Relationships of Cerebrospinal Fluid Alzheimer’s Disease Biomarkers and COMT, DBH, and MAOB Single Nucleotide Polymorphisms
Abstract
The noradrenergic and dopaminergic systems are affected in Alzheimer’s disease (AD). Polymorphisms in genes encoding enzymes and proteins that are components of these systems can affect products of transcription and translation and lead to altered enzymatic activity and alterations in overall dopamine and noradrenaline levels. Catechol-O-methyltransferase (COMT) and monoamine oxidase B (MAOB) are the enzymes that regulate degradation of dopamine, while dopamine β-hydroxylase (DBH) is involved in synthesis of noradrenaline. COMT Val158Met (rs4680), DBH rs1611115 (also called –1021C/T or –970C/T), and MAOB rs1799836 (also called A644G) polymorphisms have been previously associated with AD. We assessed whether these polymorphisms are associated with cerebrospinal fluid (CSF) AD biomarkers including total tau (t-tau), phosphorylated tau proteins (p-tau181, p-tau199, and p-tau231), amyloid-β42 (Aβ42), and visinin-like protein 1 (VILIP-1) to test possible relationships of specific genotypes and pathological levels of CSF AD biomarkers. The study included 233 subjects: 115 AD, 53 mild cognitive impairment, 54 subjects with other primary causes of dementia, and 11 healthy controls. Significant decrease in Aβ42 levels was found in patients with GG compared to AG COMT Val158Met genotype, while t-tau and p-tau181 levels were increased in patients with AA compared to AG COMT Val158Met genotype. Aβ42 levels were also decreased in carriers of A allele in MAO-B rs1799836 polymorphism, while p-tau181 levels were increased in carriers of T allele in DBH rs1611115 polymorphism. These results indicate that COMT Val158Met, DBH rs1611115, and MAOB rs1799836 polymorphisms deserve further investigation as genetic markers of AD.
INTRODUCTION
Neuropathological changes of monoaminergic systems are considered an early and clinically important feature of Alzheimer’s disease (AD) (for a review, see [1, 2]). Dopamine β-hydroxylase (DBH) is an enzyme involved in the synthesis of noradrenaline, whereas catechol-O-methyltransferase (COMT) and monoamine oxidase B (MAOB) regulate the degradation of dopamine. Polymorphisms in genes for these enzymes can lead to altered transcription and translation products, and their dysfunctional enzymatic activity consequently leads to changes in dopamine and noradrenaline levels. It is therefore not surprising that single nucleotide polymorphisms (SNPs) in genes for COMT, DBH, and MAOB are associated with neuropsychiatric disorders [3–7]. The possible association of COMT Val158Met (rs4680), DBH rs1611115 (also called –1021C/T or –970C/T), and MAOB rs1799836 (also called A644G) polymorphisms with cerebrospinal fluid (CSF) AD biomarkers has not yet been evaluated. CSF AD biomarkers can serve as intermediate quantitative traits (endophenotypes, proxy variables) of AD as they can reflect AD-related pathology [8]. Increased deposition of amyloid in brain is reflected in reduced concentration of CSF amyloid-β42 (Aβ42) [9], while phosphorylated tau proteins [10] positively correlate with formation of neurofibrillary tangles, thus reflecting the extent of neurofibrillary degeneration. Total tau (t-tau) and visinin-like protein 1 (VILIP-1) are also increased in CSF during neurodegeneration and their levels positively correlate with the cognitive impairment [11–13]. In order to determine if pathological levels of CSF biomarkers are more likely to occur in patients with certain genotypes, we measured the levels of CSF AD biomarkers (Aβ42, t-tau, p-tau181, p-tau199, p-tau231, and VILIP-1) and assessed whether they differed between patients with COMT Val158Met, DBH rs1611115, and MAOB rs1799836 genotypes.
MATERIALS AND METHODS
Subjects
The study included 233 Croatian Caucasian subjects recruited at the University Hospital Center Zagreb. While this population is clearly represeantative of a European ethnic group, by which it may not be entirely comparable to US populations investigated in comparable contexts, it is nonetheless purely Caucasian. Of note, assessing our Croatian population using a Croatian version of the Mini-Mental State Examination (MMSE) yielded outcomes entirely comparable to other population similarly assessed worldwide [14]. Out of 233 subjects recruited, 115 were AD patients, 53 had mild cognitive impairment (MCI), 54 were patients with other primary causes of dementia (14 patients had dementia due to vascular cognitive dementia [AD+VaD], three had dementia with Parkinson’s disease [PD], 7 had dementia with Lewy bodies [DLB], 23 had frontotemporal dementia [FTD], and one had corticobasal syndrome [CBS]). Eleven subjects were healthy controls (HC) (Table 1). AD was clinically diagnosed using criteria of the National Institutes on Aging - Alzheimer’s Association (NIA-AA) [15]. VaD was diagnosed by using the criteria of National Institute for Neurological Disorders and Stroke - Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINCDS-AIREN) [16] and the Hachinski Ischemic Score [17]. FTD diagnosis was made by using the criteria of Neary et al. [18], while MCI was diagnosed using criteria of Albert et al. [19] and Petersen et al. [20]. Before the enrolment in the study, patients gave informed consent for lumbar puncture and for participation in the study. They were tested neuropsychologically using the MMSE, Montreal Cognitive Assessment (MoCA), and Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog). In addition to thorough neurological examination, each patient went through complete blood tests, including serology for Lyme’s disease and syphilis, thyroid function, and levels of vitamin B12 and folic acid (B9). All procedures performed within this study were in accord with the Helsinki Declaration [21] and approved by the Central Ethical Committee of the University of Zagreb Medical School (case no. 380-59-10106-18-111/126, class 641-01/18-02/01 from June 20, 2018) and Ethical Committee of the Clinical Hospital Center Zagreb (case no. 02/21 AG, class 8.1-18/82-2 from April 24, 2018).
Table 1
Frequency of COMT Val158Met, DBH rs1611115, and MAOB rs1799836 genotypes in AD and MCI patients, HC, and in patients with other causes of dementia
| COMT | DBH | MAOB | MMSE | Age | Gender | |||||||
| AA | GG | AG | CC | TT | CT | AA | GG | AG | Mean±SD | Median (25–75th percentile) | M/F | |
| AD | 32 | 23 | 59 | 77 | 5 | 33 | 57 | 34 | 24 | 19.9±4.5 | 73 (67–77) | 53/62 |
| MCI | 9 | 14 | 30 | 35 | 3 | 15 | 23 | 18 | 12 | 25.1±2.9 | 70 (59–74) | 26/27 |
| HC | 8 | 1 | 2 | 3 | 1 | 7 | 2 | 3 | 6 | 27.8±1.9 | 54 (45–61) | 4/7 |
| VaD | 5 | 4 | 5 | 8 | 2 | 4 | 7 | 2 | 5 | 22.2±5.0 | 71 (63–77) | 8/6 |
| FTD | 5 | 3 | 15 | 13 | 1 | 8 | 8 | 11 | 3 | 16.7±5.2 | 61 (56–64) | 12/11 |
| DLB | 1 | 6 | 7 | 4 | 1 | 2 | 3 | 3 | 1 | 19.3±3.9 | 70 (68–75) | 5/2 |
| AD + VaD | 1 | 2 | 2 | 1 | 2 | 1 | 19.3±4.0 | 78 | 3/0 | |||
| PD | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 22.5±10.6 | 65 | 2/1 | ||
| CBS | 1 | 1 | 1 | 27 | 51 | 0/1 | ||||||
| ND | 1 | 2 | 2 | 1 | 2 | 1 | 20.7±5.5 | 68 | 1/2 | |||
AD, Alzheimer’s disease; AD + VaD, mixed dementia; CBS, corticobasal syndrome; COMT, catechol-O-methyltransferase; DBH, dopamine β-hydroxylase; DLB, dementia with Lewy bodies; F, female; FTD, frontotemporal dementia; HC, healthy controls; M, male; MAOB, monoamine oxidase B; MCI, mild cognitive impairment; ND, nonspecific dementia; PD, Parkinson’s disease; SD, standard deviation; VaD, vascular dementia.
Analysis of CSF biomarkers
CSF was collected by lumbar puncture between intervertebral spaces L3/L4 or L4/L5. After lumbar puncture, CSF was centrifuged for 10 min at 2,000 g and stored in polypropylene tubes at –80°C. CSF biomarkers were measured using the following enzyme-linked immunosorbent assays (ELISA): Aβ42 (Innotest β-amyloid1–42, Fujirebio, Gent, Belgium), p-tau231 (Tau [pT231] Phospho-ELISA Kit, Human, Thermo Fisher Scientific, Waltham, MA, USA), p-tau199 (TAU [pS199] Phospho-ELISA Kit, Human, Thermo Fisher Scientific), p-tau181 (Innotest Phospho-Tau [181P], Fujirebio), t-tau (Innotest hTau Ag, Fujirebio), and VILIP-1 (VILIP-1 Human ELISA, BioVendor, Brno, Czech Republic) according to the manufacturers’ instructions.
DNA analysis
Venous blood was collected in plastic syringes with 1 ml of acid citrate dextrose as an anticoagulant. Isolation of DNA from the peripheral blood was done by the salting-out method [22]. TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA, USA) were used for determination of COMT Val158Met (rs4680), DBH rs1611115 (also called –1021C/T or –970C/T), and MAOB rs1799836 SNPs. Analysis of SNPs was done using ABI Prism 7300 Real Time PCR System apparatus (Applied Biosystems).
Statistical analysis
SPSS 19.0.1 (SPSS, Chicago, IL, USA) was used for statistical analyses with level of statistical significance set at α= 0.05. Normality of data was tested using the Kolmogorov–Smirnov test. Because some groups contained small number of subjects, non-parametric statistics were also used. Non-parametric Kruskal-Wallis test was used for comparison of the CSF biomarkers’ levels among the groups. Post-hoc non-parametric test with calculation of the corrected p value was used for pairwise comparisons. One limitation of our study was the small number of HC available (n = 11). Statistical analysis was performed in all subjects combined (n = 233). Additionally, association of CSF biomarkers with SNPs was tested separately in AD subjects, MCI patients, a mixed group of AD, MCI, and HC subjects, as well as in a mixed group of MCI and HC subjects. Only statistically significant associations were reported.
RESULTS
COMT Val158Met (rs4680)
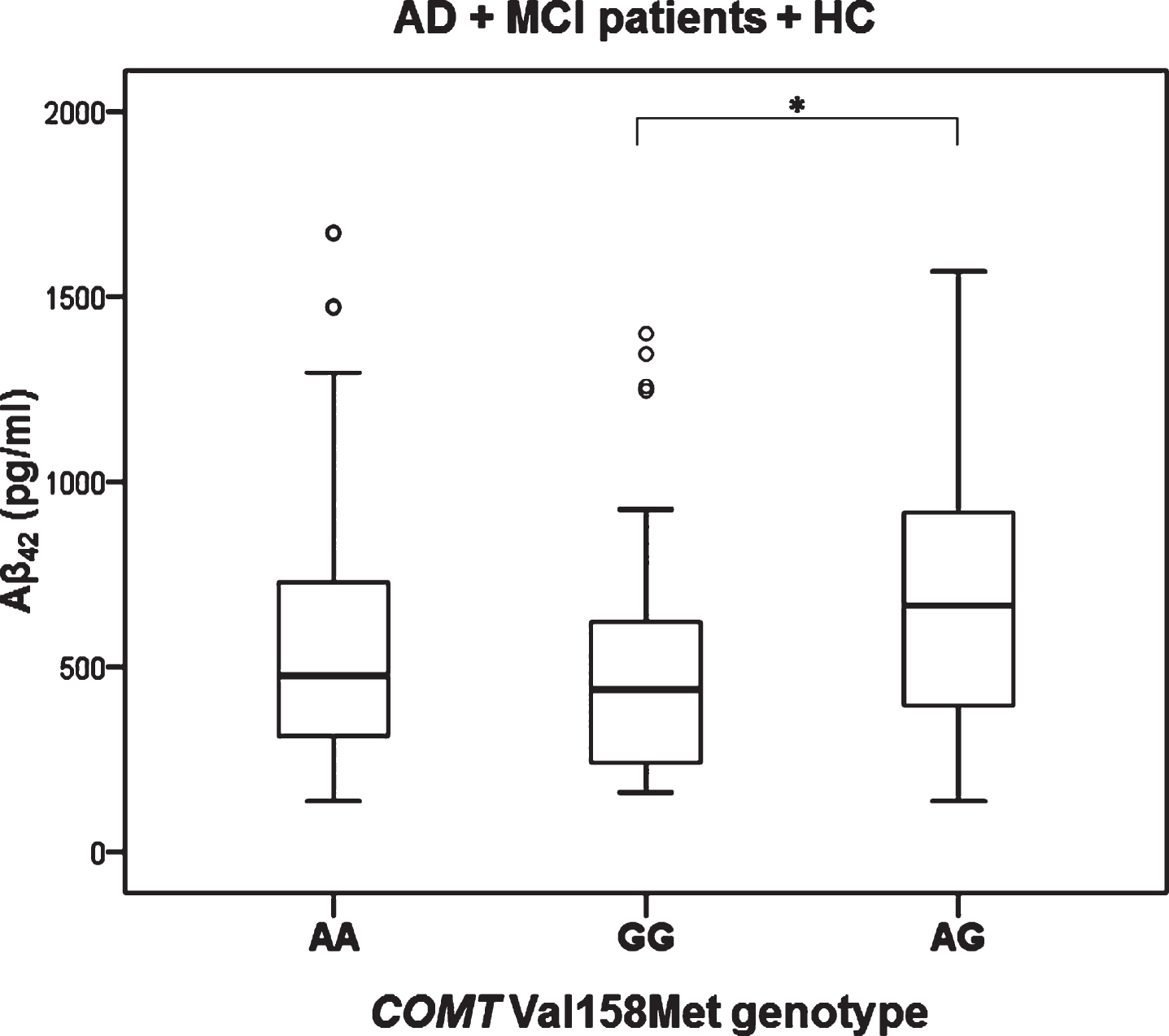
Levels of t-tau (H test = 7.657, df = 2, p = 0.022) and p-tau181 (H test = 6.348, df = 2, p = 0.042) were significantly different in patients with different COMT Val158Met genotype (in all subjects with different diagnoses; AD, MCI, VaD, FTD, DLB, AD+VaD, CBS, ND, PD, and healthy controls). Levels of t-tau (Kruskal-Wallis post hoc p = 0.017) and p-tau181 (K-W post hoc p = 0.035) were significantly increased in patients with AA compared to AG COMT Val158Met genotype (Fig. 1). Aβ42 levels were significantly different in all subjects with AD, MCI, and HC grouped together with different COMT Val158Met genotype (H test = 7.354, df = 2, p = 0.025). More precisely, Aβ42 levels were significantly decreased in patients with GG compared to AG COMT Val158Met genotype (KW post hoc p = 0.038) (Fig. 2). Patients with AG genotype had normal levels of CSF biomarkers (t-tau, p-tau181, and Aβ42), while patients with AA and GG genotype have pathological levels of CSF biomarkers (increased t-tau and p-tau181 levels and decreased Aβ42 levels) (Figs. 1 and 2).
Fig.1
Levels of A) t-tau and B) p-tau181 in patients with different COMT Val158Met genotype. *p < 0.05.
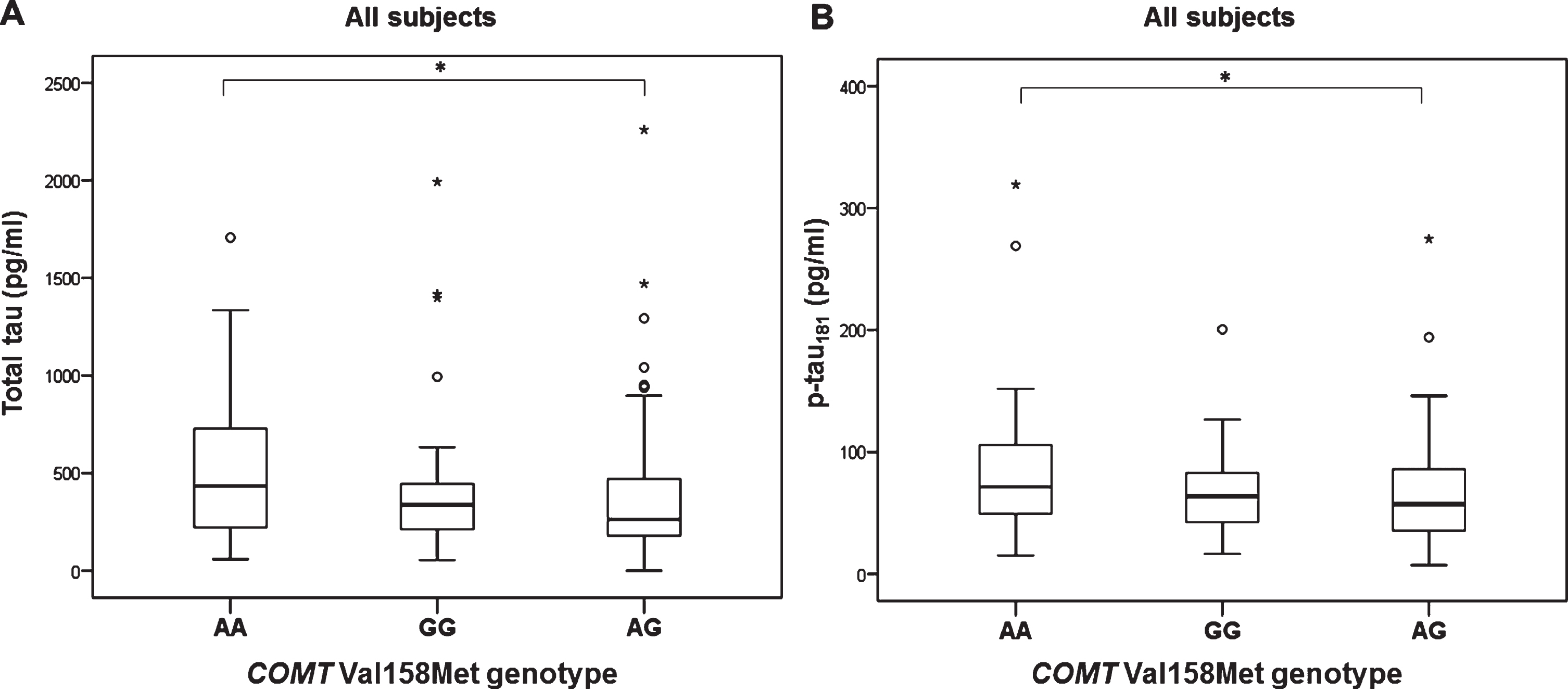
Fig.2
Levels of Aβ42 in AD, MCI patients and HC with different COMT Val158Met genotype. *p < 0.05.
DBH rs1611115
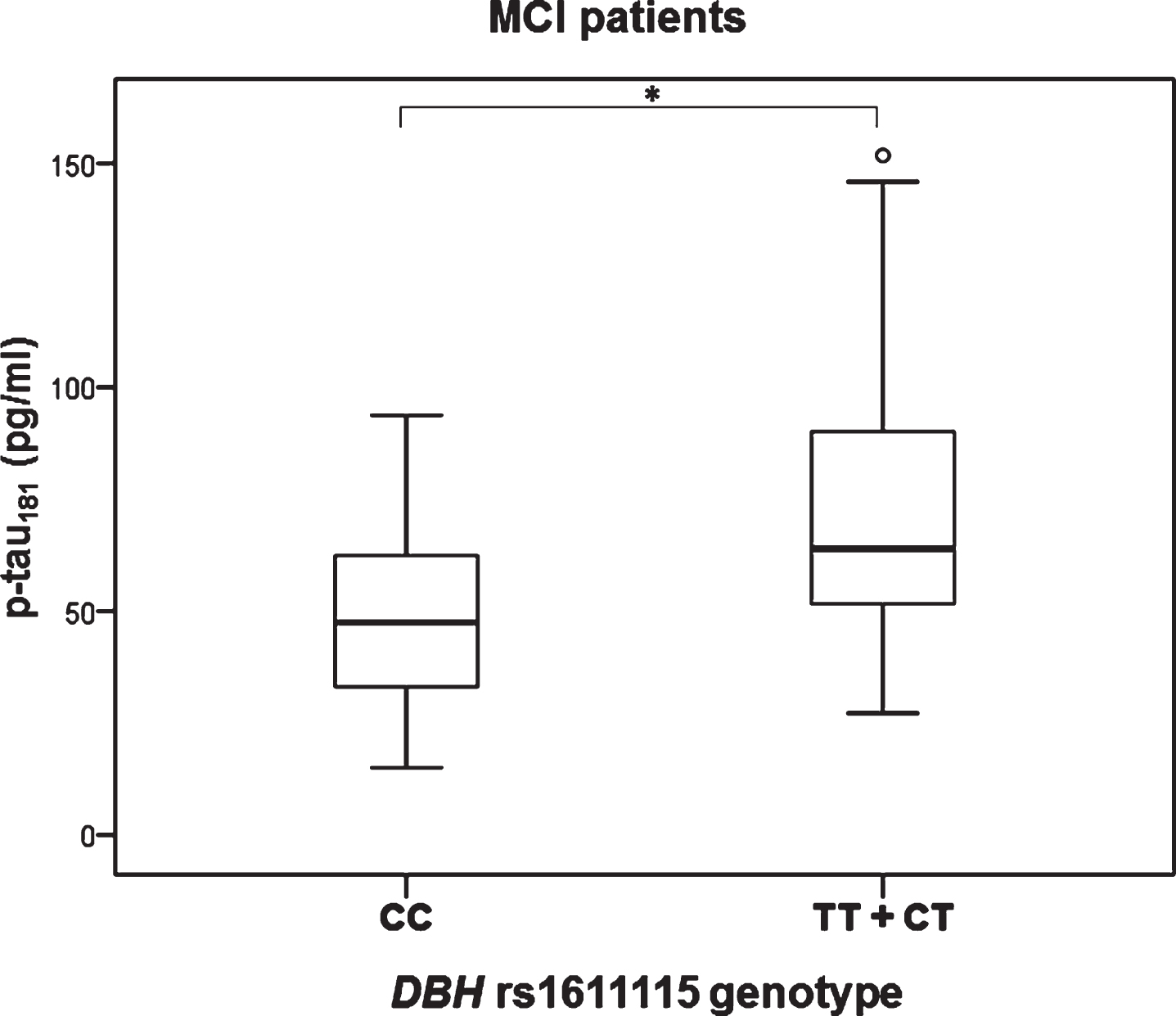
Significant difference in the levels of p-tau181 was observed in MCI patients with different DBH rs1611115 genotype (H test = 8.377, df = 2, p = 0.015). Namely, p-tau181 levels were significantly increased in patients with CT compared to CC DBH rs1611115 genotype (K-W post hoc p = 0.036) (Fig. 3). P-tau181 levels were also significantly increased in MCI patients with TT and CT compared to CC DBH rs1611115 genotype (U = 146, Z = –2.857, p = 0.004) (Fig. 4).
Fig.3
Levels of p-tau181 in MCI patients with different DBH rs1611115 genotype. *p < 0.05.
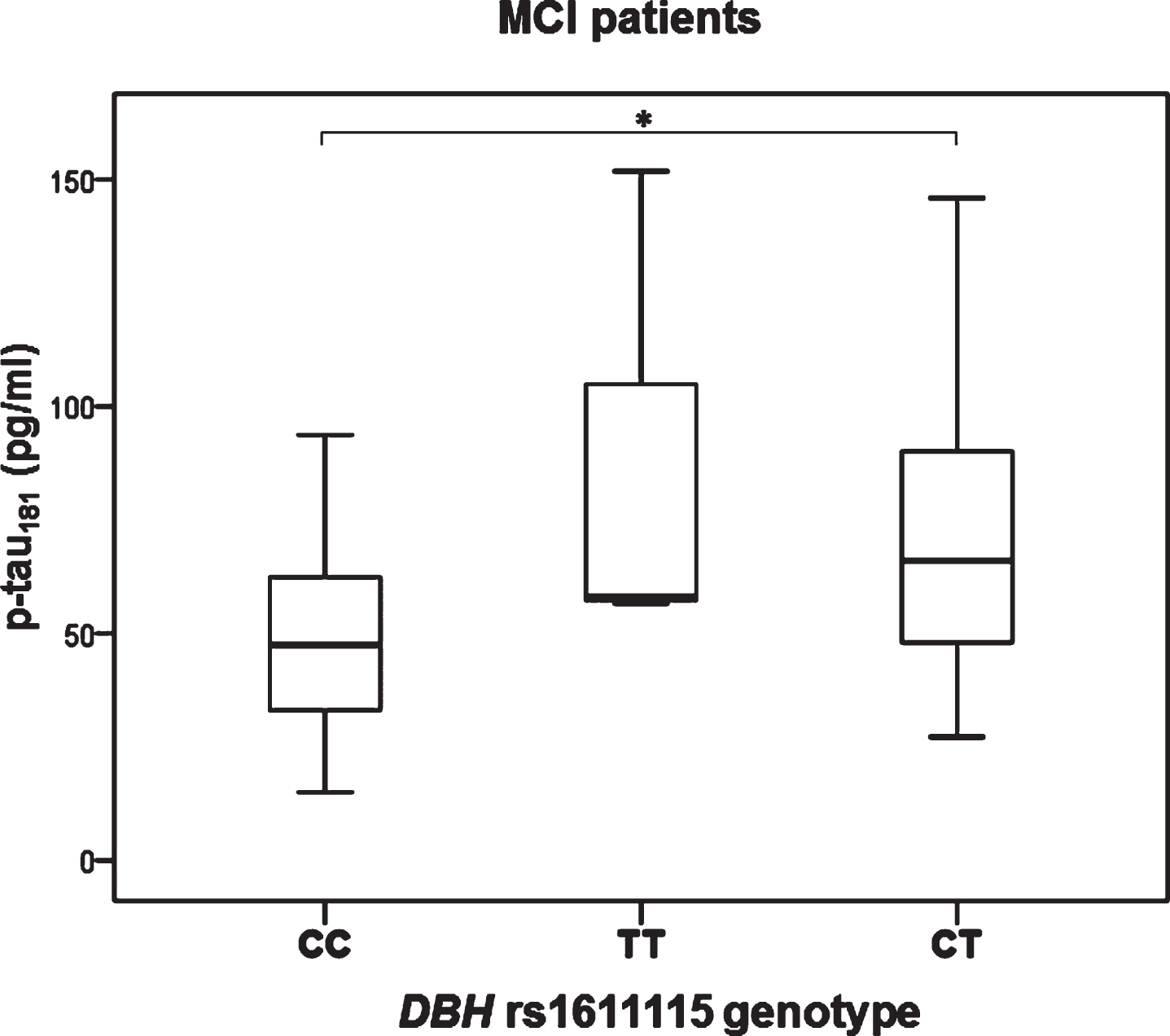
Fig.4
Levels of p-tau181 in MCI patients with different DBH rs1611115 genotype. Subjects with TT and CT genotypes are grouped together. *p < 0.05.
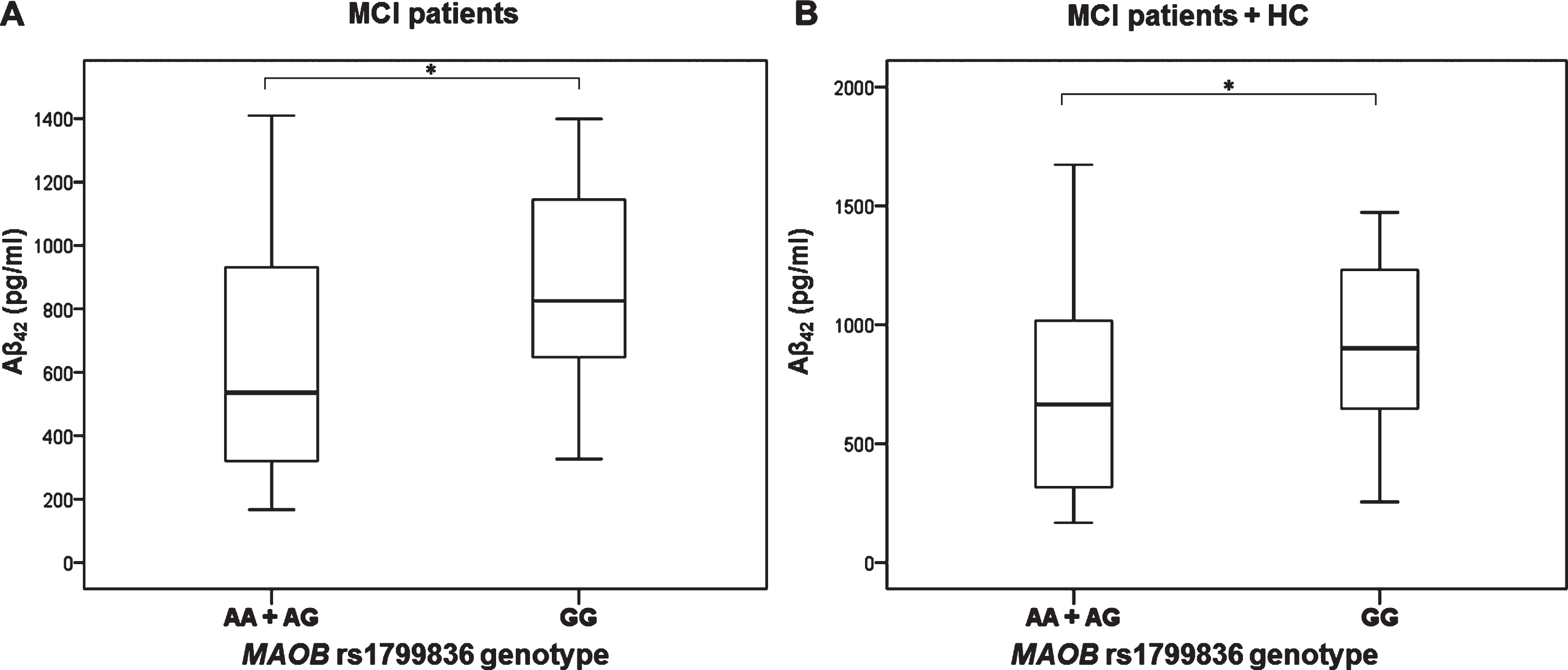
MAO-B rs1799836
Aβ42 levels were significantly decreased in MCI patients with AA and AG compared to GG MAO-B rs1799836 genotype (U = 206, Z = –2.047, p = 0.041) (Fig. 5). These results were confirmed when MCI patients and HC were grouped together (U = 313, Z = –1.980, p = 0.048) (Fig. 5).
Fig.5
Levels of Aβ42 in A) MCI patients and B) MCI patients and HC with different MAO-B rs1799836 genotype. *p < 0.05.
DISCUSSION
In this study we showed that COMT Val158Met, DBH rs1611115, and MAOB rs1799836 polymorphisms deserve further investigation as genetic markers of AD. Future research in this direction is also motivated by the occurrence of significant neuropathological alterations of noradrenergic and dopaminergic systems in AD [2, 23–26]. For example, up to 70% of locus coeruleus (LC) neurons are lost in AD brains [27–29]. A postmortem analysis of 118 brains showed that >20% of Braak stage 0 and all of Braak stage I cases have substantial neurofibrillary changes in dorsal raphe nucleus (the earliest site of neurofibrillary pathology in 6% of all AD cases) and LC (the earliest site of neurofibrillary pathology in 8% of all AD cases) [30]. These findings are paralleled by clinicopathological correlations. For example, in a retrospective review of 100 autopsy-confirmed AD cases, it was found that, on average, depression, mood change, social withdrawal, confusion, disorientation, agitation, disturbed wake-sleep cycle, and other behavioral and psychological symptoms of dementia (BPSD) were documented more than 2 years before the diagnosis of AD, whereas the first non-cognitive symptom appeared, on average, 33 months before the diagnosis [31]. Another study of 235 patients with early probable AD reported that only 8.5% of them were free of BPSD during the first three years of follow-up [32]. Perhaps the most impressive confirmation of the importance of the LC integrity to memory and cognition in aging was a recent in vivo study of Dahl and collaborators [33]. Using high-resolution, neuromelanin-sensitive magnetic resonance imaging (MRI), these authors found that individual differences across a variety of memory tasks in both 66 younger and 228 older adults strongly correlated with integrity of rostral LC [33].
Experimental work has shown that LC input to hippocampal CA3 drives single-trial learning of a novel context [26]. However, besides its role in memory consolidation and synaptic plasticity, LC neurons modulate many other different processes, such as sleep-wake cycle, blood-brain barrier permeability, and neuronal metabolism, all functions that have been impaired in AD [34, 35]. Over the past 40 years Aston-Jones and colleagues have elucidated many of the roles of noradrenaline that regulate behavior (for a review, see [36]). One of these roles is that noradrenaline is released from LC when a subject is engaged in cognitive and motor tasks in relation to novelty, interest, excitement, or effort [24]. As noradrenaline regulates the phagocytosis of Aβ by microglia and acts as a neuroprotective and anti-inflammatory agent [37], it is not surprising that enhanced noradrenergic transmission in the brain results in reduced neuroinflammation and reduced cognitive decline [35]. It was also observed that enhancement of dopaminergic transmission ameliorates cognitive deficits in AD [38, 39]. Decrease in dopamine, dopamine metabolites, and number of dopamine receptors has been reported in AD [40, 41]. Animal models of AD also showed decrease in dopamine levels in the brain [42, 43]. Also, polymorphisms in genes for the dopaminergic system proteins are associated with characteristic BPSD in early AD [44, 45].
In this study, we compared the levels of six AD CSF biomarkers (Aβ42, t-tau, p-tau181, p-tau199, p-tau231, and VILIP-1) in patients with different COMT Val158Met (rs4680), DBH rs1611115 (also called –1021C/T or –970C/T), and MAOB rs1799836 (also called A644G) polymorphisms. We observed that the levels of t-tau and p-tau181 are increased in patients with AA compared to AG COMT Val158Met genotype, while Aβ42 levels are decreased in patients with GG compared to AG COMT Val158Met genotype. P-tau181 levels are also increased in carriers of T allele in DBH rs1611115 polymorphism, while Aβ42 levels are decreased in carriers of A allele in MAO-B rs1799836 polymorphism.
As COMT is involved in degradation of dopamine, functional polymorphisms in its gene can lead to different transcription and translation products that can affect its enzymatic activity and consequently dopamine levels in the brain. Val158Met polymorphism in COMT gene involves substitution at codon 158 of amino acid Val by Met [46]. Met/Met homozygotes have four times lower COMT enzymatic activity than Val/Val homozygotes. Val allele (G allele) in COMT gene that results in lower dopamine levels in synaptic cleft was associated with increased risk for AD [47]. COMT Val158Met polymorphism was compared with genetic biomarkers of AD, such as apolipoprotein E (APOE) [48–51], and with neuroimaging biomarkers of AD [52–54]. However, the association of COMT Val158Met polymorphism with CSF AD biomarkers was not previously tested, and case-control studies on association of COMT Val158Met polymorphism and AD yielded inconsistent results. The G allele in COMT Val158Met polymorphism was associated with increased risk for AD (mostly in synergy with the effect of APOE ɛ4) [48, 49, 54–56], risk of psychosis in AD [45, 57, 58], and higher alcohol consumption in AD [52]. Several studies showed no association between COMT Val158Met polymorphism and AD [59–62], while others showed that COMT Val158Met A allele is, in fact, associated with AD [63, 64,7]. The meta-analysis of Lee and Song [47] showed association between G allele in COMT Val158Met polymorphism and AD, while other meta-analyses [6, 65, 66] found no association between COMT Val158Met polymorphism and AD. The results of our study suggest that heterozygosity in COMT Val158Met polymorphism could be protective against AD as the patients with the AA genotype had pathological levels of CSF t-tau and p-tau181, while patients with the GG genotype had pathological levels of Aβ42.
The presence of a T allele in the rs1611115 DBH polymorphism contributes to a decrease in plasma DBH (pDBH) activity [67]. Decrease in DBH activity has been detected in both brain [68, 69] and plasma [70] of AD patients. Given that pDBH activity decreases in early AD regardless of rs1611115 DBH genotype [70], AD patients carrying a T allele in rs1611115 DBH polymorphism may have even more pronounced decrease in DBH activity and consequently in noradrenaline synthesis. Combarros et al. [71] and Belbin et al. [72] reported an association between T allele in rs1611115 DBH polymorphism and AD. However, this association of rs1611115 DBH polymorphism and AD has not been confirmed in other studies [70, 73–75], although Mateo et al. [73] showed that T/T rs1611115 DBH genotype, in addition to the risk genotypes in –889 IL-1α and –174 IL6 polymorphisms, increases the risk of AD. Synergy between DBH rs1611115 and BDNF rs6265 polymorphisms was also observed, and this synergistic interaction contributed to a greater risk for AD [72]. The meta-analysis of Tang et al. [76] showed no association between rs1611115 DBH polymorphism and AD. The association of rs1611115 DBH polymorphism with CSF AD biomarkers was not previously tested. The results of our study agree with evidence of increased risk of AD in carriers of the T allele in rs1611115 DBH polymorphism and are supported by the finding of pathological CSF p-tau181 levels in patients carrying this allele.
It has been proposed that MAOB rs1799836 polymorphism affects MAOB transcription and translation, enzyme’s activity and consequently concentration of monoamines in synapses [77]. However, studies investigating influence of MAOB rs1799836 polymorphism on MAOB activity yielded conflicting results. Namely, both A allele [78] and G allele [79] in MAOB rs1799836 polymorphism were associated with lower MAOB activity. Lower MAOB activity was associated with poor impulse control, risky behavior, and behavioral disinhibition [80]. However, other studies [81–83] and a meta-analysis [4] found no association between MAOB rs1799836 polymorphism and MAOB activity. Because MAOB activity is influenced by smoking, aging, gender, ethnicity, and various medicaments [81–88], it was proposed [89] that MAOB could be a molecular link between lifestyle and AD pathogenesis. As environmental and lifestyle factors may influences epigenetic mechanisms [90], lifestyle factors could affect MAOB expression epigenetically through one-carbon metabolism that causes reduced methylation of its promoter [91]. Although there are many indices of increased MAOB activity in AD [92–95], the distribution of MAOB rs1799836 genotypes in AD patients and controls had not been analyzed. Veitinger et al. [89, 96] reported that platelet MAOB could even represent a peripheral biomarker of AD with high sensitivity and specificity. The present results and existing evidence indicate that additional investigations should consider more closely the distribution of MAOB rs1799836 genotypes between AD patients and HC, as well as the association of MAOB rs1799836 polymorphism with neuroimaging AD biomarkers and APOE genotype.
In conclusion, our study shows that carriers of different genotypes in COMT Val158Met (rs4680), DBH rs1611115 (–1021C/T or –970C/T), and MAOB rs1799836 (A644G) polymorphisms have altered levels of CSF AD biomarkers. As persons with specific genotypes in COMT, DBH, and MAOB genes are more prone to develop AD pathology (as reflected by their levels of CSF AD biomarkers), the potential of these polymorphisms as genetic biomarkers of AD is significant and should be further assessed in larger cohorts of AD patients and healthy controls.
ACKNOWLEDGMENTS
This work was funded by The Croatian Science Foundation grant IP-2014-09-9730 (“Tau protein hyperphosphorylation, aggregation, and trans-synaptic transfer in Alzheimer’s disease: cerebrospinal fluid analysis and assessment of potential neuroprotective compounds”) to GŠ and by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience CoRE-NEURO (“Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain”; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund), and in part by NIH grant P50 AG005138 to PRH.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0991r1).
REFERENCES
[1] | Šimić G , Stanić G , Mladinov M , Jovanov-Milošević N , Kostović I , Hof P ((2009) ) Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol 35: , 532–554. |
[2] | Šimić G , Babić Leko M , Wray S , Harrington CR , Delalle I , Jovanov-Milošević N , Bažadona D , Buée L , de Silva R , Di Giovanni G , Wischik CM , Hof PR ((2017) ) Monoaminergic neuropathology in Alzheimer’s disease. Prog Neurobiol 151: , 101–138. |
[3] | Camarena B , Fresán A , Aguilar A , Escamilla R , Saracco R , Palacios J , Tovilla A , Nicolini H ((2012) ) Monoamine oxidase A and B gene polymorphisms and negative and positive symptoms in schizophrenia. ISRN Psychiatry 2012: , 852949. |
[4] | Tunbridge EM , Narajos M , Harrison CH , Beresford C , Cipriani A , Harrison PJ ((2019) ) Which dopamine polymorphisms are functional? Systematic review and meta-analysis of COMT, DAT, DBH, DDC, DRD1-5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biol Psychiatry 86: , 608–620. |
[5] | Sun Z , Ma Y , Li W , He J , Li J , Yang X , Mao P , Cubells JF , Tang Y-L ((2018) ) Associations between the DBH gene, plasma dopamine β-hydroxylase activity and cognitive measures in Han Chinese patients with schizophrenia. Schizophr Res 193: , 58–63. |
[6] | Taylor S ((2018) ) Association between COMTVal158Met and psychiatric disorders: A comprehensive meta-analysis. Am J Med Genet Part B Neuropsychiatr Genet 177: , 199–210. |
[7] | Nikolac Perković M , Švob Štrac D , Tudor L , Konjevod M , Nedić Erjavec G , Pivac N ((2018) ) Catechol-O-methyltransferase, cognition and Alzheimer’s disease. Curr Alzheimer Res 15: , 408–419. |
[8] | Babić Leko M , Willumsen N , Nikolac Perković M , Klepac N , Borovečki F , Hof PR , Sonicki Z , Pivac N , de Silva R , Šimić G ((2018) ) Association of MAPT haplotype-tagging polymorphisms with cerebrospinal fluid biomarkers of Alzheimer’s disease: A preliminary study in a Croatian cohort. Brain Behav 8: , e01128. |
[9] | Grimmer T , Riemenschneider M , Förstl H , Henriksen G , Klunk WE , Mathis CA , Shiga T , Wester H-J , Kurz A , Drzezga A ((2009) ) Beta amyloid in Alzheimer’s disease: Increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry 65: , 927–934. |
[10] | Bürger K , Ewers M , Pirttila T , Zinkowski R , Alafuzoff I , Teipel SJ , DeBernardis J , Kerkman D , McCulloch C , Soininen H , Hampel H ((2006) ) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129: , 3035–3041. |
[11] | Babić M , Švob Štrac D , Mück-Šeler D , Pivac N , Stanić G , Hof PR , Šimić G ((2014) ) Update on the core and developing cerebrospinal fluid biomarkers for Alzheimer disease. Croat Med J 55: , 347–365. |
[12] | Babić Leko M , Borovečki F , Dejanović N , Hof PR , Šimić G ((2016) ) Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis 50: , 765–778. |
[13] | Babić Leko M , Krbot Skorić M , Klepac N , Borovečki F , Langer Horvat L , Vogrinc Ž , Sonicki Z , Hof PR , Šimić G ((2018) ) Event-related potentials improve the efficiency of cerebrospinal fluid biomarkers for differential diagnosis of Alzheimer’s disease. Curr Alzheimer Res 15: , 1244–1260. |
[14] | Boban M , Malojčić B , Mimica N , Vuković S , Zrilić I , Hof PR , Šimić G ((2012) ) The reliability and validity of the Mini-mental state examination in the elderly Croatian population. Dement Geriatr Cogn Disord 22: , 385–392. |
[15] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[16] | Román GC , Tatemichi TK , Erkinjuntti T , Cummings JL , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A ((1993) ) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: , 250–260. |
[17] | Hachinski VC , Iliff LD , Zilhka E , Du Boulay GH , McAllister VL , Marshall J , Russell RW , Symon L ((1975) ) Cerebral blood flow in dementia. Arch Neurol 32: , 632–637. |
[18] | Neary D , Snowden JS , Gustafson L , Passant U , Stuss D , Black S , Freedman M , Kertesz A , Robert PH , Albert M , Boone K , Miller BL , Cummings J , Benson DF ((1998) ) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51: , 1546–1554. |
[19] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[20] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[21] | World Medical Association ((2013) ) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310: , 2191–2194. |
[22] | Miller SA , Dykes DD , Polesky HF ((1988) ) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: , 1215. |
[23] | Heneka MT , Nadrigny F , Regen T , Martinez-Hernandez A , Dumitrescu-Ozimek L , Terwel D , Jardanhazi-Kurutz D , Walter J , Kirchhoff F , Hanisch U-K , Kummer MP ((2010) ) Locus coeruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A 107: , 6058–6063. |
[24] | Mather M , Harley CW ((2016) ) The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends Cogn Sci 20: , 214–226. |
[25] | Kelly SC , He B , Perez SE , Ginsberg SD , Mufson EJ , Counts SE ((2017) ) Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 5: , 8. |
[26] | Wagatsuma A , Okuyama T , Sun C , Smith LM , Abe K , Tonegawa S ((2018) ) Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc Natl Acad Sci U S A 115: , E310–E316. |
[27] | Bondareff W , Mountjoy CQ , Roth M ((1982) ) Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 32: , 164–168. |
[28] | Iversen LL , Rossor MN , Reynolds GP , Hills R , Roth M , Mountjoy CQ , Foote SL , Morrison JH , Bloom FE ((1983) ) Loss of pigmented dopamine-β-hydroxylase positive cells from locus coeruleus in senile dementia of Alzheimer’s type. Neurosci Lett 39: , 95–100. |
[29] | Zweig RM , Ross CA , Hedreen JC , Steele C , Cardillo JE , Whitehouse PJ , Folstein MF , Price DL ((1988) ) The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol 24: , 233–242. |
[30] | Grinberg LT , Rüb U , Ferretti REL , Nitrini R , Farfel JM , Polichiso L , Gierga K , Jacob-Filho W , Heinsen H ((2009) ) The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol 35: , 406–416. |
[31] | Jost BC , Grossberg GT ((1996) ) The evolution of psychiatric symptoms in Alzheimer’s disease: A natural history study. J Am Geriatr Soc 44: , 1078–1081. |
[32] | Devanand DP , Jacobs DM , Tang MX , Del Castillo-Castaneda C , Sano M , Marder K , Bell K , Bylsma FW , Brandt J , Albert M , Stern Y ((1997) ) The course of psychopathologic features in mild to moderate Alzheimer’s disease. Arch Gen Psychiatry 54: , 257. |
[33] | Dahl MJ , Mather M , Düzel S , Bodammer NC , Lindenberger U , Kühn S , Werkle-Bergner M ((2019) ) Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat Hum Behav. doi: 10.1038/s41562-019-0715-2 |
[34] | Roh JH , Huang Y , Bero AW , Kasten T , Stewart FR , Bateman RJ , Holtzman DM ((2012) ) Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med 4: , 150ra122. |
[35] | Mravec B , Lejavova K , Cubinkova V ((2014) ) Locus (coeruleus) minoris resistentiae in pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 11: , 992–1001. |
[36] | Aston-JonesG, CohenJD ((2005) ) Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 493: , 99–110. |
[37] | Heneka M , O’Banion M ((2007) ) Inflammatory processes in Alzheimer’s disease. J Neuroimmunol 184: , 69–91. |
[38] | Martorana A , Di Lorenzo F , Esposito Z , Lo Giudice T , Bernardi G , Caltagirone C , Koch G ((2013) ) Dopamine D2-agonist rotigotine effects on cortical excitability and central cholinergic transmission in Alzheimer’s disease patients. Neuropharmacology 64: , 108–113. |
[39] | Stefani A , Olivola E , Liguori C , Hainsworth AH , Saviozzi V , Angileri G , D’Angelo V , Galati S , Pierantozzi M ((2015) ) Catecholamine-based treatment in Alzheimer’s disease patients: Expectations and delusions. Front Aging Neurosci 7: , 67. |
[40] | Storga D , Vrecko K , Birkmayer JGD , Reibnegger G ((1996) ) Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett 203: , 29–32. |
[41] | Trillo L , Das D , Hsieh W , Medina B , Moghadam S , Lin B , Dang V , Sanchez MM , De Miguel Z , Ashford JW , Salehi A ((2013) ) Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neurosci Biobehav Rev 37: , 1363–1379. |
[42] | Ambrée O , Richter H , Sachser N , Lewejohann L , Dere E , de Souza Silva MA , Herring A , Keyvani K , Paulus W , Schäbitz W-R ((2009) ) Levodopa ameliorates learning and memory deficits in a murine model of Alzheimer’s disease. Neurobiol Aging 30: , 1192–1204. |
[43] | Guzman-Ramos K , Moreno-Castilla P , Castro-Cruz M , McGaugh JL , Martinez-Coria H , LaFerla FM , Bermudez-Rattoni F ((2012) ) Restoration of dopamine release deficits during object recognition memory acquisition attenuates cognitive impairment in a triple transgenic mice model of Alzheimer’s disease. Learn Mem 19: , 453–460. |
[44] | Holmes C , Smith H , Ganderton R , Arranz M , Collier D , Powell J , Lovestone S ((2001) ) Psychosis and aggression in Alzheimer’s disease: The effect of dopamine receptor gene variation. J Neurol Neurosurg Psychiatry 71: , 777–779. |
[45] | Borroni B , Agosti C , Archetti S , Costanzi C , Bonomi S , Ghianda D , Lenzi GL , Caimi L , Luca M Di , Padovani A ((2004) ) Catechol-O-methyltransferase gene polymorphism is associated with risk of psychosis in Alzheimer disease. Neurosci Lett 370: , 127–129. |
[46] | Männistö PT , Kaakkola S ((1999) ) Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51: , 593–628. |
[47] | Lee YH , Song GG ((2014) ) COMT Val158Met and PPARγ Pro12Ala polymorphisms and susceptibility to Alzheimer’s disease: A meta-analysis. Neurol Sci 35: , 643–651. |
[48] | Martínez MF , Martín XE , Alcelay LG , Flores JC , Valiente JMU , Juanbeltz BI , Beldarraín MÁG , López JM , Gonzalez-Fernández MC , Salazar AM , Gandarias RB , Borda SI , Marqués NO , Amillano MB , Zabaleta MC , de Pancorbo MM ((2009) ) The COMT Val158 Met polymorphism as an associated risk factor for Alzheimer disease and mild cognitive impairment in APOE 4 carriers. BMC Neurosci 10: , 125. |
[49] | Dixon RA , DeCarlo CA , MacDonald SWS , Vergote D , Jhamandas J , Westaway D ((2014) ) APOE and COMT polymorphisms are complementary biomarkers of status, stability, and transitions in normal aging and early mild cognitive impairment. Front Aging Neurosci 6: , 236. |
[50] | Wollam ME , Weinstein AM , Saxton JA , Morrow L , Snitz B , Fowler NR , Suever Erickson BL , Roecklein KA , Erickson KI ((2015) ) Genetic risk score predicts late-life cognitive impairment. J Aging Res 2015: , 1–8. |
[51] | Porter T , Burnham SC , Milicic L , Savage G , Maruff P , Sohrabi HR , Peretti M , Lim YY , Weinborn M , Ames D , Masters CL , Martins RN , Rainey-Smith S , Rowe CC , Salvado O , Groth D , Verdile G , Villemagne VL , Laws SM ((2019) ) COMT Val158Met is not associated with Aβ-amyloid and APOE ɛ4 related cognitive decline in cognitively normal older adults. IBRO Rep 6: , 147–152. |
[52] | Shibata N , Nagata T , Tagai K , Shinagawa S , Ohnuma T , Kawai E , Kasanuki K , Shimazaki H , Toda A , Tagata Y , Nakada T , Nakayama K , Yamada H , Arai H ((2015) ) Association between the catechol-O-methyltransferase polymorphism Val158Met and Alzheimer’s disease in a Japanese population. Int J Geriatr Psychiatry 30: , 927–933. |
[53] | Chang C-C , Tsai S-J , Chen N-C , Huang C-W , Hsu S-W , Chang Y-T , Liu M-E , Chang W-N , Tsai W-C , Lee C-C ((2018) ) Catechol-O-methyltransferase Val158Met polymorphism on striatum structural covariance networks in Alzheimer’s disease. Mol Neurobiol 55: , 4637–4649. |
[54] | Wang PN , Liu HC , Liu TY , Chu A , Hong CJ , Lin KN , Chi CW ((2005) ) Estrogen-metabolizing gene COMT polymorphism synergistic APOE ɛ4 allele increases the risk of Alzheimer’s disease. Dement Geriatr Cogn Disord 19: , 120–125. |
[55] | Thornton V , Warden D , Talbot C , Mastana SS , Bandelow S , Hogervorst E ((2011) ) Modification of estrogen’s association with Alzheimer’s disease risk by genetic polymorphisms. Brain Res 1379: , 213–223. |
[56] | Lanni C , Garbin G , Lisa A , Biundo F , Ranzenigo A , Sinforiani E , Cuzzoni G , Govoni S , Ranzani GN , Racchi M ((2012) ) Influence of COMT Val158Met polymorphism on Alzheimer’s disease and mild cognitive impairment in Italian patients. J Alzheimers Dis 32: , 919–926. |
[57] | Sweet RA , Devlin B , Pollock BG , Sukonick DL , Kastango KB , Bacanu S-A , Chowdari K V , DeKosky ST , Ferrell RE ((2005) ) Catechol-O-methyltransferase haplotypes are associated with psychosis in Alzheimer’s disease. Mol Psychiatry 10: , 1026–1036. |
[58] | Borroni B , Grassi M , Costanzi C , Zanetti M , Archetti S , Franzoni S , Caimi L , Padovani A ((2007) ) Haplotypes in cathechol-O-methyltransferase gene confer increased risk for psychosis in Alzheimer’s disease. Neurobiol Aging 28: , 1231–1238. |
[59] | Lambert J-C , Ibrahim-Verbaas CA , Harold D , Naj AC , Sims R , Bellenguez C , Jun G , DeStefano AL , Bis JC , Beecham GW , Grenier-Boley B , Russo G , Thornton-Wells TA , Jones N , Smith A V , Chouraki V , Thomas C , Ikram MA , Zelenika D , Vardarajan BN , Kamatani Y , Lin C-F , Gerrish A , Schmidt H , Kunkle B , Dunstan ML , Ruiz A , Bihoreau M-T , Choi S-H , Reitz C , Pasquier F , Hollingworth P , Ramirez A , Hanon O , Fitzpatrick AL , Buxbaum JD , Campion D , Crane PK , Baldwin C , Becker T , Gudnason V , Cruchaga C , Craig D , Amin N , Berr C , Lopez OL , De Jager PL , Deramecourt V , Johnston JA , Evans D , Lovestone S , Letenneur L , Morón FJ , Rubinsztein DC , Eiriksdottir G , Sleegers K , Goate AM , Fiévet N , Huentelman MJ , Gill M , Brown K , Kamboh MI , Keller L , Barberger-Gateau P , McGuinness B , Larson EB , Green R , Myers AJ , Dufouil C , Todd S , Wallon D , Love S , Rogaeva E , Gallacher J , St George-Hyslop P , Clarimon J , Lleo A , Bayer A , Tsuang DW , Yu L , Tsolaki M , Bossù P , Spalletta G , Proitsi P , Collinge J , Sorbi S , Sanchez-Garcia F , Fox NC , Hardy J , Naranjo MCD , Bosco P , Clarke R , Brayne C , Galimberti D , Mancuso M , Matthews F , Moebus S , Mecocci P , Del Zompo M , Maier W , Hampel H , Pilotto A , Bullido M , Panza F , Caffarra P , Nacmias B , Gilbert JR , Mayhaus M , Lannfelt L , Hakonarson H , Pichler S , Carrasquillo MM , Ingelsson M , Beekly D , Alvarez V , Zou F , Valladares O , Younkin SG , Coto E , Hamilton-Nelson KL , Gu W , Razquin C , Pastor P , Mateo I , Owen MJ , Faber KM , Jonsson P V , Combarros O , O’Donovan MC , Cantwell LB , Soininen H , Blacker D , Mead S , Mosley TH , Bennett DA , Harris TB , Fratiglioni L , Holmes C , de Bruijn RFAG , Passmore P , Montine TJ , Bettens K , Rotter JI , Brice A , Morgan K , Foroud TM , Kukull WA , Hannequin D , Powell JF , Nalls MA , Ritchie K , Lunetta KL , Kauwe JSK , Boerwinkle E , Riemenschneider M , Boada M , Hiltunen M , Martin ER , Schmidt R , Rujescu D , Wang L-S , Dartigues J-F , Mayeux R , Tzourio C , Hofman A , Nöthen MM , Graff C , Psaty BM , Jones L , Haines JL , Holmans PA , Lathrop M , Pericak-Vance MA , Launer LJ , Farrer LA , van Duijn CM , Van Broeckhoven C , Moskvina V , Seshadri S , Williams J , Schellenberg GD , Amouyel P ((2013) ) Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45: , 1452–1458. |
[60] | Zhou J , Li X-M , Jiang T , Liu Y , Chi S , Yu J-T , Tan L ((2013) ) Lack of association between COMT Val158Met polymorphism and late-onset Alzheimer’s disease in Han Chinese. Neurosci Lett 554: , 162–166. |
[61] | Corbo RM , Gambina G , Broggio E , Scarabino D , Scacchi R ((2014) ) Association study of two steroid biosynthesis genes (COMT and CYP17) with Alzheimer’s disease in the Italian population. J Neurol Sci 344: , 149–153. |
[62] | Porter T , Villemagne VL , Savage G , Milicic L , Ying Lim Y , Maruff P , Masters CL , Ames D , Bush AI , Martins RN , Rainey-Smith S , Rowe CC , Taddei K , Groth D , Verdile G , Burnham SC , Laws SM ((2018) ) Cognitive gene risk profile for the prediction of cognitive decline in presymptomatic Alzheimer’s disease. Pers Med Psychiatry 7-8: , 14–20. |
[63] | Forero DA , Benítez B , Arboleda G , Yunis JJ , Pardo R , Arboleda H ((2006) ) Analysis of functional polymorphisms in three synaptic plasticity-related genes (BDNF, COMT and UCHL1) in Alzheimer’s disease in Colombia. Neurosci Res 55: , 334–341. |
[64] | Ji Y , Shi Z , Liu M , Liu S , Liu S , Wang J ((2014) ) Association between the COMT Val158Met genotype and Alzheimer’s disease in the Han Chinese population. Dement Geriatr Cogn Dis Extra 4: , 14–21. |
[65] | Xu X , Wang Y , Wang L , Liao Q , Chang L , Xu L , Huang Y , Ye H , Xu L , Chen C , Shen X , Zhang F , Ye M , Wang Q , Duan S ((2013) ) Meta-analyses of 8 polymorphisms associated with the risk of the Alzheimer’s disease. PLoS One 8: , e73129. |
[66] | Zhang G , Li Y-C , Xu H-D , Liu X , Zhu J , Zhang F , Wang D , Wang Y , Jin C ((2015) ) Lack of association between COMT polymorphism rs4680 and risk of Alzheimer’s disease in Asians: Evidence from a meta-analysis. Psychiatry Res 228: , 979–981. |
[67] | Zabetian CP , Buxbaum SG , Elston RC , Köhnke MD , Anderson GM , Gelernter J , Cubells JF ((2003) ) The structure of linkage disequilibrium at the DBH locus strongly influences the magnitude of association between diallelic markers and plasma dopamine beta-hydroxylase activity. Am J Hum Genet 72: , 1389–1400. |
[68] | Cross AJ , Crow TJ , Perry EK , Perry RH , Blessed G , Tomlinson BE ((1981) ) Reduced dopamine β-hydroxylase activity in Alzheimer’s disease. Br Med J (Clin Res Ed) 282: , 93–94. |
[69] | Perry EK , Tomlinson BE , Blessed G , Perry RH , Cross AJ , Crow TJ ((1981) ) Neuropathological and biochemical observations on the noradrenergic system in Alzheimer’s disease. J Neurol Sci 51: , 279–287. |
[70] | Mustapić M , Presečki P , Pivac N , Mimica N , Hof PR , Šimić G , Folnegović-Šmalc V , Mück-Šeler D ((2013) ) Genotype-independent decrease in plasma dopamine beta-hydroxylase activity in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 44: , 94–99. |
[71] | Combarros O , Warden DR , Hammond N , Cortina-Borja M , Belbin O , Lehmann MG , Wilcock GK , Brown K , Kehoe PG , Barber R , Coto E , Alvarez V , Deloukas P , Gwilliam R , Heun R , Kölsch H , Mateo I , Oulhaj A , Arias-Vásquez A , Schuur M , Aulchenko YS , Ikram MA , Breteler MM , van Duijn CM , Morgan K , Smith AD , Lehmann DJ ((2010) ) The dopamine β-hydroxylase -1021C/T polymorphism is associated with the risk of Alzheimer’s disease in the Epistasis Project. BMC Med Genet 11: , 162. |
[72] | Belbin O , Morgan K , Medway C , Warden D , Cortina-Borja M , van Duijn CM , Adams HHH , Frank-Garcia A , Brookes K , Sánchez-Juan P , Alvarez V , Heun R , Kölsch H , Coto E , Kehoe PG , Rodriguez-Rodriguez E , Bullido MJ , Ikram MA , Smith AD , Lehmann DJ ((2019) ) The Epistasis Project: A multi-cohort study of the effects of BDNF, DBH, and SORT1 epistasis on Alzheimer’s disease risk. J Alzheimers Dis 68: , 1535–1547. |
[73] | Mateo I , Infante J , Rodríguez E , Berciano J , Combarros O , Llorca J ((2006) ) Interaction between dopamine beta-hydroxylase and interleukin genes increases Alzheimer’s disease risk. J Neurol Neurosurg Psychiatry 77: , 278–279. |
[74] | Komatsu M , Shibata N , Ohnuma T , Kuerban B , Tomson K , Toda A , Tagata Y , Nakada T , Shimazaki H , Arai H ((2014) ) Polymorphisms in the aldehyde dehydrogenase 2 and dopamine β hydroxylase genes are not associated with Alzheimer’s disease. J Neural Transm 121: , 427–432. |
[75] | Meng Y , Zhu Y ((2015) ) A study on the association between plasma dopamine β-hydroxylase and Alzheimer’s disease. Chin J Lab Diagn 19: , 1505–1507. |
[76] | Tang S , Yao B , Li N , Lin S , Huang Z ((2018) ) Association of dopamine β-hydroxylase polymorphisms with Alzheimer’s disease, Parkinson’s disease and schizophrenia: Evidence based on currently available loci. Cell Physiol Biochem 51: , 411–428. |
[77] | Jakubauskiene E , Janaviciute V , Peciuliene I , Söderkvist P , Kanopka A ((2012) ) G/A polymorphism in intronic sequence affects the processing of MAOB gene in patients with Parkinson disease. FEBS Lett 586: , 3698–3704. |
[78] | Garpenstrand H , Ekblom J , Forslund K , Rylander G , Oreland L ((2000) ) Platelet monoamine oxidase activity is related to MAOB intron 13 genotype. J Neural Transm 107: , 523–530. |
[79] | Balciuniene J , Emilsson L , Oreland L , Pettersson U , Jazin E ((2002) ) Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet 110: , 1–7. |
[80] | Oreland L , Hallman J ((1995) ) The correlation between platelet MAO activity and personality: Short review of findings and a discussion on possible mechanisms. Prog Brain Res 106: , 77–84. |
[81] | Pivac N , Knežević J , Mustapić M , Deželjin M , Mück-Šeler D , Kozarić-Kovačić D , Balija M , Matijević T , Pavelić J ((2006) ) The lack of association between monoamine oxidase (MAO) intron 13 polymorphism and platelet MAOB activity among men. Life Sci 79: , 45–49. |
[82] | Pivac N , Knežević J , Kozarić-Kovačić D , Deželjin M , Mustapić M , Rak D , Matijević T , Pavelić J , Mück-Šeler D ((2007) ) Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAOB activity in combat-related posttraumatic stress disorder. J Affect Disord 103: , 131–138. |
[83] | Nedić Erjavec G , Nenadić Šviglin K , Nikolac Perković M , Mück-Šeler D , Jovanović T , Pivac N ((2014) ) Association of gene polymorphisms encoding dopaminergic system components and platelet MAOB activity with alcohol dependence and alcohol dependence-related phenotypes. Prog Neuropsychopharmacol Biol Psychiatry 54: , 321–327. |
[84] | Kiive E , Eensoo D , Harro M , Harro J ((2002) ) Platelet monoamine oxidase activity in association with childhood aggressive and hyperactive behaviour: The effect of smoking? Pers Individ Dif 33: , 355–363. |
[85] | Mück-Šeler D , Šagud M , Mustapić M , Nedić G , Babić A , Mihaljević Peleš A , Jakovljević M , Pivac N ((2008) ) The effect of lamotrigine on platelet monoamine oxidase type B activity in patients with bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 32: , 1195–1198. |
[86] | Mück-Šeler D , Presečki P , Mimica N , Mustapić M , Pivac N , Babić A , Nedić G , Folnegović-Šmalc V ((2009) ) Platelet serotonin concentration and monoamine oxidase type B activity in female patients in early, middle and late phase of Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 33: , 1226–1231. |
[87] | Oreland L ((2004) ) Platelet monoamine oxidase, personality and alcoholism: The rise, fall and resurrection. Neurotoxicology 25: , 79–89. |
[88] | Pivac N , Mück-Šeler D , Šagud M , Jakovljević M , Mustapić M , Mihaljević-Peleš A ((2003) ) Long-term sertraline treatment and peripheral biochemical markers in female depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 27: , 759–765. |
[89] | Veitinger M , Oehler R , Umlauf E , Baumgartner R , Schmidt G , Gerner C , Babeluk R , Attems J , Mitulovic G , Rappold E , Lamont J , Zellner M ((2014) ) A platelet protein biochip rapidly detects an Alzheimer’s disease-specific phenotype. Acta Neuropathol 128: , 665–677. |
[90] | Alegría-Torres JA , Baccarelli A , Bollati V ((2011) ) Epigenetics and lifestyle. Epigenomics 3: , 267–277. |
[91] | Zellner M , Babeluk R , Jakobsen LH , Gerner C , Umlauf E , Volf I , Roth E , Kondrup J ((2011) ) A proteomics study reveals a predominant change in MAOB expression in platelets of healthy volunteers after high protein meat diet: Relationship to the methylation cycle. J Neural Transm 118: , 653–662. |
[92] | Adolfsson R , Gottfries C-G , Oreland L , Wiberg Å , Winblad B ((1980) ) Increased activity of brain and platelet monoamine oxidase in dementia of Alzheimer type. Life Sci 27: , 1029–1034. |
[93] | Kennedy BP , Ziegler MG , Alford M , Hansen LA , Thal LJ , Masliah E ((2003) ) Early and persistent alterations in prefrontal cortex MAO A and B in Alzheimer’s disease. J Neural Transm 110: , 789–801. |
[94] | Zellner M , Baureder M , Rappold E , Bugert P , Kotzailias N , Babeluk R , Baumgartner R , Attems J , Gerner C , Jellinger K , Roth E , Oehler R , Umlauf E ((2012) ) Comparative platelet proteome analysis reveals an increase of monoamine oxidase-B protein expression in Alzheimer’s disease but not in non-demented Parkinson’s disease patients. J Proteomics 75: , 2080–2092. |
[95] | Schedin-Weiss S , Inoue M , Hromadkova L , Teranishi Y , Yamamoto NG , Wiehager B , Bogdanović N , Winblad B , Sandebring-Matton A , Frykman S , Tjernberg LO ((2017) ) Monoamine oxidase B is elevated in Alzheimer’s disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res Ther 9: , 57. |
[96] | Veitinger M , Varga B , Guterres SB , Zellner M ((2014) ) Platelets, a reliable source for peripheral Alzheimer’s disease biomarkers? Acta Neuropathol Commun 2: , 65. |




