A Survey of Patient and Partner Outcome and Treatment Preferences in Mild Cognitive Impairment
Abstract
Background:
The patient-centered movement in health care is increasing efforts to design studies and interventions that address the outcomes that matter most to patients and their families. Research has not adequately addressed Alzheimer’s disease patient and caregiver preferences.
Objective:
To survey the outcome and treatment preferences of patients and caregivers who had completed a multicomponent behavioral intervention for mild cognitive impairment (MCI).
Methods:
Extending prior work, we conducted an online survey regarding outcome and intervention preferences. Participants were patients with MCI and partners who completed the HABIT Healthy Action to Benefit Independence & Thinking ® program.
Results:
Both patient and partner respondents ranked patient quality of life as the highest priority, followed by patient self-efficacy, functional status, patient mood, and patient memory performance. Distressing behaviors and caregiver outcomes (burden, mood, and self-efficacy) had low rankings. Regarding the importance of HABIT ® program components, memory compensation training was ranked highest and wellness education lowest by all groups.
Conclusion:
Additional research should compare patient preference for patient reported outcomes, traditional neuropsychological and clinician outcomes, and modern biomarker outcomes.
INTRODUCTION
Patient-centered care involves addressing the outcomes that matter most to patients and their caregivers [1]. Patient centered intervention research involves studying the interventions that are mostly likely to improve those outcomes. Unfortunately, neurodegenerative disease research has not traditionally been patient-centered. This appears directly related to concerns about the capacity of people with neurodegenerative diseases to state their preferences [2]. Such concerns have resulted in limited use of patient and caregiver reported outcomes, such as quality of life [3], in research on Alzheimer’s disease (AD) or related conditions [4]. However, the growing recognition and incorporation of preclinical and mild cognitive impairments (MCI) stages of neurodegenerative disease [5] presents an opportunity to better integrate patient preference into care and research with AD and related dementia. The MCI or mild neurocognitive disorder [6] phase of illness, with its concerning but isolated cognitive impairment and retained global cognition and functional capacities [7–9] provides an opportune time to establish patient (and partner) treatment and outcome preferences. Given that patients with MCI are at high risk to progress to dementia, yet retain substantial cognitive strengths, they are generally able to consider choices in an informed fashion, and establish health care preferences and otherwise participate in health care decision-making [10, 11]. Thus studies of persons with MCI and their partners provide an important opportunity to establish outcome preferences for patients, and consider those preferences relative to those of their partners.
The use of behavioral interventions for those with MCI has been on the rise [12]. We have developed a multi-component behavioral intervention called HABIT Healthy Action to Benefit Independence & Thinking ®. HABIT ® is a 10-day, group-based intervention in which persons with MCI and their partners daily receive six different1-hour interventions 1) memory support calendar training [13], 2) computerized brain fitness training (BrainHQ by Posit Science), 3) physical exercise via yoga, 4&5) separate patient and partner support groups, and 6) wellness education. A more extensive program description can be found at http://www.mayo.edu/pmts/mc2800-mc2899/mc2815-10.pdf. HABIT ® was developed with the goals of facilitating the adjustment and supporting the independence of partners and persons recently diagnosed with MCI.
We recently reported preliminary analyses of patient and caregiver priorities among seven patient and five caregiver outcomes of the HABIT ® program from two separate MCI cohorts [14]. These preliminary analyses used two methods with two cohorts that had completed the HABIT ® program 1) direct interview with 33 partners (i.e., caregivers) and 2) as face-to-face completion of paper-and-pencil reports with 16 patient and partner dyads. In each analysis, respondents rank-ordered the importance of patient and caregiver outcome measures used in the HABIT ® program. Across the methods, cohorts, and respondent types (patient or partner) quality of life (QoL) and patient self-efficacy ranked as highest priorities, ahead of patient and caregiver mood, patient functional status, patient distressing behaviors and caregiver burden. Patients and partners tended to value the outcomes for their loved ones higher than their own outcomes. The consistency of patient and partner rankings suggested partners appeared to be reasonable, but not perfect, proxies for patient report.
The present study used an online survey tool to expand our patient/partner preferences analyses to all previous dyads from the HABIT ® program willing to complete the survey. This survey was undertaken to fulfill Aim 1 of our Patient Centered Outcomes Research Institute funded project “Comparative Effectiveness of Behavioral Interventions to Prevent or Delay Dementia” (CER-1306-01897). This aim required us select the primary outcome measure for the comparative effectiveness trial based on importance of the outcomes to patients and partners.
METHODS
Participants
All HABIT ® program completers (from 2008–2014) at the time of this survey were eligible to participate in this survey. These participants were typically referred from the Neurology, Neuropsychology, or Geriatrics practices at one of the three Mayo Clinic campuses (Jacksonville, FL; Rochester, MN; or Scottsdale, AZ). There were a handful of couples who learned of the program via the internet and self-referred. Patients were required to have a medical diagnosis of mild cognitive impairment that met standard Mayo diagnostic criteria for MCI [7–9]. Partners in the HABIT ® program are required to have an MMSE score in the normal range (≥27) and to have regular (at least once weekly) contact with the person with MCI. At the time of survey distribution 269 couples had completed the HABIT ® program at Mayo Clinic Minnesota, Arizona, or Florida.
Survey
Contact information was provided to the Mayo Survey Research Center, which handled all aspects of the survey. A copy of the content is provided in the appendix. Data collection for the planned comparative effectiveness trial mirrors that of the clinical HABIT ® program but adds performance-based cognitive outcome measures. Table 1 lists the measures to be used in the comparative effectiveness trial. Patients and partners who completed the clinical HABIT ® program had completed all the measures listed in Table 1 (except for Cogstate), as part of evaluations just before, immediately after and at one-year intervals following the HABIT ® program. Most also had experience with neuropsychological evaluation (i.e., measure of cognitive performance). They were thus familiar with the constructs we were asking them to prioritize, at least as measured by these instruments. This gave them the opportunity to associate the outcome areas we were asking them to rank to a specific measure (e.g., patient’s memory-based daily function with the Everyday Cognition scale, [15] or partner burden with the Zarit Burden Short Form [21]) that they had previously completed.
All participants were contacted by an email that explained the purpose of the study and invited their participation. Participants were provided a link to complete a survey hosted on the Qualtrics survey research site (Qualtrics.com). After the initial email, the Mayo Survey Research Center sent follow-up emails at 2-week intervals, until the survey was completed, declined or until five contact attempts had been made. The survey was structured so that respondents rank ordered the 13 outcomes on a scale of most important to least important by ‘dragging and dropping’ each outcome until they were ordered from top (most important) to bottom (least important). Respondents similarly rank ordered the value of each of the six behavioral interventions included in HABIT ®.
Data analysis
Rankings were compared using simple t-tests and Wilcoxon (independent or dependent as appropriate). The parametric and non-parametric tests produced identical findings in terms of the statistical significance so we report only the outcome of the t-tests here. We examined for groups differences across all items according to respondent type. In addition, across all groups and within each group, we used dependent t-tests to compare each item with the next lower ranking item to test for the statistical significance of the item differences. If that paired comparison was not significant we then compared the item to the one that ranked next lowest. Because of the large number of comparisons (between items and across groups) we set our alpha significance level at 0.01.
RESULTS
At the time of survey distribution, 269 couples had completed HABIT ®. Of these, 39 had not provided at least one email to the program. Therefore, 230 email invitations were distributed. One hundred sixteen responses were returned. Among these, 11 had participated in our prior data collections [14] and were excluded. In addition, 10 returned blank surveys. In 13 cases, both members of the dyad responded separately. Seven surveys provided data only ranking treatment importance while 1 provided data only ranking outcomes. Recognizing that patients would be as various stages of cognitive progression in the time since participating in HABIT ®, we allowed for the possibility that partners might help patients complete the survey. Ultimately 95 usable responses were received from 82 distinct couples. Data on treatment importance was available for 94 of these respondents, while outcome ranking were available from 88 respondents. Ultimately, some form of preference data was available for 30% of couples out of the 269 HABIT ® completers at the time of the survey.
Twenty-nine of the responses were from patients, 54 were from partners and in 12 cases the patient and partner reported working together to complete the survey. Table 2 lists the demographics for patients and caregivers in the respondent dyads relative to all non-respondent dyads. No differences were found for genders, education, years since diagnosis or income level. Respondent patients and partners were on average 2-3 years younger than non-respondents. Sixty-nine percent of the patient respondents were male. Spouses constituted 87% of partners, 9% were adult children of the patient, and 4% were friends of the patient. Mean (standard deviation) age of the patient and partner groups were 72.9 (8.3) and 67.1 (8.2) years, respectively. Mean (standard deviation) education for the patient and partner groups were 16.8 (2.3) and 16.5 (2.3) years, respectively. Respondents were all non-Hispanic whites.
Table 1
Outcome measures to be used in comparative effectiveness trial
| Performance measure completed by patient (PT) | Title of measure | Example of a task from measure |
| PT Actual Memory | Cogstate [17] | One Card Learning Test |
| Outcomes patient (PT) reports about him or herself | ||
| PT Depression | Center for Epidemiological Studies-Depression [18] | I felt depressed |
| PT Quality of life | Quality of life [19] | Rate your life as whole |
| PT Self-efficacy | Self-efficacy in Mild Cognitive Impairment [20] | How confident are you that you can get your errands done despite your memory/cognitive difficulties? |
| PT Anxiety | Reach Anxiety Inventory Form [21] | I was worried |
| Outcomes partner (PR) reports about patient (PT) | ||
| PT Basic Activities of Daily Living | Functional Assessment Questionnaire [22] | Has problems functioning outside familiar environments |
| PT Memory-based activities of daily living | Everyday Cognition [15] | Ability to remember things that happened recently |
| PT Distressing behaviors | The Neuropsychiatric Inventory [23] | Frequency of Agitation/Aggression |
| Outcomes partner (PR) reports about her or himself | ||
| PR Burden | Zarit Burden-Short Form [24] | Do you feel that your relative asks for more help than he/she needs? |
| PR Self-efficacy | Pearlin Mastery [25] | I can do just about anything if I really set my mind to it |
| PR Anxiety | Reach Anxiety Inventory Form [21] | I was worried |
| PT Quality of life | Quality of life [19] | Rate your life as whole |
| PR Depression | Center for Epidemiological Studies-Depression [18] | I felt depressed |
Table 2
Demographics of non-respondents and respondents
| Non-respondent couples (n = 187) | Respondent couples (n = 82) | p | |
| Gender | 0.171 | ||
| Male | 89 (55.3% ) | 51 (64.6% ) | |
| Age | 0.0062 | ||
| Mean (SD) | 75.4 (10.1) | 73.0 (7.6) | |
| Education | 0.152 | ||
| Mean (SD) | 15.8 (2.7) | 16.6 (2.2) | |
| Marital status | 0.381 | ||
| Married | 124 (89.2% ) | 61 (93.8% ) | |
| Years since MCI diagnosis | 0.682 | ||
| Mean (SD) | 1.1 (1.2) | 1.2 (1.2) | |
| Income level/SES3 | 0.711 | ||
| missing | 62 | 24 | |
| $25,000 – $49,999 | 19 (15.2% ) | 6 (10.3% ) | |
| $50,000 – $74,999 | 18 (14.4% ) | 10 (17.2% ) | |
| $75,000 – $99,999 | 16 (12.8% ) | 6 (10.3% ) | |
| $100,000 – $149,999 | 13 (10.4% ) | 7 (12.1% ) | |
| $150,000 or more | 49 (39.2% ) | 27 (46.6% ) | |
| Decline to answer | 10 (8.0% ) | 2 (3.4% ) | |
| Partner Gender | 0.291 | ||
| Female | 92 (62.6% ) | 51 (69.9% ) | |
| Partner age | 0.032 | ||
| Mean (SD) | 71.1 (10.726) | 68.7 (8.800) | |
| Partner Education | 0.442 | ||
| Mean (SD) | 16.3 (2.1) | 16.3 (2.3) |
1Chi-Square; 2Kruskal Wallis; 3Couples reported joint income.
Outcome importance
We examined both gender effects and caregiver type (spouse vs. non-spouse) and found no significant effect of either variable on rankings. Median rank-orderings of outcome priorities are depicted graphically in Fig. 1. Not all apparent differences are statistically significant. Table 3 presents mean and standard deviations by respondent type (patient, partner, couple) and overall for the outcome rankings.
Fig.1
Median rankings of outcome priorities for patients, partners, couples, and overall. Rank ordering on a scale of 1 = most important to 13 = least important thus lower rankings equal higher priority. PT, patient with MCI; PR, partner.
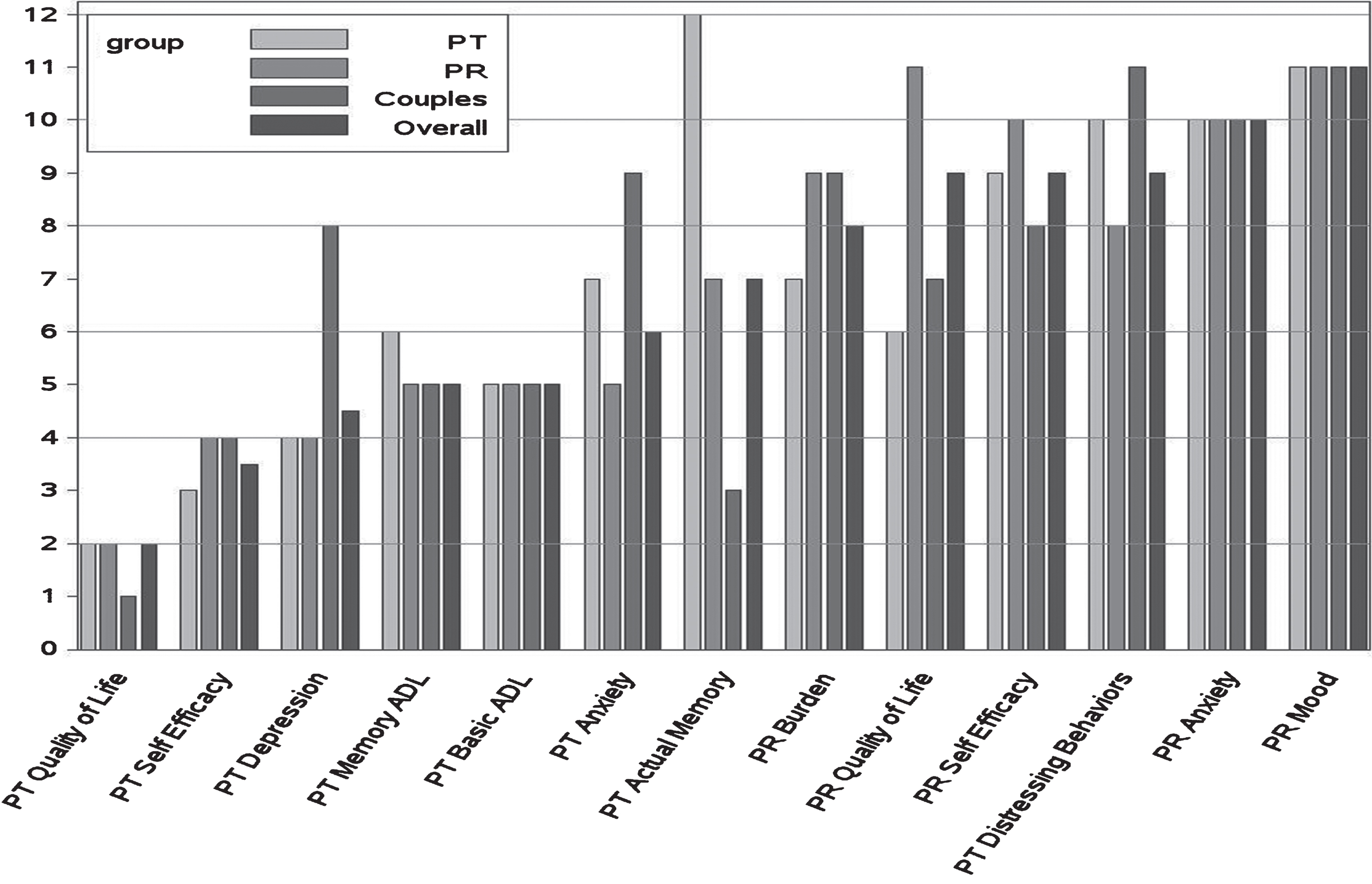
Table 3
Mean (standard deviation) ranks by respondent group and overall
| Outcomes (ordered by overall median ranks) | Patients (n = 26) | Partners (n = 51) | Couples (n = 11) | Overall (n = 88) |
| PT Quality of Life | 2.3 (1.8)b | 3.0 (2.8)b | 2.3 (2.0)b | 2.7 (2.5)b |
| PT Self-Efficacy | 4.0 (2.2)c | 4.2 (2.4) | 4.5 (1.6) | 4.2 (2.2)b |
| PT Depression | 5.6 (3.6) | 5.0 (3.0) | 7.0 (3.8) | 5.4 (3.3) |
| PT Memory Based Activities of Daily Living | 6.0 (3.1) | 5.2 (2.5) | 4.7 (2.1) | 5.3 (2.6) |
| PT Basic Activities of Daily Living | 5.8 (2.3)c | 5.0 (2.6)c | 6.2 (3.0) | 5.4 (2.6)c |
| PT Anxiety | 6.9 (3.6) | 5.4 (2.6)b | 8.2 (3.2) | 6.2 (3.1)c |
| PT Memory Function | 9.3 (4.5)b | 7.5 (4.5)c | 4.4 (3.7) | 7.7 (4.6) |
| PR Burden | 6.7 (2.6) | 8.3 (3.0) | 8.5 (2.8) | 7.8 (2.9)b |
| PR Quality of Lifea | 6.7 (4.4)c | 9.5 (3.4) | 5.8 (3.5) | 8.2 (4.0) |
| PR Self-Efficacy | 8.2 (2.8)b | 9.3 (2.2) | 8.3 (3.4) | 8.8 (2.8)c |
| PT Disruptive Behaviorsa | 10.2 (2.2) | 8.1 (2.9)b | 9.8 (2.6) | 8.9 (2.8)c |
| PR Anxiety | 9.4 (2.3) | 9.8 (2.4)b | 10.5 (2.0) | 9.8 (2.3)b |
| PR Depression | 10.0 (2.5) | 10.9 (2.4) | 10.8 (2.7) | 10.6 (2.5) |
Note: arespondent group ranks differed at p < 0.01. bItem was ranked as significantly more important than the next highest rank at p < 0.01. cItem was ranked as significantly more important than the item ranked 2 below at p < 0.01 (e.g., Overall ranking of PT Basic Activities of Daily Living Self Efficacy is not ranked as significantly more important than PT Anxiety but is ranked as significantly more important than the next item, PT Memory Function).
3.1.1Group differences
Two items showed significantly different ranks by respondent type. One was partner quality of life (QoL), where patients and couples ranked this item as more important than did partners. The second was patient distressing behaviors which partners saw as a higher priority than patients or couples. In both cases however, the items were ranked the lower half of the ranking distribution by all groups. Note that although patients ranked their actual memory function as among the least important outcomes and partners ranked this item in the upper half, this difference did not reach statistical significance.
3.1.2Item differences for the overall cohort
Patient’s QoL had the highest average ranking. Patients’ self-efficacy (in handling memory difficulties) ranked second. Next was a cluster of 4 items that were not significantly different from each other including patient depression, the two functional measures, and patient anxiety. These items were significantly different from patient’s actual cognition, and partner burden, which clustered together. Partner QoL ranked next as a single item. Partner OoL was followed by partner self-efficacy which was not different from patient distressing behaviors but was different from partner anxiety. Partner mood ranked last, distinct from anxiety. Caregiver mood (depression and anxiety) was ranked last by more than 28% of all respondents.
3.1.3Item differences with respondent groups
Within the specific groups, relative to the overall sample, there were a few differences in the mean ordering and statistical significance of item comparisons but in general the same overall pattern held.
Treatment importance
Figure 2 depicts the median rank order of the helpfulness of the six treatments provided in the HABIT ® program according to respondent type. Table 4 provides the associated means, standard deviations and statistical differences by respondent group.
Fig.2
Median treatment helpfulness rankings for patients, partners, couples, and overall. Rank ordering on a scale of 1 = most important to 6 = least important thus lower rankings equal higher priority. PT, patient with MCI; PR, partner.
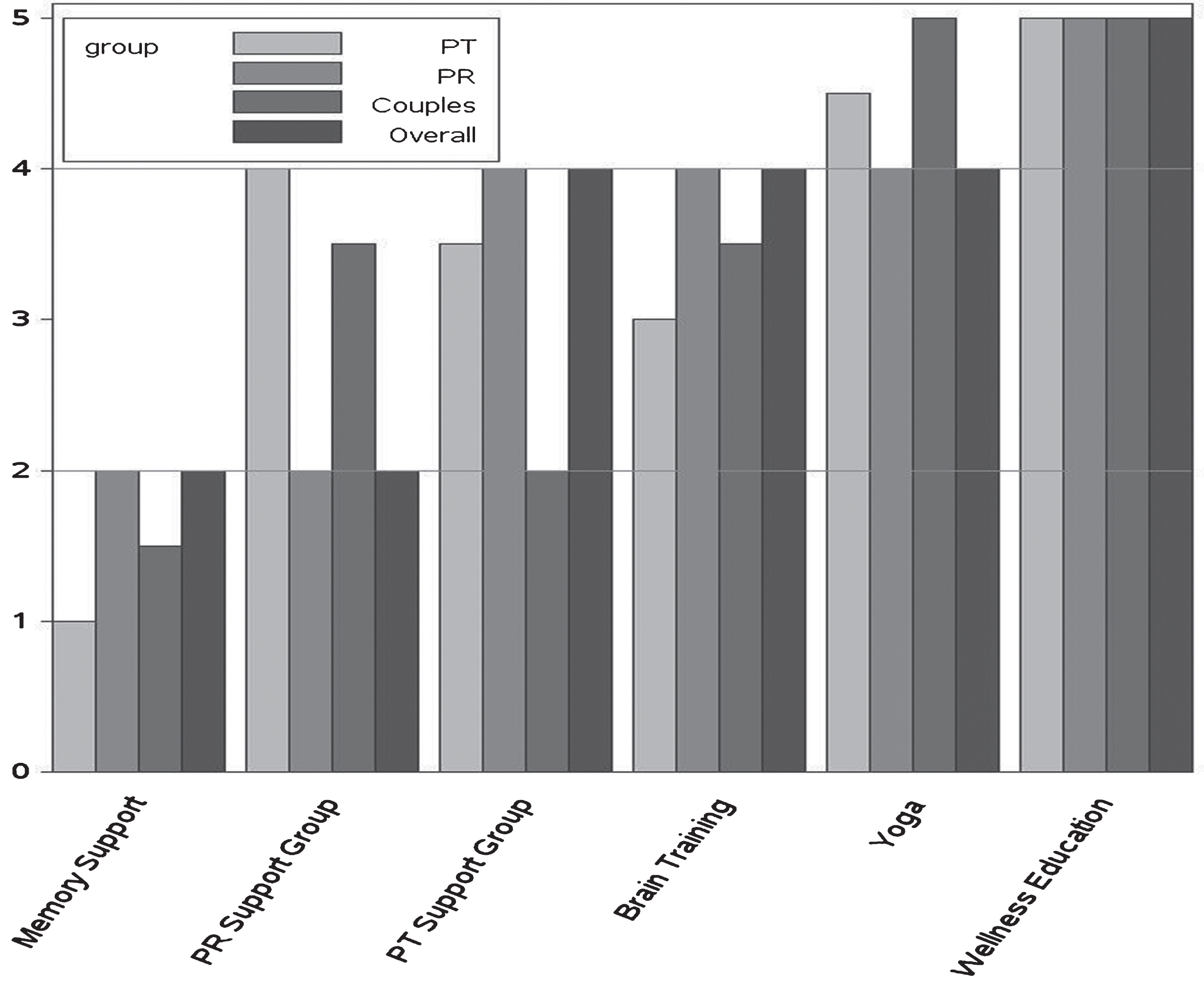
Table 4
Mean (standard deviation) ranks by respondent group and overall
| Treatments (ordered by overall median ranks) | Patients (n = 28) | Partners (n = 54) | Couples (n = 12) | Overall (n = 94) |
| Memory Support | .1.8 (1.2)b | 2.3 (1.3)b | 2.1 (1.6) | 2.1 (1.3)c |
| PR Support Groupa | 3.4 (1.4) | 2.0 (1.2)b | 3.0 (1.4)c | 2.6 (1.4)b |
| PT Support Group | 3.4 (1.7) | 4.0 (1.6) | 2.9 (1.8) | 3.7 (1.7) |
| Brain Training | 3.3 (1.5) | 4.1 (1.6) | 3.6 (1.7) | 3.8 (1.6)b |
| Yoga | 4.3 (1.6) | 4.1 (1.5) | 4.8 (1.1) | 4.2 (1.5) |
| Wellness Education | 4.8 (1.1) | 4.6 (1.4) | 4.7 (1.2) | 4.7 (1.3) |
Note: aRespondent group ranks differed at p < 0.01. bItem was ranked as significantly more important than the next highest rank at p < 0.01. cItem was ranked significantly more important than the item ranked 2 below at p < 0.01 (e.g., Overall Memory Support is not ranked as significantly more important that PR Support Group but is ranked as significantly more important than the next item PT Support Group).
3.2.1Differences by respondent group
Compared to patient ratings, partner ratings for partner support group were significantly higher (in the direction of more helpful). This was perhaps expectable since patients did not experience the partner support group.
3.2.2Item differences for the overall cohort
In the overall group, the memory support intervention and the partner support group were ranked highest followed by the patient support group and computerized brain training. Patient support group and computerized brain training were not different from each other but were seen as more important than yoga and wellness education.
3.2.3Item differences within respondent groups
Results were comparable within the patient and partner groups though the partners saw their support group as equally helpful as memory compensation training.
DISCUSSION
In this study, we extended prior pilot explorations of patient and partner preferences [14] of the outcomes they are seeking in a behavioral intervention for MCI. We surveyed a different, larger sample of patients and partners. To the menu of patient- and caregiver- reported outcomes previously studied, we added actual memory performance (See Table 1). In addition, we asked this sample of completers of the Mayo Clinic HABIT ® program to rank order the six components of the program to get a sense of how patients and partners value interventions like cognitive rehabilitation, physical or cognitive exercise, support group, and education.
Consistent with our prior findings, the present results suggest that at the MCI stage, patient QoL is the most important outcome for both patients with MCI and partners. This item was ranked most important by nearly half of all respondents. The next most important outcome is self-efficacy regarding their ability to manage memory impairment, followed by outcomes related to patients’ mood and daily function. These preferences stand in contrast to the outcomes commonly deployed in MCI clinical trials. We reviewed the 442 active MCI clinical trials listed on clinicaltrials.gov. Less than 10% of these studies (n = 43) included a QoL outcome and in only 9 cases was QoL a primary outcome measure. Only 4 studies included self-efficacy as an outcome.
Patients on average are more concerned about caregiver quality of life than are partners themselves. Partner mood outcomes were not highly prioritized by either group. We speculate that partners and patients view partner mood impacts as ‘coming with territory’, i.e., partners are entitled to their grief and worry. In any event outcomes of anxiety, depression, and daily function were not as highly prioritized as they appear to be in studies of priorities in later stages of neurodegenerative disease [16].
A new finding in this analysis is that actual cognitive function was not a high priority for patients in this cohort. Patients gave it less importance than did their partners (though the difference was not statistically significant). At the same time, the memory compensation training component noted below was given the highest importance. This finding may reflect patients’ acceptance of their memory limitations, and their focus on compensating for their memory loss. It suggests that improving actual memory ability appears less important than the desire to compensate for their memory loss in daily life. This again runs counter to the focus of much research on cognitive outcomes of behavioral interventions in MCI [12] where the focus has been on improving the memory ability itself.
Among six different behavioral treatments including wellness education, patient and partner support groups, computerized brain training and yoga, memory compensation training was seen as the most valuable by both patients and partners. Memory compensation training endorsed as most helpful by 47% of the respondents. For partners, partner support group was deemed equally helpful. Among these all respondents, wellness education was ranked least helpful. This does not mean it was seen as unhelpful, only that among the multiple components of the HABIT ® program it was seen as least helpful. Patients and partners valued a memory compensation strategy over efforts to address patient and partner mood (support group) or effort to directly improve cognition (brain training). Taken together, we speculate that partners and patients seeking an intensive multi-component behavioral intervention are focused on patients’ opportunities to gain skills that may sustain perceived quality of life and confidence or self-efficacy. However, it will be valuable to undertake further qualitative, and/or empirical analyses to further understand why participants and partners valued certain interventions over others. One such analysis will ensue from our comparative effectiveness trial, wherein we endeavor to determine the contribution of each intervention to the 13 outcomes listed in Table 1.
These analyses are limited in several respects. First, the cohorts used are not representative of the entire population of patients and partners confronted with MCI. Not only was our cohort limited in terms of ethnicity, education status, and geography, but it involved only people inclined and able to participate in an intensive multicomponent behavioral intervention. This selection factor alone may bias stated preferences. However, these findings can be seen as reflective of a subpopulation motivated to actively engage in interventional trials to address MCI.
In addition, this study focuses only the set of interventions and outcomes used by the HABIT ® program. We could have included a variety of traditional clinical trials treatments (i.e., medication) and outcomes including a range of biomarkers (brain volumes, amyloid levels, etc.) in the rankings. However, the outcomes we chose were familiar to respondents because they participated in these specific interventions and overtime have provided data on these specific measures. We were concerned that patients and partners could not provide rankings on other constructs like biomarkers unless we provide substantial education on what those measures entail. Even then the respondents would not have the same level of familiarity with those measures. In the future we hope to assess how patients and partners view the relative importance of biomarkers compared to traditional patient reported outcomes and medications relative to behavioral interventions.
Our use of the method of rank ordering is another limitation. Rank ordering constrains how outcomes may relate to each other. Rank ordering means an individual cannot report that different outcomes or interventions have equal importance. Patients and partners may value some these outcomes and treatments to be equally important (or unimportant), but our method forced them to rank one higher or lower than another. This approach could have served to magnify differences in the rankings. Conversely, rank order assumes each ranking is equally spaced in importance from the next ranking. An individual cannot indicate that the highest ranked item only slightly important than the second ranked item, which was substantially more important than the third ranked item, etc. Thus the rank ordering may have served to diminish the differences in how these outcomes and treatments are valued. Future studies using different methods are needed to more flexibly determine patient and partner outcome and treatment preferences.
These limitations notwithstanding the present findings confirm our prior findings [14] in a separate, larger sample. They affirm the primary importance of patient quality of life outcomes to patients with MCI and their partners, followed by memory self-efficacy and functional ability and mood. Interventional programs for MCI would be wise to focus efforts on directly impacting these outcomes for patients and their families. To this finding we add that from a menu of behavioral interventions, our patients with MCI and their partners report memory compensation training to be most helpful. If QoL in most important and memory compensation is most helpful, one might infer that patients and partners perceived memory compensation training to have had the most impact on QoL. However our prior small scale study comparing memory compensation training to computerized brain training showed memory compensation training to impact memory-based daily function and patient self-efficacy but not QoL [13]. Further research should focus on the direct association of treatments and patient preferred outcomes.
Based on these results we will use patient QoL as the primary outcome in the multi-center randomized comparative effectiveness trial we have underway [26]. That trial seeks to directly determine the impact of each intervention on the outcomes considered herein. Hopefully, the results of that study will provide valuable information to aid in the design and focus of tailored programs intended to impact the outcomes preferred by each individual patient and partner impacted by MCI.
ACKNOWLEDGMENTS
Research reported in this manuscript was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306-01897). The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. Partial support also provided by National Institute of Nursing Research (NR012419). This study was approved by the Mayo Clinic Institutional Review Board. A preliminary report of this data was presented at the International Neuropsychological Society, Annual Mid-year Conference, July 2017. We wish to acknowledge the efforts of Andrea Mejia in preparing a revision of this manuscript. Data set available upon request to the corresponding author. HABIT Healthy Action to Benefit Indepdendence and Thinking is a registered trademark of the Mayo Foundation for Medical Education and Research.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-1161r2).
REFERENCES
[1] | Patient Centered Outcomes Reserach Institute (PCORI). https://www.pcori.org/engagement/influencing-culture-research, Posted February 12, 2018, Accessed March 1, 2018. |
[2] | Wadekar M , Sharma A , Battaglia G ((2015) ) Patient-Centered Outcomes Research (PCOR): How can we optimize outcomes in CNS research? Innov Clin Neurosci 12: , 27–31. |
[3] | Institute of Medicine. Living well with chronic illness: A call for public health action., National Academy of Sciences, Washington DC. http://www.nationalacademies.org/hmd/ /media/Files/Report% 20Files/2012/Living-Well-with-Chronic-Illness/livingwell_chronicillness_reportbrief.pdf, Posted January 2012, Accessed March 1 (2018) . |
[4] | Riepe MW , Mittendorf T , Förstl H , Frölich L , Haupt M , Leidl R , Vauth C , von der Schulenburg MG ((2009) ) Quality of Life as an outcome in Alzheimer’s disease and other dementias- obstacles and goals, BMC Neurol 9: , 47. |
[5] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR , Kaye J , Montine TJ , Park DC ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease, Alzheimers Dement 7: , 280–292. |
[6] | American Psychiatric Association ((2013) ) American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition–Washington, DC. |
[7] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease, Alzheimers Dement 7: , 270–279. |
[8] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome, Arch Neurol 56: , 303–308. |
[9] | Smith GE , Petersen RC , Parisi JE , Ivnik RJ , Kokmen E , Tangalos EG , Waring S ((1996) ) Definition, course and outcome of mild cognitive impairment, Aging Neuropsychol Cogn 3: , 141–147. |
[10] | Okonkwo O , Griffith HR , Belue K , Lanza S , Zamrini EY , Harrell LE , Brockington JC , Clark D , Raman R , Marson DC ((2007) ) Medical decision-making capacity in patients with mild cognitive impairment, Neurology 69: , 1528–1535. |
[11] | Appelbaum PS ((2010) ) Consent in impaired populations, Curr Neurol Neurosci Rep 10: , 367–373. |
[12] | Chandler MJ , Parks AC , Marsiske M , Rotblatt LJ , Smith GE ((2016) ) Everyday impact of cognitive interventions in mild cognitive impairment: A systematic review and meta-analysis, Neuropsychol Rev 26: , 225–251. |
[13] | Greenaway MC , Duncan NL , Smith GE ((2013) ) The memory support system for mild cognitive impairment: Randomized trial of a cognitive rehabilitation intervention, Int J Geriatr Psychiatry 28: , 402–409. |
[14] | Barrios PG , González RP , Hanna SM , Lunde AM , Fields JA , Locke DE , Smith GE ((2016) ) Priority of treatment outcomes for caregivers and patients with mild cognitive impairment: Preliminary analyses, Neurol Ther 5: , 183–192. |
[15] | Farias ST , Mungas D , Reed BR , Cahn-Weiner D , Jagust W , Baynes K , DeCarli C ((2008) ) The measurement of everyday cognition (ECog): Scale development and psychometric properties, Neuropsychology 22: , 531–544. |
[16] | Mittelman MS , Brodaty H , Wallen AS , Burns A ((2008) ) A three-country randomized controlled trial of a psychosocial intervention for caregivers combined with pharmacological treatment for patients with Alzheimer disease: Effects on caregiver depression, Am J Geriatr Psychiatr 16: , 893–904. |
[17] | Maruff P , Thomas E , Cysique L , Brew B , Collie A , Snyder P , Pietrzak RH ((2009) ) Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex, Arch Clin Neuropsychol 24: , 165–178. |
[18] | Radloff LS ((1977) ) The CES-D scale: A self report depression scale for research in the general population, Appl Psychol Meas 1: , 385–401. |
[19] | Logsdon RG , Gibbons LE , McCurry SM , Teri L ((2002) ) Assessing quality of life in older adults with cognitive impairment, Psychosom Med 64: , 510–519. |
[20] | Lorig K , Ritter P , Gonzalez V , Laurent D , Lynch J ((1996) ), Outcome Measures for Health Education and other Health Care Interventions, Sage Publications, Thousand Oaks, CA. |
[21] | Wisniewski SR , Belle SH , Coon DW , Marcus SM , Ory MG , Burgio LD , Burns R , Schulz R ((2003) ) The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): Project design and baseline characteristics, Psychol Aging 18: , 375–384. |
[22] | Galasko D , Bennett DA , Sano M , Marson D , Kaye J , Edland SD ((2006) ) ADCS Prevention Instrument Project: Assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials, Alzheimer Dis Assoc Disord 20: , S152–169. |
[23] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia, Neurology 44: , 2308–2314. |
[24] | Bédard M , Molloy DW , Squire L , Dubois S , Lever JA , O’Donnell M ((2001) ) The Zarit Burden Interview: A new short version and screening version, Gerontologist 41: , 652–657. |
[25] | Pearlin LI , Mullan JT , Semple SJ , Skaff MM ((1990) ) Caregiving and the stress process: An overview of concepts and their measures, Gerontologist 30: , 583–594. |
[26] | Smith G , Chandler M , Locke DE , Fields J , Phatak V , Crook J , Hanna S , Lunde A , Morris M , Graff-Radford M , Hughes CA , Lepore S , Cuc A , Caselli M , Hurst D , Wethe J , Francone A , Eilertsen J , Lucas P , Hoffman Snyder C , Kuang L , Becker M , Dean P , Diehl N , Lofquist M , Vanderhook S , Myles D , Cochran D ((2017) ) Behavioral interventions to prevent or delay dementia: Protocol for a randomized comparative effectiveness study. JMIR Res Protoc 6: , e223. |




