , Míková B. , Kuchynka P. , Benáková H. , Zima T. , Kittnar O. , Škrha J. , Linhart A. , Kalousová M ((2009) ) Levels of circulating biomarkers at rest and after exercise in coronary artery disease patients. Physiol Res 59: , 385–392. |
[26] | Saleem M , Ratnam Bandaru VV , Herrmann N , Swardfager W , Mielke MM , Oh PI , Shammi P , Kiss A , Haughey NJ , Rovinski R , Lanctôt KL ((2013) ) Ceramides predict verbal memory performance in coronary artery disease patients undertaking exercise: A prospective cohort pilot study. BMC Geriatrics 13: , 135. |
[27] | Swardfager W , Herrmann N , Marzolini S , Saleem M , Kiss A , Shammi P , Oh PI , Lanctot KL ((2010) ) Cardiopulmonary fitness is associated with cognitive performance in patients with coronary artery disease. J Am Geriatr Soc 58: , 1519–1525. |
[28] | Bento CF , Fernandes R , Matafome P , Sena C , Seica R , Pereira P ((2010) ) Methylglyoxal-induced imbalance in the ratio of vascular endothelial growth factor to angiopoietin 2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Exp Physiol 95: , 955–970. |
[29] | Kuvin JT , Patel AR , Sliney KA , Pandian NG , Sheffy J , Schnall RP , Karas RH , Udelson JE ((2003) ) Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 146: , 168–174. |
[30] | Bonetti PO , Pumper GM , Higano ST , Holmes DR Jr. , Kuvin JT , Lerman A ((2004) ) Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 44: , 2137–2141. |
[31] | Prakash K , Chandran DS , Khadgawat R , Jaryal AK , Deepak KK ((2016) ) Correlations between endothelial function in the systemic and cerebral circulation and insulin resistance in type 2 diabetes mellitus. Diab Vasc Dis Res 13: , 49–55. |
[32] | Woo JS , Jang WS , Kim HS , Lee JH , Choi EY , Kim JB , Kim WS , Kim KS , Kim W ((2014) ) Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron Artery Dis 25: , 421–426. |
[33] | Hachinski V , Iadecola C , Petersen RC , Breteler MM , Nyenhuis DL , Black SE , Powers WJ , DeCarli C , Merino JG , Kalaria RN , Vinters HV , Holtzman DM , Rosenberg GA , Wallin A , Dichgans M , Marler JR , Leblanc GG ((2006) ) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37: , 2220–2241. |
[34] | Cogo-Moreira H , Swardfager W ((2018) ) On mediation models in clinical neurology studies. JAMA Neurol 76: , 116–117. |
[35] | Hayes AF , Rockwood NJ ((2017) ) Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther 98: , 39–57. |
[36] | Newman MF , Kirchner JL , Phillips-Bute B , Gaver V , Grocott H , Jones RH , Mark DB , Reves JG , Blumenthal JA ((2001) ) Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 344: , 395–402. |
[37] | Ottens TH , Hendrikse J , Nathoe HM , Biessels GJ , van Dijk D ((2017) ) Brain volume and cognitive function in patients with revascularized coronary artery disease. Int J Cardiol 230: , 80–84. |
[38] | Chung NA , Lydakis C , Belgore F , Li-Saw-Hee FL , Blann AD , Lip GYH ((2003) ) Angiogenesis, thrombogenesis, endothelial dysfunction and angiographic severity of coronary artery disease. Heart 89: , 1411–1415. |
[39] | Barroso MC , Boehme P , Kramer F , Mondritzki T , Koehler T , Gulker JE , Karoff M , Dinh W ((2017) ) Endostatin a potential biomarker for heart failure with preserved ejection fraction. Arq Bras Cardiol 109: , 448–456. |
[40] | Snyder HM , Corriveau RA , Craft S , Faber JE , Greenberg SM , Knopman D , Lamb BT , Montine TJ , Nedergaard M , Schaffer CB , Schneider JA , Wellington C , Wilcock DM , Zipfel GJ , Zlokovic B , Bain LJ , Bosetti F , Galis ZS , Koroshetz W , Carrillo MC ((2015) ) Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 11: , 710–717. |
[41] | Tsao CW , Seshadri S , Beiser AS , Westwood AJ , DeCarli C , Au R , Himali JJ , Hamburg N , Vita JA , Levy D , Larson MG , Benjamin EJ , Wolf PA , Vasan RS , Mitchell GF ((2013) ) Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81: , 984–991. |
[42] | Silbert BS , Scott DA , Evered LA , Lewis MS , Maruff PT ((2007) ) Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg 104: , 1023–1028. |
[43] | Sepulcre J , Masdeu JC , Sastre-Garriga J , Goni J , Velez-de-Mendizabal N , Duque B , Pastor MA , Bejarano B , Villoslada P ((2008) ) Mapping the brain pathways of declarative verbal memory: Evidence from white matter lesions in the living human brain. Neuroimage 42: , 1237–1243. |
[44] | van der Holst HM , Tuladhar AM , van Norden AG , de Laat KF , van Uden IW , van Oudheusden LJ , Zwiers MP , Norris DG , Kessels RP , de Leeuw FE ((2013) ) Microstructural integrity of the cingulum is related to verbal memory performance in elderly with cerebral small vessel disease: The RUN DMC study. Neuroimage 65: , 416–423. |
[45] | Lin L , Xue Y , Duan Q , Sun B , Lin H , Chen X , Luo L , Wei X , Zhang Z ((2015) ) Microstructural white matter abnormalities and cognitive dysfunction in subcortical ischemic vascular disease: an atlas-based diffusion tensor analysis study. J Mol Neurosci 56: , 363–370. |
[46] | Sherbet GV ((2017) ) Endogenous inhibitors of angiogenesis. In Molecular Approach to Cancer Management, Sherbet GV , ed. Academic Press, pp. 113–130. |
[47] | Ramos C , Napoleao P , Selas M , Freixo C , Viegas Crespo AM , Mota Carmo M , Cruz Ferreira R , Pinheiro T ((2014) ) Prognostic value of VEGF in patients submitted to percutaneous coronary intervention. Dis Markers 2014: , 135357. |
[48] | Ruge T , Carlsson AC , Larsson A , Arnlov J ((2017) ) Endostatin: A promising biomarker in the cardiovascular continuum? Biomark Med 11: , 905–916. |
[49] | Mitsuma W , Kodama M , Hanawa H , Ito M , Ramadan MM , Hirono S , Obata H , Okada S , Sanada F , Yanagawa T , Kashimura T , Fuse K , Tanabe N , Aizawa Y ((2007) ) Serum endostatin in the coronary circulation of patients with coronary heart disease and its relation to coronary collateral formation. Am J Cardiol 99: , 494–498. |
[50] | Ruge T , Carlsson AC , Kjoller E , Hilden J , Kolmos HJ , Sajadieh A , Kastrup J , Jensen GB , Larsson A , Nowak C , Jakobsen JC , Winkel P , Gluud C , Arnlov J ((2019) ) Circulating endostatin as a risk factor for cardiovascular events in patients with stable coronary heart disease: A CLARICOR trial sub-study. Atherosclerosis 284: , 202–208. |
[51] | Sponder M , Fritzer-Szekeres M , Litschauer B , Binder T , Strametz-Juranek J ((2015) ) Endostatin and osteopontin are elevated in patients with both coronary artery disease and aortic valve calcification. IJC Metab Endocr 9: , 5–9. |
[52] | Ruge T , Carlsson AC , Jansson JH , Soderberg S , Larsson A , Arnlov J ((2018) ) The association between circulating endostatin levels and incident myocardial infarction. Scand Cardiovasc J 52: , 315–319. |
[53] | Carlsson AC , Ruge T , Sundstrom J , Ingelsson E , Larsson A , Lind L , Arnlov J ((2013) ) Association between circulating endostatin, hypertension duration, and hypertensive target-organ damage. Hypertension 62: , 1146–1151. |
[54] | Platt MO , Shockey WA ((2016) ) Endothelial cells and cathepsins: Biochemical and biomechanical regulation. Biochimie 122: , 314–323. |
[55] | Veillard F , Saidi A , Burden RE , Scott CJ , Gillet L , Lecaille F , Lalmanach G ((2011) ) Cysteine cathepsins S and L modulate anti-angiogenic activities of human endostatin. J Biol Chem 286: , 37158–37167. |
[56] | Zhang F , Zhang Y , Li PL ((2009) ) Dependence of cathepsin L-induced coronary endothelial dysfunction upon activation of NAD(P)H oxidase. Microvasc Res 78: , 45–50. |
[57] | Zhang J , Yong L , Wang P , Huang Y , Zhu W , Li J , Huang C ((2009) ) Plasma cathepsin L and its extracellular matrix cleavage product—endostatin play useful roles in predicting poor coronary collaterals in patients with coronary artery disease. Int J Cardiol 137: , S29–S30. |
[58] | Fritz MS , Mackinnon DP ((2007) ) Required sample size to detect the mediated effect. Psychol Sci 18: , 233–239. |
[59] | Goldsmith KA , MacKinnon DP , Chalder T , White PD , Sharpe M , Pickles A ((2018) ) Tutorial: The practical application of longitudinal structural equation mediation models in clinical trials. Psychol Methods 23: , 191–207. |
[60] | Hayes AF ((2013) ) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach, Guilford Press, New York. |
[61] | Hayes AF ((2009) ) Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun Monogr 76: , 408–420. |
[62] | O’Rourke HP , MacKinnon DP ((2018) ) Reasons for testing mediation in the absence of an intervention effect: A research imperative in prevention and intervention research. J Stud Alcohol Drugs 79: , 171–181. |
[63] | Borland E , Nagga K , Nilsson PM , Minthon L , Nilsson ED , Palmqvist S ((2017) ) The Montreal Cognitive Assessment: Normative data from a large Swedish population-based cohort. J Alzheimers Dis 59: , 893–901. |
[64] | Lavi S , Gaitini D , Milloul V , Jacob G ((2006) ) Impaired cerebral CO2 vasoreactivity: Association with endothelial dysfunction. Am J Physiol Heart Circ Physiol 291: , H1856–1861. |
[65] | Targonski PV , Bonetti PO , Pumper GM , Higano ST , Holmes DR Jr. , Lerman A ((2003) ) Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation 107: , 2805–2809. |
[66] | Deininger MH , Fimmen BA , Thal DR , Schluesener HJ , Meyermann R ((2002) ) Aberrant neuronal and paracellular deposition of endostatin in brains of patients with Alzheimer’s disease. J Neurosci 22: , 10621–10626. |
[67] | Tian H-L , Chen H , Cui Y-H , Xu T , Zhou L-F ((2007) ) Increased protein and mRNA expression of endostatin in the ischemic brain tissue of rabbits after middle cerebral artery occlusion. Neurosci Bull 23: , 35–40. |
[68] | Mori K , Ando A , Gehlbach P , Nesbitt D , Takahashi K , Goldsteen D , Penn M , Chen CT , Mori K , Melia M , Phipps S , Moffat D , Brazzell K , Liau G , Dixon KH , Campochiaro PA ((2001) ) Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol 159: , 313–320. |
[69] | Eftekhari SS , Hejazi SA , Sharifipour E , Hejazi SF , Talebizadeh M , Mostafavi H , Yoosefee S ((2018) ) Cognitive impairment in patients with coronary artery disease; comparison of Montreal Cognitive Assessment (MoCA) and Mini Mental State Examination (MMSE). J Adv Med Biomed Res 26: , 12–16. |
[70] | Hendershott TR , Zhu D , Llanes S , Poston KL ((2017) ) Domain-specific accuracy of the Montreal Cognitive Assessment subsections in Parkinson’s disease. Parkinsonism Relat Disord 38: , 31–34. |
[71] | Sachdev P , Kalaria R , O’Brien J , Skoog I , Alladi S , Black SE , Blacker D , Blazer DG , Chen C , Chui H , Ganguli M , Jellinger K , Jeste DV , Pasquier F , Paulsen J , Prins N , Rockwood K , Roman G , Scheltens P , Internationlal Society for Vascular Behavioral and Cognitive Disorders ((2014) ) Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis Assoc Disord 28: , 206–218. |
[72] | Saleem M , Herrmann N , Dinoff A , Mielke MM , Oh PI , Shammi P , Cao X , Venkata SLV , Haughey NJ , Lanctot KL ((2017) ) A lipidomics approach to assess the association between plasma sphingolipids and verbal memory performance in coronary artery disease patients undertaking cardiac rehabilitation: A C18:0 signature for cognitive response to exercise. J Alzheimers Dis 60: , 829–841. |
[73] | Chan P , Saleem M , Herrmann N , Mielke MM , Haughey NJ , Oh PI , Kiss A , Lanctot KL ((2018) ) Ceramide accumulation is associated with declining verbal memory in coronary artery disease patients: an observational study. J Alzheimers Dis 64: , 1235–1246. |
[74] | Suridjan I , Herrmann N , Adibfar A , Saleem M , Andreazza A , Oh PI , Lanctot KL ((2017) ) Lipid peroxidation markers in coronary artery disease patients with possible vascular mild cognitive impairment. J Alzheimers Dis 58: , 885–896. |




