Psychophysiological Responses in People Living with Dementia after an Art Gallery Intervention: An Exploratory Study
Abstract
The use of existing public spaces by people living with dementia, such as museums and art galleries, are becoming popular due to their ability to facilitate programs which promote social engagement and inclusion. However, few studies have investigated physiological outcomes of art gallery-based programs. Using a quasi-experimental design, the present study aimed to investigate the levels of salivary biomarkers of cortisol and interleukin-6, quality of life (QoL), depressive symptoms, cognition, and wellbeing, after attending the National Gallery of Australia (NGA) Art and Dementia program. Twenty-eight people living with dementia, each supported by a carer or family member, were recruited for a six-week program and were followed up at twelve weeks. In total, 25 participants (17 female; mean age 84.6±7.27 years) completed the study, and 22 provided viable saliva samples. The waking to evening salivary cortisol ratio was higher post-intervention (p = 0.033), and returned to baseline levels at follow-up (p = 1.00), indicating a more dynamic salivary cortisol rhythm in response to the six-week program. Interleukin-6 levels remained unchanged (p = 0.664). No improvements in QoL (DEMQOL-Carer) were observed between baseline and post-intervention (p = 0.076). However, self-reported depressive symptoms decreased post-intervention compared with baseline (p = 0.015), and memory (immediate recall) (p = 0.009) and verbal fluency (p = 0.027) improved between the same timepoints. The NGA Art and Dementia program appears to have quantifiable benefits, including improved hypothalamic-pituitary-adrenal axis function, justifying a need for longer controlled trial inclusive of physiological outcomes.
INTRODUCTION
Museum and art gallery programs for people living with dementia (PLWD) can act as vehicles for social engagement and interaction that may promote improvements in quality of life (QoL), enjoyment, relaxation, new experiences, sharing of anecdotes, and the ability to reignite a sense of identity [1]. The practice of viewing and discussing art in a small group setting requires communication, attention, and promotes social inclusion [2]. Social participation is crucial for PLWD and has been identified as an important factor for caregivers [3]. Beyond these benefits, a cost-benefit analysis identified economic benefits of investment in Art and Dementia programs, such that for every $1 invested, there is a social return on investment of between $3.20 and $6.62 [4]. Yet, funding to support arts-based dementia programs is sporadic, possibly due to a lack of evidence supported by measurable physiological outcomes.
Specific salivary biomarkers have emerged as suitable non-invasive objective outcome measures of stress reduction interventions [5]. Elevated cortisol has been associated with greater cognitive impairment, brain shrinkage, and a more rapid decline in cognitive function [6–8]. However, measurement of cortisol at one time point is not reliable, and the diurnal cortisol rhythm and cortisol awakening response are more comprehensive markers of hypothalamic-pituitary-adrenal (HPA) axis function that is understudied in the context of dementia [9, 10]. Higher cortisol awakening response and daytime cortisol levels have been associated with greater tau and amyloid-β (Aβ) pathology [10]. Importantly, a flattening of the diurnal cortisol rhythm has been associated with greater frailty, decreased cognitive performance, and is a marker of psychosocial stress [10, 11]. In addition, inflammation is recognized as an important mechanism underlying the pathophysiology of dementia [12, 13]. Peripheral pro-inflammatory cytokines, such as interleukin-6 (IL-6), are elevated in PLWD and have been associated with cognitive decline in midlife [14]. In a recent meta-analysis of older people with depression and Alzheimer’s disease (AD), elevated IL-6 was the only inflammatory marker analyzed associated with depression [15]. IL-6 is associated with abnormal HPA-axis function, which stimulates cortisol production and modulates the relationship with depression [16].
Arts-based interventions have demonstrated psychosocial benefits, including improved wellbeing, sense of identity, and social connectedness [1, 3], and have been shown to affect biomarkers in adults and PLWD. In adults, art-making was found to be relaxing and enjoyable, and acutely reduced salivary cortisol levels [17]. One study of PLWD measured the salivary cortisol response before and after attendance at an art gallery and did not find a reduction in cortisol between pre- and post-art viewing [18]. To our knowledge, the effects of arts-based programs on the diurnal salivary cortisol rhythm and salivary IL-6 have yet to be investigated in PLWD. This project aims to evaluate the psychophysiological effects of attending the National Gallery of Australia (NGA) Art and Dementia program over six weeks and to determine if any potential benefits were present six weeks after the intervention. We hypothesized that the intervention will be associated with improved psychophysiological markers in saliva, improved QoL, cognitive function and wellbeing, and reduced symptoms of depression.
DESIGN AND METHODS
The study had a quasi-experimental design, involving PLWD that had not previously participated in the NGA Art and Dementia program.
Setting
The NGA Art and Dementia program commenced in 2007, and initial staff training at the NGA was informed by the Museum of Modern Art (MoMA) “Meet Me at MoMA” program [19]. The MoMA program is centered around viewing pre-selected works of art and engaging in conversation through flexible inquiry-based description, interpretation, and evaluation. The NGA limits group sizes to six PLWD, with qualified art educators leading group discussions with a focus on three to four artworks during each visit. Art educators at the NGA are trained to facilitate discussion, focusing on group members reflections, interpretations, and anecdotes. The NGA Art and Dementia program believes guests have a wealth of experience, insight, and knowledge, that allows the work of art to “tell a story” to each person.
Participants
Recruitment and consent
A convenience sample of participants were primarily recruited from five residential aged care communities in the Canberra, Australian Capital Territory region. The study was advertised through Dementia Australia, television, newspapers, radio, and social media. Study information was also distributed by email to managers and care staff at local residential aged care communities. Interested managers and care staff who replied by email were presented with details of the study intervention and procedures at informal meetings. Suitable candidates with a dementia diagnosis (any form), who were not prone to wandering, had the capacity to travel to the art gallery, and were considered likely to stay seated for the discussions at the gallery were identified by staff. Family members were contacted by care staff to become study partners with the PLWD, and then researchers contacted them to make an appointment, obtain double informed consent, and complete the baseline questionnaires. In cases where family members were not available or not located close to the participants, close friends and care staff were also permitted to support the participant with dementia. Approval for the study was obtained from the University of Canberra Human Ethics Research Committee (UC HREC 20180185), and the study was conducted in accordance with the Helsinki Declaration of 1975.
Participants and procedure
A total of 28 PLWD were recruited: 27 from five aged care providers and one community-dwelling. Groups One to Four were composed of people living full-time in residential aged care. Group Five included three participants receiving respite day care at a residential aged care facility, one living in aged care full-time, and one community-dwelling. All participants were asked to attend five of the six visits to the NGA to be eligible for data collection at the end of the intervention and follow-up.
Two art educators presented three to four works of art to each group weekly. Six weeks was identified as a suitable intervention length to both familiarize participants to the program and each week introduce more challenging works of art (Supplementary Table 1) and was the duration of a previous formative study at the NGA [20]. The visits to the art gallery lasted for approximately 90 minutes, with around 20 minutes spent discussing each work of art. Two care providers attended each group each week, except Group Five, which also included two friends of participants and two care staff from the respite day care facility. The participants sat in supportive chairs while the care staff and researchers sat behind the group and were instructed to limit their involvement in the group discussion. One researcher attended all 30 visits to the gallery, and two researchers were present at fifteen sessions to cross-validate behavioral observations.
Study outcomes
Measures
All measures were collected at the participants’ place of residence. The initial interview and baseline data collection were conducted during the week prior to the first NGA visit, and subsequent interviews were held the day following the final visit. Follow-up was completed six weeks after the final NGA visit. Following the initial screening, which included oral health screening adapted from previous literature [21], sociodemographic information and questionnaires were administered to participants and study partner by trained and experienced researchers. Height (m) and weight (kg) were measured at baseline to calculate a body mass index (kg/m2). The Bristol Activities of Daily Living Scale for dementia (BADL) was completed by the study partner to assess functional capacity [22]. The 29-item health-related quality of life questionnaire for people with dementia (DEMQOL) was administered to the PLWD, and the co-enrolled study partner completed the 32-item DEMQOL-Carer, with higher scores reflecting higher QoL [23]. The 15-item Geriatric Depression Scale (GDS) Short Form was completed by the PLWD, with higher scores reflecting greater presence of depressive symptoms [24]. The Mini-Addenbrooke’s Cognitive Examine (M-ACE) was used as a measure of cognitive performance [25]. The M-ACE evaluates orientation, memory (immediate and delayed recall), verbal fluency (animals), and clock drawing. Three Australia-specific versions allow repeated administration. The participants completed a handgrip strength test using a Dynamometer (TTM Instruments Original Smedlay’s Dynamo Meter (100 kg), Tokyo, Japan). Handgrip strength is predictive of mortality and morbidity and is recognized as a useful marker of physical status [26]. During the questionnaires, breaks were taken as required to reduce risk of fatigue and completed over a single day. Following each NGA visit, during weeks 1, 3, and 6, participants were asked to answer six questions using the six-item General Wellbeing Questionnaire (GWQ) from the museum wellbeing measures toolkit [27].
Salivary cortisol and IL-6
The collection schedule for the saliva sample for each participant was baseline (the day before the first visit), post-intervention at six weeks (the day after the final visit), and follow-up at twelve weeks (six weeks post-intervention). Participants were presented with a demonstration of the saliva collection method during enrolment. On collection days, four saliva samples were collected over the day: upon waking (M1), after 30 minutes (M2), 60 minutes after breakfast (M3), and 45 minutes after dinner (E) [11]. The collection times used were intended to standardize the effects of food intake and sleep on the analytes. Cortisol levels in saliva peak approximately 20 minutes following HPA-axis activation, hence if stress is increased during sample collection, it is unlikely to affect the sample [28].
To promote compliance, participants and study partners (where appropriate) were reminded about the saliva collection the day before. Participants were instructed to avoid eating and to refrain from brushing their teeth in the 30 minutes before each sample but were permitted to drink water and rinse their mouths until ten minutes before collection. The passive drool method was used following protocols described by McKune et al. (2014) [29]. Saliva samples for Groups One to Four were collected with a researcher and activities manager supervising. Following collection, samples were immediately frozen on-site in dry ice, kept frozen during transport, and then stored at –20°C until analysis. Participants from Group Five and their study partner were provided with clear instructions on how to collect and store the samples in their freezer until collected by the researchers. Upon collection, samples were stored in dry ice during transport, and then stored at –20°C until analysis. The time of collection was recorded for all samples. Salivary cortisol and IL-6 were measured in duplicate according to the manufacturer’s instructions (Salimetrics, LLC, State College, PA). Samples from the same participant were tested using the same analysis kit to avoid between-person variability. The intra-assay coefficients of variation (CV) were 5.09% for cortisol and 6.58% for IL-6. The inter-assay CV was 9.72% for cortisol and 3.63% for IL-6.
Behavioral observations and exit questionnaire
Researchers observed the behavior of participants during each visit and each occasion where a participant spoke was recorded using a standardized template. Prior to the gallery visits, researchers evaluated a mock experience until agreement was reached. A single letter code was used for each observation of speech: ‘U’ represented “unprompted” when the participant spoke spontaneously, ‘P’ represented where speech was “prompted” by the educator or another group member. Only comments of three or more consecutive words were recorded. Instances of laughter or other evidence of overt happiness were also recorded, in addition to “sleeping” or “napping”, and any negative emotions, such as “anger” or “upset”. Due to the conversational nature of the discussions, it was not possible to reconcile differences in the observations between researchers following each visit. A brief exit questionnaire was designed to determine what participants remembered six weeks after the final visit and only participants that indicated they remembered the visits were administered the questionnaire. Participants were asked if they remembered the visits to the NGA. If they answered affirmatively, the questionnaire continued, and they were asked to describe three things they remembered about their visits. If the content of their response was unrelated to the art gallery, the researchers did not continue the questionnaire, and the participant was deemed not to remember the visits. Using 5-item Likert scales, participants who remembered the visits were asked whether they found the intervention to be memorable, and how much they looked forward to the visits to the art gallery. Participants who remembered the intervention were also asked to rate on a scale of 1 to 10, with 1 being horrible and 10 representing wonderful, how much did they enjoy their experience of going to the NGA. Study partners were also asked how beneficial they thought the visits were, using a 5-item Likert scale.
Statistical methods
We aimed to recruit 35 participants based on the feasibility of recruitment within the timeframe and resources of the NGA. To our knowledge, this is the first study of its kind to investigate the salivary diurnal cortisol rhythm in response to a psychosocial intervention for PLWD, and we determined this sample size to be suitable to provide useful data to inform future studies. A similar pragmatic approach has been used in a large Art and Dementia study in the United Kingdom [30]. All variables were examined prior to analysis to determine suitability for parametric or non-parametric methods using histograms and both Kolmogorov-Smirnov and Shapiro-Wilk tests of normality. Descriptive statistics for normally distributed continuous variables are reported as a mean (±standard deviation), and not normally distributed variables as median values (1st, 3rd quartiles). Student’s t-test for independent samples was used to evaluate differences between groups for normally distributed variables and the Mann–Whitney test for non-parametric variables. Chi-square test of independence was performed to examine relationships between forms of dementia and time since dementia diagnosis. Repeated measures ANOVA was used for normally distributed variables (DEMQOL-Carer and Hand Grip Strength) and the Friedman test used for non-parametric variables (Salivary Cortisol, Salivary IL-6, DEMQOL, M-ACE (and all subdomains), and Behavioral Observations), followed by the Wilcoxon sign-rank test where appropriate. Raw salivary cortisol levels were non-normally distributed, and log transformation did not induce normality. Thus, differences in absolute changes in the raw values were calculated using non-parametric methods. The ratios of M1 to E (M1/E) and M2 to E (M2/E) were also calculated. The area under the curve with respect to ground (AUCG) was calculated following the method described by Fekedulegn et al. (2007) [31]. Cohen’s κ was used to evaluate the inter-rater agreement of the observations during the Art and Dementia program [32]. The level of significance was defined at α= 0.05, and Bonferroni adjustments were made post-hoc for multiple comparisons. All statistical analysis was performed using IBM SPSS version 25 (Armonk, NY: IBM Corp).
RESULTS
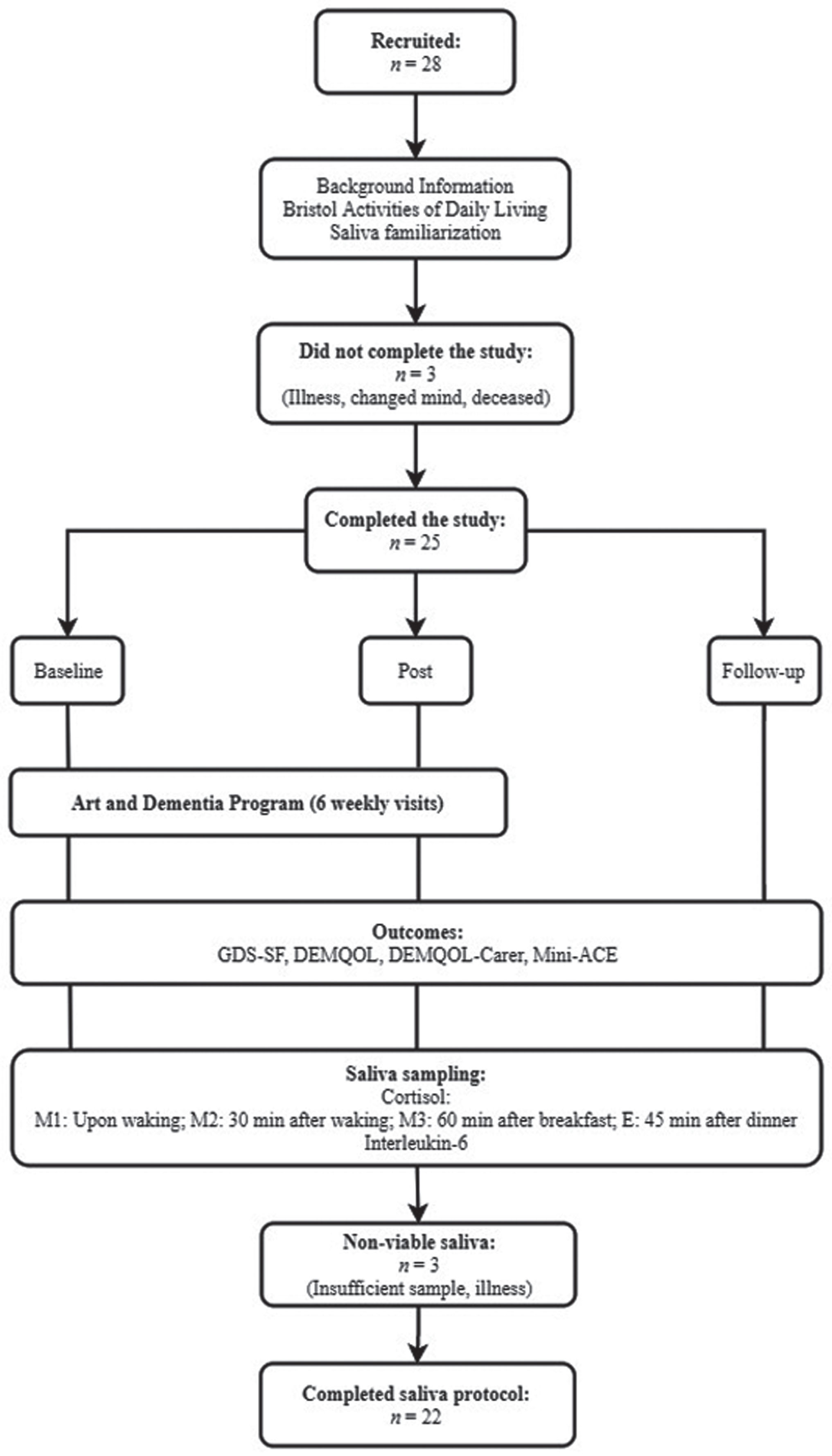
Of the 28 participants that commenced the visits to the NGA, one did not attend at least five sessions due to illness, and one voluntarily withdrew from the study before the first visit. One participant passed away between post-intervention and follow-up. In total, 25 participants (17 female, 8 male) completed the study protocol and completed questionnaires at baseline, six weeks, and follow-up. One participant did not complete the DEMQOL but was included in the rest of the analysis. Of the 25 participants, viable saliva samples were collected from 22 participants (16 female, 6 male). The study flow is presented in Fig. 1. Recruitment ceased prior to the Southern hemisphere summer period when the NGA have limited capacity to facilitate Art and Dementia programs.
Fig.1
Study flow diagram
Baseline data
The participant characteristics are displayed in Table 1. The mean age of the participants was 84.7 (±7.42) years, and 68.0% were female (n = 17). Most (68.0%) described their ethnicity as Australian, were diagnosed with Alzheimer’s disease (68.0%), and completed a median of 10.0 (8.00, 15.0) years of formal education. The time since the formal dementia diagnosis differed with ten diagnosed over four years ago, eight between one and two years ago, four within the previous year, and three between two and three years ago. There was no difference in age, body mass index, and activities of daily living between females and males (all, ps > 0.05). Participants maintained a moderate level of functional independence as rated by the BADL.
Table 1
Baseline information (n = 25)
| All | Female | Male | p | |
| Baseline information | ||||
| n | 25 | 17 | 8 | <0.001 |
| Age | 84.6±7.27 | 86.4±6.30 | 80.9±8.20 | 0.078 |
| Body Mass Index (kg/m2) | 26.1±5.09 | 26.5±5.80 | 24.8±2.79 | 0.446 |
| Education (y) | 10.0 (8.00, 15.0) | 10.0 (8.00, 13.5) | 11.0 (10.0, 15.0) | 0.189 |
| Type of dementia: | <0.001 | |||
| Alzheimer’s disease | 17 | 11 | 6 | |
| Vascular | 3 | 3 | 0 | |
| Parkinson’s dementia | 2 | 1 | 1 | |
| Not known/mixed | 3 | 2 | 1 | |
| Time since dementia diagnosis: | 0.155 | |||
| Under 1 year | 4 | 4 | 0 | |
| 1-2 years | 8 | 4 | 4 | |
| 2-3 years | 3 | 2 | 1 | |
| >4 years | 10 | 7 | 3 | |
| Bristol Activities of Daily Living (Range: 0–80*) | 19.0 (15.0, 31.0) | 19.0 (15.0, 29.5) | 24.0 (13.3, 32.0) | 0.726 |
| Hand Grip Strength (kg) | 16.3±6.83 | 13.1±5.41 | 23.2±3.73 | <0.001 |
| Physiology | ||||
| n | 22 | 16 | 6 | <0.001 |
| Salivary Cortisol (nmol/L): | ||||
| Waking | 8.99 (7.46, 14.3) | 8.57 (7.22, 10.2) | 11.9 (7.39, 14.9) | 0.555 |
| Pre-breakfast | 10.9 (9.01, 14.3) | 10.0 (8.27, 12.9) | 13.6 (10.4, 19.0) | 0.077 |
| Morning | 8.33 (5.85, 11.3) | 7.15 (5.59, 9.20) | 11.8 (11.0, 13.1) | 0.002 |
| Evening | 5.45 (3.82, 6.16) | 4.02 (3.13, 5.67) | 6.97 (6.11, 10.2) | 0.002 |
| Area Under the Curve | 192 (146, 263) | 176 (138, 211) | 275 (210, 307) | 0.033 |
| Interleukin-6 (pg/mL) | 29.2 (15.6, 92.8) | 28.8 (15.5, 85.2) | 43.0 (15.9, 247.5) | 0.658 |
| Questionnaires | ||||
| n | 25 | 17 | 8 | <0.001 |
| Geriatric Depression Scale (Short-form) (Range: 0–15) | 3.00 (2.00, 4.50) | 3.00 (1.50, 4.50) | 3.00 (2.25, 4.50) | 0.509 |
| Health-related Quality of Life (Range: 28–112) | 91.5 (80.3, 95.8) | 94.0 (81.0, 96.5) | 90.0 (70.0, 92.0) | 0.098 |
| Health-related Quality of Life (Proxy) (Range: 31–124) | 94.4±16.0 | 94.5±15.8 | 94.0±17.3 | 0.940 |
| Mini Addenbrooke’s Cognitive Examination (Range: 0–30) | 10.5±7.85 | 11.2±8.17 | 9.00±7.42 | 0.519 |
| Attention (Range 0–4) | 1.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | 0.00 (0.00, 2.00) | 0.455 |
| Memory (Range: 0–7) | 5.00 (0.00, 7.00) | 5.00 (1.00, 7.00) | 4.50 (0.00, 6.75) | 0.546 |
| Verbal Fluency (Animals) (Range: 0–7) | 2.00 (0.00, 3.00) | 2.00 (0.00, 4.00) | 1.50 (0.00, 2.75) | 0.716 |
| Clock Drawing (Range: 0–5) | 2.00 (1.00, 4.00) | 3.00 (1.00, 4.25) | 2.00 (0.25, 3.50) | 0.317 |
| Memory Recall (Range: 0–7) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.00 (0.00, 0.00) | 1.00 |
*Lower Score indicates higher functional independence. Continuous normally distributed variables are presented as mean±standard deviation and not normally distributed variables are displayed as median (1st, 3rd quartile). Outcomes were considered to be statistically significant at p < 0.05.
Salivary cortisol at waking and pre-breakfast and IL-6 levels did not differ between males and females at baseline (n = 22) (all, p > 0.05). However, males had higher M3 (z = –3.096, p = 0.002), E (z = –3.097, p = 0.002), and AUCG (z = –2.138, p = 0.033). No differences were observed at baseline for the DEMQOL, DEMQOL-Proxy, GDS, M-ACE, and all subdomains of the M-ACE between females and males (all, ps > 0.05). Participants reported moderate depressive symptoms on the GDS and moderate to high scores in both the DEMQOL and DEMQOL-Carer, indicating a general contentedness with their overall QoL. Participants scores on the M-ACE were indicative of cognitive functioning typical of moderate cognitive symptoms of dementia.
Physiological outcomes
Twenty-two of the 25 participants completing the study protocol provided twelve viable saliva samples. Two participants were excluded due to not providing enough sample during the baseline collection, and one participant was ill. The results are presented in Table 2. The raw salivary cortisol levels followed a similar pattern at M1 and M2, increasing from baseline to post-intervention, and decreasing at follow-up (both, p = 0.057). No changes at M3 or E or with IL-6 were observed across timepoints (all, ps > 0.05). We calculated both the M1/E and M2/E ratios due to non-significant increases in the raw M1 and M2 salivary cortisol levels, and decreases in E levels observed between baseline and post-intervention. A significant effect of time was observed for the M1/E ratio (χ2(2) = 8.273, p = 0.016). The M1/E ratio increased from baseline to post-intervention (z = –2.549, p = 0.033), indicating a more dynamic diurnal salivary cortisol rhythm at post-intervention. The AUCG did not change (p = 0.554). Handgrip strength (n = 25) measurements did not differ across timepoints (p = 0.07) (Table 3).
Table 2
Physiological outcomes (n = 22)
| Baseline | Post | Follow-up | p | p1 | p2 | p3 | |
| Cortisol (nmol/L) | |||||||
| M1 - Upon waking | 8.99 (7.46, 14.3) | 12.4 (9.92, 15.8) | 9.99 (7.56, 12.7) | 0.057 | |||
| M2 - 30 min after waking | 10.9 (9.01, 14.3) | 12.6 (10.9, 16.5) | 10.0 (7.88, 13.9) | 0.057 | |||
| M3 - 60 min post-breakfast | 8.33 (5.85, 11.3) | 7.94 (6.22, 10.2) | 9.59 (7.11, 12.3) | 0.664 | |||
| E - 45 min post-dinner | 5.45 (3.82, 6.16) | 4.43 (2.81, 5.46) | 4.43 (3.53, 6.94) | 0.170 | |||
| M1 to E Ratio | 1.35 (1.19, 1.63) | 1.72 (1.54, 1.96) | 1.44 (1.22, 1.79) | 0.016 | 0.033 | 0.060 | 1.00 |
| M2 to E Ratio | 1.45 (1.25, 1.85) | 1.73 (1.58, 2.04) | 1.52 (1.28, 1.80) | 0.113 | |||
| AUCG | 192 (146, 263) | 213 (180, 258) | 199 (165, 259) | 0.554 | |||
| Interleukin-6 (pg/mL) | 29.2 (15.6, 92.8) | 28.4 (15.5, 48.7) | 24.8 (11.5, 68.4) | 0.664 |
AUC, Area Under Curve. Not normally distributed variables are displayed as median (1st, 3rd quartile). Categorical variables = p1: between “Baseline” and “Post” timepoints, p2: between “Post” and “Follow-up” timepoints, p3: between “Baseline” and “Follow-up” timepoints. Outcomes were considered to be statistically significant at p < 0.05. p values for p1, p2, and p3 are corrected for the inflation of Type-I error with the Bonferroni rule.
Table 3
Questionnaire outcomes and handgrip strength (n = 25)
| Range | Baseline | Post | Follow-up | p | p1 | p2 | p3 | |
| Geriatric Depression Scale (Short-form) | 0–15 | 3.00 (2.00, 4.50) | 2.00 (1.00, 2.00) | 3.00 (1.00, 4.00) | 0.002 | 0.015 | 0.021 | 1.00 |
| Health-related Quality of Life (n = 24) | 28–112 | 91.5 (80.3, 95.8) | 101 (92.5, 104) | 98.5 (86.0, 102) | <0.001 | <0.001 | 0.072 | 0.101 |
| Health-related Quality of Life (Proxy) | 31–124 | 94.4±16.0 | 101±9.59 | 95.5±14.2 | 0.013 | 0.076 | 0.042 | 1.00 |
| Mini Addenbrooke’s Cognitive Examination | 0–30 | 9.50 (3.00, 17.5) | 14.0 (9.00, 19.0) | 12.0 (8.00, 18.8) | <0.001 | <0.001 | 0.045 | 0.036 |
| Attention | 0–4 | 1.00 (0.00, 2.00) | 1.00 (0.00, 3.00) | 1.00 (0.00, 3.00) | 0.212 | |||
| Memory | 0–7 | 5.00 (0.00, 7.00) | 6.00 (5.00, 7.00) | 6.00 (4.00, 7.00) | 0.021 | 0.009 | 0.333 | 0.117 |
| Verbal Fluency (Animals) | 0–7 | 2.00 (0.00, 3.00) | 2.00 (0.500, 4.00) | 2.00 (0.00, 3.00) | 0.015 | 0.027 | 0.021 | 1.00 |
| Clock Drawing | 0–5 | 2.00 (1.00, 4.00) | 3.00 (1.00, 5.00) | 3.50 (1.50, 4.25) | 0.313 | |||
| Memory Recall | 0–7 | 0.00 (0.00, 1.00) | 0.00 (0.00, 2.00) | 0.00 (0.00, 2.50) | 0.290 | |||
| Hand Grip Strength (kg) | 16.3±6.83 | 17.4±6.89 | 16.1±6.34 | 0.070 |
Continuous normally distributed variables are presented as mean±standard deviation and not normally distributed variables are displayed as median (1st, 3rd quartile). Categorical variables = p1: between “Baseline” and “Post” timepoints, p2: between “Post” and “Follow-up” timepoints, p3: between “Baseline” and “Follow-up” timepoints. Outcomes were considered to be statistically significant at p < 0.05. p values for p1, p2, and p3 are corrected for the inflation of Type-I error with the Bonferroni rule.
Symptoms of depression and quality of life
Changes in symptoms of depression, QoL, and cognitive function were observed between baseline and post-intervention (n = 25) (Table 3). Symptoms of depression measured by the GDS-SF were ranked differently across timepoints (χ2 = 12.2, p = 0.002), decreasing between baseline and post-intervention (z = –2.822, p = 0.015), and returning to baseline levels at follow-up (z = –2.689, p = 0.021). Rankings of QoL varied significantly for both the DEMQOL (χ2 = 18.8, n = 24, p < 0.001) and DEMQOL-Carer (F(2,48) = 4.74, p = 0.013). However, only higher scores in the DEMQOL between baseline and post-intervention (z = –3.845, p < 0.001), and lower scores between post-intervention and follow-up, were observed (z = –2.264, p = 0.042).
Cognitive function
Cognitive function, measured by the M-ACE, varied across timepoints (χ2 = 18.3, p < 0.001), improving between baseline and post-intervention (z = –3.657, p < 0.001), and decreasing between post-intervention and follow-up (z = –2.438, p = 0.045). In subdomains of memory (immediate recall) and verbal fluency (animals), scores varied between timepoints (χ2 = 7.72, p < 0.021; χ2 = 8.37, p < 0.015, respectively). Memory (immediate recall) improved between baseline and post-intervention (z = –2.995, p = 0.009). The verbal fluency (animals) score also increased from baseline to post-intervention (z = –2.601, p = 0.027), and decreased from post-intervention to follow-up (z = –2.693, p = 0.021). No differences in attention, visuospatial ability (clock drawing), or memory (delayed recall) were observed (all, ps > 0.05).
General wellbeing
The six-item GWQ (scored /30) was administered to participants (n = 25) following each visit to the NGA program during weeks 1, 3, and 6. A one-way repeated measures ANOVA results indicated wellbeing was different across timepoints (F(2,48) = 6.53, p = 0.007). Pairwise comparisons revealed GWQ scores increased from week 1 (21.1(±4.32)) to week 3 (23.5(±4.29)) (t = –2.750, p = 0.033) and were maintained at week 6 (23.5(±4.43)) (p = 0.932).
Behavioral observations and exit questionnaire
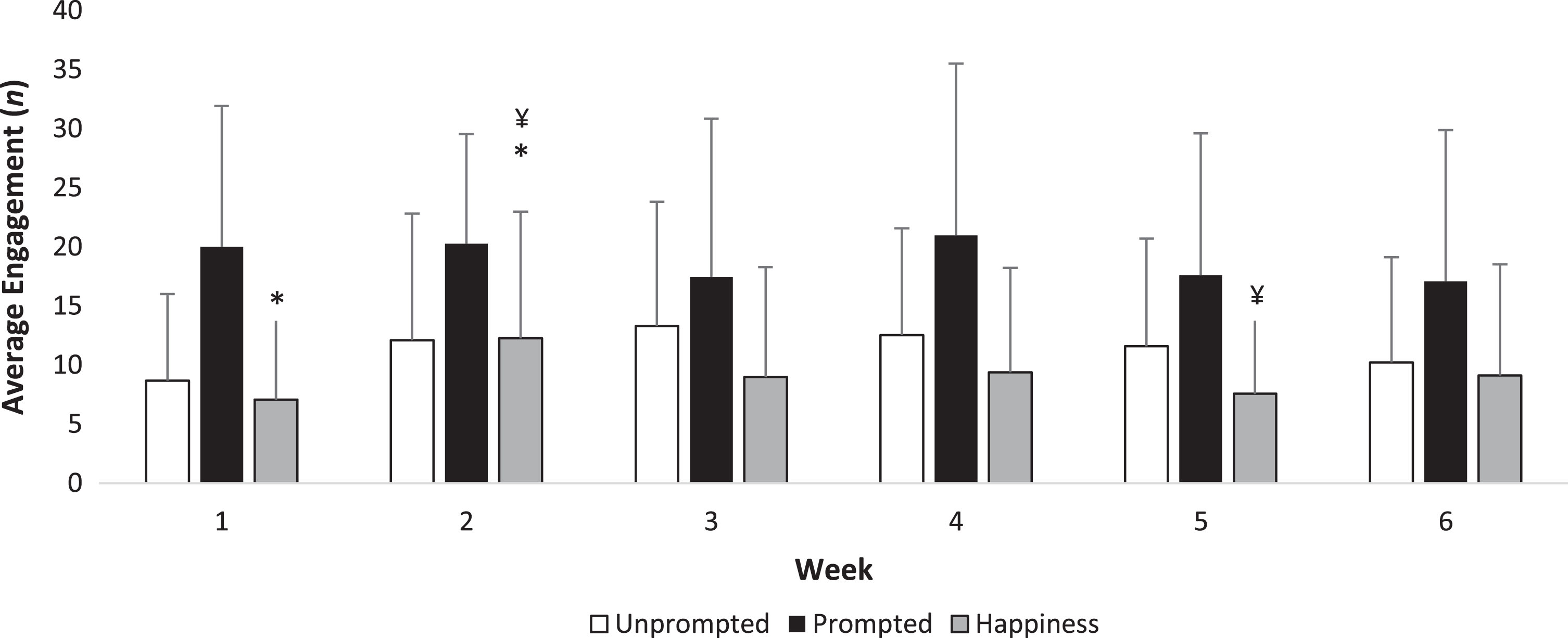
One researcher collected observations at the NGA at all 30 visits and cross validation was performed at 15 visits by another researcher (Fig. 2 and Supplementary Table 2). Agreement between the observers was rated as fair using Cohen’s κ analysis (κ= 0.282, p < 0.001). No scores differed across the six weeks for unprompted discussion, prompted discussion, sleeping and negative emotions (all, ps > 0.05). Expressions of happiness and laughter increased (χ2 = 18.3, p = 0.013) between week one and two (z = –2.797, p = 0.015) and decreased in week five compared with week two (z = –2.845, p = 0.012).
Fig.2
Weekly engagement at the art gallery. Mean±standard deviation are presented. *p = 0.015; ¥p = 0.012. Outcomes were considered to be statistically significant at p < 0.05. p values are corrected for the inflation of Type-I error with the Bonferroni rule.
In total, twelve participants (48.0%) remembered the visits at follow-up and completed the exit questionnaire. Participants rated the visits as “Extremely memorable” (33.3%), “Very memorable” (50.0%), “Neutral” (8.33%), and “Slightly memorable” (8.33%). The same participants looked forward to attending the NGA, rated as “Extremely” (66.6%), “Very” (25.0%), and “Neutral” (8.33%). On a scale of 1–10, with one being horrible and ten being wonderful, the twelve participants rated their experience on average as 8.12(±1.95) out of a possible 10.0. The study partners were asked if they found the intervention to be beneficial to the participants. They responded with “Extremely beneficial” (24.0%), “Very beneficial” (48.0%), “Neutral” (12.0%), “Slightly beneficial” (8.00%), and “Unsure” (8.00%).
DISCUSSION
The findings of this study propose that attending an Art and Dementia program at the NGA may have several measurable benefits. The most novel finding was a change in the M1/E salivary cortisol ratio, indicating that HPA-axis function may be altered following engagement in a specific and tailored program for PLWD. Depressive symptoms, self-reported QoL, and cognitive function also improved between baseline and post-intervention, but no differences between timepoints were found for IL-6, the QoL carer measure, and handgrip strength. Behavioral observations of the participants revealed increased happiness and laughter between week one and two suggesting that participants acclimatized quickly to the new experience and sustained engagement across the intervention period. However, the study design, including the absence of a control group and possible selection bias, limit the overall generalizability of the results.
Salivary cortisol has emerged as a marker of psychosocial stress as cortisol is associated with impaired cognition, wellbeing, and inflammatory responses [9–11], as it is a surrogate for free serum cortisol levels due to a very good correlation [33]. To our knowledge, the present study is the second to measure the salivary cortisol in response to an art intervention in PLWD [18]. However, previous studies have investigated the salivary stress response in music interventions of older people [5]. To detect a meaningful change in salivary cortisol levels, it is important to measure the response in the diurnal cortisol rhythm which is resistant to acute changes and characterized by a negative slope initiated by peaking morning cortisol levels and a subsequent decline throughout the day [11]. Our findings suggest the intervention resulted in a more dynamic salivary cortisol rhythm represented through an increased M1/E ratio. The ratio of the evening to morning salivary cortisol is highest in PLWD with both depression and dementia, compared with either condition alone [34]. This aligns with our findings both with the M1/E ratio and reduced depressive symptoms following the intervention. A low morning to evening ratio suggests flattening of the diurnal cortisol variation, which is associated with inflammation and functional limitations during aging [35], and poorer mental and physical health outcomes [36]. In PLWD, a flat diurnal cortisol rhythm is associated with sundowning, frailty, cognitive impairment, and decreased resiliency [11, 37]. Therefore, psychosocial interventions which can lead to less flattening of the diurnal cortisol rhythm can potentially improve a broad range of outcomes to promote healthier aging of PLWD.
Several studies have reported associations between higher cortisol levels, decreased cognitive performance, and the pathophysiology of AD [7, 38]. However, these studies measured cortisol at one-time point, and higher cortisol has also been associated with a decreased rate of cognitive decline in individuals with mild cognitive impairment [39]. Cortisol has beneficial effects, and diurnal variation promotes synaptic formation and improves the adaptive immune function [40, 41]. A potential mechanistic explanation for the changes observed in the M1/E ratio, increased morning cortisol levels, and cognitive performance, involves higher binding of cortisol to the mineralocorticoid receptors in the brain. Impaired mineralocorticoid receptor function is related to the aging process, inhibiting HPA-axis activity via receptors in the hippocampus. Activation of these receptors is associated with positive effects on cognitive performance, but only until glucocorticoid receptors are activated, which occurs once mineralocorticoid receptors are saturated [38]. Glucocorticoid receptor activation occurs when cortisol levels are chronically elevated and lead to detrimental effects on cognitive performance, particularly in executive functioning [42], and cause alterations in hippocampal functioning [43]. Excess cortisol can be damaging, particularly if elevated across the day as indicated by a flat diurnal cortisol rhythm, however, cortisol reactivity and an ability to respond to stressors or inflammation are important to promote resiliency and recovery [41]. Higher mineralocorticoid activity in the brain may also reverse depressive symptoms [44]. Participants may have benefitted from several aspects of the intervention, such as leaving their residence, and the increased social engagement and interaction. A controlled trial measuring cortisol may help clarify the importance of the change in the diurnal cortisol rhythm relative to the NGA program.
Additional benefits were observed following engagement in the intervention. Symptoms of depression decreased, and laughter and smiling were consistently observed during the visits to the NGA. In healthy older adults, loneliness has been associated with HPA-axis dysfunction, poorer cognitive function and higher evening cortisol levels [45], and we have previously mentioned the association between depression in PLWD and the diurnal cortisol rhythm [34]. The QoL in our sample was improved; however, while the QoL carer results also suggested improvements, this was not statistically significant, weakening the self-reported finding. Previous QoL findings of similar interventions are inconsistent. Both interventions, in a trial of 59 people with mild AD randomized to either twelve weeks of singing or painting, improved QoL, mood, and some cognitive abilities while reducing depressive symptoms in only the painting group [46]. A larger three-month study involving the visual arts and art-making found improvements in only the DEMQOL carer measure and not for PLWD; however, this was in contrast to wellbeing measures and a qualitative analysis which revealed improved social connectedness and inner-strength [47]. Similarly, DEMQOL results did not improve in a study of twelve PLWD and their carers who participated in eight art-viewing and art-making sessions at two different sites [48]. However, in this study, the thematic analysis revealed benefits due to social inclusion and self-reported cognitive benefits. Positive changes in mood and sociability have also been observed over 40 weeks in a multi-center study comparing use of art therapy with normal recreational activities [49].
Several factors enriched the experience of the NGA visits for the participants. The art was selected to transition the participants into art and art discussion. After a few weeks of attendance, once they felt more confident with art-related terms, the gallery and the educators, participants were challenged with new ideas, new art and different cultural terms and experiences (Supplementary Table 1). By design, it was expected the group would feel safer to talk about color, composition, and the artists’ ideas each week. The form of art differed each week and included a mix of paintings, posters, textiles, sculpture, and lamps. The size of the art also changed throughout, from very large works of art to quite small in some instances. The changes were designed and intended to stimulate new thoughts and perceptions of art and to encourage different types of discussion. The art was also intended to be relatable to the age of the participants. For example, “The Music Lesson” by Alexandra Exter was relatable to participants as many in their generation learned to play a musical instrument. All educators at the NGA are trained to facilitate groups with PLWD, and it is plausible that the reduction in depressive symptoms was influenced by the educators who were sensitive to individuals needs while still promoting interaction and engagement between group members. Moreover, the NGA holds an aura of prestige that may not be evident at other art galleries. Participants are made to feel valued when entering the large and open gallery and integrate seamlessly with other patrons, and visits were conducted on weekdays where the NGA is neither empty nor busy.
Limitations
The present study has some limitations. Due to the population under investigation, it is possible that some saliva collection protocols were not followed precisely, such as brushing of teeth while unsupervised. Groups One to Four were collected with a researcher and care staff supervising. However, it was not possible to confirm that all participants followed the protocol precisely. The present study collected passive drool samples despite previous studies recommending the use of oral swab collection methods. In the pilot and feasibility study of PLWD by Bourne et al. (2019), the researchers had difficulty with using oral swabs due to chewing and recommended passive drool sample collection in future studies to facilitate greater volume for analysis. Indeed, the present study excluded two participants from the saliva analysis due to insufficient volumes provided. As such, recommendations have been made to conduct diurnal cortisol sampling across three consecutive days for the most reliable results [50]; however, the practicality with PLWD is questionable. Our sampling schedule did not include an afternoon or pre-dinner sample, meaning the potential presence of sundowning could not be evaluated. We did not ask participants about their medication regime, nor did we exclude participants based on their medications, as it is expected the majority of participants receive pharmacological treatment, which may have included glucocorticoids. We also requested study partners to complete an events diary to assess stress-related confounders. However, several study partners did not provide comprehensive information, and we could not report this data.
The study is also limited by the lack of a control group, potential selection bias, and the use of self-report measures in this population. Recruitment was challenging due to the requirement that all participants possess a formal dementia diagnosis, despite four of the five facilities being high care. The majority of participants were identified by recreational officers based on whether they would respond to the intervention by cheering them up and give them something to do, and as such, selection bias was likely. There are also inherent problems with self-report measures, especially QoL questionnaires. The DEMQOL is widely used due to its proxy version; however, other measures have shown greater reliability and validity for PLWD [51]. Some participants and their study partners responded as not concerned with their cognitive function, and as such, may have received higher scores in the DEMQOL. Also, a learning effect may have influenced results of the M-ACE irrespective of the multiple versions administered. Finally, while the GDS-SF is widely used with PLWD, its reliability is questionable for people with more severe dementia. Despite these limitations, the novel and exploratory nature of the study represent an important step towards more robust and rigorous, suitably powered trials inclusive of biological outcomes.
Implications and future directions
Wider implementation of art-based programs for PLWD holds the potential to benefit a wide range of people, including carers, with additional social and economic benefits [4]. While non-pharmacological interventions for PLWD are acknowledged as an important area of research, they are yet to be prioritized by policymakers and government officials. Art contributes to every aspect of society, from strengthening communities to promoting individual mental health. According to the Australia Council for the Arts, in 2012-2013, the arts contributed $4.2 billion to the national gross domestic product, but only received $1.3 billion in government support with the majority of operating income coming from consumer spending. Investment to enable greater use of public spaces is warranted to provide programs for PLWD which may help reduce stigma, promote social integration, and provide a social return on investment [4], particularly as more people in Australia are being diagnosed with dementia [52]. On a global scale, the arts should be encouraged as low-cost non-invasive interventions for PLWD, which should be embraced by community organizations and within dementia care. While acceptance of the potential for multidisciplinary rehabilitation in dementia care has several barriers [53], the present study supports a potential beneficial role of museum and art gallery-based programs in prolonging the QoL of PLWD within the usual care pathway. Art- and music-based interventions for PLWD may offer similar benefits to pharmacological treatments such as anti-depressants, and more studies with over 100 participants are required despite inherent methodological barriers [54, 55].
Several additional measures may provide more in-depth insight into benefits that can be attributed to Art and Dementia programs, in particular, greater use of proxy-rated measures. The present study did not measure levels of anxiety or apathy. Anxiety has been identified as an under-researched outcome in nonpharmacological interventions for PLWD in nursing homes [56]. While carer burden has been measured previously for carers participating with PLWD in an art gallery intervention [48], an evaluation of respite received when not attending the NGA would have been valuable. For example, anecdotal reports from study partners suggested behavioral disturbances were not as frequent during the intervention period. Heart-rate variability also represents another valid non-invasive method to receive biofeedback and provides a more convenient data collection method than saliva [5, 18]. However, biomarker-based evaluation of arts-based interventions for PLWD is still a relatively new and evolving concept which can serve to complement qualitative and mixed-methods research [5]. Research of this nature is inherently complex. Future research may focus on consultation with PLWD to determine the type of artworks that are considered interesting for group programs. Further evaluation of associations between biomarkers and individual reactions through video analysis may identify the most appropriate types of art to be enjoyable and engaging for PLWD. While personal preferences and potential for reminiscence will vary when viewing art, future research may seek to understand common types of art which promote engagement. A greater understanding of the differences between types of dementia and reactions to these experiences should also be pursued [5]. Finally, art gallery and museum-based experiences for PLWD are only available to those living within the vicinity of these institutions who can safely travel. Further research and program development are required to facilitate art-based programs into aged care communities and rural areas, potentially using assistive technology [57].
ACKNOWLEDGMENTS
Nathan M D’Cunha is supported by a PhD scholarship awarded by the Dementia Australia Research Foundation and the Australian Government Research Training program. The laboratory analysis was supported by an Australian Association of Gerontology R.M. Gibson Research Fund Award.
The authors would like to thank the participants for their enthusiasm, time, and commitment to the research project. We would also like to thank the NGA and the long-running and globally recognized NGA Art and Dementia program for supporting the delivery of the groups for the research. In particular, the study would not have been possible without the tireless work of Adriane Boag, who organized and facilitated the groups. We want to thank the NGA group educators Margie Kevin, John Carey, Penelope Low, Anne-Marie Turner, and volunteers Marianela Aguilera and Brit Helgeby. The authors would like to acknowledge the cooperation and support of BaptistCare, Illawarra Retirement Trust Group, Southern Cross Care, Villaggio S’ant Antonio, and St Andrew’s Village. The study would also like to acknowledge the energy, kindness, and time commitment provided by recreational officers and activities managers Jill Segaert, Maria Tallon, Rebecca Luongo, Peter Stevenson, Vicki Hackett, Rosie Clince, and Bev Webb. We would also like to thank Stephanie Mulhall, Michelle Minehan, Daniela Castro de Jong, and Ian Drayton for their insights towards the project. Lastly, the authors would like to thank Abdeljalil Lahiouel and Kelly Ng for their assistance with data collection.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0784r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-190784.
REFERENCES
[1] | Cousins E , Tischler V , Garabedian C , Dening T ((2019) ) A taxonomy of arts interventions for people with dementia. Gerontologist, doi: https://doi.org/10.1093/geront/gnz024 |
[2] | Halpin-Healy C ((2017) ) Well-chosen objects support well-being for people with dementia and their care partners. J Museum Edu 42: , 224–235. |
[3] | Camic PM , Baker EL , Tischler V ((2015) ) theorizing how art gallery interventions impact people with dementia and their caregivers. Gerontologist 56: , 1033–1041. |
[4] | Jones C , Windle G , Edwards RT ((2018) ) Dementia and imagination: A social return on investment analysis framework for art activities for people living with dementia. Gerontologist, doi: https://doi.org/10.1093/geront/gny147 |
[5] | Thomas GEC , Crutch SJ , Camic PM ((2018) ) Measuring physiological responses to the arts in people with a dementia. Int J Psychophysiol 123: , 64–73. |
[6] | Echouffo-Tcheugui JB , Conner SC , Himali JJ , Maillard P , DeCarli CS , Beiser AS , Vasan RS , Seshadri S ((2018) ) Circulating cortisol and cognitive and structural brain measures. Neurology 91: , e1961–e1970. |
[7] | Pietrzak RH , Laws SM , Lim YY , Bender SJ , Porter T , Doecke J , Ames D , Fowler C , Masters CL , Milicic L , Rainey-Smith S , Villemagne VL , Rowe CC , Martins RN , Maruff P ((2017) ) Plasma cortisol, brain amyloid-β, and cognitive decline in preclinical Alzheimer’s disease: A 6-year prospective cohort study. Biol Psychiatry Cogn Neurosci Neuroimaging 2: , 45–52. |
[8] | Jaroudi W , Garami J , Garrido S , Hornberger M , Keri S , Moustafa AA ((2017) ) Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev Neurosci 28: , 705–714. |
[9] | Savla J ((2018) ) Salivary cortisol: Is it still the canary in the coal mine? J Gerontol B Psychol Sci Soc Sci 73: , 435–436. |
[10] | Sindi S , Holleman J , Enstedt S , Kåreholt I , Kivipelto M , Solomon A ((2017) ) Salivary cortisol, Alzheimer’s disease biomarkers and cognition among memory clinic patients. Psychoneuroendocrinology 83: , 50. |
[11] | Kovach CR , Woods DL , Logan BR , Raff H ((2011) ) Diurnal variation of cortisol in people with dementia: Relationship to cognition and illness burden. Am J Alzheimers Dis Other Demen 26: , 145–150. |
[12] | Kinney JW , Bemiller SM , Murtishaw AS , Leisgang AM , Salazar AM , Lamb BT ((2018) ) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement 4: , 575–590. |
[13] | Forloni G , Balducci C ((2018) ) Alzheimer’s disease, oligomers, and inflammation. J Alzheimers Dis 62: , 1261–1276. |
[14] | Singh-Manoux A , Dugravot A , Brunner E , Kumari M , Shipley M , Elbaz A , Kivimaki M ((2014) ) Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 83: , 486–493. |
[15] | Ng A , Tam WW , Zhang MW , Ho CS , Husain SF , McIntyre RS , Ho RC ((2018) ) IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: Systematic review and meta-analysis. Sci Rep 8: , 12050. |
[16] | Maes M , Scharpe S , Meltzer HY , Bosmans E , Suy E , Calabrese J , Cosyns P ((1993) ) Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res 49: , 11–27. |
[17] | Kaimal G , Ray K , Muniz J ((2016) ) Reduction of cortisol levels and participants’ responses following art making. Art Ther 33: , 74–80. |
[18] | Bourne P , Camic P , Crutch S , Hulbert S , Firth N , Harding E , Created Out of Mind team ((2019) ) Using psychological and physiological measures in arts-based activities in a community sample of people with a dementia and their caregivers: A feasibility and pilot study. J Aging Stud Ther 1: . doi: 10.16966/jast.102 |
[19] | Rosenberg F ((2009) ) The MoMA Alzheimer’s Project: Programming and resources for making art accessible to people with Alzheimer’s disease and their caregivers. Arts Health 1: , 93–97. |
[20] | MacPherson S , Bird M , Anderson K , Davis T , Blair A ((2009) ) An art gallery access programme for people with dementia: ‘You do it for the moment’. Aging Ment Health 13: , 744–752. |
[21] | Bhattarai KR , Kim HR , Chae HJ ((2018) ) Compliance with saliva collection protocol in healthy volunteers: Strategies for managing risk and errors. Int J Med Sci 15: , 823–831. |
[22] | Bucks RS , Ashworth DL , Wilcock GK , Siegfried K ((1996) ) Assessment of activities of daily living in dementia: Development of the Bristol Activities of Daily Living Scale. Age Ageing 25: , 113–120. |
[23] | Smith SC , Lamping DL , Banerjee S , Harwood R , Foley B , Smith P , Cook JC , Murray J , Prince M , Levin E , Mann A , Knapp M ((2005) ) Measurement of health-related quality of life for people with dementia: Development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 9: , 1–93, iii-iv. |
[24] | Yesavage JA , Sheikh JI ((1986) ) Geriatric Depression Scale (GDS). Clin Gerontol 5: , 165–173. |
[25] | Hsieh S , McGrory S , Leslie F , Dawson K , Ahmed S , Butler CR , Rowe JB , Mioshi E , Hodges JR ((2015) ) The Mini-Addenbrooke’s Cognitive Examination: A new assessment tool for dementia. Dement Geriatr Cogn Disord 39: , 1–11. |
[26] | Norman K , Stobäus N , Gonzalez MC , Schulzke J-D , Pirlich M ((2011) ) Hand grip strength: Outcome predictor and marker of nutritional status. Clin Nutr 30: , 135–142. |
[27] | Thomson LJ , Chatterjee HJ ((2013) ) UCL museum wellbeing measures toolkit. Arts & Humanities Research Council, London. |
[28] | Warth M , Koehler F , Weber M , Bardenheuer HJ , Ditzen B , Kessler J ((2019) ) “Song of Life (SOL)” study protocol: A multicenter, randomized trial on the emotional, spiritual, and psychobiological effects of music therapy in palliative care. BMC Palliat Care 18: , 14. |
[29] | McKune AJ , Bach CW , Semple SJ , Dyer BJ ((2014) ) Salivary cortisol and α-amylase responses to repeated bouts of downhill running. Am J Hum Biol 26: , 850–855. |
[30] | Windle G , Newman A , Burholt V , Woods B , Brien D , Baber M , Hounsome B , Parkinson C , Tischler V ((2016) ) Dementia and Imagination: A mixed-methods protocol for arts and science research. BMJ Open 6: , e011634. |
[31] | Fekedulegn DB , Andrew ME , Burchfiel CM , Violanti JM , Hartley TA , Charles LE , Miller DB ((2007) ) Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med 69: , 651–659. |
[32] | Altman DG ((1999) ) Practical statistics for medical research, Chapman & Hall/CRC Press, New York. |
[33] | Estrada-Y-Martin RM , Orlander PR ((2011) ) Salivary cortisol can replace free serum cortisol measurements in patients with septic shock. Chest 140: , 1216–1222. |
[34] | Barca ML , Eldholm RS , Persson K , Bjorklof GH , Borza T , Telenius E , Knapskog AB , Braekhus A , Saltvedt I , Selbaek G , Engedal K ((2018) ) Cortisol levels among older people with and without depression and dementia. Int Psychogeriatr 31: , 1–5. |
[35] | Piazza JR , Dmitrieva NO , Charles ST , Almeida DM , Orona GA ((2018) ) Diurnal cortisol profiles, inflammation, and functional limitations in aging: Findings from the MIDUS study. Health Psychol 37: , 839–849. |
[36] | Adam EK , Quinn ME , Tavernier R , McQuillan MT , Dahlke KA , Gilbert KE ((2017) ) Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 83: , 25–41. |
[37] | Venturelli M , Sollima A , Ce E , Limonta E , Bisconti AV , Brasioli A , Muti E , Esposito F ((2016) ) Effectiveness of exercise- and cognitive-based treatments on salivary cortisol levels and sundowning syndrome symptoms in patients with Alzheimer’s disease. J Alzheimers Dis 53: , 1631–1640. |
[38] | Ouanes S , Popp J ((2019) ) High cortisol and the risk of dementia and Alzheimer’s disease: A review of the literature. Front Aging Neurosci 11: , 43. |
[39] | Peavy GM , Salmon DP , Jacobson MW , Hervey A , Gamst AC , Wolfson T , Patterson TL , Goldman S , Mills PJ , Khandrika S , Galasko D ((2009) ) Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am J Psychiatry 166: , 1384–1391. |
[40] | Clow A , Thorn L , Evans P , Hucklebridge F ((2004) ) The awakening cortisol response: Methodological issues and significance. Stress 7: , 29–37. |
[41] | McEwen BS ((2019) ) What is the confusion with cortisol? Chronic Stress 3: , 2470547019833647. |
[42] | Lupien SJ , Maheu F , Tu M , Fiocco A , Schramek TE ((2007) ) The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65: , 209–237. |
[43] | Kim EJ , Pellman B , Kim JJ ((2015) ) Stress effects on the hippocampus: A critical review. Learn Memory 22: , 411–416. |
[44] | ter Heegde F , De Rijk RH , Vinkers CH ((2015) ) The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 52: , 92–110. |
[45] | Montoliu T , Hidalgo V , Salvador A ((2019) ) The relationship between loneliness and cognition in healthy older men and women: The role of cortisol. Psychoneuroendocrinology 107: , 270–279. |
[46] | Pongan E , Tillmann B , Leveque Y , Trombert B , Getenet JC , Auguste N , Dauphinot V , El Haouari H , Navez M , Dorey JM , Krolak-Salmon P , Laurent B , Rouch I ((2017) ) Can musical or painting interventions improve chronic pain, mood, quality of life, and cognition in patients with mild Alzheimer’s disease? Evidence from a randomized controlled trial. J Alzheimers Dis 60: , 663–677. |
[47] | Windle G , Gregory S , Howson-Griffiths T , Newman A , O’Brien D , Goulding A ((2017) ) Exploring the theoretical foundations of visual art programmes for people living with dementia. Dementia 17: , 702–727. |
[48] | Camic PM , Tischler V , Pearman CH ((2014) ) Viewing and making art together: A multi-session art-gallery-based intervention for people with dementia and their carers. Aging Ment Health 18: , 161–168. |
[49] | Rusted J , Sheppard L , Waller D ((2006) ) A Multi-centre randomized control group trial on the use of art therapy for older people with dementia. Group Analysis 39: , 517–536. |
[50] | Hulett JM , Fessele KL , Clayton MF , Eaton LH ((2019) ) Rigor and reproducibility: A systematic review of salivary cortisol sampling and reporting parameters used in cancer survivorship research. Biol Res Nurs 21: , 318–334. |
[51] | Li L , Nguyen K-H , Comans T , Scuffham P ((2018) ) Utility-based instruments for people with dementia: A systematic review and meta-regression analysis. Value Health 21: , 471–481. |
[52] | Dementia Australia, Dementia statistics: Key facts and statistics, https://www.dementia.org.au/statistics, Last updated April 2019, Accessed on August 29, 2019. |
[53] | Cations M , May N , Crotty M , Low L-F , Clemson L , Whitehead C , McLoughlin J , Swaffer K , Laver KE ((2019) ) Health professional perspectives on rehabilitation for people with dementia. Gerontologist, doi: https://doi.org/10.1093/geront/gnz007 |
[54] | de Medeiros K , Basting A ((2014) ) Shall I compare thee to a dose of donepezil?: Cultural arts interventions in dementia care research. Gerontologist 54: , 344–353. |
[55] | Garrido S , Dunne L , Chang E , Perz J , Stevens CJ , Haertsch M ((2017) ) The use of music playlists for people with dementia: A critical synthesis. J Alzheimers Dis 60: , 1129–1142. |
[56] | Brown Wilson C , Arendt L , Nguyen M , Scott TL , Neville CC , Pachana NA ((2019) ) Nonpharmacological interventions for anxiety and dementia in nursing homes: A systematic review. Gerontologist, doi: https://doi.org/10.1093/geront/gnz020 |
[57] | D’Cunha NM , Nguyen D , Naumovski N , McKune AJ , Kellett J , Georgousopoulou EN , Frost J , Isbel S ((2019) ) A mini-review of virtual reality-based interventions to promote well-being for people living with dementia and mild cognitive impairment. Gerontology 65: , 430–440. |




