Gender and Educational Differences in the Association between Lifestyle and Cognitive Decline over 10 Years: The Doetinchem Cohort Study
Abstract
Background:
Several modifiable risk factors for cognitive decline have been identified, but whether differences by gender and educational level exist is unclear.
Objective:
The present study aims to clarify this by prospectively investigating the relationship between health and lifestyle factors and cognitive functioning in different subgroups defined by gender and educational level.
Methods:
2,347 cognitive healthy individuals (mean age = 54.8, SD = 6.8, range: 41–71; 51.8% female; 26.2% low education) from the Doetinchem Cohort Study were examined for cognitive function at baseline, and at 5- and 10-year follow-up. Health- and lifestyle factors were captured by a poly-environmental risk score labelled ‘LIfestyle for BRAin Health’ (LIBRA). This score consists of 12 modifiable risk and protective factors for cognitive decline and dementia, with higher scores indicating greater risk (range: –2.7 to +12.7). Heterogeneity in associations between LIBRA and decline in verbal memory, cognitive flexibility, and mental speed between males and females and individuals with different levels of education were assessed in linear mixed models.
Results:
Overall, higher LIBRA scores predicted faster decline in verbal memory, cognitive flexibility, and mental speed over 10 years. Higher LIBRA scores were further associated with increased risk for incident cognitive impairment (one-point increase in LIBRA: HR = 1.09, 1.04–1.14, p = 0.001). In general, these effects were similar across gender and educational level.
Conclusion:
A composite risk score comprising unhealthy lifestyle and relatively poor health in midlife is significantly associated with a worse course of cognition 10 years later. These associations were for the most part unrelated to gender or educational differences.
INTRODUCTION
The increasing number of people with dementia worldwide will majorly affect the burden on the health care system and society as a whole [1, 2]. While investing in the quest to find a disease-modifiable drug for dementia is important, primary prevention is likely the best strategy to promote brain health in late life [3]. One-third of all Alzheimer’s disease cases worldwide have been attributed to common modifiable risk factors under the causality assumption [4, 5]. The incidence of cognitive disorders like dementia might thus be reduced through effective primary prevention strategies such as health education and lifestyle interventions [3, 6]. Yet, evidence from dementia intervention studies is scarce due to unresolved methodological issues such as the identification of risk factors with the biggest impact, the optimal time window when factors have the best potential to positively or negatively affect brain health, and the intensity of interventions [6]. Additionally, the current literature on risk factors for cognitive decline or dementia is not clear on specific gender- and education-effects. Female gender has been associated with increased risk of developing dementia in several European cohort studies [7–9], which partly can be related to increased life expectancy in women [10]. However, more recent population-based studies from the United States found no gender differences with regard to dementia prevalence and incidence [11–14]. Differences by educational level are often related to a greater brain reserve capacity in people with a high educational level [15], but can also be linked to differences in general health and lifestyle. Having a better understanding of the gender and educational differences in dementia risk factor profiles should inform the design of (future) personalized prevention programs.
Therefore, the aim of the present study is to investigate the relationship between a compound score of modifiable health and lifestyle factors and change in cognitive functioning over 10 years in the general population. More specifically, heterogeneity in associations between different subgroups defined by gender and educational level will be studied.
METHODS
Study population
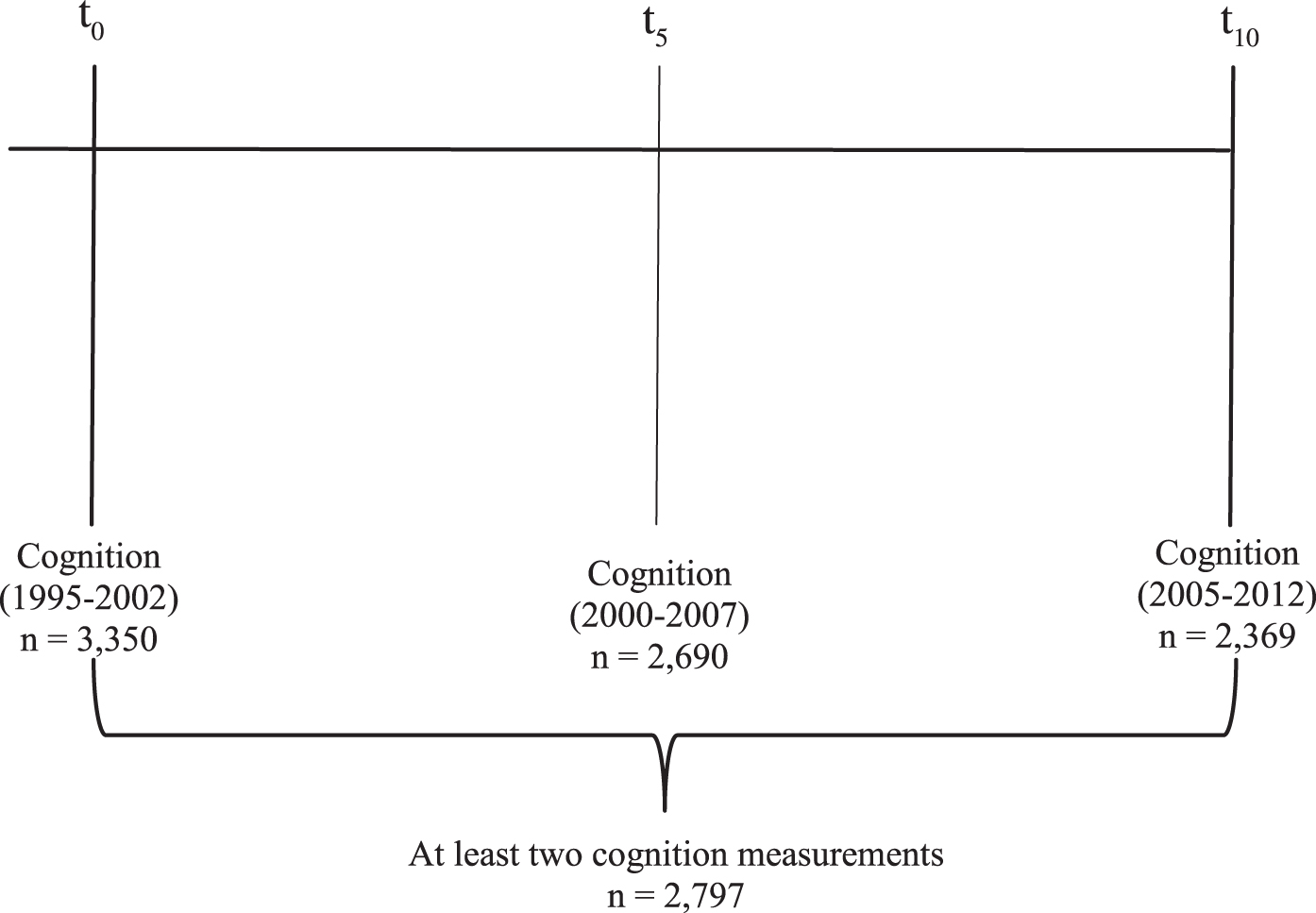
The Doetinchem Cohort Study is a Dutch ongoing prospective cohort study developed to evaluate the impact of lifestyle and biological risk factors on various aspects of health. It consists of a random sample of 7,769 men and women from the 12,405 respondents in the baseline study (1987–1991) [16, 17]. At baseline, an age-and gender-stratified random sample from the general population aged 20–59 years was invited to participate. The cohort is re-examined every 5 years (1993–1997, 1998–2002, 2003–2007, 2008–2012, 2013–2017). In 1995, a random sample of one-third of participants aged≥45 years was invited for cognitive testing, of which only 2% refused to participate. Between 1995 and 2002, a total of 3,350 respondents participated in cognitive testing for the first time. Five years later (2000–2007), 2,690 of them participated again in cognitive testing and 10 years later 2,369 persons participated (2005–2012). There were no differences in mean age (55.7 years versus 56.2 years; p = 0.56) and distributions of gender (50.1% versus 50.8% women; p = 0.91) and level of education (low: 33.7% versus 43.9%, medium: 41.9% versus 40.9%, high: 24.4% versus 15.2%; p = 0.12) between the participants who did and did not complete cognitive testing. A total of 2,797 (1,409 women; 1,388 men) individuals with at least two cognition measurements were included for the present analyses (Fig. 1). The study was approved by the external Medical Ethics Committee of the Dutch Organization of Applied Scientific Research (TNO) according to the guidelines of the Helsinki Declaration. All participants gave written informed consent.
Fig. 1.
Timeline of the cognitive tests.
Demographics
Information on age, gender, and educational level was collected using standardized questionnaires that were administered at baseline. Educational level was evaluated as the highest level reached and classified into low (primary school and low vocational education), medium (intermediate secondary education and intermediate vocational or higher secondary education) or high (higher vocational education or university). Vocational education is education directed at a particular occupation and its skills.
Cognitive tests
A neuropsychological test battery was administered by trained investigators at baseline and the 5- and 10-year follow-up measurements. For the present study, the 15 Words Verbal Learning Test (VLT), the Stroop Color Word Test (SCWT), and the Letter Digit Substitution Test (LDST) were used. These neuropsychological tests have been described in more details elsewhere [18]. Distributions of scores on the SCWT were normalized (log-transformation). For each neuropsychological test, a standardized z-score was calculated at each time point, on the basis of the mean and standard deviation of the total sample at baseline. By doing this, we were able to evaluate cognitive changes over time. Standardized scores of the SCWT were inverted, so that higher scores represent better cognitive performance. The present study focuses on three cognitive functions: verbal memory (z-score of VLT delayed recall), cognitive flexibility (i.e., high-order information processing; z-score of the interference score of the SCWT; interference score: SCWT card III – ((SCWT card I + SCWT card II) / 2)), and mental speed (z-score SCWT card I + z-score SCWT card II + z-score LDST / 3). Cognitive decline was defined as the difference between the compound scores at baseline and follow-up. Participants with a score of <1.5 standard deviation below the mean of the baseline measurement on any of the three cognitive functions mentioned above were classified as cognitively impaired.
Health and lifestyle factors
Health and lifestyle factors were captured by a poly-environmental risk score labelled the ‘LIfestyle for BRAin health’ index (LIBRA) [19]. LIBRA consists of twelve modifiable risk and protective factors for cognitive decline and dementia that can be targeted by tailored lifestyle interventions and primary prevention. Risk factors are coronary heart disease, diabetes, hypercholesterolemia, hypertension, depression, obesity, smoking, physical inactivity, and renal disease. Protective factors are low-to-moderate alcohol use, high cognitive activity, and healthy diet. LIBRA was developed after triangulation of results from a systematic literature review on risk and protective factors for dementia and an expert consensus study [19], as part of the European (FP7) INnovative, Midlife INtervention for Dementia Deterrence (In-MINDD) project [20]. A weight was assigned to each factor, based on the factor’s relative risk [19]. Weights were then standardized and summed to yield the final LIBRA score (LIBRA range from –5.9 to +12.7), with higher scores indicating greater risk. It has been shown to explain variance in cognitive functioning and dementia risk in previous (population- and patient-based) cohort studies [21–23].
In the Doetinchem Cohort Study, status information at baseline was available for all LIBRA factors, except for high cognitive activity, with a theoretical LIBRA score range from –2.7 to +12.7. Health and lifestyle factor status was based on clinical data from physical examination (including non-fasting blood plasma samples) performed at the research center and/or self-reported information (e.g., self-completion questionnaire). Each measure was dichotomized according to established cut-offs as explained below. The presence of coronary heart disease was based on self-reported myocardial infarction (“Did you ever had a heart attack?”) or self-reported cardiac interventions (“Have you ever had bypass surgery?”; “Have you ever had cardiac catheterization?”; “Have you ever had coronary angioplasty?”). Diabetes was defined on the basis of self-report or random blood glucose level ≥ 11.0 mmol/l. Hypercholesterolemia was based on total cholesterol ≥ 6.5 mmol/l and/or self-reported use of cholesterol-lowering medication. Hypertension was defined as systolic blood pressure≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg (using a random-zero sphygmomanometer), and/or the use of antihypertensive medication. Depressive symptoms were assessed using the Dutch version of the MOS 36-Item Short-Form Health Survey (SF-36). The scales ‘mental health’ and ‘vitality’ evaluate symptoms of depression. Scores on both scales range from 0–100, in which higher scores represent better (mental) health [24, 25]. For the purpose of the present study, the sum score of both scales was taken and the lowest 10% of the respondents were classified as depressed based on Dutch population trends [26]. Body mass index (BMI) was calculated, from measured weight (minus 1 kilogram to adjust for clothing) and height, as weight in kilograms divided by height in meters squared (kg/m2). The cut-off point for obesity (BMI≥30 kg/m2) was used according to the World Health Organization guidelines [27]. Smoking status was defined based on cigarette smoking status at baseline (smokers: less or more than once a month; non-smokers: former smokers and never smokers). The level of physical activity was classified into four categories based on the European Prospective Investigation into Cancer and Nutrition (EPIC) questionnaire on physical activity: inactive, moderately inactive, moderately active, and active [28, 29]. For the purpose of the present study, it was further dived into low (inactive, moderately inactive) and high (moderately active, active) physical activity. Renal dysfunction was based on a combined creatinine-cystatin C equation for estimated glomerular filtration rate (eGFRcc) [30]. The cut-off for renal dysfunction was eGFRcc < 60 mL/min per 1.73 m2. Alcohol consumption was defined as the intake of a total number of glasses of beer, wine, and liquor per week. The cut-off for low-to-moderate alcohol consumption was a maximum of one alcohol consumption per day for both men and women and was based on the Dutch dietary guidelines [31]. The habitual consumption of 178 food items during the previous year was assessed with a validated food frequency questionnaire [32, 33]. A healthy diet was based on the modified Mediterranean Diet Score (MDS) [34]. This score consists of the following nine nutritional components: vegetables, fruits, legumes and nuts, grains, fish and seafood, meat products, unsaturated to saturated fatty acid ratio, dairy products, and alcohol. A value of 0 or 1 was assigned to each component, using gender-specific medians in the study population as cut-off. Since the traditional Mediterranean diet is characterized by low meat and dairy intake, the scores for these two components were inverted. Furthermore, alcohol consumption is a separate LIBRA factor and therefore it was not included in the MDS. The MDS ranged from zero (minimal adherence) to 8 (maximal adherence). A MDS score of ≥ 5 was used as the cut-off for healthy diet [35].
Statistical analyses
To examine baseline differences (in health and lifestyle factors and demographic variables) between males and females and between participants with different levels of education, independent samples t-test, χ2-tests, and one-way ANOVA were used. Linear mixed models tested the association between LIBRA and change in cognition over time (using continuous cognitive function scores at the three time points). The models included a random intercept and random slope with an independent correlation matrix, because likelihood-ratio testing suggested the best fit for this approach. First, the association between LIBRA and change in cognition in the total sample was tested. Interaction terms between LIBRA and a discrete time variable were included by using dummy variables for the two follow-ups (1 = baseline to 5 years; 2 = baseline to 10 years). The LIBRA-by-time interaction was tested using a likelihood ratio-test with 2 degrees of freedom. Next, the three-way interaction was tested using a likelihood ratio-test with 4 (gender) or 8 (education) degrees of freedom, followed by stratified analyses in case of statistical significance (p < 0.05). Cox proportional hazards regression models were used to examine the association between LIBRA and time to cognitive impairment (and whether this possible association was moderated by gender or educational level), resulting in hazard ratios (HR) and their 95% confidence interval (CI). The proportional hazard assumption was assessed based on the Schoenfeld residuals. Cognitive impairment was treated as the failure event and date of birth was used as the origin of the time axis. Survival time was calculated from study entry (first cognitive measurement (t0)) until study exit (date of cognitive measurement where cognitive impairment was present or last cognitive measurement (t5 or t10)), whichever came first. Analyses were adjusted for age (at baseline), gender (in analyses on educational level), and educational level (in analyses on gender). First, analyses were performed for the continuous LIBRA score. Further, participants were classified in three risk groups based on tertiles of the LIBRA score (i.e., tertile 1 = low-risk, tertile 2 = intermediate-risk, tertile 3 = high-risk). All analyses were done in Stata/SE 15 (StataCorp, TX) and the level of statistical significance was p < 0.05 in two-sides tests.
RESULTS
Sample characteristics
After exclusion of individuals with baseline cognitive impairment (n = 386), an incomplete neuropsychological assessment at baseline (n = 26), and persons with incomplete LIBRA factors (n = 38 (excluding 8 individuals with baseline cognitive impairment); Supplementary Table 1), the sample included in these analyses consisted of 2,347 individuals. Baseline characteristics are summarized by gender and educational level in Table 1. Males were on average higher educated, had higher presence of coronary heart disease and hypertension, had less depressive symptoms, were less often low-to-moderate alcohol drinkers, had higher LIBRA scores, and had lower scores on all three cognitive functions in comparison with females. Individuals with a low educational level were on average older, more often female, had higher presence of hypercholesterolemia, obesity, and hypertension, had more depressive symptoms, were more often smokers, were more often low-to-moderate alcohol drinkers, adhered less often to a healthy diet, had higher LIBRA scores, and had lower scores on all three cognitive functions in comparison with persons with a medium or high educational level.
Table 1
Baseline characteristics of the study population (n = 2,347) by gender and educational level
| Gender | Educational level | ||||||
| Males (n = 1,132) | Females (n = 1,215) | p | Low (n = 614) | Medium (n = 1,047) | High (n = 686) | p | |
| Demographics | |||||||
| Age, mean (SD) | 55.0 (6.8) | 54.7 (6.7) | 0.234 | 55.8 (6.9) | 54.8 (6.8) | 54.1 (6.5) | <0.001 |
| Female, n (%) | – | – | – | 397 (64.7) | 532 (50.8) | 286 (41.7) | <0.001 |
| Educational level, n (%) | <0.001 | – | – | – | – | ||
| Low | 217 (19.2) | 397 (32.7) | |||||
| Middle | 515 (45.5) | 532 (43.8) | |||||
| High | 400 (35.3) | 286 (23.5) | |||||
| Health and lifestyle factors | |||||||
| Coronary heart disease, n (%) | 72 (6.4) | 34 (2.8) | <0.001 | 28 (4.6) | 49 (4.7) | 29 (4.2) | 0.905 |
| Diabetes, n (%) | 30 (2.7) | 26 (2.1) | 0.418 | 14 (2.3) | 31 (3.0) | 11 (1.6) | 0.190 |
| Hypercholesterolemia, n (%) | 301 (26.6) | 340 (28.0) | 0.449 | 196 (31.9) | 276 (26.4) | 169 (24.6) | 0.009 |
| Hypertension, n (%) | 463 (40.9) | 421 (34.7) | 0.002 | 253 (41.2) | 408 (39.0) | 223 (32.5) | 0.003 |
| Depressive symptoms, n (%) | 74 (6.5) | 136 (11.2) | <0.001 | 69 (11.2) | 93 (8.9) | 48 (7.0) | 0.028 |
| Obesity, n (%) | 127 (11.2) | 164 (13.5) | 0.094 | 134 (21.8) | 102 (9.7) | 55 (8.0) | <0.001 |
| Smoking, n (%) | 227 (20.1) | 284 (23.4) | 0.051 | 139 (22.6) | 254 (24.3) | 118 (17.2) | 0.002 |
| Low-to-moderate alcohol use, n (%) | 443 (39.1) | 827 (68.1) | <0.001 | 414 (67.4) | 554 (52.9) | 302 (44.0) | <0.001 |
| Physical inactivity, n (%) | 290 (25.6) | 284 (23.4) | 0.206 | 141 (23.0) | 247 (23.6) | 186 (27.1) | 0.151 |
| Renal dysfunction, n (%) | 10 (0.9) | 12 (1.0) | 0.793 | 4 (0.7) | 13 (1.2) | 5 (0.73) | 0.386 |
| Healthy diet, n (%) | 434 (38.3) | 475 (39.1) | 0.707 | 201 (32.7) | 382 (36.5) | 326 (47.5) | <0.001 |
| LIBRA score, mean (SD) * | 0.99 (1.97) | 0.73 (2.00) | 0.001 | 1.14 (2.09) | 0.91 (1.94) | 0.52 (1.92) | <0.001 |
| Cognitive function scores | |||||||
| Verbal memory, mean (SD) | –0.01 (0.85) | 0.30 (0.92) | <0.001 | –0.14 (0.80) | 0.15 (0.87) | 0.40 (0.95) | <0.001 |
| Cognitive flexibility, mean (SD) | 0.15 (0.74) | 0.22 (0.87) | 0.029 | –0.02 (0.93) | 0.16 (0.75) | 0.41 (0.74) | <0.001 |
| Mental speed, mean (SD) | 0.13 (0.83) | 0.27 (0.82) | <0.001 | –0.17 (0.70) | 0.22 (0.80) | 0.52 (0.82) | <0.001 |
SD, standard deviation; LIBRA, LIfestyle for BRAin health. *LIBRA score theoretical range: –2.7 to 12.7; observed range: –2.7 to 9.3.
Participants who dropped out during follow-up (n = 981) were on average older (57.6 years versus 54.9 years; p < 0.001), were lower educated (43.8% versus 29.2% low education), had lower LIBRA scores (1.67 versus 0.84; p < 0.001), and had lower cognitive scores (0.3–0.4 lower z-scores on all three cognitive functions; p < 0.001) at baseline than participants who still were in the study at t10 (n = 2,369). No differences between the groups were observed for gender (p = 0.86). Associations between LIBRA and cognitive functions at baseline were comparable between the follow-up and drop-out group. However, for verbal memory, differences between men and women were stronger in the drop-out group and the association between LIBRA and verbal memory was especially present in persons with high educational level in the drop-out group.
Lifestyle and cognitive decline
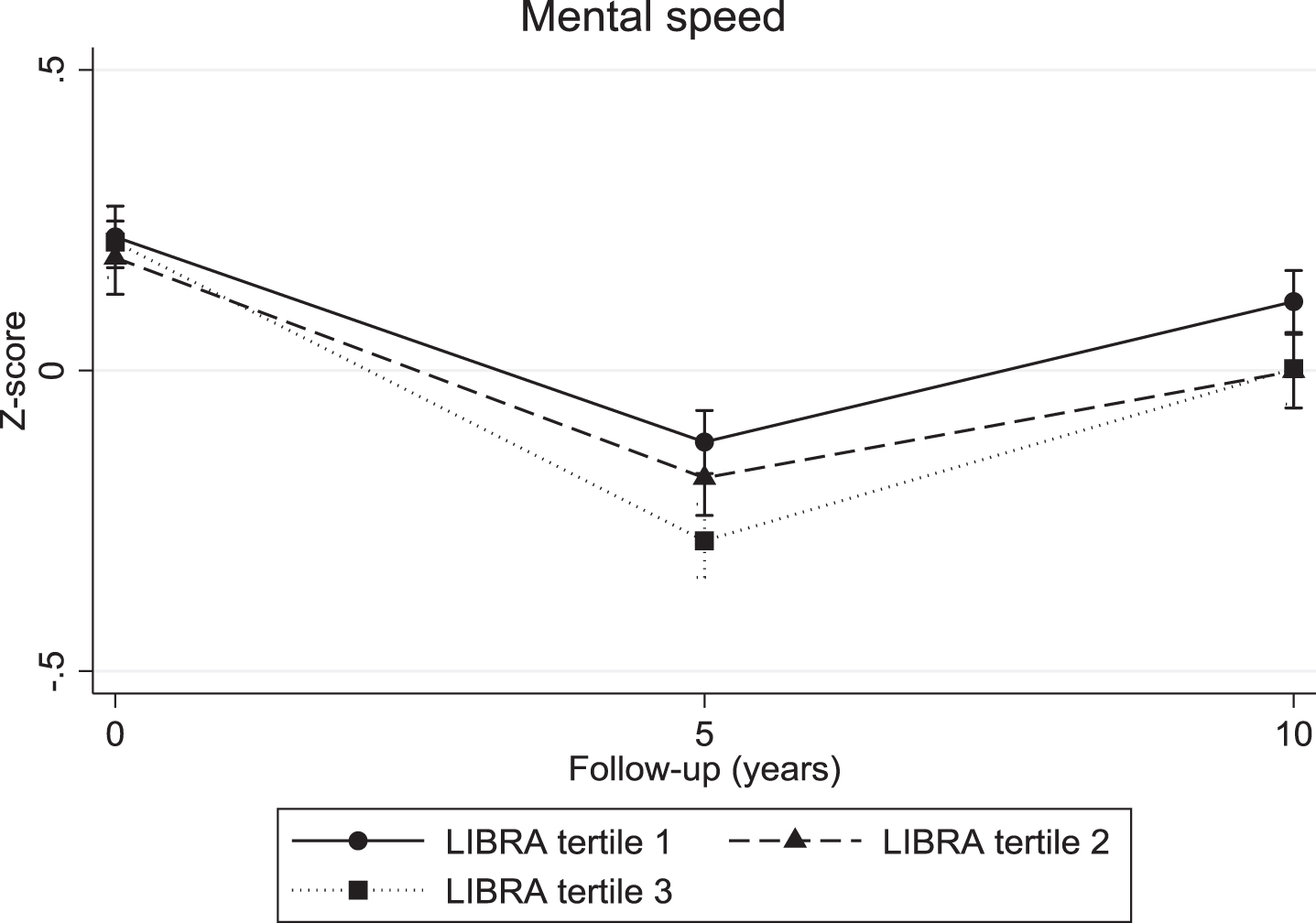
The continuous LIBRA score was associated with a significant decline in verbal memory (overall interaction: χ2 = 10.04; df = 2; p = 0.007; 5-year LIBRA-by-time interaction: B = –0.03, χ2 = 8.82, df = 1, p = 0.003; 10-year LIBRA-by-time interaction: B = –0.02, χ2 = 5.71, df = 1, p = 0.017) and mental speed (overall interaction: χ2 = 32.62; df = 2; p < 0.001; 5-year LIBRA-by-time interaction: B = –0.03, χ2 = 31.29, df = 1, p < 0.001; 10-year LIBRA-by-time interaction: B = –0.02, χ2 = 12.93, df = 1, p < 0.001), but not with cognitive flexibility (overall interaction: χ2 = 5.10; df = 2; p = 0.078; 5-year LIBRA-by-time interaction: B = –0.02, χ2 = 5.10, df = 1, p = 0.024; 10-year LIBRA-by-time interaction: B = –0.01, χ2 = 1.06, df = 1, p = 0.302). Individuals in the intermediate- and high-risk groups showed significantly more decline on all three cognitive functions over the 10-year study period in comparison with persons from the low-risk group (Table 2 and Figs. 2–4). There were no significant differences between the intermediate- and high-risk groups.
Fig. 2.
Verbal memory trajectories for individuals in the three LIBRA risk groups, by gender.
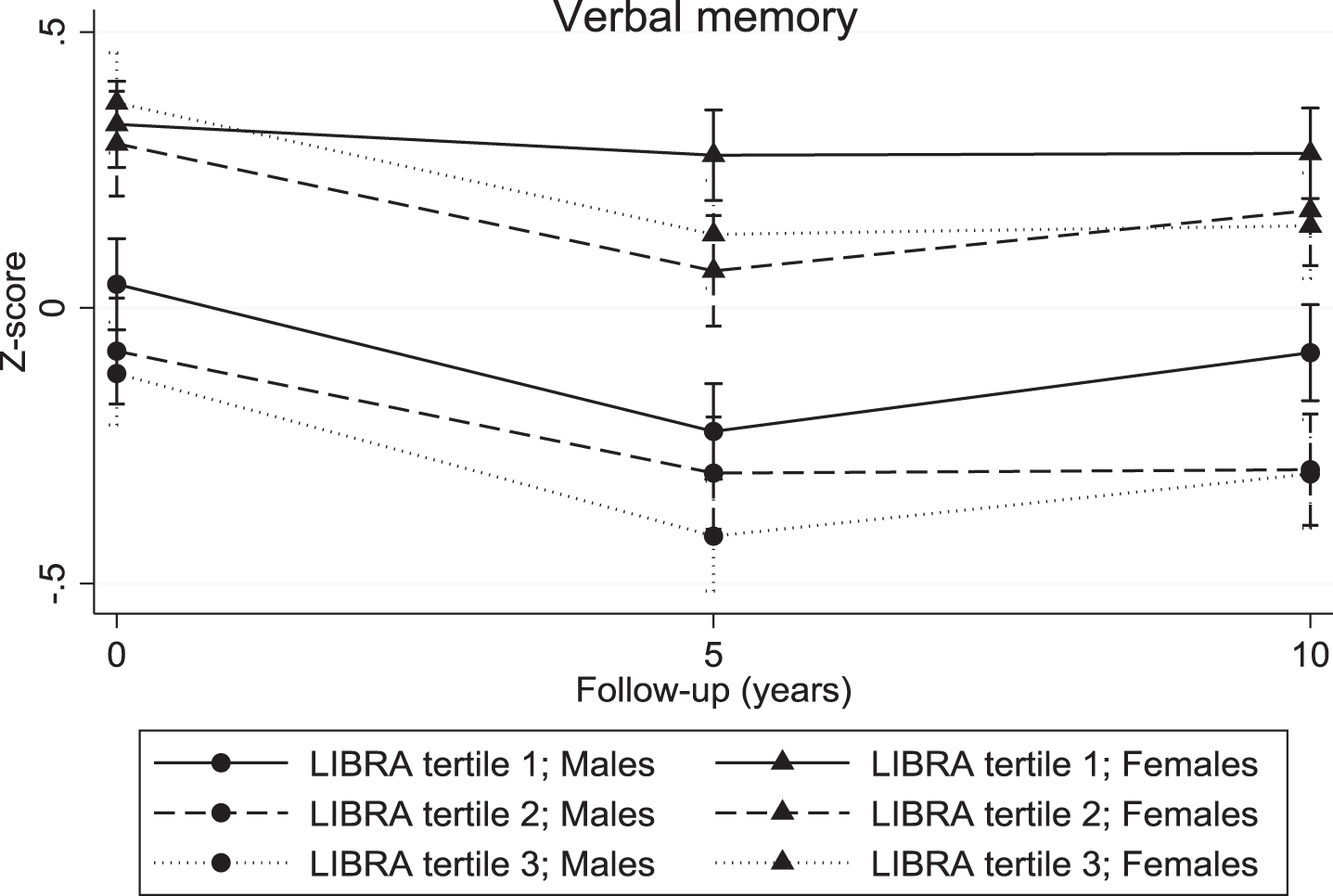
Fig. 3.
Cognitive flexibility trajectories for individuals in the three LIBRA risk groups.
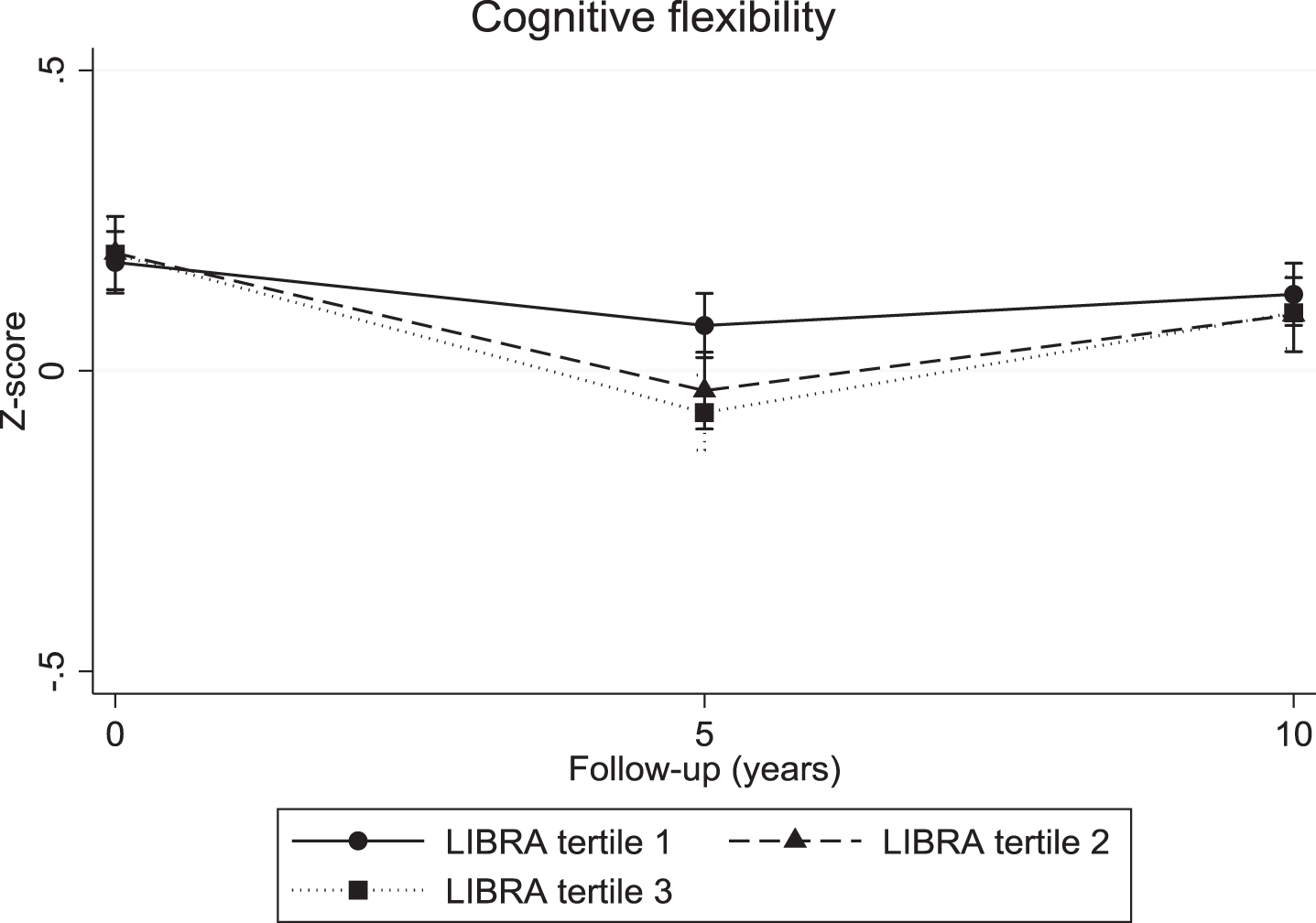
Fig. 4.
Mental speed trajectories for individuals in the three LIBRA risk groups.
Gender and educational differences
For the continuous LIBRA score, there were no significant differences in cognitive decline within the subgroups defined by gender and educational level. The differences in 10-year decline in cognitive flexibility and mental speed between the three LIBRA risk groups were similar across gender. However, there was a significant difference in decline on verbal memory between males and females as a function of baseline LIBRA risk status (χ2 = 11.15; df = 4; p = 0.025). Men in the intermediate- and high-risk groups had significant lower verbal memory scores in comparison with males from the low-risk group, but showed no significantly different decline over the 10-year study period (Table 2). On the other hand, there were no baseline differences between women across the risk groups, but a steeper decline was found in women from the higher risk groups in comparison with women from the low-risk group. The association between LIBRA risk groups and cognitive decline did not differ across educational level.
Table 2
Change in cognitive function over time across LIBRA tertiles. Associations by gender rather than population mean effects are shown in case of significant interaction by gender in the primary analyses
| Parameter | n | Time | |||||||
| Baseline | Rate of decline from baseline to 5-year follow-up | Rate of decline from baseline to 10-year follow-up | LIBRA x Time χ2 (df = 2) | p | |||||
| Difference * | 95% CI | Difference * | 95% CI | Difference * | 95% CI | ||||
| Verbal memory | 2,347 | ||||||||
| Males | 1,132 | ||||||||
| LIBRA tertile 2 | 331 | –0.13 | –0.25 to –0.003 | 0.04 | –0.08 to 0.17 | –0.09 | –0.21 to 0.03 | 4.90 | 0.086 |
| LIBRA tertile 3 | 351 | –0.18 | –0.30 to –0.06 | –0.03 | –0.15 to 0.09 | –0.06 | –0.18 to 0.06 | 0.88 | 0.645 |
| Females | 1,215 | ||||||||
| LIBRA tertile 2 | 338 | –0.02 | –0.15 to 0.11 | –0.17 | –0.30 to –0.05 | –0.07 | –0.19 to 0.06 | 7.34 | 0.025 |
| LIBRA tertile 3 | 377 | 0.07 | –0.06 to 0.19 | –0.18 | –0.31 to –0.06 | –0.17 | –0.29 to –0.05 | 10.59 | 0.005 |
| Cognitive flexibility | 2,347 | ||||||||
| LIBRA tertile 2 | 669 | 0.02 | –0.06 to 0.10 | –0.12 | –0.20 to –0.05 | –0.05 | –0.13 to 0.03 | 9.54 | 0.009 |
| LIBRA tertile 3 | 728 | 0.01 | –0.06 to 0.09 | –0.16 | –0.24 to –0.08 | –0.04 | –0.12 to 0.03 | 16.60 | <0.001 |
| Mental speed (N | 2,347 | ||||||||
| LIBRA tertile 2 | 669 | –0.03 | –0.11 to 0.05 | –0.03 | –0.08 to 0.03 | –0.08 | –0.14 to –0.03 | 8.78 | 0.012 |
| LIBRA tertile 3 | 728 | –0.01 | –0.09 to 0.07 | –0.16 | –0.21 to –0.10 | –0.10 | –0.16 to –0.05 | 31.62 | <0.001 |
*Difference in cognitive performance between the group of interest and the reference group (LIBRA tertile 1; low-risk group); Analyses were adjusted for age (at baseline), gender, educational level and LIBRA x Time. CI, confidence interval; df, degrees of freedom; LIBRA, LIfestyle for BRAin health.
Lifestyle and incident cognitive impairment
During the 10-year study period, 440 individuals (18.8% of total sample; 45.2% female; 41.4% low education) developed cognitive impairment (incidence rate = 212.2 (95% CI, 193.3–233.0) per 10,000 person-years). Higher LIBRA scores were significantly associated with increased risk of incident cognitive impairment (one-point increase in LIBRA: HR = 1.09, 1.04–1.14, p = 0.001). More specifically, the high-risk group had an increased risk for incident cognitive impairment compared to the low-risk group (high-risk group: HR = 1.47, 1.17–1.5, p = 0.001; intermediate-risk group: HR = 1.26, 0.99–1.61, p = 0.061). There was no significant interaction between LIBRA (continuous and risk groups) and gender or educational level.
Sensitivity analyses
Three sensitivity analyses were performed (same approach as primary analyses). As cardiovascular accidents are related to health and lifestyle factors and can have detrimental effects on cognitive performance, we investigated the effects of previous reported cardiovascular accidents (n = 29) on our association of interest. Adjusting for cardiovascular accidents did not alter any of the results (data not shown). Second, we investigated the relationship between the continuous LIBRA score and cognitive decline in persons with cognitive impairment at baseline (n = 378 after exclusion of 8 individuals with incomplete LIBRA scores; 41.0% female; 56.9% low education). At baseline, there were no gender or educational differences in LIBRA scores. Males had lower scores on verbal memory at baseline in comparison with females (p < 0.001), while individuals with a low and medium educational level had lower scores on mental speed at baseline in comparison with individuals with a high education level (all pairwise comparisons p < 0.001). A higher LIBRA score was associated with more decline in mental speed over the 10-year study period (this effect was particularly driven by a steep decline from baseline to 5-year follow-up), but not with verbal memory or cognitive flexibility. There were no significant differences in the course of cognition within the subgroups defined by gender and educational level (no further subdivision in LIBRA risk groups due to limited sample size; data not shown). Third, we investigated the association of interest in individuals with cognitive function scores on all three measurements (verbal memory: n = 1,936; cognitive flexibility: n = 1,929; mental speed: n = 1,930) in order to examine the potential influence of drop-outs. Results for the continuous LIBRA score were comparable to the primary analyses. Looking at the LIBRA risk groups, the difference in decline on verbal memory between males and females as a function of baseline LIBRA risk status was no longer significant (χ2 = 8.61; df = 4; p = 0.072). Individuals in the high-risk group showed significantly more decline on all three cognitive functions over the 10-year study period in comparison with persons from the low-risk group. Individuals in the intermediate-risk group showed only more decline in cognitive flexibility compared with those from the low-risk group. Again, the association between the LIBRA risk groups and cognitive decline did not differ across educational level (data not shown).
DISCUSSION
In this prospective cohort study, we studied the relationship between a modifiable risk score and cognitive functioning in different subgroups defined by gender and educational level. In general, our results suggest that a higher LIBRA score predicted faster decline in verbal memory, cognitive flexibility, and mental speed over 10 years. These effects were similar across educational groups. A steeper decline in verbal memory was found in women from the higher risk groups in comparison with women from the low-risk group. On the other hand, men in the higher risk groups presented lower scores on verbal memory at baseline already. A faster decline in cognitive flexibility and mental speed in the higher risk groups was not gender-specific. Higher LIBRA scores were further associated with increased risk for incident cognitive impairment. Yet, this association was not moderated by gender or educational level. Sensitivity analyses adjusting for cardiovascular accidents showed similar results. To further explore the relationship between health and lifestyle factors and the course of cognition, we also looked at a subgroup with baseline cognitive impairment and found that higher LIBRA scores were predictive of a steep decline in mental speed (especially in the first five years after cognitive impairment). These findings suggest the potential usefulness of lifestyle programs in secondary prevention of cognitive disorders and require further investigation.
Numerous studies investigated the individual effects of health and lifestyle factors on cognitive functioning or dementia risk [5, 19, 36, 37]. Besides, the associations between (female) gender and (low) educational level and the risk for cognitive impairment or dementia are well-known [7, 8, 38]. Several studies looked at the effect of gender differences in relation to distribution and prediction of dementia risk factors [39, 40], of which some showing differences for risk factors like midlife hypertension [41] and marital status [42]. One of these studies conducted in a sample of older adults from South Korea showed that high blood pressure, poor hearing, regular exercise, normal weight, and having limitations in instrumental activities of daily living (IADL) were significant predictors of cognitive impairment in women, whereas age and limitations in activities in daily living (ADL) were associated with cognitive impairment in men [39]. Yet, to our knowledge, the present study is the first study that investigated the relationship between a compound score of health and lifestyle factors and cognitive decline in middle-aged subgroups defined by gender and educational level. It is important to note that LIBRA is a composite risk score. Therefore, differences by gender and educational level could have been present on the level of individual risk and protective factors. Yet, the aim of the present study was to investigate whether there were differences in dementia risk factors profiles between males and females and between individuals with different levels of education. LIBRA is a suitable tool for this purpose since it assesses an individual’s potential for dementia prevention by flagging their personal ‘room for improvement’. Despite our findings, future studies are encouraged to further investigate gender and educational differences in dementia risk profiles.
Several mechanisms have been proposed to explain gender and educational differences in cognitive functioning or dementia risk. For gender differences, the first and most obvious one is the fact that women outlive men [10] and therefore have been longer exposed to the detrimental effects of risk factors on brain health. Other mechanisms that might be involved are hormonal differences, genetic factors, differences in brain networks, and differences in social, economic, societal, and cultural roles and relationships. For educational differences, the concept of brain reserve might play an important role. It suggests that inborn intelligence and a mentally stimulating lifestyle, such as educational and occupational attainments, may delay cognitive decline or dementia onset by increasing an individual’s brain reserve [15, 43, 44]. Several studies have found that people with more years of formal education have lower risk of cognitive decline or dementia than those with fewer years of education [5, 19, 36]. Other possible mechanisms (and in some way related to each other) include differences in health choices, health literary, financial resources, and access to health services.
Our study has a number of strengths including the prospective design, the fact that the sample represents the target population of middle-aged and healthy individuals, the availability of several health and lifestyle factors (including Mediterranean diet and renal function), and the long follow-up period with repeated assessment of cognitive functioning with a sensitive neuropsychological test battery. Yet, there are also some limitations to our study. First, as an inevitable part of all longitudinal studies, not all participants who entered the study returned for the follow-up measurements. Although an 71% response rate at the last follow-up is quite high, this still might have led to some selection of a healthier sample and therefore may results in an underestimation of the “true” association. Indeed, differences between participants who still were in the study at t10 and participants who dropped out during follow-up were evident. Selection bias was reduced by use of linear mixed models using all available data with maximum likelihood to estimate data points of individuals with missing data. One of the major strengths of linear mixed models is that data are missing at random if covariates (e.g., age, educational level, LIBRA) related to missingness are included in the model. In addition, sensitivity analyses in a subsample of individuals with cognition data on all three time points showed only minor changes for the decline in verbal memory between males and females, suggesting that selection bias was not exceptional. Secondly, participants with missing data (e.g., incomplete LIBRA scores (n = 38) and incomplete neuropsychological assessment at baseline (n = 26)) were excluded from the analyses (Supplementary Table 1). Although these numbers were quite small (±2%) and analyses showed no significant differences between individuals with (n = 64) and without (n = 2,347) missing data with regard to demographics, health and lifestyle factors (except for obesity: 16 out of 64 (25.0%) versus 291 out of 2,347 (12.4%); p = 0.003), and cognitive function scores at baseline, this could have led to an under- or overestimation of the observed effects. Third, the ascertainment of some health and lifestyle factors was based on self-report or suboptimal operationalization (e.g., depressive symptoms), which could have resulted in response bias or non-differential exposure misclassification. Other, namely body weight, height, blood pressure, cholesterol levels, blood glucose levels, and eGFRcc were objectively measured with standardized protocols and instruments. Fourth, the classification of cognitive impairment (<1.5 standard deviation below the mean criterion) is challenging and could have led to identification of more false positives given the cognitive health and relatively young age captured in this sample.
Our results might help in identifying (risk) groups that benefit from preventative measures in the general population. Future studies should investigate the possible mediation effects of various biological factors (e.g., genetics, hormones, brain networks) in relation to the observed associations and more studies need to assess gender differences rather than just adjusting for gender in analyses. Given the double aging of our society and the worldwide increase in sedentary lifestyle behavior, policy makers, insurance companies, and public health campaigns should place more emphasis on brain-healthy lifestyle changes in middle-aged and younger individuals to promote optimal brain ageing and prevention of dementia in the long-term.
In conclusion, our research showed that a composite risk score comprising unhealthy lifestyle and relatively poor health in midlife is significantly associated with a worse course of cognition 10 years later. These associations were for the most part unrelated to gender or educational differences.
ACKNOWLEDGMENTS
This work was supported by a fellowship (Kay Deckers) from Alzheimer Nederland and the Alzheimer’s Society UK [grant number WE.15-2015-01]. The Doetinchem Cohort Study is financially supported by the Ministry of Public Health, Welfare and Sport of The Netherlands and the National Institute for Public Health and the Environment. The data up to and including 1997, including the dietary assessment method, were additionally financially supported by the Europe against Cancer programme of the European Commission (DG SANCO). The authors thank the respondents and the epidemiologists and fieldworkers of the Municipal Health Service in Doetinchem for their contribution to the data collection for this study. Principal investigator is Prof WMM Verschuren. Logistic management was provided by J Steenbrink and P Vissink, and administrative support by EP van der Wolf. Data management was provided by A Blokstra, AWD van Kessel and PE Steinberger.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0492r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180492.
REFERENCES
[1] | Prince M , Bryce R , Albanese E , Wimo A , Ribeiro W , Ferri CP ((2013) ) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9: , 63–75 e62. |
[2] | Wimo A , Jonsson L , Bond J , Prince M , Winblad B ((2013) ) The worldwide economic impact of dementia 2010. Alzheimers Dement 9: , 1–11 e13. |
[3] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbaek G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[4] | Barnes DE , Yaffe K ((2011) ) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10: , 819–828. |
[5] | Norton S , Matthews FE , Barnes DE , Yaffe K , Brayne C ((2014) ) Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 13: , 788–794. |
[6] | Prince M , Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina M ((2015) ) The global impact of dementia. World Alzheimer’s Report, Alzheimer’s Disease International (ADI), London. |
[7] | Gao S , Hendrie HC , Hall KS , Hui S ((1998) ) The relationships between age, sex, and the incidence of dementia and Alzheimer disease: A meta-analysis. Arch Gen Psychiatry 55: , 809–815. |
[8] | Launer LJ , Andersen K , Dewey ME , Letenneur L , Ott A , Amaducci LA , Brayne C , Copeland JRM , Dartigues J-F , Kragh-Sorensen P , Lobo A , Martinez-Lage JM , Stijnen T , Hofman A ((1999) ) Rates and risk factors for dementia and Alzheimer’s disease: Results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 52: , 78–84. |
[9] | Lobo A , Saz P , Marcos G , Dia JL , De-la-Camara C , Ventura T , Montanes JA , Lobo-Escolar A , Aznar S ((2007) ) Prevalence of dementia in a southern European population in two different time periods: The ZARADEMP Project. Acta Psychiatr Scand 116: , 299–307. |
[10] | World Health Organization (2018) Global Health Estimates 2016: Deaths by cause, age, sex, by country and by region, 2000-2016, World Health Organization, Geneva. |
[11] | Katz MJ , Lipton RB , Hall CB , Zimmerman ME , Sanders AE , Verghese J , Dickson DW , Derby CA ((2012) ) Age and sex specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer’s dementia in Blacks and Whites: A report from the Einstein Aging Study. Alzheimer Dis Assoc Disord 26: , 335–343. |
[12] | Langa KM , Larson EB , Crimmins EM , Faul JD , Levine DA , Kabeto MU , Weir DR ((2017) ) A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 177: , 51–58. |
[13] | Mayeda ER , Glymour MM , Quesenberry CP , Whitmer RA ((2016) ) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12: , 216–224. |
[14] | Satizabal CL , Beiser AS , Chouraki V , Chene G , Dufouil C , Seshadri S ((2016) ) Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 374: , 523–532. |
[15] | Valenzuela MJ , Sachdev P ((2006) ) Brain reserve and dementia: A systematic review. Psychol Med 36: , 441–454. |
[16] | Picavet HSJ , Blokstra A , Spijkerman AMW , Verschuren WMM ((2017) ) Cohort Profile Update: The Doetinchem Cohort Study 1987-2017: Lifestyle, health and chronic diseases in a life course and ageing perspective. Int J Epidemiol 46: , 1751–1751g. |
[17] | Verschuren WM , Blokstra A , Picavet HS , Smit HA ((2008) ) Cohort profile: The Doetinchem Cohort Study. Int J Epidemiol 37: , 1236–1241. |
[18] | Nooyens AC , Bueno-de-Mesquita HB , van Boxtel MP , van Gelder BM , Verhagen H , Verschuren WM ((2011) ) Fruit and vegetable intake and cognitive decline in middle-aged men and women: The Doetinchem Cohort Study. Br J Nutr 106: , 752–761. |
[19] | Deckers K , van Boxtel MP , Schiepers OJ , de Vugt M , Munoz Sanchez JL , Anstey KJ , Brayne C , Dartigues JF , Engedal K , Kivipelto M , Ritchie K , Starr JM , Yaffe K , Irving K , Verhey FR , Kohler S ((2015) ) Target risk factors for dementia prevention: A systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry 30: , 234–246. |
[20] | O'Donnell CA , Browne S , Pierce M , McConnachie A , Deckers K , van Boxtel MP , Manera V , Köhler S , Redmond M , Verhey FR , van den Akker M , Power K , Irving K ; In-MINDD Team ((2015) ) Reducing dementia risk by targeting modifiable risk factors in mid-life: Study protocol for the Innovative Midlife Intervention for Dementia Deterrence (In-MINDD) randomised controlled feasibility trial. Pilot Feasibility Stud 1: , 40. |
[21] | Pons A , LaMonica HM , Mowszowski L , Kohler S , Deckers K , Naismith SL ((2018) ) Utility of the LIBRA Index in relation to cognitive functioning in a clinical health seeking sample. J Alzheimers Dis 62: , 373–384. |
[22] | Schiepers OJG , Kohler S , Deckers K , Irving K , O'Donnell CA , van den Akker M , Verhey FRJ , Vos SJB , de Vugt ME , van Boxtel MPJ ((2018) ) Lifestyle for Brain Health (LIBRA): A new model for dementia prevention. Int J Geriatr Psychiatry 33: , 167–175. |
[23] | Vos SJB , van Boxtel MPJ , Schiepers OJG , Deckers K , de Vugt M , Carriere I , Dartigues JF , Peres K , Artero S , Ritchie K , Galluzzo L , Scafato E , Frisoni GB , Huisman M , Comijs HC , Sacuiu SF , Skoog I , Irving K , O'Donnell CA , Verhey FRJ , Visser PJ , Kohler S ((2017) ) Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: Validation of the LIBRA Index. J Alzheimers Dis 58: , 537–547. |
[24] | Van der Zee KI , Sanderman R ((1993) ) Het meten van de gezondheidstoestand met de RAND-36: Een handleid-ing (Measuring General Health Status with the RAND-36. Users Manual). Noordelijk Centrum voor Gezondhei-dsvraagstukken, Groningen. |
[25] | Ware JE Jr , Sherbourne CD ((1992) ) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30: , 473–483. |
[26] | Statistics Netherlands (2013) Population trends 2013: Depression and antidepressants in the Netherlands, Statistics Netherlands, Den Haag/Heerlen. |
[27] | World Health Organization (2008) Waist circumference and waist-hip ratio: Report of a WHO expert consultation, World Health Organization, Geneva. |
[28] | Pols MA , Peeters PH , Ocke MC , Slimani N , Bueno-de-Mesquita HB , Collette HJ ((1997) ) Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int J Epidemiol 26: , S181–189. |
[29] | Wareham NJ , Jakes RW , Rennie KL , Schuit J , Mitchell J , Hennings S , Day NE ((2003) ) Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 6: , 407–413. |
[30] | Inker LA , Schmid CH , Tighiouart H , Eckfeldt JH , Feldman HI , Greene T , Kusek JW , Manzi J , Van Lente F , Zhang YL , Coresh J , Levey AS ((2012) ) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: , 20–29. |
[31] | Health Counsil of the Netherlands (2015) Dutch dietary guidelines 2015, Health Counsil of the Netherlands, The Hague. |
[32] | Ocke MC , Bueno-de-Mesquita HB , Goddijn HE , Jansen A , Pols MA , van Staveren WA , Kromhout D ((1997) ) The Dutch EPIC food frequency questionnaire. I. Description of the questionnaire, and relative validity and reproducibility for food groups. Int J Epidemiol 26: , S37–48. |
[33] | Ocke MC , Bueno-de-Mesquita HB , Pols MA , Smit HA , van Staveren WA , Kromhout D ((1997) ) The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int J Epidemiol 26: , S49–58. |
[34] | Trichopoulou A , Orfanos P , Norat T , Bueno-de-Mesquita B , Ocke MC , Peeters PH , van der Schouw YT , Boeing H , Hoffmann K , Boffetta P , Nagel G , Masala G , Krogh V , Panico S , Tumino R , Vineis P , Bamia C , Naska A , Bene-tou V , Ferrari P , Slimani N , Pera G , Martinez-Garcia C , Navarro C , Rodriguez-Barranco M , Dorronsoro M , Spencer EA , Key TJ , Bingham S , Khaw KT , Kesse E , Clavel-Chapelon F , Boutron-Ruault MC , Berglund G , Wirfalt E , Hallmans G , Johansson I , Tjonneland A , Olsen A , Over-vad K , Hundborg HH , Riboli E , Trichopoulos D ((2005) ) Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 330: , 991. |
[35] | Hulsegge G , Looman M , Smit HA , Daviglus ML , van der Schouw YT , Verschuren WMM ((2016) ) Lifestyle changes in young adulthood and middle age and risk of cardiovascular disease and all-cause mortality: The Doetinchem Cohort Study. J Am Heart Assoc 5: , e002432. |
[36] | Baumgart M , Snyder HM , Carrillo MC , Fazio S , Kim H , Johns H ((2015) ) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11: , 718–726. |
[37] | Plassman BL , Williams JW Jr , Burke JR , Holsinger T , Benjamin S ((2010) ) Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 153: , 182–193. |
[38] | Sharp ES , Gatz M ((2011) ) Relationship between education and dementia: An updated systematic review. Alzheimer Dis Assoc Disord 25: , 289–304. |
[39] | Lyu J , Kim HY ((2016) ) Gender-specific incidence and predictors of cognitive impairment among older Koreans: Findings from a 6-year prospective cohort study. Psychiatry Investig 13: , 473–479. |
[40] | Azad NA , Al Bugami M , Loy-English I ((2007) ) Gender differences in dementia risk factors. Gend Med 4: , 120–129. |
[41] | Gilsanz P , Mayeda ER , Glymour MM , Quesenberry CP , Mungas DM , DeCarli C , Dean A , Whitmer RA ((2017) ) Female sex, early-onset hypertension, and risk of dementia. Neurology 89: , 1886–1893. |
[42] | Sundstrom A , Westerlund O , Kotyrlo E ((2016) ) Marital status and risk of dementia: A nationwide population-based prospective study from Sweden. BMJ Open 6: , e008565. |
[43] | Scarmeas N , Stern Y ((2003) ) Cognitive reserve and lifestyle. J Clin Exp Neuropsychol 25: , 625–633. |
[44] | Sattler C , Toro P , Schonknecht P , Schroder J ((2012) ) Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res 196: , 90–95. |




