Preoperative Phosphorylated Tau Concentration in the Cerebrospinal Fluid Can Predict Cognitive Function Three Years after Shunt Surgery in Patients with Idiopathic Normal Pressure Hydrocephalus
Abstract
Background:
Idiopathic normal pressure hydrocephalus (iNPH) is commonly treated by cerebrospinal fluid (CSF) shunting. However, the long-term efficacy of shunt intervention in the presence of comorbid Alzheimer’s disease (AD) pathology is debated.
Objective:
To identify AD-associated CSF biomarkers predictive of shunting surgery outcomes in patients with iNPH.
Methods:
Preoperative levels of total and phosphorylated Tau (p-Tau) were measured in 40 patients with iNPH divided into low (<30 pg/mL) and high (≥30 pg/mL) p-Tau groups and followed up for three years after lumboperitoneal shunting. The modified Rankin Scale (mRS), Mini-Mental State Examination (MMSE), Frontal Assessment Battery, and iNPH Grading Scale scores were compared between the age-adjusted low (n = 24; mean age 75.7 years [SD 5.3]) and high (n = 11; mean age 76.0 years [SD 5.6]) p-Tau groups.
Results:
Cognitive function improved early in the low p-Tau group and was maintained thereafter (p = 0.005). In contrast, the high p-Tau group showed a gradual decline to baseline levels by the third postoperative year (p = 0.040). Although the p-Tau concentration did not correlate with the preoperative MMSE score, a negative correlation appeared and strengthened during follow-up (R2 = 0.352, p < 0.001). Furthermore, the low p-Tau group showed rapid and sustained mRS grade improvement, whereas mRS performance gradually declined in the high p-Tau group.
Conclusions:
Preoperative CSF p-Tau concentration predicted some aspects of cognitive function after shunt intervention in patients with iNPH. The therapeutic effects of shunt treatment were shorter-lasting in patients with coexisting AD pathology.
INTRODUCTION
Idiopathic normal pressure hydrocephalus (iNPH) is a neurodegenerative condition characterized by gait disturbance, dementia, and urinary incontinence [1]. Independence in everyday life and improvements in cognitive functions in patients with iNPH can be achieved through shunt treatment. In Japan, an iNPH guideline was published ahead of the Western world in 2004 [2], and revised in 2011 [3]. This guideline noted that cerebrospinal fluid (CSF) shunt intervention in patients with possible iNPH on MRI resulted in an improvement in the modified Rankin Scale (mRS) score in >80% of the patients within a year [4]. Additional improvements were also observed in the following symptoms: gait disturbance with a wide short-stepped gait; cognitive impairment resulting in decreased psychomotor speed; urinary urgency; overactive bladder manifesting mainly as urinary incontinence; ventricular enlargement; narrowing of the high convexity in the subarachnoid space; and disproportionately enlarged subarachnoid-space hydrocephalus (DESH) with CSF retention in the subarachnoid space under the sylvian fissure [5]. These positive results support the use of the diagnosis techniques recommended in the iNPH guideline. CSF shunting is currently approved as the only treatment method for iNPH with evidence of efficacy [6]; however, the long-term effects of shunt intervention are still in question [7, 8]. Additionally, the treatment outcome can be affected by the presence of various complications in the elderly [9].
CSF diagnosis by the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) has been used in Alzheimer’s Disease Neuroimaging Initiative (ADNI) studies to differentiate patients with Alzheimer’s disease (AD) and mild cognitive impairment (MCI) from healthy controls [10, 11]. Based on these studies, we used CSF biomarkers to verify the presence of AD comorbidity in patients with iNPH [12].
The diagnosis of AD comorbidity based on radiographic findings alone is often difficult [13]. Patel and colleagues [14] showed that the levels of amyloid-β peptide (Aβ42) and tau protein phosphorylated at threonine 181 (p-Tau) were the most significant CSF biomarkers for predicting the presence of cortical tissue AD pathology; in particular, high p-Tau/Aβ42 ratios in the ventricular CSF were correlated with cortical AD pathology. The Aβ42 levels in the lumbar CSF (L-CSF) of iNPH patients are lower than those in healthy seniors [25]; however, they are often even lower among patients with AD [15]. The total Tau and p-Tau protein levels in patients with iNPH are reported to be similar to or lower than those in healthy individuals [16], and, unlike Aβ42, clearly lower than those in patients with AD [15]. Shunt intervention in iNPH patients with high total Tau or p-Tau levels shows poor improvement [17, 18], which is considered an indicator of neuronal disorder or AD comorbidity. Previous reports have shown that although the CSF biomarkers of iNPH patients can be affected by concomitant Tau or amyloid pathology, the CSF p-Tau concentration is highly useful for the differentiation of iNPH and AD [19, 20].
In the ADNI cohort, p-Tau is an advanced-stage marker related to dementia symptoms and disease progression [21]. The threshold value for discrimination between AD patients and age-matched cognitively normal elderly subjects is 23–25 pg/mL (sensitivity, 68–87%; specificity 68–73%) [10, 22]. In the present study, as a specific marker for AD, subjects were grouped by the p-Tau concentration alone to evaluate AD pathology [23, 24]. At present, there is no p-Tau cutoff value that determines whether AD pathology is coexistent in the L-CSF of iNPH patients. A previous study using the xMAP showed that the p-Tau levels in an iNPH group were lower than or similar to those in a control group [16]. We used the p-Tau cutoff value of 30 pg/mL obtained from the results of the Japanese ADNI [10] to follow iNPH patients long-term.
Increased p-Tau concentrations in the CSF reflect AD pathology [24]. CSF shunting in iNPH patients with coexisting AD is believed to have smaller and shorter-lasting therapeutic effects than in patients without comorbidity. Thus, the efficacy of shunt intervention in patients with iNPH is debated [25]. We aimed to examine whether the cognitive improvements due to shunt treatment were sustained for three years in patients diagnosed with iNPH based on symptom examination and imaging findings, particularly in iNPH patients with AD.
MATERIALS AND METHODS
Study populations
The present cohort included 40 patients with iNPH, 29 males and 11 females, with a mean age of 74.3 years (SD 6.6). These patients received lumboperitoneal (LP) shunt intervention from February 2010 to February 2015 at the Juntendo University Department of Neurosurgery. The diagnostic criteria were symptoms of iNPH in accordance with the iNPH Guidelines [3] and ventriculomegaly with an Evans Index of >0.3. The patients were followed up for three years. A retrospective analysis of the detailed course of clinical symptoms, including long-term progress, was then performed. The Mini–Mental State Examination (MMSE) [26, 27], Frontal Assessment Battery (FAB) [28], mRS, and Japan iNPH Grading Scale (iNPHGS; assessing gait disturbance, cognitive impairment, and urinary incontinence) [29] were used for evaluation.
Biomarkers were measured in CSF sampled by lumbar tap before shunt intervention. The patients were assessed for coexisting AD using a p-Tau cutoff of 30 pg/mL and divided into high p-Tau (≥30 pg/mL, n = 12) and low p-Tau (<30 pg/mL, n = 28) groups. Differences in long-term prognosis were compared between groups. The p-Tau cutoff value of ≥30 pg/mL was determined based on L-CSF data obtained from cognitively normal subjects with negative 11C-Pittsburgh compound B (PiB) amyloid positron emission tomography (PET) scans (n = 22) and patients with AD with positive PiB PET scans (n = 17) [11]. An age-adjusted control study was conducted with 11 high p-Tau and 24 low p-Tau patients (Table 1). Four cases of patients aged 65 or under were excluded from the low p-Tau group, and one case where third-year examination data could not be taken was removed from the high p-Tau group. Comparison between groups showed no significant difference in age after removing these five cases (Fig. 1).
Fig.1
Study patient flow chart. Forty patients with a diagnosis of iNPH who had three years of follow-up after lumboperitoneal shunting were divided into two groups according to their CSF p-Tau levels: 28 patients with p-Tau levels <30 pg/mL, and 12 patients with p-Tau levels ≥30 pg/mL. After adjustment for age (range 65–85 years old), 24 patients were included in a low p-Tau group, and 11 patients in a high p-Tau group. Outcome measures obtained at the indicated points during follow-up were compared between the groups and with the preoperative values.
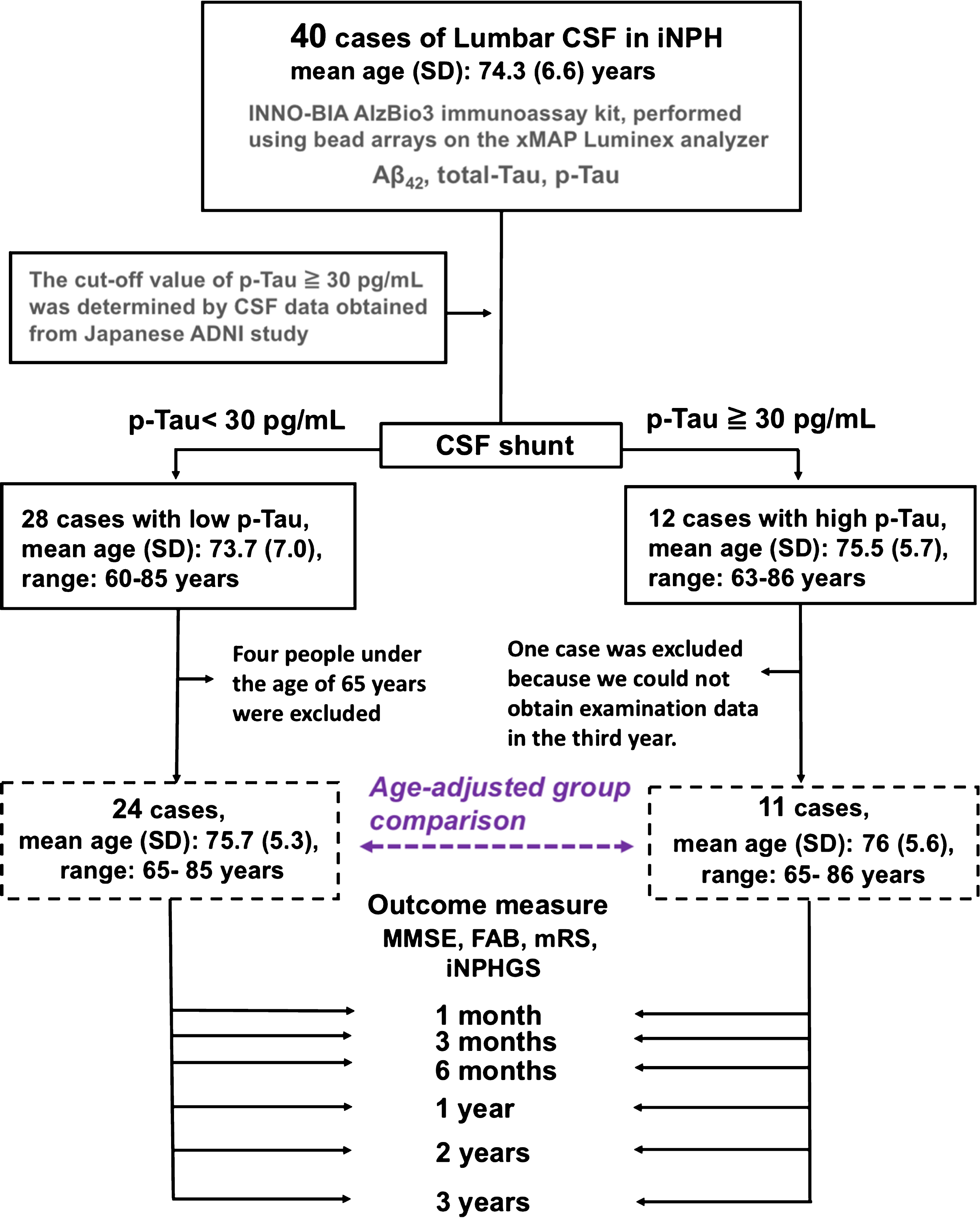
Table 1
Baseline characteristics of the iNPH patients
| Age-unadjusted (n = 40) | Age-adjusted (n = 35) | |||||
| Low p-Tau <30 pg/mL (n = 28) | High p-Tau ≥30 pg/mL (n = 12) | p-value | Low p-Tau <30 pg/mL (n = 24) | High p-Tau ≥30 pg/mL (n = 11) | p | |
| Male, number (%) | 21 (75%) | 8 (66.7%) | 0.589 | 17 (70.8%) | 8 (72.7%) | 0.908 |
| Age, mean (SD), (y, range) | 73.7 (7.0), (60–85) | 75.5 (5.7), (63–86) | 0.391 | 75.7 (5.3), (65–85) | 76 (5.6), (65–85) | 0.605 |
| DESH, number (%) | 24 (86%) | 10 (83%) | 0.847 | 21 (88%) | 9 (82%) | 0.656 |
| Evans Index, mean (SD) | 0.35 (0.036) | 0.34 (0.041) | 0.393 | 0.35 (0.037) | 0.34 (0.037) | 0.668 |
| Comorbidities, number (%) | ||||||
| Hypertension | 13 (46%) | 7 (58%) | 0.490 | 12 (50%) | 6 (55%) | 0.803 |
| Diabetes | 6 (21%) | 3 (25%) | 0.740 | 6 (25%) | 3 (27%) | 0.942 |
| Hyperlipidemia | 9 (32%) | 5 (42%) | 0.482 | 9 (38%) | 5 (45%) | 0.726 |
| Stroke | 4 (14%) | 0 (0%) | 0.168 | 3 (13%) | 0 (0%) | 0.220 |
| CSF biomarker, mean (SD) | ||||||
| p-Tau (pg/mL) | 21.7 (3.2) | 40.1 (10.8) | <0.001 | 22.0 (3.0) | 40.2 (11.3) | <0.001 |
| total-Tau (pg/mL) | 49.1 (12.2) | 71.0 (20.7) | <0.001 | 50.5 (12.0) | 69.6 (21.1) | 0.002 |
| Aβ42(pg/mL) | 182.4 (57.5) | 176.0 (85.1) | 0.409 | 188.8 (56.8) | 175.6 (89.2) | 0.256 |
| p-Tau/ Aβ42 | 0.128 (0.034) | 0.284 (0.162) | <0.001 | 0.124 (0.029) | 0.290 (0.168) | <0.001 |
| Total Tau/ Aβ42 | 0.278 (0.040) | 0.188 (0.182) | 0.004 | 0.274 (0.036) | 0.488 (0.297) | <0.001 |
| Clinical findings, mean (SD) | ||||||
| MMSE | 22.2 (4.3) | 21.8 (3.4) | 0.941 | 22.2 (4.1) | 21.7 (3.6) | 0.844 |
| FAB | 10.9 (3.4) | 11.3 (3.2) | 0.941 | 10.9 (3.5) | 11.6 (3.2) | 0.761 |
| mRS | 2.93 (0.81) | 2.67 (0.49) | 0.271 | 2.88 (0.85) | 2.80 (0.76) | 0.375 |
| iNPHGS: Gait disturbance | 2.43 (0.69) | 2.08 (0.52) | 0.134 | 2.46 (0.72) | 2.09 (0.54) | 0.148 |
| iNPHGS: Cognitive impairment | 1.86 (0.97) | 2.08 (0.67) | 0.524 | 1.75 (0.94) | 2.09 (0.70) | 0.311 |
| iNPHGS: Urinary incontinence | 1.57 (0.93) | 1.83 (1.03) | 0.698 | 1.54 (0.93) | 1.91 (1.04) | 0.489 |
Differences in the number of females, disproportionately enlarged subarachnoid-space hydrocephalus (DESH) on brain imaging, and patients with comorbidities were evaluated using Pearson’s chi-squared test. The other characteristics were compared using the Mann–Whitney U test.
This study was approved by the Ethics Committee of Juntendo University and Niigata University. All patients who participated in the study were briefed on the details of the study and provided written informed consent.
CSF sampling
Lumbar punctures and lumboperitoneal shunts with a programmable valve (STRATA, Medtronic Neurosurgery, Goleta, CA) were performed using previously reported methods [30, 31]. CSF samples were collected preoperatively through lumbar puncture performed in the L3–L4 or L4–L5 interspace using an 18-gauge spinal needle in all patients. All CSF samples were centrifuged at 4°C and 1690 G for 10 min to remove the cells and debris. Then, the samples were aliquoted and stored in polypropylene tubes at –80°C until biochemical analysis. Spinal fluid was dispensed into polypropylene containers at the storage site at Juntendo University, and then transported to the Department of Molecular Genetics, Brain Research Institute, Niigata University, Niigata, Japan, on dry ice to avoid thawing. Each aliquot contained 1 mL of CSF.
CSF analyses
We used the multiplex xMAP Luminex platform with Innogenetics (INNO-BIA ALzBio3, Ghent, Belgium) immunoassay kit reagents for the CSF biomarker measurements. The platform is capable of measuring p-Tau, total Tau, and Aβ42 concentrations at the same time [10, 32, 33]. Briefly, the Innogenetics kit reagents include well-characterized capture monoclonal antibodies specific for Aβ42 (4D7A3), total Tau (AT120), and p-Tau181p (AT270), each chemically bonded to a unique set of color-coded beads, and analyte-specific detector antibodies (HT7, 3D6). All analyses were performed by the same platform of J-ADNI biomarker measurement [23] and blinded to the clinical data and, batch-wise on one occasion, by board-certified laboratory technicians at the Department of Molecular Genetics, Brain Research Institute, Niigata University, Niigata, Japan. The intra-assay coefficients of variation were below 10%.
Statistical analysis
We used the Shapiro-Wilk test to verify the normal distributions of the Aβ42, total Tau, and p-Tau levels, total Tau/Aβ42 and p-Tau /Aβ42 level ratios, and MMSE, FAB, and iNPHGS scores (Fig. 1, Table 1). Age, Evans Index, CSF biomarkers, clinical findings, and the MMSE, FAB, mRS, and iNPHGS scores were assessed using the Mann-Whitney U test. The numbers of males, patients with DESH on brain imaging, and patients with comorbidity differences were compared between the groups by Pearson’s chi-squared test. Then, we performed simple linear regression analyses of the correlations between preoperative CSF biomarkers (p-Tau, total Tau, Aβ42, p-Tau/Aβ42, and total Tau/Aβ42) and the MMSE and FAB scores to identify biomarkers that could predict cognitive function. We evaluated differences between before and after shunt intervention using the Wilcoxon signed-rank test. The level of significance chosen was 0.05, unless otherwise stated. Statistical analyses were performed using IBM SPSS Statistics for Windows version 18 (SPSS, Chicago, IL).
RESULTS
After adjusting for age, significant differences were found between the high (11 patients) and low (24 patients) p-Tau groups in total Tau and p-Tau CSF levels (i.e., before surgical intervention; Table 1); however, cognitive function, mRS score, FAB, iNPHGS, and comorbidities, were not significantly different between the two groups.
Cognitive function
Following shunting, the MMSE score was improved after three years (p = 0.005) in the low p-Tau group (Fig. 2, Table 2). The FAB score was also improved at one-month follow-up (p = 0.027), and remained significantly improved for one year (p = 0.004). While the improvement in FAB score decreased slightly at three years post-treatment (p = 0.224), both cognitive functions were improved compared with prior to treatment. In the high p-Tau group, the MMSE and FAB scores had not improved. Further, they tended to decline to below the preoperative values after three years, although this decrease was not statistically significant. A statistically significant difference between groups was found three years after the shunt treatment for both MMSE (p = 0.040) and FAB (p = 0.02) scores.
Fig.2
Comparison of MMSE performance in the low and high p-Tau groups. MMSE performance progress in individual patients from before shunting to three years after shunting is shown for the low (<30 pg/mL, n = 24) and high (≥30 pg/mL, n = 11) p-Tau groups. The black line shows the average value in each group. A temporary improvement was observed in the high p-Tau group, while the improvement in the low p-Tau group was maintained throughout follow-up.
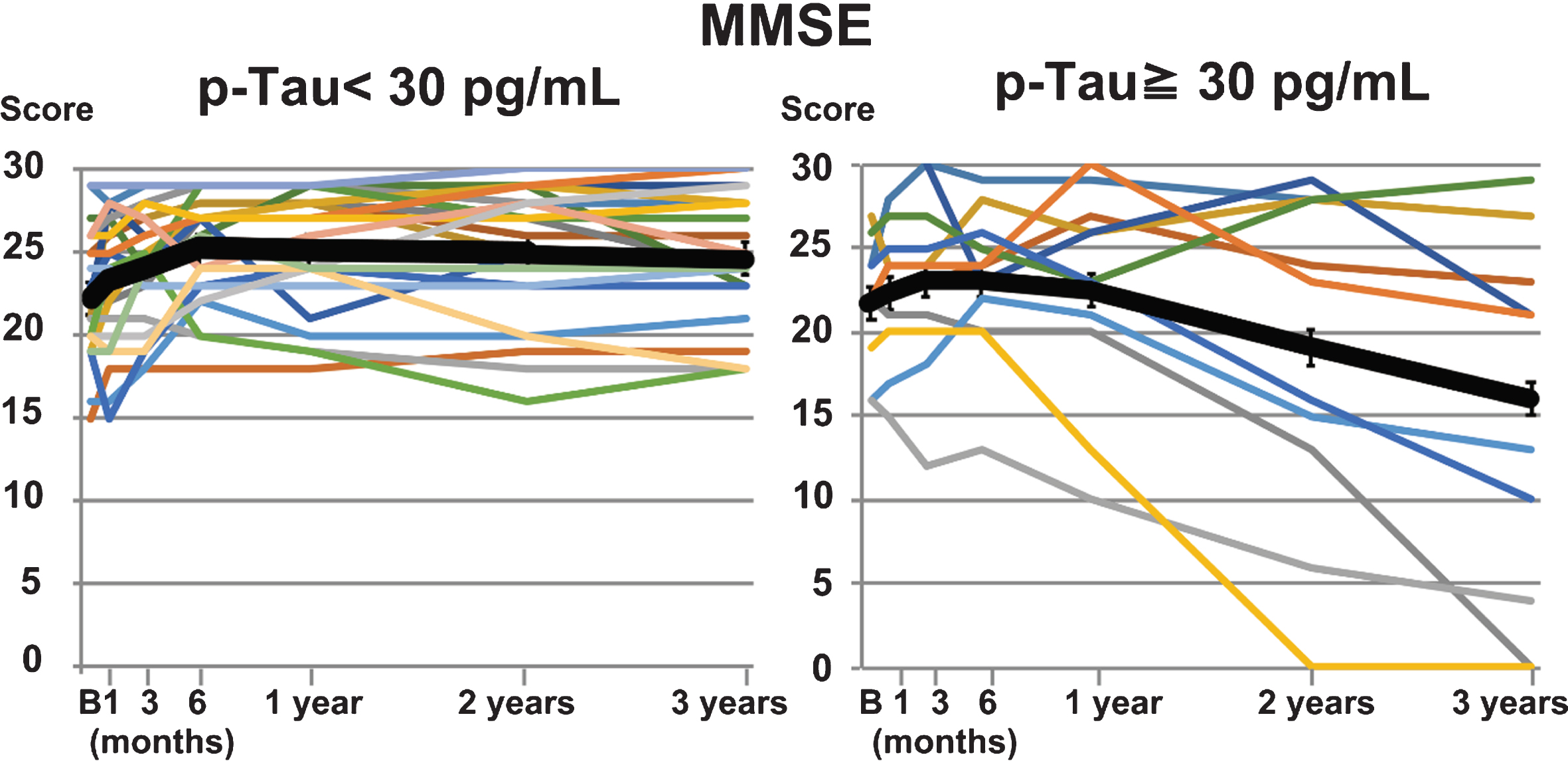
Table 2
Cognitive function in iNPH patients during three-year follow-up after shunt intervention
| Low p-Tau <30 pg/mL (n = 24) | High p-Tau ≥30 pg/mL (n = 11) | Low versus High p-Tau (p) | |||
| MMSE score, mean (SD) | |||||
| Before versus After (p) | Before versus After (p) | ||||
| Before | 22.2 (4.1) | – | 21.7 (3.6) | – | 0.844 |
| 1 month | 23.2 (4.4) | *0.041 | 22.3 (4.2) | 0.359 | 0.519 |
| 3 months | 23.9 (3.5) | *0.003 | 23.1 (5.3) | 0.243 | 0.748 |
| 6 months | 25.1 (3.4) | *<0.001 | 23.1 (4.4) | 0.150 | 0.210 |
| 1 year | 25.0 (3.7) | *0.000 | 22.6 (6.3) | 0.721 | 0.309 |
| 2 years | 24.9 (4.1) | *0.001 | 19.1 (9.9) | 0.562 | 0.116 |
| 3 years | 24.6 (4.0) | *0.005 | 16.1 (11.2) | 0.138 | *0.040 |
| FAB score, mean (SD) | |||||
| Before | 10.9 (3.5) | – | 11.6 (3.2) | – | 0.761 |
| 1 month | 12.2 (3.2) | *0.027 | 10.8 (2.8) | 0.758 | 0.188 |
| 3 months | 12.4 (3.3) | *0.024 | 11.6 (2.7) | 0.590 | 0.345 |
| 6 months | 12.6 (2.9) | *0.006 | 10.7 (2.7) | 0.327 | 0.069 |
| 1 year | 12.9 (2.8) | *0.004 | 11.6 (2.7) | 0.859 | 0.142 |
| 2 years | 12.2 (3.1) | 0.102 | 10.6 (3.5) | 0.667 | 0.180 |
| 3 years | 11.9 (3.3) | 0.224 | 8.4 (3.9) | 0.096 | *0.020 |
Differences in MMSE and FAB scores before and after shunt intervention were evaluated using the Wilcoxon signed-rank test. Differences between the low and high p-Tau groups were evaluated using the Mann–Whitney U test. *p < 0.05.
Predictor for cognition
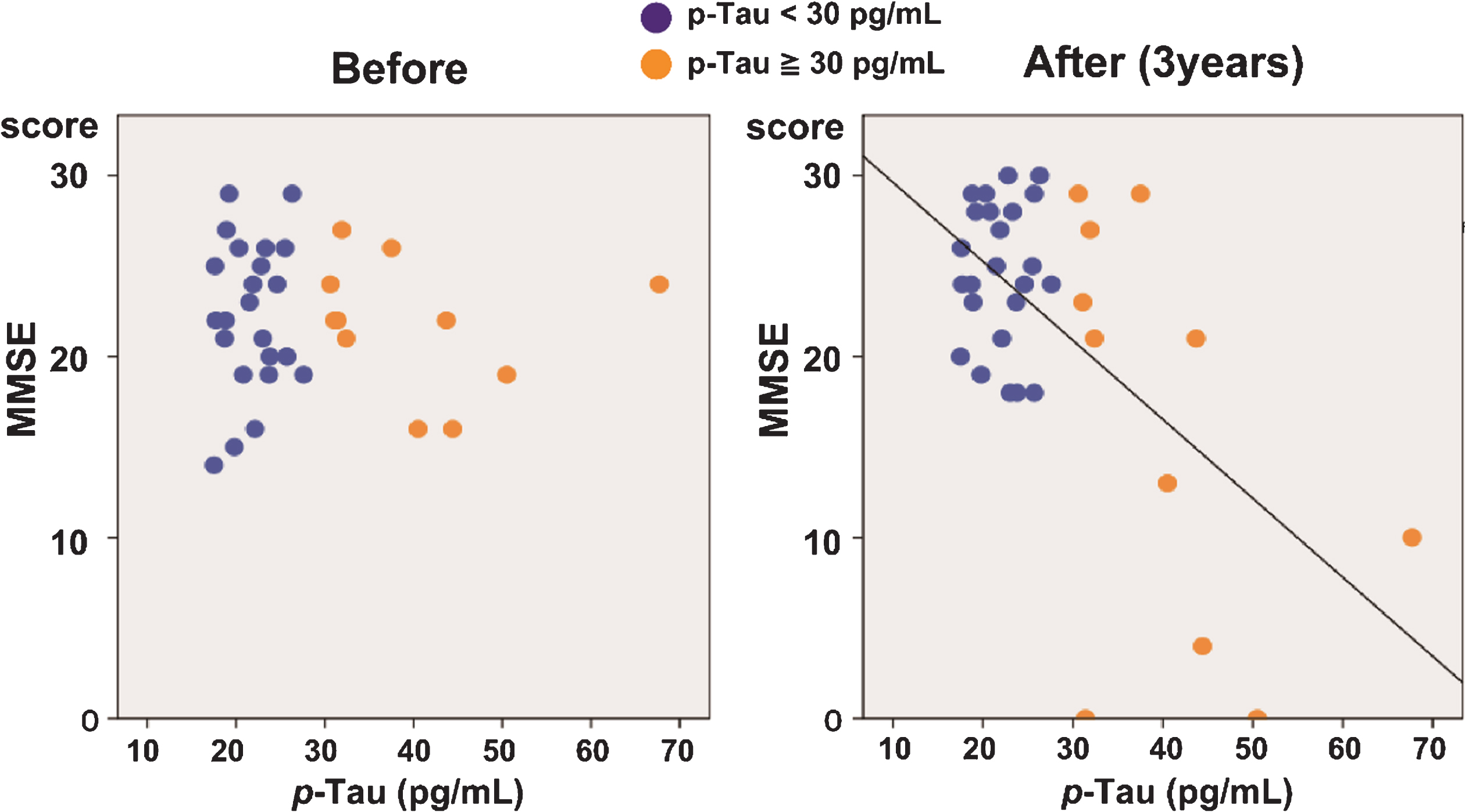
The regression analysis of the correlation between the MMSE scores and p-Tau levels showed that, while no correlation was observed before the surgery, a negative correlation appeared and gradually increased after surgery, reaching a high level of significance after three years (R2 = 0.352, p < 0.001; Table 3, Fig. 3). A negative correlation was also observed between the FAB scores and p-Tau levels at three years after the surgery (R2 = 0.190, p = 0.011). Total Tau levels could also predict MMSE (R2 = 0.168, p = 0.014) and FAB (R2 = 0.122, p = 0.046) performance three years after shunt intervention, whereas Aβ42 levels could not (MMSE: R2 = 0.107, p = 0.055; FAB: R2 = 0.010, p = 0.575).
Table 3
Regression analysis of the correlations between preoperative CSF biomarkers and postoperative neuropsychological test scores
| MMSE | FAB | ||||
| R2 | p | R2 | p | ||
| p-Tau | 1 month | 0.016 | 0.472 | 0.019 | 0.433 |
| 3 months | 0.035 | 0.284 | 0.006 | 0.905 | |
| 6 months | 0.077 | 0.106 | 0.113 | 0.052 | |
| 1 year | 0.137 | *0.028 | 0.009 | 0.590 | |
| 2 years | 0.305 | *0.001 | 0.073 | 0.117 | |
| 3 years | 0.352 | *<0.001 | 0.190 | *0.011 | |
| Total Tau | 1 month | 0.001 | 0.886 | 0.002 | 0.828 |
| 3 months | 0.007 | 0.632 | 0.023 | 0.387 | |
| 6 months | 0.071 | 0.121 | 0.010 | 0.574 | |
| 1 year | 0.138 | *0.028 | 0.000 | 0.915 | |
| 2 years | 0.173 | *0.013 | 0.030 | 0.318 | |
| 3 years | 0.168 | *0.014 | 0.122 | *0.046 | |
| Aβ42 | 1 month | 0.033 | 0.304 | 0.021 | 0.413 |
| 3 months | 0.069 | 0.127 | 0.086 | 0.092 | |
| 6 months | 0.012 | 0.535 | 0.109 | 0.057 | |
| 1 year | 0.004 | 0.704 | 0.013 | 0.516 | |
| 2 years | 0.069 | 0.127 | 0.036 | 0.274 | |
| 3 years | 0.107 | 0.055 | 0.010 | 0.575 | |
| p-Tau/Aβ42 | 1 month | 0.034 | 0.296 | 0.048 | 0.214 |
| 3 months | 0.083 | 0.094 | 0.023 | 0.388 | |
| 6 months | 0.068 | 0.131 | 0.173 | *0.015 | |
| 1 year | 0.093 | 0.075 | 0.010 | 0.561 | |
| 2 years | 0.290 | *0.001 | 0.099 | 0.066 | |
| 3 years | 0.354 | *<0.001 | 0.121 | *0.047 | |
The preoperative concentration of p-Tau was the most accurate predictor of the MMSE and FAB scores after shunting intervention, showing significant negative correlations with the MMSE score at one, two, and three years, and the FAB score at three years after shunt intervention. R, coefficient of determination. *p < 0.05.
Fig.3
Correlation between p-Tau levels and MMSE scores in iNPH patients. Correlation between the MMSE scores and p-Tau levels before (left) and three years after (right) shunt surgery. While no significant correlation was observed before the treatment, a significant negative correlation was observed three years after surgery (R2 = 0.352, p < 0.001).
mRS
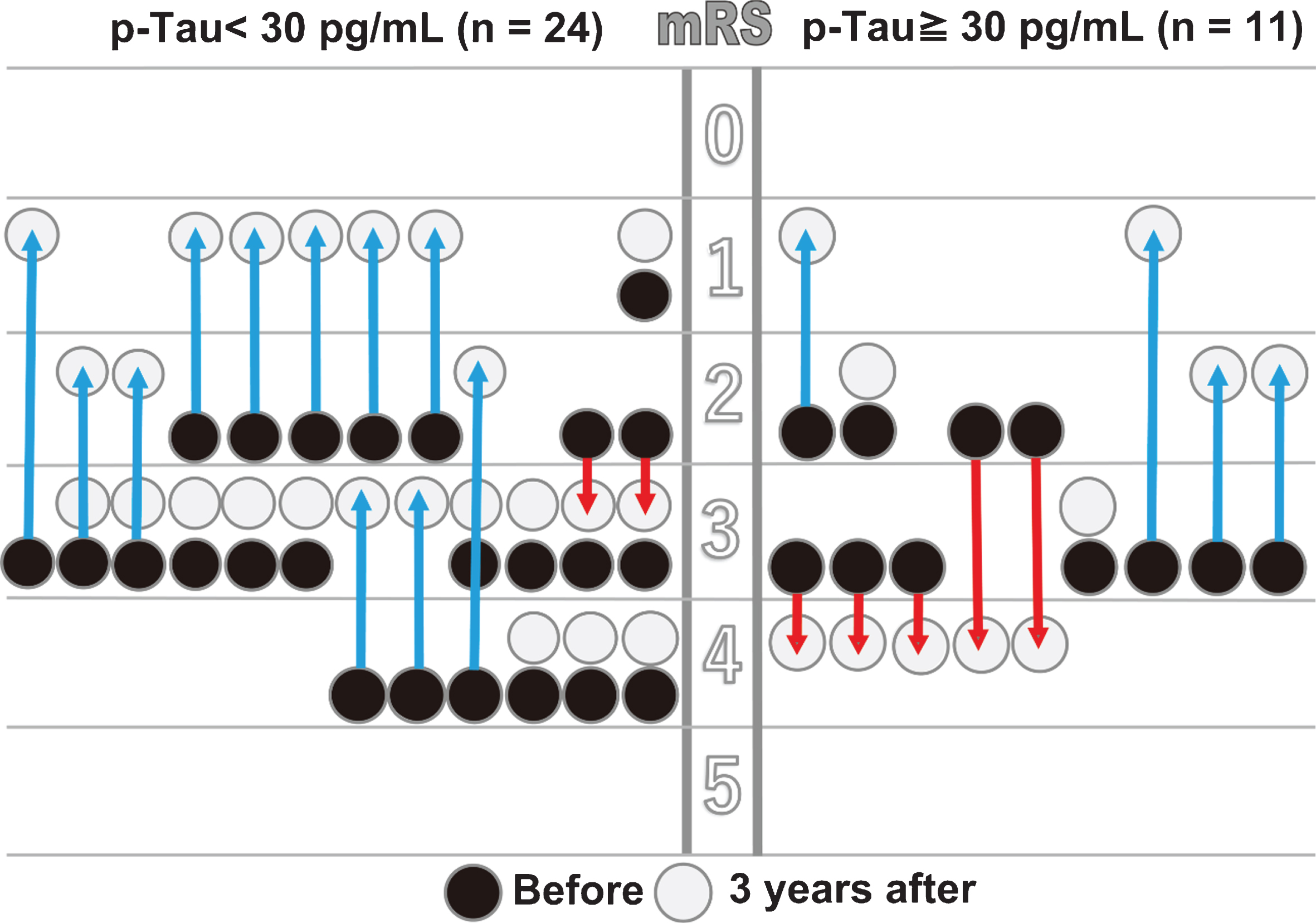
A statistically significant improvement was observed in the mRS score in the low p-Tau group at 1-month post-treatment (p = 0.011), but the improvement was not significant in the high p-Tau group (p = 0.083; Table 4). There was no statistically significant difference between the groups after three years (p = 0.293); however, an improved mRS score was maintained in the low p-Tau group. In contrast, the mRS score in the high p-Tau group gradually declined to below the preoperative value at three years after intervention. After three years, 8.3% of the patients in the low p-Tau group and 45.5% in the high p-Tau group showed decreased mRS scores, and this percentage difference was statistically significant between groups (p = 0.011; Fig. 4).
Table 4
Changes in mRS performance during three-year follow-up after shunt intervention
| p-Tau <30 pg/mL (n = 24) | p-Tau ≥30 pg/mL (n = 11) | Low versus High p-Tau (p) | |||
| mRS grade (SD) | Before versus After (p) | mRS score (SD) | Before versus After (p) | ||
| Before | 2.88 (0.85) | – | 2.80 (0.76) | – | 0.375 |
| 1 month | 2.54 (0.93) | *0.011 | 2.36 (0.51) | 0.083 | 0.423 |
| 3 months | 2.38 (0.97) | *0.001 | 2.36 (0.51) | 0.083 | 0.970 |
| 6 months | 2.25 (1.03) | *0.001 | 2.36 (0.51) | 0.083 | 0.666 |
| 1 year | 2.33 (1.09) | *0.013 | 2.45 (0.69) | 0.315 | 0.724 |
| 2 years | 2.25 (1.11) | *0.002 | 2.55 (1.21) | 0.755 | 0.497 |
| 3 years | 2.42 (1.06) | *0.012 | 2.82 (1.25) | 0.623 | 0.293 |
| Worse/Improved, number (worse %) | Worse/Improved, number (worse %) | ||||
| 1 month | 0/24 (0%) | 0/11 (0%) | Not available | ||
| 3 months | 0/24 (0%) | 0/11 (0%) | Not available | ||
| 6 months | 0/24 (0%) | 0/11 (0%) | Not available | ||
| 1 year | 1/23 (4.2%) | 1/10 (9.1%) | 0.560 | ||
| 2 years | 1/23 (4.2%) | 5/6 (45.5%) | *0.003 | ||
| 3 years | 2/22 (8.3%) | 5/6 (45.5%) | *0.011 | ||
Differences in mRS score before and after surgery were evaluated using the Wilcoxon signed-rank test. Differences between low and high p-Tau groups were assessed using the Mann–Whitney U test. The worse/improved patient ratios were evaluated using Pearson’s chi-squared test. *p < 0.05.
Fig.4
Comparison of mRS performance between the low and high p-Tau groups. Changes in the individual patients’ mRS scores from before to three years after shunt treatment are shown for the low (<30 pg/mL, n = 24) and high (≥30 pg/mL, n = 11) p-Tau groups. The arrows show individual improvements (blue) or declines (red).
iNPHGS
A significant difference was observed in gait disturbance between the low and high p-Tau groups after three months (p = 0.024; Table 5, Fig. 5). Gait improved early, and remained improved throughout the three-year follow-up period, in the low p-Tau group. In contrast, the improvement in the high p-Tau group was poor and dropped below the preoperative value after three years.
Table 5
Changes in iNPHGS scores during three-year follow-up after shunt intervention
| p-Tau <30 pg/mL (n = 24) | p-Tau ≥30 pg/mL (n = 11) | Low versus High p-Tau | |||
| iNPHGS | mean (SD) | Before versus After (p) | mean (SD) | Before versus After (p) | (p) |
| Gait disturbance | 2.46 (0.72) | – | 2.64 (0.51) | – | 0.343 |
| : Before | |||||
| : 1 month | 2.00 (0.78) | *0.001 | 2.36 (0.51) | 0.083 | 0.194 |
| : 3 months | 1.75 (0.79) | *<0.001 | 2.36 (0.51) | 0.083 | *0.024 |
| : 6 months | 1.75 (0.79) | *<0.001 | 2.36 (0.51) | 0.083 | *0.024 |
| : 1 years | 1.75 (0.79) | *<0.001 | 2.46 (0.69) | 0.317 | *0.020 |
| : 2 years | 1.75 (0.94) | *<0.001 | 2.55 (1.21) | 0.755 | 0.076 |
| : 3 years | 1.79 (0.98) | *0.001 | 2.82 (1.25) | 0.623 | *0.030 |
| Cognitive impairment | 1.75 (0.94) | – | 2.09 (0.70) | – | 0.311 |
| : Before | |||||
| : 1 month | 1.46 (0.83) | 0.058 | 1.82 (0.98) | 0.180 | 0.275 |
| : 3 months | 1.29 (0.75) | *0.013 | 1.73 (1.04) | *0.046 | 0.142 |
| : 6 months | 1.21 (0.78) | *0.003 | 1.55 (1.21) | *0.034 | 0.308 |
| : 1 year | 1.21 (0.93) | *0.005 | 1.64 (1.33) | 0.096 | 0.325 |
| : 2 years | 1.13 (1.04) | *0.008 | 1.82 (1.38) | 0.366 | 0.136 |
| : 3 years | 1.29 (1.08) | 0.053 | 2.09 (1.04) | 1.000 | 0.096 |
| Urinary incontinence | 1.54 (0.93) | – | 1.91 (1.04) | – | 0.489 |
| : Before | |||||
| : 1 month | 1.50 (0.66) | 0.854 | 1.73 (0.65) | 0.739 | 0.467 |
| : 3 months | 1.08 (0.88) | *0.008 | 1.36 (0.81) | 0.084 | 0.316 |
| : 6 months | 1.00 (0.66) | *0.003 | 1.18 (0.98) | 0.123 | 0.840 |
| : 1 year | 1.04 (0.81) | *0.001 | 1.55 (1.29) | 0.459 | 0.331 |
| : 2 years | 0.83 (0.82) | *0.001 | 1.64 (1.37) | 0.550 | 0.194 |
| : 3 years | 0.96 (0.81) | *0.013 | 1.91 (1.30) | 0.856 | *0.029 |
Differences in iNPHGS score before and after shunting were evaluated using the Wilcoxon signed-rank test. Differences in iNPHGS score between the low and high p-Tau groups were evaluated using the Mann–Whitney U test. *p < 0.05.
Fig.5
Heat map of iNPHGS impairments in the low and high p-Tau groups over three-year follow-up. Scores on the three iNPHGS subcategories (gait disturbance, urinary disturbance, and dementia) in the low (<30 pg/mL, n = 24; lower panel) and high (≥30 pg/mL, n = 11; upper panel) p-Tau groups during the follow-up period are colored by the degree of severity.
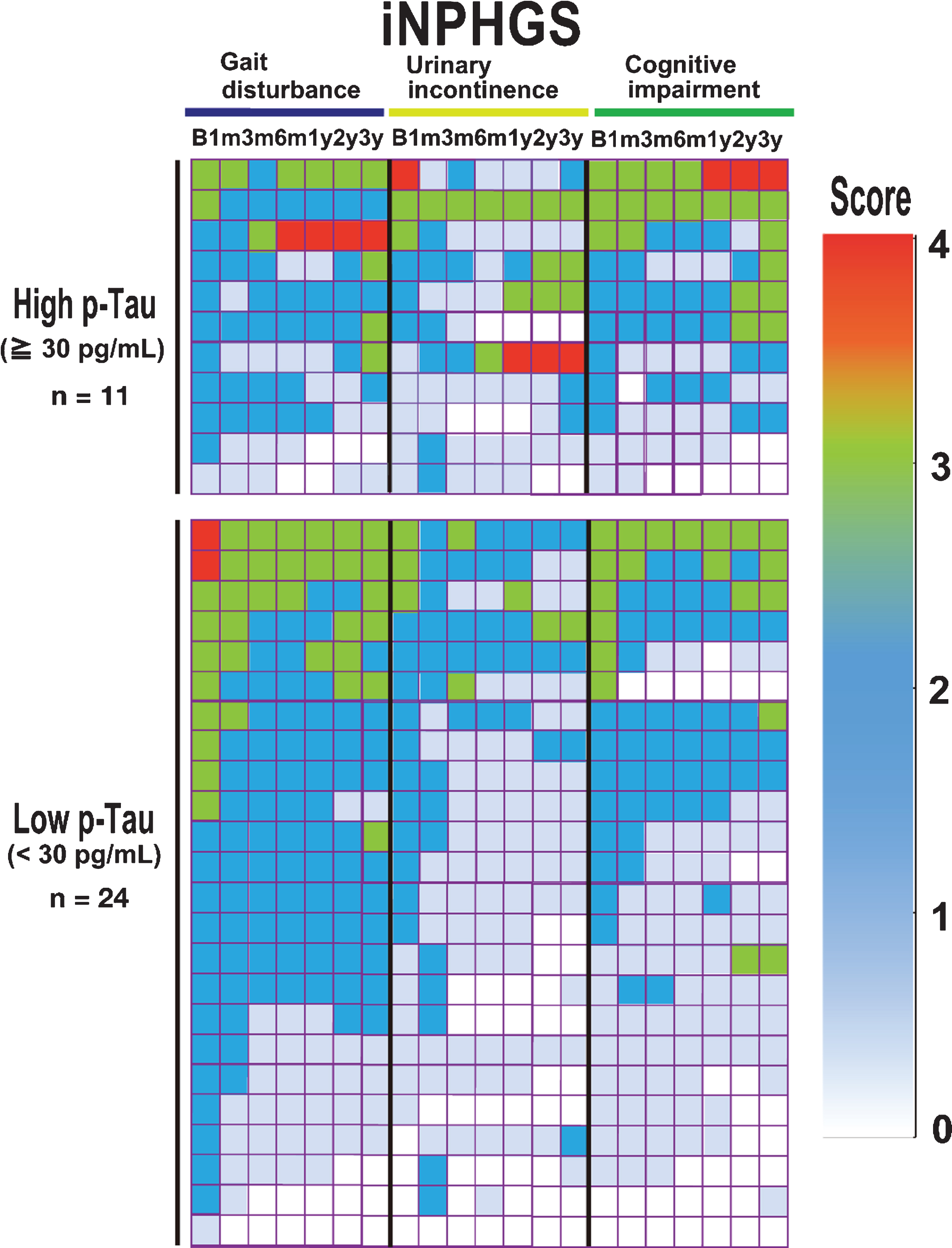
An improvement in cognitive function was observed in the early postoperative stages in both groups, but gradually declined after peaking at six months. Moreover, after three years, the cognitive iNPHGS items were at the same levels as pretreatment in the high p-Tau group. No significant difference was detected between the two groups (p = 0.096; Table 4).
Urinary function as assessed by the iNPHGS improved gradually from three months to six months in the low p-Tau group, and this improvement was maintained after three years. A similar gradual improvement was observed from three to six months in the high p-Tau group; however, it was not statistically significant. Moreover, urinary function in the high p-Tau group gradually worsened after a year. A statistically significant difference was observed between the two groups after three years (p = 0.029; Table 4).
DISCUSSION
Whether shunt treatment for iNPH is economically justified is a matter of debate. According to the Laupacis standards [34], for the treatment to be cost-effective, a treated group needs to maintain the mRS score for 18 months after ventriculoperitoneal shunt, and 21 months after LP shunt surgery, with grade 1 evidence [35]. For this requirement to be realized, the treatment effect of shunt intervention for patients with iNPH should be maintained for at least two years. According to Kazui and colleagues, attenuation of cognitive impairment is a major factor contributing to the caregiver analysis through Zarit burden interviews [36, 37]. The diagnosis of iNPH remains challenging, especially at early stages, when presenting symptoms that largely overlap with those of other neurodegenerative diseases [38]. In particular, cognitive function is affected most strongly by AD comorbidity, leading to a poor response to shunting intervention [25, 39]. Therefore, we consider the identification of AD comorbidity to be crucial for successful shunt intervention in iNPH.
While a diagnosis of iNPH is typically based on symptom evaluation and radiographic findings [3], identifying patients who are more likely to respond to specific treatments by adding a CSF biomarker adjunctive diagnostic test can increase the effectiveness of our limited medical resources [17, 18, 40, 41]. To develop such a test in the present study, we categorized patients with iNPH based on biochemical analyses of CSF collected before shunt intervention, and then retrospectively tracked cognitive function, gait disturbance, urinary incontinence, and mRS performance for three years after the surgery. Group analyses showed that the duration of shunt-induced improvements in these functions was shorter in the high p-Tau group compared with the low p-Tau group. To the best of our knowledge, this is the first report of a correlation between preoperative p-Tau CSF levels (determined using the xMAP Luminex platform) and three-year prognosis after shunt intervention in patients with iNPH. We used MMSE, FAB, mRS, and iNPHGS scores as cognitive outcome measures. Notably, the results of cognitive function evaluation with the MMSE and FAB differed from those obtained with the iNPHGS. This difference is likely attributable to the iNPHGS including multiple subjective elements.
Our results showed that the current practice of using radiographic findings and symptom assessment only for diagnosis is not sufficient to predict the long-term success of shunt treatment for iNPH. In addition to conventional diagnosis, preoperative CSF parameters, measured by the xMAP Luminex platform, were useful for a more accurate long-term prognosis. There may be temporary relief following a restoration of CSF flow, but the presence of underlying neurodegenerative changes more directly correlates with long-term outcomes. There may also be a direct correlation between altered CSF dynamics and both metabolic clearance and the development of dementia [42].
A significant correlation between total Tau and p-Tau levels was observed; however, including total Tau in our data did not improve analysis accuracy. In addition, there was no correlation between Aβ42 concentration and cognition after surgery. In this study, the participants were grouped by p-Tau concentration alone to evaluate the effects of comorbid AD pathology [34]. However, it may be premature to conclude that the observed negative correlation between cognitive outcomes and p-Tau levels reflects the advancement of AD because cerebral tissue pathology [43, 44] and Tau PET findings [45, 46] were not considered. CSF shunting may have been partially effective in the high p-Tau group because favorable outcomes, albeit non-significant when compared with preoperative values, were achieved in the short term. The non-significant mRS and iNPHGS improvements may be attributed to the small number of cases in the high p-Tau group. In the short-term follow-up, absolute improvements appeared to be similar between the groups. As discussed above, shunt intervention requires nearly two years of sustained improvement to be cost-effective. This condition was sufficiently satisfied in the low p-Tau group; however, a clear judgment cannot be made on the economic prudence of this treatment in patients with high p-Tau levels.
This report has several limitations. Selection bias may have affected the results of this single-center retrospective study. In addition, arguments have been put forth against using CSF biomarkers as evidence of AD comorbidity in patients with iNPH. Various factors, such as CSF retention and diffusion impairment, affect the levels of CSF biomarkers obtained from lumbar puncture, and may reflect disturbances in the transition or clearance of Aβ and Tau proteins from the interstitial cell fluid to the L-CSF [47]. Parkinson’s-related neurodegenerative diseases, which are similar to AD and thought to have high rates of complications, cannot be identified by p-Tau, total Tau, or Aβ42 CSF levels. In cases of iNPH where AD coexists, comorbidities in Parkinson’s-related neurodegenerative diseases have a strong impact on the long-term prognosis of iNPH [48]. Therefore, incorporating the results of adjunctive diagnostic tests, such as the DaT scan (dopamine transporter visualization using single-photon emission computed tomography brain imaging), into the diagnosis [49, 50] and adding the details of complications for Parkinson’s-related neurodegenerative disease to the analysis, may also aid in deciding whether shunt intervention is advisable.
In conclusion, preoperative CSF p-Tau levels predicted the long-term prognosis after shunt intervention in patients with iNPH. Improvements across all evaluated items, including mRS score, were maintained for three years after treatment in the low p-Tau group. In the high p-Tau group, improvement was observed in the early stage, peaked at six months, and gradually declined to or below preoperative levels. Therefore, shunt intervention in patients with iNPH with coexisting AD may be effective in the short term. The indication of shunt intervention for patients with iNPH whose symptoms improve for a limited period of time needs further study and discussion.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Tsukie from the Department of Molecular Genetics, Brain Research Institute, Niigata University, Niigata, Japan, for their assistance with data management.
This work was supported in part by the Ministry of Health, Labor, and Welfare of Japan (2014-Nanchi-General-052), and in part by Grants-in-Aid for Scientific Research (grant numbers 16KK0187, 17K10908, and 18H02916) from the Japan Society for the Promotion of Science and by AMED JP18dk0207028 and JP18dm0107143.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0557r1).
REFERENCES
[1] | Hakim S , Adams RD ((1965) ) The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 2: , 307–327. |
[2] | Ishikawa M , Hashimoto M , Kuwana N , Mori E , Miyake H , Wachi A , Takeuchi T , Kazui H , Koyama H ((2008) ) Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 48: (Suppl), S1–23. |
[3] | Mori E , Ishikawa M , Kato T , Kazui H , Miyake H , Miyajima M , Nakajima M , Hashimoto M , Kuriyama N , Tokuda T , Ishii K , Kaijima M , Hirata Y , Saito M , Arai H , Japanese Society of Normal Pressure H ((2012) ) Guidelines for management of idiopathic normal pressure hydrocephalus: Second edition. Neurol Med Chir (Tokyo) 52: , 775–809. |
[4] | Hashimoto M , Ishikawa M , Mori E , Kuwana N , Study of Ioni ((2010) ) Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: A prospective cohort study. Cerebrospinal Fluid Res 7: , 18. |
[5] | Kitagaki H , Mori E , Ishii K , Yamaji S , Hirono N , Imamura T ((1998) ) CSF spaces in idiopathic normal pressure hydrocephalus: Morphology and volumetry. AJNR Am J Neuroradiol 19: , 1277–1284. |
[6] | Kazui H , Miyajima M , Mori E , Ishikawa M , Investigators S ((2015) ) Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): An open-label randomised trial. Lancet Neurol 14: , 585–594. |
[7] | Saper CB ((2017) ) Is there even such a thing as “Idiopathic normal pressure hydrocephalus”? Ann Neurol 82: , 514–515. |
[8] | Espay AJ , Lang AE ((2017) ) Is there even such a thing as “idiopathic normal pressure hydrocephalus”? Ann Neurol 82: , 1032. |
[9] | Espay AJ , Da Prat GA , Dwivedi AK , Rodriguez-Porcel F , Vaughan JE , Rosso M , Devoto JL , Duker AP , Masellis M , Smith CD , Mandybur GT , Merola A , Lang AE ((2017) ) Deconstructing normal pressure hydrocephalus: Ventriculomegaly as early sign of neurodegeneration. Ann Neurol 82: , 503–513. |
[10] | Shaw LM , Vanderstichele H , Knapik-Czajka M , Figurski M , Coart E , Blennow K , Soares H , Simon AJ , Lewczuk P , Dean RA , Siemers E , Potter W , Lee VM , Trojanowski JQ , Alzheimer’s Disease Neuroimaging Initiative ((2011) ) Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 121: , 597–609. |
[11] | Iwatsubo T , Iwata A , Suzuki K , Ihara R , Arai H , Ishii K , Senda M , Ito K , Ikeuchi T , Kuwano R , Matsuda H , Japanese Alzheimer’s Disease Neuroimaging Initiative, Sun CK , Beckett LA , Petersen RC , Weiner MW , Aisen PS , Donohue MC , Alzheimer’s Disease Neuroimaging Initiative ((2018) ) Japanese and North American Alzheimer’s Disease Neuroimaging Initiative studies: Harmonization for international trials. Alzheimers Dement 14: , 1077–1087. |
[12] | Golomb J , Wisoff J , Miller DC , Boksay I , Kluger A , Weiner H , Salton J , Graves W ((2000) ) Alzheimer’s disease comorbidity in normal pressure hydrocephalus: Prevalence and shunt response. J Neurol Neurosurg Psychiatry 68: , 778–781. |
[13] | Ott BR , Cohen RA , Gongvatana A , Okonkwo OC , Johanson CE , Stopa EG , Donahue JE , Silverberg GD , Alzheimer’s Disease Neuroimaging Initiative ((2010) ) Brain ventricular volume and cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis 20: , 647–657. |
[14] | Patel S , Lee EB , Xie SX , Law A , Jackson EM , Arnold SE , Clark CM , Shaw LM , Grady MS , Trojanowski JQ , Hamilton RH ((2012) ) Phosphorylated tau/amyloid beta 1-42 ratio in ventricular cerebrospinal fluid reflects outcome in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 9: , 7. |
[15] | Pyykko OT , Lumela M , Rummukainen J , Nerg O , Seppala TT , Herukka SK , Koivisto AM , Alafuzoff I , Puli L , Savolainen S , Soininen H , Jaaskelainen JE , Hiltunen M , Zetterberg H , Leinonen V ((2014) ) Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One 9: , e91974. |
[16] | Jeppsson A , Zetterberg H , Blennow K , Wikkelso C ((2013) ) Idiopathic normal-pressure hydrocephalus: Pathophysiology and diagnosis by CSF biomarkers. Neurology 80: , 1385–1392. |
[17] | Moriya M , Miyajima M , Nakajima M , Ogino I , Arai H ((2015) ) Impact of cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus on the amyloid cascade. PLoS One 10: , e0119973. |
[18] | Nakajima M , Miyajima M , Ogino I , Akiba C , Sugano H , Hara T , Fusegi K , Karagiozov K , Arai H ((2015) ) Cerebrospinal fluid biomarkers for prognosis of long-term cognitive treatment outcomes in patients with idiopathic normal pressure hydrocephalus. J Neurol Sci 357: , 88–95. |
[19] | Jingami N , Asada-Utsugi M , Uemura K , Noto R , Takahashi M , Ozaki A , Kihara T , Kageyama T , Takahashi R , Shimohama S , Kinoshita A ((2015) ) Idiopathic normal pressure hydrocephalus has a different cerebrospinal fluid biomarker profile from Alzheimer’s disease. J Alzheimers Dis 45: , 109–115. |
[20] | Akiba C , Nakajima M , Miyajima M , Ogino I , Motoi Y , Kawamura K , Adachi S , Kondo A , Sugano H , Tokuda T , Irie K , Arai H ((2018) ) Change of amyloid-beta 1-42 toxic conformer ratio after cerebrospinal fluid diversion predicts long-term cognitive outcome in patients with idiopathic normal pressure hydrocephalus. J Alzheimers Dis 63: , 989–1002. |
[21] | De Meyer G , Shapiro F , Vanderstichele H , Vanmechelen E , Engelborghs S , De Deyn PP , Coart E , Hansson O , Minthon L , Zetterberg H , Blennow K , Shaw L , Trojanowski JQ , Alzheimer’s Disease Neuroimaging Initiative ((2010) ) Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67: , 949–956. |
[22] | Vemuri P , Wiste HJ , Weigand SD , Shaw LM , Trojanowski JQ , Weiner MW , Knopman DS , Petersen RC , Jack CR Jr , Alzheimer’s Disease Neuroimaging Initiative ((2009) ) MRI and CSF biomarkers in normal, MCI, and AD subjects: Diagnostic discrimination and cognitive correlations. Neurology 73: , 287–293. |
[23] | Blennow K , Hampel H ((2003) ) CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2: , 605–613. |
[24] | Buerger K , Ewers M , Pirttila T , Zinkowski R , Alafuzoff I , Teipel SJ , DeBernardis J , Kerkman D , McCulloch C , Soininen H , Hampel H ((2006) ) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129: , 3035–3041. |
[25] | Hamilton R , Patel S , Lee EB , Jackson EM , Lopinto J , Arnold SE , Clark CM , Basil A , Shaw LM , Xie SX , Grady MS , Trojanowski JQ ((2010) ) Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol 68: , 535–540. |
[26] | Folstein MF , Robins LN , Helzer JE ((1983) ) The Mini-Mental State Examination. Arch Gen Psychiatry 40: , 812. |
[27] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[28] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB: A Frontal Assessment Battery at bedside. Neurology 55: , 1621–1626. |
[29] | Kubo Y , Kazui H , Yoshida T , Kito Y , Kimura N , Tokunaga H , Ogino A , Miyake H , Ishikawa M , Takeda M ((2008) ) Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr Cogn Disord 25: , 37–45. |
[30] | Nakajima M , Miyajima M , Akiba C , Ogino I , Kawamura K , Sugano H , Hara T , Tange Y , Fusegi K , Karagiozov K , Arai H ((2018) ) Lumboperitoneal shunts for the treatment of idiopathic normal pressure hydrocephalus: A comparison of small-lumen abdominal catheters to gravitational add-on valves in a single center. Oper Neurosurg (Hagerstown). doi: 10.1093/ons/opy044 |
[31] | Nakajima M , Miyajima M , Ogino I , Sugano H , Akiba C , Domon N , Karagiozov KL , Arai H ((2015) ) Use of external lumbar cerebrospinal fluid drainage and lumboperitoneal shunts with Strata NSC valves in idiopathic normal pressure hydrocephalus: A single-center experience. World Neurosurg 83: , 387–393. |
[32] | Olsson A , Vanderstichele H , Andreasen N , De Meyer G , Wallin A , Holmberg B , Rosengren L , Vanmechelen E , Blennow K ((2005) ) Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem 51: , 336–345. |
[33] | Vanderstichele H , De Meyer G , Andreasen N , Kostanjevecki V , Wallin A , Olsson A , Blennow K , Vanmechelen E ((2005) ) Amino-truncated beta-amyloid42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin Chem 51: , 1650–1660. |
[34] | Laupacis A , Feeny D , Detsky AS , Tugwell PX ((1992) ) How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 146: , 473–481. |
[35] | Kameda M , Yamada S , Atsuchi M , Kimura T , Kazui H , Miyajima M , Mori E , Ishikawa M , Date I , Sinphoni Investigators S ((2017) ) Cost-effectiveness analysis of shunt surgery for idiopathic normal pressure hydrocephalus based on the SINPHONI and SINPHONI-2 trials. Acta Neurochir (Wien) 159: , 995–1003. |
[36] | Zarit SH , Reever KE , Bach-Peterson J ((1980) ) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20: , 649–655. |
[37] | Kazui H , Mori E , Hashimoto M , Ishikawa M , Hirono N , Takeda M ((2011) ) Effect of shunt operation on idiopathic normal pressure hydrocephalus patients in reducing caregiver burden: Evidence from SINPHONI. Dement Geriatr Cogn Disord 31: , 363–370. |
[38] | Schirinzi T , Sancesario GM , Di Lazzaro G , D’Elia A , Imbriani P , Scalise S , Pisani A ((2018) ) Cerebrospinal fluid biomarkers profile of idiopathic normal pressure hydrocephalus. J Neural Transm (Vienna) 125: , 673–679. |
[39] | Lim TS , Choi JY , Park SA , Youn YC , Lee HY , Kim BG , Joo IS , Huh K , Moon SY ((2014) ) Evaluation of coexistence of Alzheimer’s disease in idiopathic normal pressure hydrocephalus using ELISA analyses for CSF biomarkers. BMC Neurol 14: , 66. |
[40] | Miyajima M , Nakajima M , Ogino I , Miyata H , Motoi Y , Arai H ((2013) ) Soluble amyloid precursor protein alpha in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. Eur J Neurol 20: , 236–242. |
[41] | Nakajima M , Miyajima M , Ogino I , Watanabe M , Miyata H , Karagiozov KL , Arai H , Hagiwara Y , Segawa T , Kobayashi K , Hashimoto Y ((2011) ) Leucine-rich alpha-2-glycoprotein is a marker for idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 153: , 1339–1346; discussion 1346. |
[42] | Pomeraniec IJ , Taylor DG , Bond AE , Lopes MB ((2018) ) Concurrent Alzheimer’s pathology in patients with clinical normal pressure hydrocephalus. J Neurosurg Sci. doi: 10.23736/S0390-5616.18.04350-3 |
[43] | Bech-Azeddine R , Hogh P , Juhler M , Gjerris F , Waldemar G ((2007) ) Idiopathic normal-pressure hydrocephalus: Clinical comorbidity correlated with cerebral biopsy findings and outcome of cerebrospinal fluid shunting. J Neurol Neurosurg Psychiatry 78: , 157–161. |
[44] | Leinonen V , Koivisto AM , Savolainen S , Rummukainen J , Tamminen JN , Tillgren T , Vainikka S , Pyykko OT , Molsa J , Fraunberg M , Pirttila T , Jaaskelainen JE , Soininen H , Rinne J , Alafuzoff I ((2010) ) Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann Neurol 68: , 446–453. |
[45] | Xia CF , Arteaga J , Chen G , Gangadharmath U , Gomez LF , Kasi D , Lam C , Liang Q , Liu C , Mocharla VP , Mu F , Sinha A , Su H , Szardenings AK , Walsh JC , Wang E , Yu C , Zhang W , Zhao T , Kolb HC ((2013) ) [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 9: , 666–676. |
[46] | Marquie M , Normandin MD , Vanderburg CR , Costantino IM , Bien EA , Rycyna LG , Klunk WE , Mathis CA , Ikonomovic MD , Debnath ML , Vasdev N , Dickerson BC , Gomperts SN , Growdon JH , Johnson KA , Frosch MP , Hyman BT , Gomez-Isla T ((2015) ) Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 78: , 787–800. |
[47] | Graff-Radford NR ((2014) ) Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology 83: , 1573–1575. |
[48] | Malm J , Graff-Radford NR , Ishikawa M , Kristensen B , Leinonen V , Mori E , Owler BK , Tullberg M , Williams MA , Relkin NR ((2013) ) Influence of comorbidities in idiopathic normal pressure hydrocephalus - research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 10: , 22. |
[49] | Catafau AM , Tolosa E , DaTSCAN Clinically Uncertain Parkinsonian Syndromes Study Group, ((2004) ) Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord 19: , 1175–1182. |
[50] | Tolosa E , Borght TV , Moreno E , DaTSCAN Clinically Uncertain Parkinsonian Syndromes Study Group, ((2007) ) Accuracy of DaTSCAN (123I-Ioflupane) SPECT in diagnosis of patients with clinically uncertain parkinsonism: 2-year follow-up of an open-label study. Mov Disord 22: , 2346–2351. |




