Post Traumatic Stress Disorder Heralding the Onset of Semantic Frontotemporal Dementia
Abstract
Background:
Post traumatic stress disorder (PTSD) is associated with cognitive decline. The dementia type following PTSD is unclear.
Objective:
To assess whether PTSD is associated with a specific dementia.
Methods:
Prospective study: 46 PTSD patients (DSM-IV-TR) were followed for 6–10 years with clinical, neuropsychological, imaging evaluations for possible development of dementia.
Retrospective study:
849 dementia patients followed during 1999–2014 (509 Alzheimer’s disease, AD; 207 dementia with Lewy bodies, DLB; 90 vascular dementia, VaD; 43 frontotemporal dementia, FTD) and 287 patients with any neurological condition (including patients with/without dementia) were evaluated for the presence of PTSD in their history.
Results:
Prospective study: 8 patients developed dementia; 1 AD, 1 DLB, 6 semantic FTD (13.0% of the PTSD population). Retrospective study: 38 patients (4.5%) had a history of PTSD; 3.5% of AD, 4.3% of DLB, 14.0% of FTD, 5.6% of VaD. The percentage was higher in FTD than in AD or DLB (χ2 = 10, p = 0.001, and χ2 = 6, p = 0.02). At difference with AD, DLB, or VaD, FTD incidence among dementia patients with PTSD history (38 patients) was higher than in the dementia population overall (16% versus 5%, χ2 = 8, p = 0.005). The impact of possible demographical/clinical confounders (age, gender, MMSE) was excluded by Poisson regression. PTSD prevalence in the comparative group without dementia matched the prevalence in the Italian general population (1.1%). PTSD prevalence in the demented comparative group matched the prevalence in our dementia retrospective cohort, 3.7%).
Discussion:
PTSD was associated with the development of semantic FTD.
INTRODUCTION
A coexistence between post-traumatic stress disorder and a variety of neuropsychiatric conditions, ranging from depression to the so-called “syndrome de glissement” of the French authors, and dementia, has been extensively studied [1–5]. The majority of the reports comes from observation of holocaust and war survivors [2, 6].
A markedly increased risk of dementia with different clinical phenotypes, i.e., Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), and vascular dementia (VaD), in veterans suffering from post-traumatic stress disorder (PTSD) was recently observed [2]. In that cohort study [2], veterans diagnosed with PTSD were at a nearly 2-fold higher risk of developing dementia than those without. Reasons for this finding include poorer premorbid cognitive performance as a risk factor, chronic stress damaging the hippocampus [7–11], alterations in the hypothalamic-pituitary-adrenal axis and proinflammatory cytokines [12], or an increased nonspecific vulnerability to several neuropsychiatric disorders caused by PTSD [13].
The clinical observations have been supported by neuroimaging studies demonstrating PTSD-induced functional abnormalities, including hyper-responsive amygdala, hypoactive medial prefrontal cortex (MPFC), anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex dysfunction [14].
A great number of animal studies strongly supports the hypothesis that stress can decrease dendritic branching and neurogenesis in MPFC volume and can damage hippocampal formation [15]. MPFC and pregenual ACC are highly interconnected with the amygdala, and structural or functional abnormalities in these regions have been reported, in humans, to markedly impair fear extinction, which is considered as a basic mechanism in the development of PTSD [16]. Some researchers emphasized that not only has research in humans found that ACC volumes and functional activation in PTSD are altered as compared to trauma-exposed individuals without PTSD, but has also found that these alterations correlate with severity of PTSD symptoms [17, 18].
These data underline a possible common pathway of neurodegeneration for PTSD and dementia. Despite the amount of literature data on the co-occurrence of PTSD and dementia [1–5], a causal connection between the two conditions has not been clarified. For example, a study on identical twins discordant for combat exposure in Vietnam found that, although the combat veterans with PTSD had smaller hippocampi than the combat veterans without PTSD, the hippocampal diminution was shared by the formers’ identical twins who had not been exposed to combat and did not have PTSD [19]. These findings suggest that hippocampal diminution represents a familial risk factor for PTSD, rather than an acquired consequence and even if a temporal sequence is ascertainable with dementia coming after PTSD, still “post hoc does not necessarily imply propter-hoc” [2].
Therefore, the authors suggest that if there is commonality between PTSD and dementia it should be looked for as a susceptibility trait to both disorders recognizable in pre-morbid brain alterations (e.g., hippocampal volume diminution) [14, 19].
The prevalence of PTSD in primary care and in consultation liaison psychiatry ranges from 11% to 18% [20] yet PTSD prevalence in adult population varies from Country to Country based on war exposure and cultural influence. In Italy it was reported to be 0.7% [21]. Dementia prevalence in people aged 60 years and over now range from 4.6% in Central Europe to 8.7% in North Africa and the Middle East [22].
The study of a possible association between the two conditions is, indeed, of clinical and social interest for its implications to prevention.
With the present study we tried to understand whether there is a prevalent clinical phenotype for the dementia associated with PTSD. In order to answer this question, we designed a prospective and a retrospective study, with a population of patients affected by PTSD followed for years until signs of cognitive decline occurred, and a population of patients affected by different forms of dementia, whose medical history was retrospectively analyzed for the presence of PTSD.
METHODS
The study was conducted following the Helsinki recommendations and subsequent revisions and was approved by our local ethical committee. The patients (or their caregivers, for patients with dementia) had to sign a written informed consent to participate to the studies.
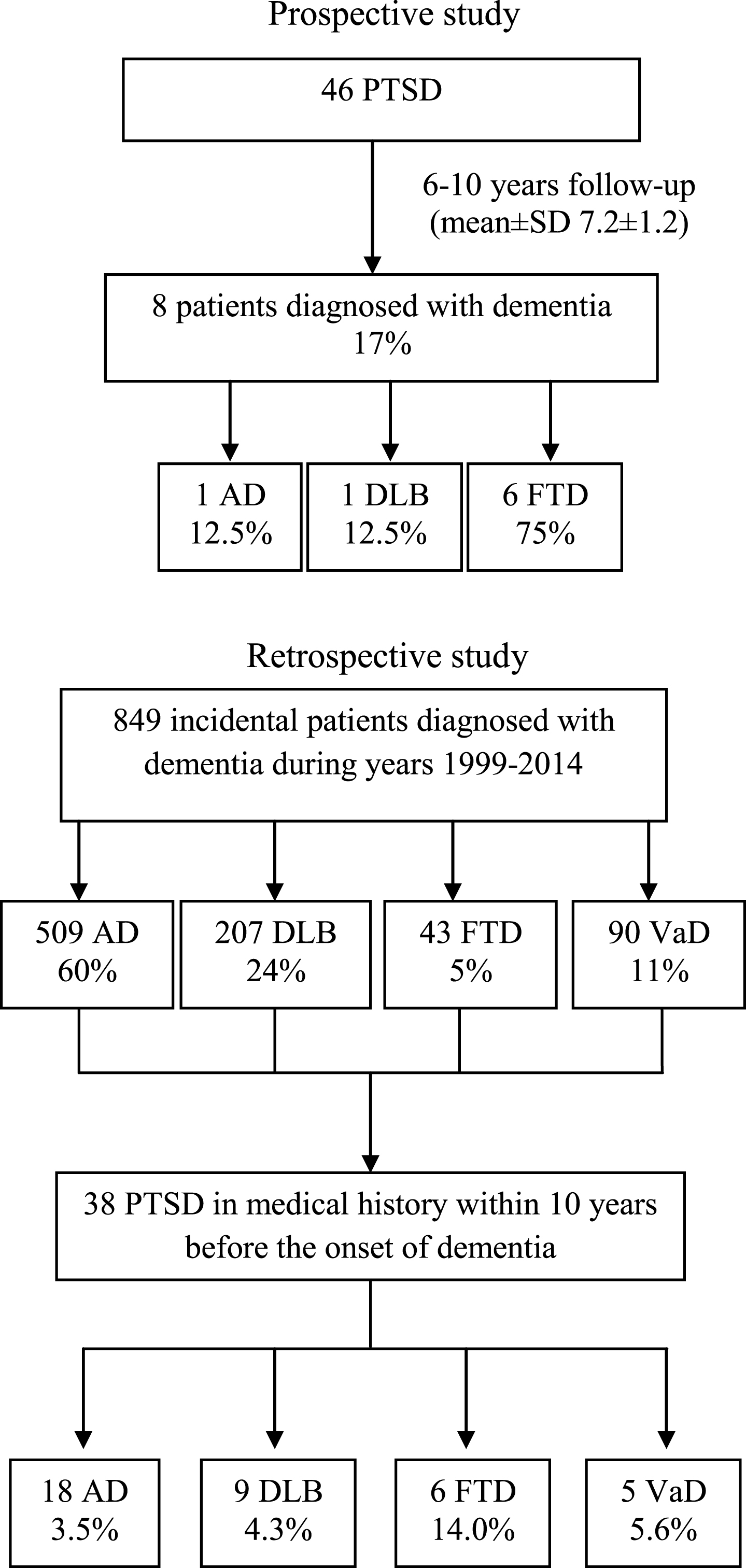
Figure 1 shows a flow chart of our study populations.
Fig.1
Flow chart of study populations. PTSD = post-traumatic stress disorder; AD = Alzheimer’s disease; DLB = Dementia with Lewy bodies; FTD = Frontotemporal dementia; VaD=Vascular Dementia.
PTSD diagnosis
PTSD was diagnosed according to DSM-IV TR criteria [23], by means of semi-structured interviews.
The Clinician-Administered PTSD Scale for DSM-IV-TR (CAPS-IV-TR) [24] was finally applied to confirm the diagnosis [25, 26].
Prospective study
Forty-six patients with PTSD were clinically and neuropsychologically followed for 6 to 10 years on a six-month basis. Probable AD diagnosis was based on NINCDS-ADRDA criteria [27]. DLB diagnosis was based on the revision of Consensus Criteria [28]. Frontotemporal dementia (FTD) diagnosis was based on standardized clinical criteria [29]. Vascular Dementia was diagnosed based on NINDS-AIREN criteria [30].
All patients underwent Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB) [31], Neuropsychiatric Inventory (NPI) [32], Clinician Assessment of Fluctuations (CAF) [33], tests for language (Token Test) [34] reasoning (Raven Colored Progressive Matrices [35], letter and category fluency test, [36]), short-term verbal memory (Digit Span Forward, [6 visuo-spatial short-term memory (Corsi Test 37]), verbal long-term memory (RAVLT, delayed recall [38]), long-term visual spatial memory (Rey-Osterrieth Complex Figure recall [39]), visuoconstructive abilities (Rey-Osterrieth Complex Figure copy [39]), and attention (Attentive Matrices, [40]). Beck Depression Inventory (BDI) was also administered [41]. The presence of REM sleep Behavior Disorder (RBD) was evaluated according to minimal International Classification of Sleep Disorders (ICSD) criteria for RBD [42] and confirmed by polysomnographic recordings.
Neuroimaging studies in all patients were performed at baseline and once a year during follow-up. They included 1.5 Tesla MRI of the brain with quantitative assessment of regional brain atrophy, including hippocampal, and frontal atrophy and assessment of sub-cortical vascular burden (leukoencephalopathy) according to Hachinski [43].
Diagnosis of AD or non-AD (DLB, FTD, VaD) dementia was supported by the measurement of specific protein levels (Aβ42, tau, and Ptau) in the cerebrospinal fluid (CSF) [44]. A CSF AD profile was defined as follows: Aβ level <800 pg/mL, total tau >300 pg/mL, and phosphorylated-tau protein >60 pg/mL. A CSF profile suggestive of DLB was defined as follows: Aβ level <800 pg/mL, total tau >300 pg/mL, and P-tau<60 pg/ mL [43]. A CSF profile suggestive of non-AD and non-DLB dementia was defined as follows: Aβ 1-42 level >800 pg/mL, total tau >300 pg/mL, and P-tau>60 pg/ mL [44].
Retrospective study
849 patients regularly followed in our dementia tertiary clinic (509 AD, 207 DLB, 90 VaD, 43 FTD) in the years 1999–2014 and a population of 287 patients afferent in the year 2016 to our Neurology Clinic for any neurological condition, were retrospectively evaluated for the presence in their medical history (within 10 years before the onset of dementia) of a life-threatening trauma with PTSD as for DSM-IV-TR criteria and CAPS-IV-TR. Interviews of patients, family members and General Practioners were conducted by clinicians (MDG, GM, FV, AT) unaware of the patients status and of the purpose of the study.
In particular, PTSD was investigated as reported in PTSD diagnosis paragraph and included also information from prior hospital records related to traumas or physical assaults and reports from patient’s GPs and family members.
Statistics
In the retrospective study, age and MMSE score were statistically compared across groups by means of ANOVA with Bonferroni post-hoc test, whereas the gender variable was compared by means of the non parametric Kruskal Wallis Test. The same statistical analyses were performed for the comparison of the dementia cohort of the retrospective study (cohort 1) and the demented cohort of the comparative group (cohort 2).
Comparisons between prevalences of all the categorical variables were performed by χ2 test. Bilateral confidence intervals (C.I.) for normal distribution without continuity corrections were also performed with 95% confident level, taking into account the percentage and sample size.
For the retrospective study of the cohort 1, Poisson regression was performed in the dementia cohort overall, using PTSD as the dependent variable and age, gender, and MMSE as independent possible demographic and clinical confounders.
RESULTS
Prospectively followed PTSD patient population
None of the 46 PTSD patients were lost to follow-up. Mean follow-up duration was 7.2±1.2 years.
18 patients were followed for 6 years, 12 patients were followed for 7 years, 9 patients for 8 years, 5 patients for 9 years, 2 patients for 10 years.
Eight patients with PTSD were diagnosed with dementia within 4 years follow-up but were followed for 6 years.
CAPS-IV-TR score was 9.3±2.2. Mean age was 65±4.3 years. Male gender represented 54% of the PTSD population (Table 1).
Table 1
Prospective study: baseline characteristics of PTSD followed for 6–10 years. PTSD patients (n = 46)
| Age (mean±S.D.) | 65±4.3 |
| Gender (% male) | 54% |
| MMSE(mean±S.D.) | 29.3±1.0 |
| CAPS IV-TR score | 9.3±2.2 |
CAPS-IV-TR, Clinician-Administered PTSD Scale for DSM-IV-TR; MMSE, Mini-Mental State Examination; PTSD, post traumatic stress disorder.
Twelve out of the 46 patients with PTSD were survivors from the catastrophic earthquake which stroke the city of L’Aquila in 2009.
In 28 of the 46 PTSD patients, PTSD symptoms appeared 3 to 5 months after the traumatic event (15 of these patients were initially diagnosed as affected by acute stress disorder [22]). In the remaining 18 patients symptoms had a delayed onset (in 10 patients the symptoms onset dated 6 to 10 months after the trauma, in 8 patients onset occurred between 1 and 2 years after the trauma).
Twenty-two patients completely recovered after 1 to 8 months after the symptoms onset. 10 of them were diagnosed as acute PTSD, since duration of symptoms was 1 to 3 months.
While typology of the trauma greatly varied among 34 patients, going from physical assault, to rape, to armed robbery, 12 patients shared the same trauma represented by a disastrous earthquake which hit the city of L’Aquila (the main city of Abruzzo Region) in the night between the 5 and 6 April 2009, causing the destruction of large parts of the town and the death of 309 people.
Eight patients with PTSD (17.4% of the total 46 patients) matched criteria for dementia within 4 years follow-up. In particular, one patient (female, 72 years old, educational level of 8 years, CAPS-IV-TR score 10) matched the criteria for AD dementia in 4 years (MMSE 20), one (male, 75 years old, CAPS-IV-TR score 10) for DLB dementia in 3 years (MMSE 22), 6 patients developed semantic FTD (with a latency of 2.7±1.0 years from PTSD occurrence), representing 75% of the PTSD patients who developed dementia and 13% of the total PTSD observed patients.
Table 2 reports demographical, clinical and neuropsychological test scores in the 6 patients who developed semantic FTD.
Table 2
Demographical, clinical and neuropsychological test scores of the six patients with post traumatic stress disorder (PTSD) who developed semantic frontotemporal dementia (FTD)
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | |
| Age | At the time of PTSD diagnosis | 69 | 73 | 69 | 75 | 70 | 66 |
| At onset of dementia | 72 | 77 | 72 | 78 | 71 | 68 | |
| Gender | F | M | M | M | M | F | |
| Education (y) | 13 | 13 | 17 | 5 | 3 | 5 | |
| BDI | 21 | 25 | 12 | 15 | 20 | 16 | |
| CAPS-IV-TR | 12 | 10 | 10 | 7 | 8 | 6 | |
| MMSE | At the time of PTSD diagnosis | 30 | 29 | 30 | 28 | 30 | 27 |
| At onset of dementia | 24 | 22 | 21 | 23 | 20 | 25 | |
| FAB | 10 | 11 | 9 | 11 | 10 | 11 | |
| DRS-2 | 107 | 115 | 98 | 103 | 99 | 100 | |
| NPI | 25 | 25 | 20 | 30 | 32 | 27 | |
| Token Test | 18 | 20 | 16 | 17 | 13 | 17 | |
| Letter fluency | 11 | 17 | 12 | 18 | 20 | 19 | |
| Raven Colored progressive matrices | 20 | 30 | 28 | 21 | 25 | 24 | |
| Digit span forward | 6 | 5 | 5 | 4 | 4 | 5 | |
| Clock drawing | 1 | 2 | 1 | 2 | 2 | 1 |
BDI, Beck Depression Inventory; CAPS-IV-TR, Clinician-Administered PTSD Scale for DSM-IV-TR; MMSE, Mini-Mental State Examination; PTSD, post traumatic stress disorder; FAB, Frontal Assessment Battery; DRS-2, Dementia Rating Scale-2; NPI, Neuropsychiatric Inventory. FAB, DRS-2, NPI, Token Test, Letter fluency, Raven Colored progressive matrices, Digit span forward, Clock drawing were performed at onset of dementia.
Case descriptions
Patient 1 is a 69-year-old female, with 13 years of education, a retired dealer, amateur mountain climber, and participated in 2003 in a guided climbing expedition to Mount Everest. During the ascent, a sudden storm isolated her from the rest of the group and she remained alone for 10 hours in the cold until the guide rescued her. Back to Italy she returned to her routine life without any apparent consequence from the adventure. After seven months, however, she started feeling unusually generically anxious about her 20-year-old son, who was in perfect health; she abandoned her passion for climbing and she became agoraphobic. After two months of unsuccessful attempt to sedate her anxiety with benzodiazepines, she was referred by the GP to our Psychiatry Clinic where she was diagnosed as affected by PTSD (CAPS-IV-TR total score of 12). When asked about her experience on mount Everest she started sweating and asked not to talk about that event anymore. Even mentioning it caused her a sense of thoracic constriction. She reported that during the last three months she had dreamed several times about being abandoned in the cold.
An MRI scan at that time was normal. No atrophy and unspecific mild signs of leukoencephalopathy were reported. Neck vessels Doppler ultrasound gave normal results. MMSE was 30. Beck Depression Inventory rated the presence of depression (BDI = 11).
She was successfully treated with sertraline (50 mg/day) for 3 years. She went back to climbing again, but with her bewilderment she was unable to use climbing hooks. Her family members reported also that the patient’s spontaneous speech was fluent but empty. She was then referred to our dementia center to be tested for the possible onset of a cognitive disorder.
At the end of 2006 her MMSE resulted to be 24. Her naming and comprehension appeared progressively impaired, with paraphasias. An MRI scan showed the presence of initial regional left-sided atrophy in the temporal lobe and mild signs of leukoencephalopathy, unmodified as compared to first MRI scan. EEG recording at rest showed a dominant frequency stable at 8.0 Hz in posterior derivations and theta activity at 5-6 Hz in temporal derivations.
Lumbar puncture for the dosage of CSF tau and amyloid proteins showed the following pattern: increased phospho-tau with normal amyloid levels: total tau 448 pg/ml, phosphorylated-tau protein 98 pg/ml, Aβ 1-42 level 857 pg/mL).
The patient was diagnosed as affected by semantic FTD.
She was followed in our dementia center with regular clinical and neuropsychological examinations. Dysexecutive symptoms appeared with difficulties in reasoning and planning daily activities.
An attempt to treat the patient with memantine caused the appearance of aggressiveness.
The last evaluation was performed in 2011. It was not possible to perform a neuropsychological evaluation: the patient was completely aphasic and apathetic. Fecal incontinence was reported by family members. The last MRI, performed in June 2011, showed marked frontal and temporal symmetric atrophy. EEG recording at rest showed a dominant frequency in the delta-theta bands in temporal derivations.
Patient 2 is a 73-year-old man, with 13 years of education, and a retired director of post office.
Between 2003 and 2005 he underwent 3 robbery attempts. During the first of these episodes three criminals broke in the post office shooting with their guns against the ceiling. During the second and third episode the criminals ran inside the office threatening the employees with guns.
He seemed to have reacted well in the first 4 months after the last episode. He reported that the employees felt heartened by his rational and calm behavior. But after a while he started waking up at morning with a new sense of anxiety, fearing the moment to step into his work place. He was particularly anxious when people entered the office. He became sleepless at night thinking about possible ways to make his post office more secure.
He was diagnosed as PTSD (CAPS-IV-TR = 10) and treated with mirtazapine 30 mg with good efficacy.
He spent 4 years in good health, until he started having difficulties in mathematical calculations. Neuropsychological tests showed word finding and comprehension difficulties and semantic paraphasias. MMSE was 22. He underwent an MRI scan which showed bilateral temporal atrophy, more evident on the left side. CSF tau and amyloid proteins showed: total tau 455 pg/ml, phosphorylated-tau protein 89 pg/ml, Aβ 1-42 level 915 pg/mL). The patients received the diagnosis of semantic variant of primary progressive aphasia.
Patient 3 is a 69-year-old man, with 17 years of education, and a retired middle school teacher.
In 1999, he was giving his chemistry lecture at school, when a foolish ran into his class screaming and pointing a gun against the patient and his schoolchildren, claiming that he had been fired by his company and he was then able to do anything that passed into his mind.
The patient and children were forced to listen to crazy lectures by the madman for several hours, until the police came and saved them, arresting the criminal.
The patient reported to have been extremely stricken by this experience and from the very first moments after the trauma he was unable to run his normal life. He was anxious, agitated, sleepless, often compelled to cry. He came to our center where he received the diagnosis of acute PTSD (CAPS-IV-TR = 10). He was treated with benzodiazepines and sertraline 100 mg daily.
He went back to work and felt better for 3 years, after which he reported to experience episodes of “lack of words” during teaching and of forgetfulness while carrying out normal activities of daily living: he happened to lose his keys, to forget where he had parked his car. He started also experiencing difficulties in performing sequential tasks, such as preparing a meal, or driving his car. We prescribed an MRI, which showed frontal atrophy more prominent in the left hemisphere, and an EEG which showed posterior dominant frequency stable at 10 Hz, with inscription of theta sequences and sharp waves in temporal derivations. MMSE was 21.
The patient was diagnosed as affected by semantic FTD. Complete neuropsychological test scores are reported in Table 2. He underwent lumbar puncture which showed increased level of tau and phospho-tau proteins (total tau 385 pg/ml, phosphorylated-tau protein 77 pg/ml, Aβ 1-42 level 878 pg/mL).
Patient 4 is a 75-year-old man, with 5 years of education, and a dealer. In 1999 he had a car accident while he was driving along an overpass with his 5-year-old grand-child. His car remained suspended over a canyon for one hour, before the breakdown service came, secured the car and rescued the two passengers.
The patients never stopped dreaming the accident for 3 months. He became anxious and could not drive any longer.
He came to our Psychiatry Clinic where he was diagnosed as affected by PTSD (CAPS-IV-TR score = 7).
The patient symptoms, treated with antidepressants, benzodiazepines and psychotherapy subsided in one year. He returned to a normal life and started driving again.
After three years, however, the patient’s language became poor with occasional anomia. And in the following year the patient developed dyscalculia and a dysexecutive syndrome characterized by difficulties in planning the orders of foodstuffs for his grocery store.
The patient was referred to our memory clinic. An MRI scan showed the presence of initial regional left-sided atrophy in the temporal and frontal lobe. EEG recording at rest showed a dominant frequency stable at 8.0 Hz in posterior derivations and delta-theta activity at 3–6 Hz in temporal derivations. MMSE was 23, DRS-2 total score was 103.
He underwent lumbar puncture which showed increased level of tau and phospho-tau proteins (total tau 401 pg/ml, phosporylated-tau protein 98 pg/ml, Aβ 1-42 level 856 pg/mL).
The patient was diagnosed as affected by semantic FTD and treated with transdermic rivastigmine 4.6 mg daily.
Patient 5 is a 70-year-old man, with 3 years of education, and a farmer. In 2001 the patient had a quarrel with his neighbor about the boundaries of their respective adjacent properties. During the argue his neighbor, increasingly upset, repeatedly poke the patient with a pitchfork, causing severe wounds to the patient face, neck and chest.
Conspicuously bleeding, the patient was transported to the emergency room where his wounds were sutured. A brain CT scan resulted to be normal. An X ray of the chest showed no signs of pneumothorax. He recovered in one month.
After 7 months the patient started feeling anxious with inexplicable sense of fear which caused withdrawal of social activities. He had occasional nightmares about the fight with his neighbor. He was referred by his GP to our Psychiatry Clinic where he was diagnosed as affected by PTSD (CAPS-IV-TR = 8).
These symptoms lasted for 6 months. He spent one year of fairly normal life, after which, as reported by his wife, the patient started showing progressive difficulties in recognizing familiar places. Prominent language impairment with impaired word comprehension became apparent. The patient was therefore referred to our memory clinic, where he was studied with MRI scan, EEG, complete neuropsychological test battery.
MRI showed bilateral temporal atrophy. EEG recording showed a dominant frequency stable in the alpha band (9.0 Hz) in posterior derivations and slow wave activity in temporal derivations more evident in the left hemisphere characterized by delta-theta activity at 3–5 Hz with sporadic sharp waves, MMSE was 20, DRS-2 total score was 99.
He underwent lumbar puncture which showed increased level of tau and phospho-tau proteins (total tau 397 pg/ml, phosphorylated-tau protein 82 pg/ml, Aβ 1-42 level 822 pg/mL).
The patient was diagnosed as affected by semantic FTD and treated with transdermal rivastigmine 4.6 mg.
At present the patient has a MMSE of 10 and is treated with rivastigmine 9.5 mg.
Patient 6 is a 66-year-old woman, with 5 years of education, and a farmer. On April 6th, 2009, she was sleeping in her two-storey house in the city of L’Aquila when at 3.00 a.m. a violent earthquake stroke the city, causing destruction of several buildings. The patient was transported by her son outside her house, unharmed but frightened, and spent that night in the street in her pajama with an outdoor temperature of 5°C. She was compelled with many other citizens of L’Aquila to live in a tent for three weeks before being transferred to a hotel on the coast.
A few days after, the patient, unusually silent and secluded to family members, started reporting frequent intrusive recollections of destroyed buildings and falling stones and bricks. These images appeared also at night as terrifying dreams, where the patient reported to feel lonely and helpless. At day 10 from the relocation (which lasted 4 months) the patient was found by her family members in confusional state and she was admitted to our Neurology Clinic.
EKG and laboratory exams were all normal.
EEG dominant frequency was stable at 9.0 Hz. An MRI scan showed mild signs of age-related unspecific diffuse atrophy. She was treated with benzodiazepines and quetiapine, with good clinical response, with resolution of confusion. MMSE was 27. The patient was therefore diagnosed as affected by PTSD (CAPS-IV-TR score = 6).
In June 2011 the patient’s daughter-in-law contacted our Dementia Center, concerned by a change in the patient behavior, characterized by difficulty to recall names of family members, fluent but senseless speech followed by incongruous attitude, with inappropriate language and vain hilarity.
MMSE resulted to be 25. A second MRI scan showed the presence of modest signs of leukoencephalopathy, clear signs of frontal atrophy, initial signs of bilateral temporal atrophy, with increase of lateral ventricles size.
She underwent lumbar puncture which showed increased level of tau and phospho-tau proteins (total tau 401 pg/ml, phosphorylated-tau protein 91 pg/ml, Aβ 1-42 level 813 pg/mL).
The patient was diagnosed as affected by semantic FTD.
EEG dominant frequency was still characterized by alpha dominant frequency (9 Hz) in posterior derivations.
Retrospective study
PTSD in the dementia cohorts
Based on a patient population of 849 patients regularly followed in our dementia tertiary clinic (509 AD, 207 DLB, 90 VaD, 43 FTD) in the years 1999–2014, 38 patients (22 males) resulted to be suffering from PTSD. Table 3 reports demographic and neuropsychological data of the dementia populations.
Table 3
Demographic, clinical and cognitive status in the dementia population followed in the years 1999–2014
| Retrospective study: patients with dementia (n = 849) | |||||
| AD (n = 509) | DLB (n = 207) | FTD (n = 43) | VaD (n = 90) | p | |
| Age (mean±SE) | 72.5±0.2 | 74.2±0.3 | 60.6±0.5 | 82.4±0.5 | <10-6 |
| Age range | 65–80 | 67–82 | 55–65 | 75–90 | |
| Gender (% male) | 35% | 65% | 49% | 51% | <10-4 |
| MMSE (mean±SE) | 14.2±0.3 | 17.0±0.3 | 10.4±0.5 | 18.1±0.4 | <10-4 |
| MMSE range | 5–24 | 10–24 | 5–15 | 12–24 | |
| PTSD prevalence | 18 (3.5%) | 9 (4.3%) | 6 (14.0%) | 5 (5.6%) | |
MMSE, Mini-Mental State Examination; PTSD, post traumatic stress disorder. p values refer to main effect of ANOVA analysis. Post-hoc analysis showed that age was significantly different among all groups (p < 10-4 for the comparison between AD and DLB, p < 10-6 for all the other comparisons). Gender was significantly different between AD and DLB (p < 10-6). MMSE score was significantly different for all comparisons between groups (p < 10-4) except for the DLB versus VaD comparison.
Significant differences among groups were found for age (p < 10-6), MMSE (p < 10-6) and gender p < 10-4).
The 38 patients who developed PTSD were representing 4.5% of our population affected by dementia.
Traumas described by patients were physical assaults in 14, life threatening car accidents in 6 patients, life threatening workplace injuries in 8, sexual violence in 2, robbery in 8 patients.
The mean age of PTSD symptoms appearance was 65±4.3 years.
Specifically, the percentage of patients with dementia who had a history of PTSD was as follows: 3.5% (2.7–4.3 C. I.) of AD patients, 4.3% (2.9–5.7%) of DLB, 14.0% (8.4–19.6%) of FTD, 5.6% (3.2–8.0%) of VaD (Table 3). The percentage was significantly higher in FTD than in AD or DLB (χ2 = 10, p = 0.001, and χ2 = 6, p = 0.02 respectively). Furthermore, the incidence of FTD among the dementia patients with a history of PTSD (38 patients) was higher than in the dementia population overall (16% (9.6–22.4% C. I.) of FTD patients who had a history of PTSD as compared to 5% (4.2–5.8%) of FTD in the overall 849 patients with dementia, χ2 = 8, p = 0.005). Among the dementia patients with a history of PTSD the incidence of AD (47%, 36–58% C. I.), DLB (24%, 16–32%) or VaD (13%, 7–19%), was not significant different to the incidence of AD (60%, 57–63%), DLB (24%, 22–26%) and VaD (11%, 10–12%) in the dementia population overall.
Poisson regression showed no significant effect of the demographic (age, gender) and clinical (MMSE) variables on the group of demented patients which had PTSD in their history or not.
Poisson regression results on PTSD effect on dementia did not change whether taking into account (p = 0.05) or not (p = 0.03) the aforementioned confounders (Supplementary Material).
PTSD in the comparative group
Demographical and clinical data of the 287 neurological patients of the comparative group admitted to our Neurology Unit in the first six months of the year 2016 are reported in Table 4. Among them, 107 patients (37%) resulted to be affected by dementia (46 AD (43%), 45 VaD (42%), 14 DLB (13%), 2 FTD (2%). A history of PTSD was present in 4 patients (3.7%, 2 AD, 2 VaD).
Table 4
Demographic, clinical and cognitive status in patients afferent to our Neurology Clinic for any neurological condition
| all patients (n = 287) | non demented patients (n = 180) | demented patients (n = 107) | |
| Diagnosis ad admission: | |||
| Cerebral ischemia n (%) | 138 (48%) | 88 (49%) | 50 (47%) |
| Cerebral hemorrhages* n (%) | 40 (14%) | 26 (14%) | 14 (13%) |
| Brain tumor§ n (%) | 26 (9%) | 18 (10%) | 8 (7%) |
| Other† n (%) | 83 (29%) | 48 (27%) | 35 (33%) |
| Age (mean±SE) | 68.8±0.1 | 63.9±0.1 | 77.0±0.1 |
| Age range | 21–95 | 21–95 | 29–95 |
| Gender (% male) | 52% | 52% | 52% |
| MMSE (mean±SE) | 24.0±0.0 | 27.8±0.1 | 18.4±0.1 |
| MMSE range | 1–30 | 24–30 | 1–23 |
| PTSD prevalence n (%) | 6 (2.1%) | 2 (1.1%) | 4 (3.7%) |
MMSE, Mini Mental State Examination; PTSD, post traumatic stress disorder. *including post-traumatic hematoma, subarachnoid haemorrhage, intracerebral haemorrhages. §Including both primary and metastases. †18 patients (pts) with epilepsy, 13 pts with myasthenia gravis, 9 pts with central nervous system vasculitis, 5 pts with motor neuron disease, 5 pts with global amnesia, 5 pts with viral encephalitis, 4 pts with Parkinson Disease, 4 pts with polineuropathy, 3 pts with spinal ataxia, 3 with dystonia, 2 pts with progressive supranuclear palsy, 2 pts with muscular dystrophy, 2 pt with chronic paraplegia, 1 pt with retrobulbar optic neuritis, 1 pt with Creutzfeld-Jacobs disease, 1 pt with chorea, 1 pt with inflammatory myelopathy, 1 pt with limbic encephalitis, 1 pt with hydrocephalus, 1 pt with medullary spinal cord ischemia, 1 pt with Guillain-Barrè Syndrome.
On the population of 180 patients without dementia (63%), a history of PTSD was present in 2 patients (1.1%, 1 patient with ischemic stroke, 1 with brain tumor).
Statistical comparison between the demented cohort of the comparative group (cohort 2) and the retrospective cohort (cohort 1) showed that age and MMSE were higher in the cohort 2 (p < 10-5; p < 10-6, respectively).
DISCUSSION
We found an association between PTSD and FTD. In the prospective study on 46 PTSD patients, 6 developed FTD (13%) and in the retrospective observation on 38 patients which resulted to be suffering from PTSD, 6 patients had FTD (16%).
PTSD prevalence in adult population varies from Country to Country based on war exposure and cultural influence. In Italy it was reported to be 0.7% [21]. Specifically, the percentage of PTSD found in our Neurology Clinic, in patients without dementia was 1.1%, very similar to percentage reported in the Italian general population (Table 4). The percentage of PTSD was higher in patients with dementia afferent in the same observational period in the same Neurology Clinic (3.7%) (Table 4) and in our retrospective dementia cohorts (4.5%). The association between PTSD and dementia is well reported in literature [1–5, 45], even though not specific association has been evaluated with dementia subtypes.
Literature data are available on a population from the same Italian region, which was hit by a earthquake in 2009, which led to 300 deaths. A prospective study on survivors from the earthquake [46] evaluated that PTSD was developed in 4.1% of that specific population, a percentage very close to the one that we found in our retrospective cohort of dementia patients (4.5%), and higher than the prevalence or incidence of PTSD in the Italian general population, as reported in literature [21]. This underlines that the prevalence of PTSD in dementia is similar to incidence found in an at risk population.
The mean age of our PTSD population (65±4.3 years) was higher than the corresponding index in the general population [23], as children do not refer to our tertiary clinic.
An age range of >60 years was considered by the World Mental Health Survey Initiative as a risk factor for PTSD in the Italian population [47], as Italian people older than 60 were exposed to trauma related to World War II. However, incidence of PTSD in our Neurology clinics, in non demented patients, did not show such a difference as compared to patients younger than 60 years, even though the small number of patients with PTSD in that population did not allow any statistical comparison.
Furthermore, Poisson regression in our retrospective dementia cohort (cohort 1) showed no effect of age (and gender and MMSE) on the association between dementia subgroups and a history of PTSD.
At difference with previous reports [48], which underline a higher incidence of PTSD in the female population, there was no gender effect in the occurrence of PTSD in our patient population (as also confirmed by Poisson regression).
The incidence of FTD cases in the PTSD population was surprisingly high, if considering that FTD is considered a rare disease, with an overall prevalence among dementia subtypes of about 10%.
Data on prospective cohorts of patients followed in our region independently of a history of PTSD showed that incidences of different dementia types were inside the variability ranges reported in prior epidemiological surveys and by other centers [49]. It is actually interesting to note that FTD incidence was 3.5% among patients with dementia, matching the percentage found in our retrospective cohort (5% on a population of 849 demented patients).
FTD is now considered as an umbrella definition including different clinical and neuroimaging subtypes.
From a nosological perspective, semantic dementia, also known as the temporal variant is one of the three clinical forms of frontotemporal lobar degeneration, along with the frontal or behavioural variant and progressive non-fluent aphasia [50].
Semantic dementia represents about 45% of FTD cases and is characterized by prominent and early language impairment.
Our FTD cases with PTSD presented all with early language dysfunction, suggesting an involvement of dorsolateral frontal and temporal cortex in the genesis of the PTSD symptoms. This was surprising because alterations of amygdala related to PTSD would have suggested an involvement of mesial orbito-frontal cortex [14].
A common concept that links PTSD and FTD dementia is the lack of resilience [51, 52]. Resilience is defined as individual’s ability to properly adapt to stress and adversity and medial frontal lobe and the prefrontal cortex are the structures of the brain that support response flexibility, being able to shift perspectives, discern options, and make wise choices, essential elements of resilience [52].
We suggest that a possible lack of resilience could be a sign of the tendency to develop FTD and therefore to make the subject who will eventually become demented, more prone to also develop PTSD in his history.
A genetic assessment for genes related to FTD in PTSD population could help defining the presence of a shared milieu between the two conditions, expressed for example as atrophy of hippocampus which is present in both PTSD and FTD patients.
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that serves as survival factor for selected populations of central nervous system (CNS) neurons and plays a role in the limbic system by regulating synaptic plasticity, memory processes and behaviour. Impaired BDNF production in the brain can lead to a variety of CNS dysfunctions including symptoms associated with PTSD [53].
A recent study found that BDNF serum levels were lower in PTSD patients as compared to related control subjects [54]. Genetic studies showed evidence for an association between a single nucleotide polymorphism in the BDNF gene (Val66Met) and PTSD [55, 56]. This mutation is thought to alter BDNF stability and activity dependent secretion, leading to dysfunctional BDNF signaling and altered memory function [57]. These abnormalities have been described in different psychiatric disorders.
Impaired cognition has been described in PTSD patients, in comparison with subjects exposed to trauma who did not develop PTSD [58]. Given the role of BDNF in cognitive function [59] it is possible that BDNF reduction or, in case of Val66Met polymorphism, dysfunction, may contribute to the cognitive disturbance in PTSD patients. Interestingly, low levels of serum BDNF have been recently associated with cognitive deficits in other pathologies such as schizophrenia, depression [60–65] and FTD [62].
We hypothesize that a common genetic environment together with lower levels of BDNF could provide a possible link between PTSD and FTD.
The importance of the recognition of a common basis between PTSD and dementia, specifically of FTD type, resides in the possibility to successfully treat PTSD with possible positive repercussion on dementia symptoms onset or severity.
Study limitations
The retrospective part of the study is, undoubtedly,flawed by the typical biases of any retrospective study, including recall bias which might explain the lower incidence of PTSD among the dementia population (4%) as compared to incidence of dementia found in PTSD population evaluated in the prospective study (17%). Another possible limitation is that the prevalence of specific diagnoses is higher in a tertiary center as our University center, which is endowed with several advanced diagnostic tools ranging from SPECT, PET, MRI, fMRI 1.5–3 T, to laboratories of proteomics for assessment of CSF proteins, to genetic assessments of neurodegenerative disease to neuroimmunological essays, to complex neuropsychological evaluations [49].
Furthermore, it is also likely that our patients population was spontaneously selected because of the severity of symptoms which often lead the patients to refer to tertiary centers. Therefore further studies in different centers should be augured.
We strictly applied, however, DSM-IV-TR classification methods, and interviews were performed by clinicians unaware of diagnosis of patients and purpose of the study.
All in all,the two parts of the study suggest a significant relationship between PTSD and FTLD,an association deserving further multicenter studies.
Strengths and limitations
One strength of our study lays in the complementary approach conferred by the prospective and retrospective observation of the association between PTSD and FTD. In particular, the retrospective study was conducted in a very large population of dementia patients and could confirm the surprising association between the two conditions, observed in the prospective study.
Further prospective or retrospective studies, from multiple centers, conducted with multivariate analyses are, undoubtedly, needed in order to confirm the relevance of our observations.
Despite this major limitation our study has a strength, as it identifies the distinct FTD phenotype, with the semantic variant, as a possible negative outcome of PTSD. Preceding literature did not categorize dementia after PTSD beyond a generic definition or anecdotal reports [1–5]. A minor strength, not overcoming the limitations reported above, is also that our observations are based on a tertiary center referral, with a wide catchment area: during the study period the incidences of different dementia types were inside the variability ranges reported in prior epidemiological surveys [49] and by other centers. Thus, we believe that our observation indicates an unexpected and unprecedented possible link between PTSD and FTD phenotype, and may constitute a preliminary background for further improved studies.
We believe that it is worthy to strictly follow patients with PTSD for the possible development of signs of cognitive decline, in order to identify these signs early in the course of dementia and allocate patients to early pharmacological and non pharmacological interventions.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-1134r2).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-171134.
REFERENCES
[1] | Brewin CR , Kleiner JS , Vasterling JJ , Field AP ((2007) ) Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. J Abnorm Psychol 116: , 448–463. |
[2] | Yaffe K , Vittinghoff E , Lindquist K , Barnes D , Covinsky KE , Neylan T , Kluse M , Marmar C ((2010) ) Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry 67: , 608–613. |
[3] | Yehuda R , Tischler L , Golier JA , Grossman R , Brand SR , Kaufman S , Harvey PD ((2006) ) Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol Psychiatry 60: , 714–721. |
[4] | Vasterling JJ , Proctor SP , Amoroso P , Kane R , Heeren T , White RF ((2006) ) Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA 296: , 519–529. |
[5] | Johnsen GE , Asbjørnsen AE ((2008) ) Consistent impaired verbal memory in PTSD: A meta-analysis. J Affect Disord 111: , 74–82. |
[6] | Sperling W , Kreil SK , Biermann T ((2011) ) Posttraumatic stress disorder and dementia in Holocaust survivors. J Nerv Ment Dis 199: , 196–198. |
[7] | Bremner JD , Randall P , Scott TM , Bronen RA , Seibyl JP , Southwick SM , Delaney RC , McCarthy G , Charney DS , Innis RB ((1995) ) MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 152: , 973–981. |
[8] | Bremner JD , Randall P , Vermetten E , Staib L , Bronen RA , Mazure C , Capelli S , McCarthy G , Innis RB , Charney DS ((1997) ) MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse-A preliminary report. Biol Psychiatry 41: , 23–32. |
[9] | Gurvits TV , Shenton ME , Hokama H , Ohta H , Lasko NB , Gilbertson MW , Orr SP , Kikinis R , Jolesz FA , McCarley RW , Pitman RK ((1996) ) Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 40: , 1091–1109. |
[10] | Stein MB , Koverola C , Hanna C , Torchia MG , McClarty B ((1997) ) Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 27: , 951–959. |
[11] | Schuff N , Neylan TC , Lenoci MA , Du AT , Weiss DS , Marmar CR , Weiner MW ((2001) ) Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry 50: , 952–959. |
[12] | Stratta P , Bonanni RL , Sanitá P , de Cataldo S , Angelucci A , Origlia N , Domenici L , Carmassi C , Piccinni A , Dell’Osso L , Rossi A ((2013) ) Plasma brain-derived neurotrophic factor in earthquake survivors with full and partial post-traumatic stress disorder. Psychiatry Clin Neurosci 67: , 363–364. |
[13] | Brady KT , Killeen TK , Brewerton T , Lucerini S ((2000) ) Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry 61: (Suppl 7), 22–32. |
[14] | Liberzon I , Sripada CS ((2008) ) The functional neuroanatomy of PTSD: A critical review. Prog Brain Res 167: , 151–169. |
[15] | Whitaker AM , Gilpin NW , Edwards S ((2014) ) Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol 25: , 398–409. |
[16] | Andero R , Ressler KJ ((2012) ) Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav 11: , 503–512. |
[17] | Pitman RK , Rasmusson AM , Koenen KC , Shin LM , Orr SP , Gilbertson MW , Milad MR , Liberzon I ((2012) ) Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13: , 769–787. |
[18] | Kennis M , Rademaker AR , van Rooij SJ , Kahn RS , Geuze E ((2015) ) Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum Brain Mapp 36: , 99–109. |
[19] | Pitman RK , Gilbertson MW , Gurvits TV , May FS , Lasko NB , Metzger LJ , Shenton ME , Yehuda R , Orr SP , Harvard VA , Twin Study PTSD Investigators ((2006) ) Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci 1071: , 242–254. |
[20] | Hidalgo RB , Davidson JR ((2000) ) Posttraumatic stress disorder: Epidemiology and health-related considerations. J Clin Psychiatry 61: (Suppl 7), 5–13. |
[21] | Burri A , Maercker A ((2014) ) Differences in prevalence rates of PTSD in various European countries explained by war exposure, other trauma and cultural value orientation. BMC Res Notes 7: , 407. |
[22] | Prince M , Wimo AGM , Ali GC , Wu YT , Prina M ((2015) ) World Alzheimer Report 2015: The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London. |
[23] | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fifth ed.). American Psychiatric Publishing, (2013) Arlington, VA . |
[24] | Weathers FW , Ruscio AM , Keane TM ((1999) ) Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess 11: , 124–133. |
[25] | Bliese PD , Wright KM , Adler AB , Cabrera O , Castrol CA , Hoge CW ((2008) ) Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. J Consult Clin Psychol 76: , 272–281. |
[26] | https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp. |
[27] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[28] | McKeith IG , Dickson DW , Lowe J , Emre M , O’Brien JT , Feldman H , Cummings J , Duda JE , Lippa C , Perry EK , Aarsland D , Arai H , Ballard CG , Boeve B , Burn DJ , Costa D , Del Ser T , Dubois B , Galasko D , Gauthier S , Goetz CG , Gomez-Tortosa E , Halliday G , Hansen LA , Hardy J , Iwatsubo T , Kalaria RN , Kaufer D , Kenny RA , Korczyn A , Kosaka K , Lee VM , Lees A , Litvan I , Londos E , Lopez OL , Minoshima S , Mizuno Y , Molina JA , Mukaetova-Ladinska EB , Pasquier F , Perry RH , Schulz JB , Trojanowski JQ , Yamada M , Consortium on DLB ((2005) ) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium . Neurology 65: , 1863–1872. Erratum in: Neurology 65, 1992. |
[29] | McKhann GM , Albert MS , Grossman M , Miller B , Dickson D , Trojanowski JQ , Work Group on Frontotemporal Dementia, Pick’s Disease ((2001) ) Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on frontotemporal dementia and Pick’s disease. Arch Neurol 58: , 1803–1809. |
[30] | Roman GC , Tatemichi TK , Erkinjuntti T , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A ((1993) ) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: , 250–260. |
[31] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB: A Frontal Assessment Battery at bedside. Neurology 55: , 1621–1626. |
[32] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbeinm J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: , 2308–2314. |
[33] | Walker MP , Ayre GA , Cummings JL ((2000) ) The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 177: , 252–256. |
[34] | De Renzi A , Vignolo LA ((1962) ) Token test: A sensitive test to detect receptive disturbances in aphasics. Brain 85: , 665–678. |
[35] | Basso A , Capitani E , Laiacona M ((1987) ) Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Funct Neurol 2: , 189–194. |
[36] | Novelli G , Papagno C , Capitani E , Laiacona M , Cappa S , Vallar G ((1986) ) Three clinical tests for the assessment of verbal long term memory function. Norms from 320 normal subjects. Arch Psicol Neurol Psichiatr 47: , 278–296. |
[37] | Orsini A , Grossi D , Capitani E , Laiacona M , Papagno C , Vallar G ((1987) ) Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8: , 537–548. |
[38] | Carlesimo GA , Caltagirone C , Gainotti G , Nocentini U ((1995) ) Batteria per la valutazione del deterioramento mentale (PARTE II): Standardizzazione e affidabilitá diagnostica nell’identificazione di pazienti affetti da sindrome demenziale. Arch Psicol Neurol Psichiatr 56: , 1–18. |
[39] | Caffarra P , Vezzadini G , Dieci F , Zonato F , Venneri A ((2002) ) Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol Sci 22: , 443–447. |
[40] | Spinnler H , Tognoni G ((1987) ) Taratura e standardizzazione italiana di test neuropsicologici. Ital J Neurol Sci 278: (Suppl 8), 1–120. |
[41] | Beck AT , Ward CH , Mendelson M , Mock J , Erbaugh J ((1961) ) An inventory for measuring depression. Arch Gen Psychiatry 4: , 561–571. |
[42] | World Health Organization ((1992) ) The ICD-10 classification of mental and behavioural disorders WHO, Geneva. |
[43] | Hachinski VC , Iliff LD , Zilhka E , DuBoulay GH , McAllister VL , Marshall J , Russell RW , Symon L ((1975) ) Cerebral blood flow in dementia. Arch Neurol 32: , 632–637. |
[44] | Parnetti L , Lanari A , Amici S , Gallai V , Vanmechelen E , Hulstaert F , Phospho-Tau International Study Group ((2001) ) CSF phosphorylated tau is a possible marker for discriminating Alzheimer’s disease from dementia with Lewy bodies. Phospho-Tau International Study Group. Neurol Sci 22: , 77–78. |
[45] | Roncone R , Giusti L , Mazza M , Bianchini V , Ussorio D , Pollice R , Casacchia M ((2013) ) Persistent fear of aftershocks, impairment of working memory, and acute stress disorder predict post-traumatic stress disorder: 6-month follow-up of help seekers following the L’Aquila earthquake. Springerplus 2: , 636. |
[46] | Gigantesco A , Mirante N , Granchelli C , Diodati G , Cofini V , Mancini C , Carbonelli A , Tarolla E , Minardi V , Salmaso S , D’Argenio P ((2013) ) Psychopathological chronic sequelae of the 2009 earthquake in L’Aquila, Italy. J Affect Disord 148: , 265–271. |
[47] | Carmassi C , Dell’Osso L , Manni C , Candini V , Dagani J , Iozzino L , Koenen KC , de Girolamo G ((2014) ) Frequency of trauma exposure and post-traumatic stress disorder in Italy: Analysis from the World Mental Health Survey Initiative. J Psychiatr Res 59: , 77–84. |
[48] | Ditlevsen DN , Elklit A ((2010) ) The combined effect of gender and age on post traumatic stress disorder: Do men and women show differences in the lifespan distribution of the disorder? Ann Gen Psychiatry 9: , 32. |
[49] | Bonanni L , Bontempo G , Borrelli I , Bifolchetti S , Buongarzone MP , Carlesi N , Carolei A , Ciccocioppo F , Colangelo U , Colonna G , Desiderio M , Ferretti S , Fiorelli L , D’Alessio O , D’Amico A , D’Amico MC , De Lucia R , Del Re L , Di Blasio F , Di Giacomo R , Di Iorio A , Di Santo E , Di Giuseppe M , Felice N , Litterio P , Gabriele A , Mancino E , Manzoli L , Maruotti V , Mearelli S , Molino G , Monaco D , Nuccetelli F , Onofrj M , Perfetti B , Sacchet C , Sensi F , Sensi S , Sucapane P , Taylor JP , Thomas A , Viola P , Viola S , Zito M , Zhuzhuni H ((2013) ) Ascertainment bias in dementias: A secondary to tertiary centre analysis in Central Italy and conceptual review. Aging Clin Exp Res 25: , 265–274. |
[50] | Neary D , Snowden JS , Gustafson L , Passant U , Stuss D , Black S , Freedman M , Kertesz A , Robert PH , Albert M , Boone K , Miller BL , Cummings J , Benson DF ((1998) ) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 51: , 1546–1554. |
[51] | Olff M , Polak AR , Witteveen AB , Denys D ((2014) ) Executive function in posttraumatic stress disorder (PTSD) and the influence of comorbid depression. Neurobiol Learn Mem 112: , 114–121. |
[52] | Zannas AS , West AE ((2014) ) Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 264: , 157–170. |
[53] | Angelucci F , Ricci V , Gelfo F , Martinotti G , Brunetti M , Sepede G , Signorelli M , Aguglia E , Pettorruso M , Vellante F , Di Giannantonio M , Caltagirone C ((2014) ) BDNF serum levels in subjects developing or not post-traumatic stress disorder after trauma exposure. Brain Cogn 84: , 118–122. |
[54] | Matsuoka Y , Nishi D , Noguchi H , Kim Y , Hashimoto K ((2013) ) Longitudinal changes in serum brain-derived neurotrophic factor in accident survivors with posttraumatic stress disorder. Neuropsychobiology 68: , 44–50. |
[55] | Felmingham KL , Dobson-Stone C , Schofield PR , Quirk GJ , Bryant RA ((2013) ) The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry 3223: , 1034–1037. |
[56] | Hemmings SM , Martin LI , Klopper M , van der Merwe L , Aitken L , de Wit E , Black GF , Hoal EG , Walzl G , Seedat S ((2013) ) BDNF Val66Met and DRD2 Taq1A polymorphisms interact to influence PTSD symptom severity: A preliminary investigation in a South African population. Prog Neuropsychopharmacol Biol Psychiatry 10: , 273–280. |
[57] | Dennis NA , Cabeza R , Need AC , Waters-Metenier S , Goldstein DB , LaBar KS ((2010) ) Brain-derived neurotrophic factor val66met polymorphism and hippocampal activation during episodic encoding and retrieval tasks. Hippocampus 21: , 980–989. |
[58] | Qureshi SU , Long ME , Bradshaw MR , Pyne JM , Magruder KM , Kimbrell T , Hudson TJ , Jawaid A , Schulz PE , Kunik ME ((2011) ) Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci 23: , 16–28. |
[59] | Park H , Poo MM ((2013) ) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14: , 7–23. |
[60] | Teixeira AL , Barbosa IG , Diniz BS , Kummer A ((2010) ) Circulating levels of brain-derived neurotrophic factor: Correlation with mood, cognition and motor function. Biomark Med 4: , 871–887. |
[61] | Zhang XY , Liang J , Chen da C , Xiu MH , Yang FD , Kosten TA , Kosten TR ((2012) ) Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology (Berl) 222: , 277–284. |
[62] | Belrose JC , Masoudi R , Michalski B , Fahnestock M ((2014) ) Increased pro-nerve growth factor and decreased brain-derived neurotrophic factor in non-Alzheimer’s disease tauopathies. Neurobiol Aging 35: , 926–933. |
[63] | Martinotti G , Di Iorio G , Marini S , Ricci V , De Berardis D , Di Giannantonio M ((2012) ) Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: A review. J Biol Regul Homeost Agents 26: , 347–356. |
[64] | Mandelli L , Mazza M , Martinotti G , Tavian D , Colombo E , Missaglia S , Di Nicola M , De Ronchi D , Negri G , Colombo R , Janiri L , Serretti A ((2010) ) Further evidence supporting the influence of brain-derived neurotrophic factor on the outcome of bipolar depression: Independent effect of brain-derived neurotrophic factor and harm avoidance. J Psychopharmacol 24: , 1747–1754. |
[65] | Spalletta G , Morris DW , Angelucci F , Rubino IA , Spoletini I , Bria P , Martinotti G , Siracusano A , Bonaviri G , Bernardini S , Caltagirone C , Bossù P , Donohoe G , Gill M , Corvin AP ((2010) ) BDNF Val66Met polymorphism is associated with aggressive behavior in schizophrenia. Eur Psychiatry 25: , 311–313. |




