Dual-Task Performance and Neurodegeneration: Correlations Between Timed Up-and-Go Dual-Task Test Outcomes and Alzheimer’s Disease Cerebrospinal Fluid Biomarkers
Abstract
Background:
Tools to identify individuals at preclinical stages of dementia disorders are needed to enable early interventions. Alterations in dual-task performance have been detected early in progressive neurodegenerative disorders. Hence, dual-task testing may have the potential to screen for cognitive impairment caused by neurodegeneration. Exploring correlations between dual-task performance and biomarkers of neurodegeneration is therefore of interest.
Objective:
To investigate correlations between Timed Up-and-Go dual-task (TUGdt) outcomes and Alzheimer’s disease (AD) cerebrospinal fluid (CSF) biomarkers amyloid-β 42 (Aβ42), total tau (t-tau), and phosphorylated tau (p-tau).
Methods:
This cross-sectional cohort study included 90 participants (age range 49–84 years) undergoing memory assessment, who were subsequently diagnosed with AD, other dementia disorders, mild cognitive impairment, or subjective cognitive impairment. TUG combined with “Naming Animals” (TUGdt NA) and “Months Backwards” (TUGdt MB), respectively, were used to assess dual-task performance. The number of correct words and time taken to complete the tests were measured. The CSF biomarkers were analysed by ELISA. Spearman’s rank correlation was used for analyses between TUGdt outcomes (TUGdt NA and TUGdt MB), and CSF biomarkers, adjusted for age, gender, and educational level.
Results:
The number of correct words, as well as the number of correct words/10 s during TUGdt NA correlated negatively to CSF t-tau and p-tau. No correlations were found between any time scores and CSF biomarkers.
Conclusion:
The correlations between TUGdt NA and t-tau and p-tau may indicate that neurodegeneration affects dual-task performance. Longitudinal studies are needed to further explore dual-task testing in screening for cognitive impairment due to neurodegeneration.
INTRODUCTION
Walking while simultaneously performing another task (dual-task performance) challenges attentional reserves and executive functions [1]. The two tasks interfere with and compete for brain cortical resources [2], which is why dual-task testing can be regarded as a “brain stress” test [3]. The two most widely accepted models of motor-cognitive interference in the human brain are the central capacity sharing model [4], where limited resources must be distributed between the simultaneously performed tasks, and the bottleneck model [4], which states that certain tasks are impossible to carry out concurrently. In previous studies, dual-task performance has been researched to explore its potential as a screening tool for cognitive impairment [5–7] and its predictive capacity of future cognitive decline and fall risk [8–11]. The tasks used in dual-task testing vary between studies. Most commonly, straight-line walking [12, 13] or the mobility test Timed Up-and-Go (TUG) [7, 14], are combined with a verbal task [15].
Alterations in dual-task performance can be detected early in the progression of the most frequently occurring neurodegenerative disorders; Alzheimer’s disease (AD) and Parkinson’s disease (PD) [8], as well as in mild cognitive impairment (MCI) [6, 16], which can be a precursor of AD [17]. Research has shown that in cohorts of individuals with MCI, 13–16% progress to fulfil the criteria for AD over the course of a year [18, 19]. Additionally, subjective cognitive impairment (SCI) is regarded as a possible forerunner of MCI, with an annual conversion rate of around 7% [20, 21].
Neurodegenerative disorders such as AD and PD are characterized by degenerative processes in the central nervous system, which may be reflected in cerebrospinal fluid (CSF). Specific AD CSF biomarkers can be used to support the diagnosis of AD [17, 22, 23]. The levels of the AD CSF biomarkers amyloid-β 42 (Aβ42), total tau (t-tau), and phosphorylated tau (p-tau) reflect abnormal aggregation of Aβ deposits, neurofibrillary tangles, and axonal neurodegeneration in the brain, respectively [24]. Total tau is regarded as a marker of neurodegeneration, where high concentrations of t-tau rather than a decrease of Aβ42 or an increase of p-tau are associated with a rapid disease decline in patients with MCI due to incipient AD [25]. There are indications that the AD CSF biomarker profile at group level becomes increasingly abnormal in the range from SCI, to MCI, and further to AD [26], while none or minimal changes are seen during the clinical phase of AD [27, 28]. Concentrations of Aβ42 in CSF deviate years or even decades before the appearance of clinical symptoms and then remain stable, while levels of t-tau and p-tau have been reported to increase later in the disease progression [29].
Research on the clinical use of dual-task performance and how to interpret different dual-task test outcomes in neurodegenerative disorders is highly topical [1, 30–32]. However, studies focusing on correlations between dual-task performance and AD CSF biomarkers are still rare. To our knowledge, only one study has explored such associations [14], in which correlations between TUG in combination with counting backwards from 100 by ones (TUGdt C) and AD CSF biomarkers were investigated. The results showed positive correlations between certain TUGdt C outcomes and Aβ42, as well as negative correlations between certain TUGdt C outcomes and t-tau and p-tau in a study population consisting of individuals with mild AD or MCI, and controls.
Hence, dual-task performance seems to be related to neurodegeneration. We therefore hypothesized that there are correlations between TUGdt outcomes and AD CSF biomarkers, which might contribute toward establishing the potential value of the TUGdt test as a simple and non-strenuous screening tool for identifying cognitive impairment caused by neurodegenerative disorders. Our aim with this study was thus to examine the correlations between TUGdt outcomes and AD CSF biomarkers.
MATERIALS AND METHODS
Design and study participants
This cross-sectional study was based on a subgroup of participants from the Uppsala-Dalarna Dementia and Gait (UDDGait) project. Approval was granted from the Regional Ethical Review Board in Uppsala (dnr: 2014/068). Signed, informed consent was attained from all participants during enrollment.
Participants for the present study were consecutively recruited from two specialist clinics for memory assessment in Sweden; Uppsala University Hospital in Uppsala and Falu Hospital in Falun. All patients referred during the periods April 2015 to February 2017 in Uppsala and September 2015 to June 2016 in Falun were eligible for inclusion. The exclusion criteria were: inability to walk three meters back and forth or to rise up from a sitting position, indoor use of a walker, inability to carry out instructions given in Swedish, and current or recent (within the last two weeks) hospitalization. Only patients who, according to the clinical routine, had undergone determination of AD CSF biomarkers were subject to inclusion in the present study, a total of 94. Three individuals who subsequently were diagnosed with diagnoses other than dementia disorders, MCI, or SCI were excluded, as was one individual who discontinued both TUGdt tests, leaving totally 90 individuals for the statistical analyses.
Data collection and data preparation
All participants went through the same assessment procedure at one of the two specialist clinics, before they received any diagnosis. Demographic characteristics were collected orally. A battery of cognitive and motor function assessments was carried out and participants were screened for depressive symptoms. Among the tests conducted, two were used for analyses in the present study: The Verbal Fluency test, in which the participants were asked to name as many different animals as possible during 60 seconds while sitting down [33], and the Mini-Mental State Examination (MMSE), which is the most widely used test for providing an overall measure of cognitive impairment in clinical and research settings [34, 35]. Results from these tests were used to compare levels of correlations between dual-task performance and CSF biomarkers versus correlations between these cognitive tests and CSF biomarkers.
Timed Up-and-Go tests
The TUG test is a well-established clinical test of one mobility sequence: rising from an armchair, walking straight ahead, passing a line marked on the floor, turning around, walking back, and sitting down again. The TUG time score correlates well with gait speed, and is reliable for testing people with and without cognitive impairment [36, 37]. In the current study, TUG was performed first, followed by two TUGdt tests: TUG in combination with naming different animals (TUGdt NA) and TUG in combination with reciting months backwards (TUGdt MB), respectively. The choice of verbal tasks was based on the notion that they should be at or near the threshold of participants’ ability [38]. The verbal tasks were based on two well-established tests of cognitive function: The Verbal Fluency test of naming animals [33] and the Months Backward test [39], in which the subject recites the months of the year in reverse order starting with December, until reaching January or discontinuing. Verbal Fluency tests are among the most widely used measures to assess cognitive functioning following neurological damage [40], as well as one of the most common verbal tasks used in dual-task testing in the AD continuum [12, 41–43]. The Verbal Fluency test of naming animals challenges semantic memory and executive function, such as effortful retrieval [40, 44]. The Months Backward test has not, to our knowledge, been used in previous studies of dual-task performance. Nevertheless, the original Months Backward test relies on working memory, attention, semantic memory, and executive function [39, 45], and has a significant diagnostic classificatory power regarding individuals with mild AD, MCI, and SCI [46].
When performing TUGdt NA, the participants were instructed to name different animals while simultaneously completing TUG. In TUGdt MB, the participants were instructed to recite months in reverse order while completing TUG, starting from the last month of the year and recite backwards until seated again. The participants were instructed to walk at a self-selected, comfortable speed during the TUG and TUGdt tests, and to prioritize walking over the verbal task during TUGdt tests. This was done to enable a standardized test procedure and to reduce the stress of the test for the participants, as they could complete the test without the feeling of failure or bruised pride.
The TUG and TUGdt tests were timed with a stopwatch to an accuracy of 0.01 seconds, from the participant’s rising up (the back leaving the backrest) to sitting down (the posterior touching the seat). They were documented by two cameras, one which was placed in front of and one to the side of the set-up, in order to capture mobility and verbal performance. Using the video recordings, the number of animals recited during the TUGdt NA tests and the number of correct months recited during TUGdt MB were counted. As comprehensive measures of dual-task performance, the average number of correct animals per time unit, and the average number of correct months per time unit during the TUGdt tests were calculated. The measure “correct words per 10 seconds” was calculated as 10*(TUGdt number of correct words/TUGdt time). Dual-task cost in the sense of relative time difference was calculated as 100*(TUGdt time – TUG time)/TUG time.
Lumbar puncture and cerebrospinal fluid biomarkers
Following the clinical routine, lumbar puncture was performed when there was reason to suspect AD and more information than the standard assessment provided was required. Contraindications for performing lumbar puncture were anticoagulant treatment or signs of increased intracranial pressure. Since the AD biomarker profile becomes less informative in older individuals [47], lumbar puncture is generally not performed in patients of advanced age. Moreover, in individuals who are severely overweight, lumbar puncture is not carried out because of technical difficulties. The CSF sample was obtained by lumbar puncture between the L3/L4, L4/L5, or L5/S1 intervertebral spaces, with the patient in a lying or upright position. All procedures were carried out following the same routine in both specialist clinics. The samples were sent to the same laboratory; the Clinical Neurochemistry Laboratory at the University of Gothenburg, Mölndal, Sweden, where the concentrations of Aβ42, t-tau, and p-tau were measured. Sandwich ELISAs (INNOTEST, Fujirebio, Ghent, Belgium) were used for the analyses. The laboratory technicians were blinded to clinical data.
Statistical analyses
Because data were not normally distributed, medians and interquartile ranges were used to present test results. All variables were analyzed as continuous variables except for gender and educational level (university education or not). All statistical tests were two-tailed and the significance level for all analyses was set at p < 0.05. Non-parametric correlation coefficients (Spearman’s rank test) were used to assess relationships. In all correlation analyses, adjustments were made for age, gender, and educational level to control for potentially confounding variables. Scatter plots with color-coded points showing diagnoses were generated in order to create an overview of the correlations between TUGdt outcomes and AD CSF biomarkers.
Due to the relatively small diagnostic groups, no correlation analyses were carried out within groups. In order to assure that the obtained results were not merely explained by the presence of specific diagnoses, a sensitivity analysis that included only participants diagnosed with AD and MCI was subsequently carried out. Correlations between TUGdt outcomes (i.e., time score, dual-task cost, correct words, and correct words per 10 seconds) and CSF biomarkers, versus correlations between cognitive tests (MMSE and Verbal Fluency test scores) and CSF biomarkers, were analyzed. All statistical analyses were carried out using SPSS version 25 (IBM Corp., Armonk, NY, USA) and SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Descriptive data relating to the diagnoses of the participants’ cognitive impairment and demographic characteristics are presented in Table 1.
Table 1
Study participants’ characteristics (N = 90)
| Characteristic | Value |
| Age in years, mean/±SD/min.–max. | 70.6/±7.1/49–84 |
| Female, n (%) | 38 (42) |
| University educated, n (%) | 41 (46) |
| Alzheimer’s disease, n (%) | 21 (23) |
| Other dementia disorders, n (%) | 9 (10) |
| Mild cognitive impairment, n (%) | 52 (58) |
| Subjective cognitive impairment, n (%) | 8 (9) |
Thirty participants were diagnosed with dementia disorders (21 AD, two vascular dementia, two unspecified dementia, two PD with dementia, and three frontotemporal dementia). The individuals diagnosed with dementia disorders had a mean age of 71.3 years (range 55–84), MCI 71.2 years (range 50–83), and SCI 63.5 years (range 49–74). In the entire sample, the participants’ mean age was 70.6 years, the majority were male, and nearly 50% had university-level education.
On average, the participants required more time to complete the TUGdt tests than the original TUG. Medians of the total number of correct words recited were the same for TUGdt NA and TUGdt MB. Similarly, comparable levels were shown concerning the number of correct words per 10 seconds during TUGdt NA and TUGdt MB. The medians of the AD CSF biomarker concentrations were on non-pathological levels, but ranges were wide (Table 2).
Table 2
The clinical test results and concentrations of Alzheimer’s disease cerebrospinal (CSF) biomarkers (N = 90)
| Test result | Md (IQRs) min.–max. |
| TUG (s) | 12.6 (11.1–15.2) 8.3–22.4 |
| TUGdt naming animals (s) | 14.4 (12.0–17.5) 8.9–31.7 |
| TUGdt naming animals, cost (%) | 12.1 (3.2–23.9) – 17.5–69.0 |
| TUGdt naming animals, correct words | 5 (4–7) 0–10 |
| TUGdt naming animals, correct words per 10 s | 3.7 (2.4–5.5) 0–9.8 |
| TUGdt months backward* (s) | 15.6 (12.7–20.6) 9.3–28.9 |
| TUGdt months backward*, cost (%) | 18.8 (8.2–39.2) – 21.6–114.3 |
| TUGdt months backward*, correct words | 5 (3–8) 0–12 |
| TUGdt months backward*, correct words per 10 s | 3.2 (1.5–4.9) 0–9.3 |
| MMSE score | 25 (23–27) 16–30 |
| Verbal Fluency test† score | 13 (10.8–19.0) 3–29 |
| AD CSF biomarker (ng/l) | Md (IQRs) min.–max. |
| Aβ42 | 400 (324.8–563.8) 125–1040 |
| t-tau | 357 (228.5–556.0) 75–1570 |
| p-tau | 44 (30.0–65.3) 16–144 |
TUG, Timed Up-and-Go; TUGdt, Timed Up-and-Go dual-task; MMSE, Mini Mental State Examination; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; ng/l, nanogram per litre; Aβ42, amyloid-β 42; t-tau, total tau; p-tau, phosphorylated tau.
*n = 89, one participant was unable to complete the TUGdt MB.
†n = 86 because of missing data.
(Aβ42 >450, t-tau <400, p-tau <80 are considered to be non-pathologic).
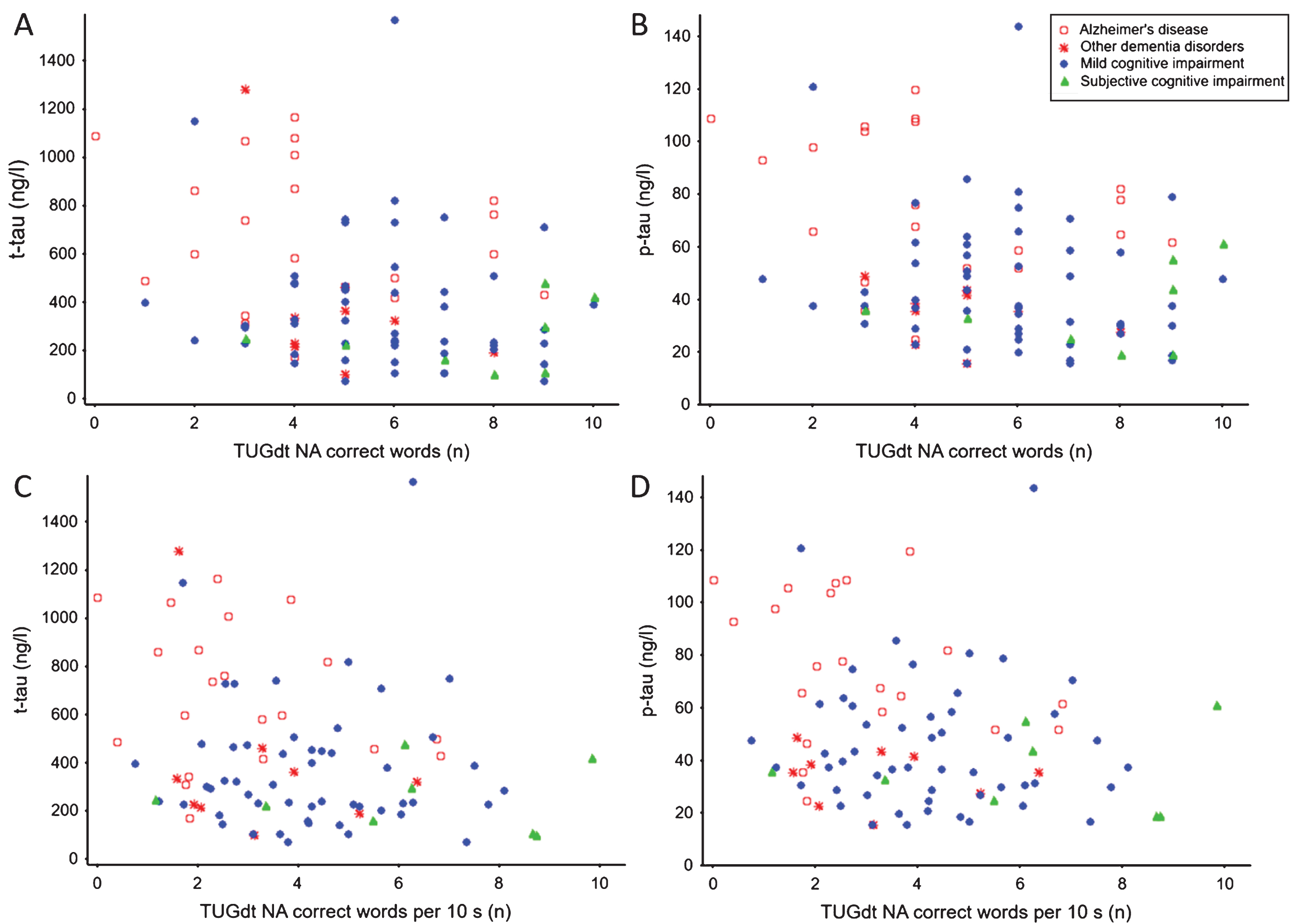
The total number of correct words recited during TUGdt NA was negatively correlated with t-tau and p-tau, as was the number of correct words per 10 seconds of TUGdt NA (Table 3). An overview of these associations, including the distribution of diagnostic groups, is shown in Fig. 1.
Table 3
Spearman’s rank correlation coefficients between Timed Up-and-Go (TUG) test results and Alzheimer’s disease fluid biomarkers (N = 90)
| TUG test result | Aβ42, ng/l | t-tau, ng/l | p-tau, ng/l |
| r, p | r, p | r, p | |
| TUG (s) | 0.104, 0.340 | 0.017, 0.876 | 0.016, 0.885 |
| TUGdt naming animals (s) | 0.136, 0.211 | 0.086, 0.431 | 0.068, 0.534 |
| TUGdt naming animals, cost (%) | 0.084, 0.439 | 0.107, 0.323 | 0.076, 0.485 |
| TUGdt naming animals correct words | –0.041, 0.708 | –0.281, 0.008* | –0.267, 0.012* |
| TUGdt naming animals, correct words per 10 s | 0.152, 0.229 | –0.267, 0.012* | –0.249, 0.020* |
| TUGdt months backward† (s) | 0.018, 0.872 | 0.130, 0.233 | 0.106, 0.334 |
| TUGdt months backward†, cost (%) | –0.146, 0.182 | 0.141, 0.195 | 0.097, 0.374 |
| TUGdt months backward†, correct words | 0.006, 0.959 | –0.123, 0.260 | –0.157, 0.148 |
| TUGdt months backward†, correct words per 10 s | 0.023, 0.831 | –0.164, 0.132 | –0.187, 0.085 |
Adjusted for age, gender, and educational level. TUG, Timed Up-and-Go; TUGdt, Timed Up-and-Go dual-task; Aβ42, amyloid-β 42; ng/l, nanogram per litre, t-tau, total tau; p-tau, phosphorylated tau; r, Spearman’s rho. *Significant, p < 0.05. †n = 89, one participant was unable to complete the TUGdt MB.
Fig.1
Associations between A) t-tau concentration and number of correct words during Timed Up-and-Go dual-task naming animals (TUGdt NA); B) p-tau concentration and number of correct words during TUGdt NA; C) t-tau concentration and number of correct words per 10 s during TUGdt NA; D) p-tau concentration and number of correct words per 10 s during TUGdt NA.
A sensitivity analysis showed that the correlation coefficients for the associations between TUGdt outcomes and CSF biomarker levels in a subgroup of AD and MCI were similar to those in the entire sample. Furthermore, significant negative correlations were found between the MMSE score and t-tau (r = –0.303, p = 0.004), MMSE score and p-tau (r = –0.302, p = 0.004), and the Verbal Fluency test score and t-tau (r = –0.244, p = 0.025).
No significant correlations were found between any TUGdt MB outcomes, TUGdt NA time score, or TUGdt NA cost, and any of the AD CSF biomarkers. Neither were any correlations found between Aβ42 and any of the TUGdt NA or TUGdt MB outcomes.
DISCUSSION
The results of the present study show that certain TUGdt outcomes correlate with CSF t-tau and p-tau. The number of correct words recited during TUGdt NA was negatively correlated to t-tau and p-tau, as was the number of correct words per 10 seconds of TUGdt NA. On the other hand, there were no significant correlations found between any of the TUGdt outcomes or the cognitive test results and CSF Aβ42. However, significant negative correlations between MMSE score and t-tau, p-tau, as well as between Verbal Fluency test score and t-tau were found, at similar levels to those between TUGdt NA and t-tau.
The current findings showing that TUGdt NA correlated with t-tau and p-tau but not with Aβ42 were not surprising, since CSF t-tau and p-tau have previously been found to reflect the level of neurodegeneration [48]. Concentrations of CSF Aβ42, on the other hand, are viewed as more specific for AD and deviate before the appearance of cognitive symptoms, then remain stable throughout the disease progression [29].
The significant correlations found between t-tau and p-tau and certain TUGdt NA outcomes, but not to TUGdt MB outcomes, could be worth considering due to the different central functions that the Verbal Fluency test of naming animals and Months Backward test challenge. The Verbal Fluency test relies mainly on semantic memory and executive function [44], while the Months Backward test additionally depends on working memory and attention [39, 45]. Presumably, an explanation to why TUGdt NA was more highly associated with neurodegeneration than TUGdt MB, may be due to an impaired ability to retrieve items from the semantic network [49]. Still, it should be noted that verbal outcomes generated from the TUGdt tests are not comparable to results from the single-task Verbal Fluency test or the Months Backward test. It may further be argued that TUGdt tests cannot be treated as versions of single-task cognitive tests since they are designed to assess the ability of dual-tasking, which implies that the load of two tasks performed simultaneously is expected to reveal more information than a cognitive or motor test performed separately.
The present results did not confirm any of the correlations between TUGdt time score or TUGdt cost, and the AD CSF biomarkers, which were previously found in a study population of individuals with mild AD, or MCI, and controls performing TUGdt C (counting backwards from 100 by ones) [14]. This discrepancy may be in part due to the different setups of the TUGdt tests. The complexity of the verbal tasks as well as the instructions given to the participants differed substantially. The verbal tasks used in the present study may be viewed as more demanding, since counting backwards by ones is rhythmic and may cue step pattern [38, 50]. Also, the participants in the present study were instructed to walk at “a self-selected, comfortable speed”, prioritizing walking over the verbal task, as opposed to the instructions to walk “as quickly and safely as possible”, without prioritizing one task over the other. The instruction to prioritize walking, used in the present study, most likely shortened the TUGdt time scores and thereby also influenced TUGdt cost. The differences in the TUGdt procedures make it difficult to compare results between these two studies.
The significant negative correlations found—at approximately the same levels—between t-tau and TUGdt NA, the MMSE scores, and the Verbal Fluency test scores, may suggest that certain TUGdt outcomes and results of these cognitive tests are influenced by neurodegeneration. Since the capacity of the biomarkers to function as indicators of cognition is not fully understood, interpreting these correlations is difficult. The results of previous studies are not consistent in demonstrating significant associations between cognition and AD CSF biomarkers in the AD continuum. Research on the relationship between MMSE and AD CSF biomarkers has provided inconsistent results [51, 52], as do studies on associations between Verbal Fluency test score and AD CSF biomarkers [53–55]. In order to provide a sensitivity analysis, the significant correlations found between TUGdt performance and CSF biomarkers, as well as between the cognitive tests and biomarkers, were analyzed in a subgroup of the sample consisting of individuals diagnosed with AD or MCI. The correlation coefficients were found to be similar to the ones in the entire sample, thus the associations could not merely be explained by the influence of certain diagnoses.
The present study is one of the very first that investigates correlations between dual-task performance and AD CSF biomarkers. However, some limitations must be taken into consideration when interpreting the results. The inclusion of multiple test results in the correlation analysis is a weakness in this study, as it implies a risk of inflated Type 1 errors. The explorative outset implied numerous analyses and may have led to significant correlations by chance. Another limitation concerns the generalizability of the results. The sample may not be representative, since only a small proportion (11%) of all patients going through memory assessment were included during the period of recruitment. One explanation for the low inclusion rate is that only individuals with the level of mobility required to carry out the TUGdt tests could be included, which resulted in a selection of only the physically fittest individuals. Moreover, only individuals selected for lumbar puncture, according to the clinical routine, were included. For this reason, the sample comprised fewer individuals with SCI, as well as fewer individuals with apparent AD, in particular those of advanced age, than the entire population going through memory assessment. Since the AD CSF biomarker profile becomes less informative in individuals of advanced age, the oldest individuals were omitted from the sample. Still, six participants did exceed the age of 80 by up to four years. The fairly limited number of participants is also considered to be a limitation, since correlations within each diagnostic subgroup could not be carried out.
The methodology used has several strengths, primarily because of its standardized procedures. The lumbar puncture and laboratory analyses, as well as the TUGdt testing procedures used, including equipment, instructions, and validation of the video material were strictly standardized.
Once all the limitations and strengths of the study are taken into consideration, the results indicate that neurodegeneration may influence TUGdt performance. Nonetheless, caution is required before further generalizations of the results are made.
Conclusion
In summary, the correlations found in the present study between TUGdt NA outcomes and CSF t-tau and p-tau may indicate that neurodegeneration affects dual-task performance. Although the results suggest that TUGdt may be used as a screening tool for cognitive impairment due to neurodegeneration, data from longitudinal studies will be needed to validate these tests.
ACKNOWLEDGMENTS
This study was supported by grants from: The Swedish Research Council, the Alzheimer Foundation Sweden, The Swedish Society of Medicine, The Promobilia Foundation, The Uppsala-Örebro Regional Research Council, The County Council of Uppsala, The Geriatric Research Foundation, The Thuréus fund for Geriatric Research, and The Commemorative Foundation of Ragnhild & Einar Lundström.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-1265r1).
REFERENCES
[1] | Laske C , Sohrabi HR , Frost SM , Lopez-de-Ipina K , Garrard P , Buscema M , Dauwels J , Soekadar SR , Mueller S , Linnemann C , Bridenbaugh SA , Kanagasingam Y , Martins RN , O’Bryant SE ((2015) ) Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimers Dement 11: , 561–578. |
[2] | Yogev-Seligmann G , Hausdorff JM , Giladi N ((2008) ) The role of executive function and attention in gait. Mov Disord 23: , 329–342; quiz 472. |
[3] | Cullen S , Montero-Odasso M , Bherer L , Almeida Q , Fraser S , Muir-Hunter S , Li K , Liu-Ambrose T , McGibbon CA , McIlroy W , Middleton LE , Sarquis-Adamson Y , Beauchet O , McFadyen BJ , Morais JA , Camicioli R ((2018) ) Guidelines for gait assessments in the Canadian Consortium on Neurodegeneration in Aging (CCNA). Can Geriatr J 21: , 157–165. |
[4] | Pashler H ((1994) ) Dual-task interference in simple tasks: Data and theory. Psychol Bull 116: , 220–244. |
[5] | Bahureksa L , Najafi B , Saleh A , Sabbagh M , Coon D , Mohler MJ , Schwenk M ((2017) ) The impact of mild cognitive impairment on gait and balance: A systematic review and meta-analysis of studies using instrumented assessment. Gerontology 63: , 67–83. |
[6] | MacAulay RK , Wagner MT , Szeles D , Milano NJ ((2017) ) Improving sensitivity to detect mild cognitive impairment: Cognitive load dual-task gait speed assessment. J Int Neuropsychol Soc 23: , 493–501. |
[7] | Borges Sde M , Radanovic M , Forlenza OV ((2015) ) Functional mobility in a divided attention task in older adults with cognitive impairment. J Mot Behav 47: , 378–385. |
[8] | Belghali M , Chastan N , Cignetti F , Davenne D , Decker LM ((2017) ) Loss of gait control assessed by cognitive-motor dual-tasks: Pros and cons in detecting people at risk of developing Alzheimer’s and Parkinson’s diseases. Geroscience 39: , 305–329. |
[9] | Montero-Odasso MM , Sarquis-Adamson Y , Speechley M , Borrie MJ , Hachinski VC , Wells J , Riccio PM , Schapira M , Sejdic E , Camicioli RM , Bartha R , McIlroy WE , Muir-Hunter S ((2017) ) Association of dual-task gait with incident dementia in mild cognitive impairment: Results from the Gait and Brain Study. JAMA Neurol 74: , 857–865. |
[10] | Bridenbaugh SA , Kressig RW ((2015) ) Motor cognitive dual tasking: Early detection of gait impairment, fall risk and cognitive decline. Z Gerontol Geriatr 48: , 15–21. |
[11] | Lundin-Olsson L , Nyberg L , Gustafson Y ((1997) ) “Stops walking when talking” as a predictor of falls in elderly people. Lancet 349: , 617. |
[12] | Cedervall Y , Halvorsen K , Aberg AC ((2014) ) A longitudinal study of gait function and characteristics of gait disturbance in individuals with Alzheimer’s disease. Gait Posture 39: , 1022–1027. |
[13] | Montero-Odasso M , Speechley M , Muir-Hunter SW , Sarquis-Adamson Y , Sposato LA , Hachinski V , Borrie M , Wells J , Black A , Sejdic E , Bherer L , Chertkow H , Canadian Gait and Cognition Network ((2018) ) Motor and cognitive trajectories before dementia: Results from Gait and Brain Study. J Am Geriatr Soc 66: , 1676–1683. |
[14] | Nielsen MS , Simonsen AH , Siersma V , Hasselbalch SG , Hoegh P ((2018) ) The diagnostic and prognostic value of a dual-tasking paradigm in a memory clinic. J Alzheimers Dis 61: , 1189–1199. |
[15] | Al-Yahya E , Dawes H , Smith L , Dennis A , Howells K , Cockburn J ((2011) ) Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci Biobehav Rev 35: , 715–728. |
[16] | Klotzbier TJ , Schott N ((2017) ) Cognitive-motor interference during walking in older adults with probable mild cognitive impairment. Front Aging Neurosci 9: , 350. |
[17] | Blennow K , Dubois B , Fagan AM , Lewczuk P , de Leon MJ , Hampel H ((2015) ) Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement 11: , 58–69. |
[18] | Petersen RC , Aisen PS , Beckett LA , Donohue MC , Gamst AC , Harvey DJ , Jack CR Jr , Jagust WJ , Shaw LM , Toga AW , Trojanowski JQ , Weiner MW ((2010) ) Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 74: , 201–209. |
[19] | Tifratene K , Robert P , Metelkina A , Pradier C , Dartigues JF ((2015) ) Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology 85: , 331–338. |
[20] | Garcia-Ptacek S , Eriksdotter M , Jelic V , Porta-Etessam J , Kareholt I , Manzano Palomo S ((2016) ) Subjective cognitive impairment: Towards early identification of Alzheimer disease. Neurologia 31: , 562–571. |
[21] | Mitchell AJ , Beaumont H , Ferguson D , Yadegarfar M , Stubbs B ((2014) ) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand 130: , 439–451. |
[22] | Palmqvist S , Zetterberg H , Mattsson N , Johansson P , Minthon L , Blennow K , Olsson M , Hansson O ((2015) ) Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 85: , 1240–1249. |
[23] | Ritchie C , Smailagic N , Noel-Storr AH , Ukoumunne O , Ladds EC , Martin S ((2017) ) CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 3: , CD010803. |
[24] | Jack CR Jr , Holtzman DM ((2013) ) Biomarker modeling of Alzheimer’s disease. Neuron 80: , 1347–1358. |
[25] | Degerman Gunnarsson M , Ingelsson M , Blennow K , Basun H , Lannfelt L , Kilander L ((2016) ) High tau levels in cerebrospinal fluid predict nursing home placement and rapid progression in Alzheimer’s disease. Alzheimers Res Ther 8: , 22. |
[26] | Colijn MA , Grossberg GT ((2015) ) Amyloid and tau biomarkers in subjective cognitive impairment. J Alzheimers Dis 47: , 1–8. |
[27] | Blennow K , Zetterberg H , Minthon L , Lannfelt L , Strid S , Annas P , Basun H , Andreasen N ((2007) ) Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett 419: , 18–22. |
[28] | Mattsson N , Portelius E , Rolstad S , Gustavsson M , Andreasson U , Stridsberg M , Wallin A , Blennow K , Zetterberg H ((2012) ) Longitudinal cerebrospinal fluid biomarkers over four years in mild cognitive impairment. J Alzheimers Dis 30: , 767–778. |
[29] | Jack CR Jr. , Knopman DS , Jagust WJ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Trojanowski JQ ((2010) ) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: , 119–128. |
[30] | Belghali M , Chastan N , Davenne D , Decker LM ((2017) ) Improving dual-task walking paradigms to detect prodromal Parkinson’s and Alzheimer’s diseases. Front Neurol 8: , 207. |
[31] | Fritz NE , Kegelmeyer DA , Kloos AD , Linder S , Park A , Kataki M , Adeli A , Agrawal P , Scharre DW , Kostyk SK ((2016) ) Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture 50: , 1–7. |
[32] | Montero-Odasso M , Pieruccini-Faria F , Bartha R , Black SE , Finger E , Freedman M , Greenberg B , Grimes DA , Hegele RA , Hudson C , Kleinstiver PW , Lang AE , Masellis M , McLaughlin PM , Munoz DP , Strother S , Swartz RH , Symons S , Tartaglia MC , Zinman L , Strong MJ , McIlroy W ((2017) ) Motor phenotype in neurodegenerative disorders: Gait and balance platform study design protocol for the Ontario Neurodegenerative Research Initiative (ONDRI). J Alzheimers Dis 59: , 707–721. |
[33] | Tallberg IM , Ivachova E , Jones Tinghag K , Ostberg P ((2008) ) Swedish norms for word fluency tests: FAS, animals and verbs. Scand J Psychol 49: , 479–485. |
[34] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[35] | Arevalo-Rodriguez I , Smailagic N , Roque IFM , Ciapponi A , Sanchez-Perez E , Giannakou A , Pedraza OL , Bonfill Cosp X , Cullum S ((2015) ) Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev, CD010783. |
[36] | Podsiadlo D , Richardson S ((1991) ) The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: , 142–148. |
[37] | Ries JD , Echternach JL , Nof L , Gagnon Blodgett M ((2009) ) Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther 89: , 569–579. |
[38] | Muir SW , Speechley M , Wells J , Borrie M , Gopaul K , Montero-Odasso M ((2012) ) Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture 35: , 96–100. |
[39] | Meagher J , Leonard M , Donoghue L , O’Regan N , Timmons S , Exton C , Cullen W , Dunne C , Adamis D , Maclullich AJ , Meagher D ((2015) ) Months backward test: A review of its use in clinical studies. World J Psychiatry 5: , 305–314. |
[40] | Henry JD , Crawford JR , Phillips LH ((2004) ) Verbal fluency performance in dementia of the Alzheimer’s type: A meta-analysis. Neuropsychologia 42: , 1212–1222. |
[41] | Sheridan PL , Solomont J , Kowall N , Hausdorff JM ((2003) ) Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc 51: , 1633–1637. |
[42] | Montero-Odasso M , Bergman H , Phillips NA , Wong CH , Sourial N , Chertkow H ((2009) ) Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr 9: , 41. |
[43] | Beauchet O , Dubost V , Aminian K , Gonthier R , Kressig RW ((2005) ) Dual-task-related gait changes in the elderly: Does the type of cognitive task matter? J Mot Behav 37: , 259–264. |
[44] | Vaughan RM , Coen RF , Kenny R , Lawlor BA ((2018) ) Semantic and phonemic verbal fluency discrepancy in mild cognitive impairment: Potential predictor of progression to Alzheimer’s Disease. J Am Geriatr Soc 66: , 755–759. |
[45] | Ostberg P , Hansson V , Haagg S ((2012) ) Adult norms and test-retest reliability for the Months Backward test: Durational and response accuracy measures. Logoped Phoniatr Vocol 37: , 11–17. |
[46] | Ostberg P , Fernaeus SE , Bogdanovic N , Wahlund LO ((2008) ) Word sequence production in cognitive decline: Forward ever, backward never. Logoped Phoniatr Vocol 33: , 126–135. |
[47] | Velickaite V , Giedraitis V , Strom K , Alafuzoff I , Zetterberg H , Lannfelt L , Kilander L , Larsson EM , Ingelsson M ((2017) ) Cognitive function in very old men does not correlate to biomarkers of Alzheimer’s disease. BMC Geriatr 17: , 208. |
[48] | Spillantini MG , Goedert M ((2013) ) Tau pathology and neurodegeneration. Lancet Neurol 12: , 609–622. |
[49] | Rogers SL , Friedman RB ((2008) ) The underlying mechanisms of semantic memory loss in Alzheimer’s disease and semantic dementia. Neuropsychologia 46: , 12–21. |
[50] | Beauchet O , Allali G , Poujol L , Barthelemy JC , Roche F , Annweiler C ((2010) ) Decrease in gait variability while counting backward: A marker of “magnet effect”? J Neural Transm (Vienna) 117: , 1171–1176. |
[51] | Rami L , Fortea J , Bosch B , Sole-Padulles C , Llado A , Iranzo A , Sanchez-Valle R , Molinuevo JL ((2011) ) Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J Alzheimers Dis 23: , 319–326. |
[52] | Wallin AK , Blennow K , Andreasen N , Minthon L ((2006) ) CSF biomarkers for Alzheimer’s Disease: Levels of beta-amyloid, tau, phosphorylated tau relate to clinical symptoms and survival. Dement Geriatr Cogn Disord 21: , 131–138. |
[53] | Mirandez RM , Aprahamian I , Talib LL , Forlenza OV , Radanovic M ((2017) ) Multiple category verbal fluency in mild cognitive impairment and correlation with CSF biomarkers for Alzheimer’s disease. Int Psychogeriatr 29: , 949–958. |
[54] | Reijs BLR , Ramakers I , Kohler S , Teunissen CE , Koel-Simmelink M , Nathan PJ , Tsolaki M , Wahlund LO , Waldemar G , Hausner L , Vandenberghe R , Johannsen P , Blackwell A , Vanderstichele H , Verhey F , Visser PJ ((2017) ) Memory correlates of Alzheimer’s disease cerebrospinal fluid markers: A longitudinal cohort study. J Alzheimers Dis 60: , 1119–1128. |
[55] | Rolstad S , Berg AI , Bjerke M , Johansson B , Zetterberg H , Wallin A ((2013) ) Cerebrospinal fluid biomarkers mirror rate of cognitive decline. J Alzheimers Dis 34: , 949–956. |



