Detection of Dementia Cases in Two Swedish Health Registers: A Validation Study
Abstract
Background:
Population-based health registers are potential assets in epidemiological research; however, the quality of case ascertainment is crucial.
Objective:
To compare the case ascertainment of dementia, from the National Patient Register (NPR) and the Cause of Death Register (CDR) with dementia diagnoses from six Swedish population based studies.
Methods:
Sensitivity, specificity, and positive predictive value (PPV) of dementia identification in NPR and CDR were estimated by individual record linkage with six Swedish population based studies (n = 19,035). Time to detection in NPR was estimated using data on dementia incidence from longitudinal studies with more than two decades of follow-up.
Results:
Barely half of the dementia cases were ever detected by NPR or CDR. Using data from longitudinal studies we estimated that a record with a dementia diagnosis appears in the NPR on average 5.5 years after first diagnosis. Although the ability of the registers to detect dementia cases was moderate, the ability to detect non-dementia cases was almost perfect (99%). When registers indicate that there is a dementia diagnosis, there are very few instances in which the clinicians determined the person was not demented. Indeed, PPVs were close to 90%. However, misclassification between dementia subtype diagnoses is quite common, especially in NPR.
Conclusions:
Although the overall sensitivity is low, the specificity and the positive predictive value are very high. This suggests that hospital and death registers can be used to identify dementia cases in the community, but at the cost of missing a large proportion of the cases.
INTRODUCTION
Population-based health registers are potentially useful assets in dementia research. Swedish national health registers, in particular the National Patient Register (NPR) and the Cause of Death Register (CDR), are frequently used as sources for health data in epidemiological studies. The NPR and CDR are population-based with possible follow-up approaching 50 years, making them very useful as data sources. However, for conditions such as dementia, which are primarily clinically diagnosed, non-acute, and rarely the primary cause for hospitalization or death, detection in the registers may be biased. Therefore, it is essential to investigate the validity of register diagnoses compared to clinical diagnoses made in high-quality population-based studies. However, validation of register-based diagnosis of dementia has been seldom done [1–4]. Those studies have reported a probability of dementia detection ranging between 26% and 55% in NPR and between 28% and 53% in CDR [1–4]. Moreover, high specificity was detected in both registers (over 97%) [1–3]. As epidemiological studies generally depend on knowing the precise time of an event or outcome, the estimated time to detection from true onset of disease is another measure of interest that is closely related to sensitivity. Jin et al. [2] reported an average time from onset of disease to detection in NPR for dementia cases of 4.1 years with a standard deviation (SD) of 4.3.
The aims of this study are twofold: 1) to assess whether there have been any improvements in the detection of dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) cases in the NPR and CDR using much larger study populations (n = 19,035), longer follow-up, and better differential dementia diagnoses than previously available; 2) to investigate time from onset of clinically-diagnosed dementia to detection in NPR using a novel approach and longitudinal data from studies of dementia incidence.
METHODS
National Swedish samples
The study of dementia in Swedish twins (HARMONY)
The Swedish Twin Register (STR) is a population-based register of twins born in Sweden since 1886. Studies conducted within the STR include the cross-sectional study HARMONY conducted between 1998 and 2002, which constituted a cognitive screening phase followed by a clinical phase, described in detail previously [5]. Briefly, all twins aged 65 years and older (n = 20,269) were eligible to be screened, of whom 13,786 (68.5%) participated. Interviews were conducted with a Telephone screening protocol called TELE and, in cases where the twin performed poorly, an informant was interviewed with the Blessed Dementia Rating Scale (BDRS). The TELE and BDRS were combined into an ordinal scale with scores ranging from 0 (cognitively intact) to 3 (cognitive dysfunction, i.e., positive for dementia suspicion). All twins who screened positive, together with their co-twin and a control sample of healthy twin pairs were referred for clinical workup for dementia. In total, 1,752 participants were referred to the clinical phase, and work-up was completed for 1,513 participants. For the present analyses, we excluded participants who screened positive but were lost to follow-up due to death or refusal to participate in the clinical phase (n = 676); who received a diagnosis of questionable or secondary dementia (e.g., due to alcoholism, brain tumor, or hydrocephalus, n = 204); or who previously participated in one of the longitudinal studies described below (n = 1,008). The remaining 12,120 participants were included in the current study.
The clinical work-up protocol followed the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [6] criteria and included, among other tests, physical and neurological examination, a complete medical history including onset and sequence of memory and cognitive symptoms, and a neuropsychological assessment. Clinical diagnoses of dementia were set using a consensus procedure, and followed the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria (DSM-IV) criteria [7]. In total, after applying the exclusion criteria, 494 participants were diagnosed with dementia. Differential diagnoses were made according to NINCDS-ADRDA [8] criteria for AD (n = 316) and NINDS-AIREN [9] criteria for VaD (n = 121). Other differential dementia diagnoses included were frontotemporal dementia (n = 10), Lewy body dementia (n = 5), dementia in Parkinson’s disease (n = 8), dementia in multiple sclerosis (n = 1), and dementia of unknown cause (n = 33).
The Swedish Adoption/Twin Study of Aging (SATSA), the Origins of Variance among the Oldest-old (OCTO-Twin), and the Study of Gender Differences in Health Behavior and Health among Elderly (GENDER)
Three prospective studies conducted with the STR were combined: 1) SATSA [10, 11], a study of same-sex twin pairs aged 50 and older who had participated in up to eight waves of in-person testing (IPT) since 1984; 2) OCTO-Twin [12], a study of same-sex twin pairs aged 80 years or older with up to five waves of IPT between 1991 and 2002; 3) GENDER [1, 13], a study of unlike-sex twin pairs born 1916–1925 who participated in up to three waves of IPT between 1995 and 2005. The IPTs of these studies included the Mini-Mental State Examination (MMSE) and a large cognitive test battery. Dementia suspicion was based on low MMSE scores, low scores for spatial or verbal cognitive abilities or a significant decline in cognitive ability from the previous IPT. In total, the combined cohort included 1,913 participants assessed prospectively for dementia.
In SATSA, all participants who screened positive for dementia suspicion were invited to clinical work-up along with their twin partners up until the 4th wave of IPT. In OCTO-Twin and GENDER and from the 5th wave of IPT in SATSA, dementia diagnoses were based on the extensive cognitive tests of the IPT, the research nurse’s evaluation of the participants, and review of medical records. Final diagnoses were set in multidisciplinary consensus conferences according to Diagnostic and Statistical Manual of Mental Disorders, revised Third Edition (DSM-III-R) [14] or DSM-IV criteria. Differential diagnoses were made using the same protocol as in HARMONY. Prevalent cases were excluded, i.e., those who received a diagnosis of dementia at the first IPT wave of each respective study, or participants who received their first clinical diagnosis through the HARMONY study. In total 260 incident cases of dementia (123 AD, 85 VaD, and 52 OD) were identified. OD included were dementia in Parkinson’s disease (n = 2), Lewy body dementia (n = 1), and dementia of unknown cause (n = 49).
Regional Swedish samples
Kungsholmen Project (KP)
All inhabitants of the Kungsholmen district, central area of Stockholm, who were aged 75 years or older in October 1987 were invited to participate in the longitudinal study KP (n = 2,368) [15]. The baseline examination included 1,810 (response rate 76.4%) participants who underwent MMSE testing. First, the whole population was screened with the MMSE and then all the participants who screened positive (MMSE score ≤23) and a sample of those who screened negative (MMSE score ≥24) were clinically examined. Of those, 110 were excluded from the analysis because they declined to participate in the clinical phase that aimed at identifying prevalent cases of dementia. At baseline 14 people received a diagnosis of questionable dementia of which six received a diagnosis of dementia during the follow-up examination. The remaining eight people were excluded, leaving 1,692 participants for the current analysis. After baseline examination, four follow-ups with approximately three-year intervals were completed before the data collection was terminated in the 1998.
Dementia and dementia type was diagnosed according to DSM-III-R [14] by using data obtained at the clinical examinations. At baseline and at each of the three follow-up occasions, participants underwent a structured interview, medical examination, and psychological assessment. The diagnosis followed a three-step procedure [15]; two physicians independently made preliminary diagnoses, and a third opinion was asked in case of disagreement. Prevalent cases included all participants who received a diagnosis of dementia at baseline (n = 211), incident cases included all individuals who developed dementia during the eleven years of follow-up (n = 389). Differential diagnosis included AD (121 prevalent cases and 308 incident cases), VaD (14 incident cases), and OD (90 prevalent cases and 67 incident cases). OD included were dementia of unknown cause (n = 156) and dementia in Parkinson’s disease (n = 1).
The Swedish National study on Aging and Care in Kungsholmen (SNAC-K)
SNAC-K involves a random sample of people aged ≥60 years who lived either at home or in institutions in the same geographic area as the KP participants [16]. The sampling was stratified by age cohort and time interval of assessments. In total, 11 age-specific cohorts where chosen, and a six-year assessment interval was used for the younger cohorts (60, 66, 72, and 78 years) and a three-year assessment interval for the older cohorts (>78 years). At baseline in 2001–2004, out of the 4,590 eligible individuals randomly selected for SNAC-K, 3,363 (73.3%) undertook a one-phase examination. Information necessary for diagnosis of dementia was missing for 10 participants. At baseline 70 people received a diagnosis of questionable dementia out of whom 27 received a diagnosis of dementia during the follow-up examination. The remaining 43 people were excluded, leaving 3310 participants for the current analysis.
In SNAC-K, dementia cases were identified by a one-phase procedure for all participants that included a structured interview, a clinical examination, and cognitive testing. Dementia diagnoses were made using the same protocol as in KP. In total 240 prevalent (193 AD, 25 VaD, and 22 OD), and 160 incident (117 AD, 19 VaD, and 24 OD) dementia cases were identified. All OD included were dementia of unknown cause.
National registry data have not been used to assist in making dementia diagnoses in any of the included national and regional samples.
Swedish health registers
Sweden has a decentralized unified health care system with universal coverage that ensures equal access to inpatient hospital care. The study participants were linked to the health registers by the unique 10-digit personal identification number assigned to all residents of Sweden [17]. The key for linking the serial numbers to the personal identification number is saved by the databases’ administrators and researchers under no circumstances have access to this key.
The National Patient Register (NPR)
The NPR [18] is composed of the In-Patient Register (IPR) and the Out-Patient Register (OPR). IPR began in 1964 and has had full national coverage since 1987. The OPR for specialized care was initiated in 2001 and collects data on specialized out-patient care (coverage of data is approximately 80%) [19]. In the OPR data from private caregivers are missing (coverage of data from public caregivers in OPR is almost 100%). It is mandatory for all physicians, private and publicly funded, to deliver data to the NPR. NPR variables can be divided into four categories: 1) patient related data (e.g., the personal identification number, sex, and the county where the patient permanently resides); 2) data about the place of care, i.e., the hospital and the type of department; 3) administrative data such as admission and discharge date, duration of admission, and mode of admission and discharge; and 4) medical data (e.g., primary and additional diagnoses). The primary diagnosis should be the condition responsible for the patient’s need for treatment. The additional diagnoses may or may not contribute to the primary diagnosis. The NPR includes up to seven additional diagnoses. All the diagnoses are coded at discharge from each hospitalization using the edition of the International Classification of Diseases (ICD) relevant at the time of diagnosis.
The Cause of Death Register (CDR)
The CDR [20] contains information from death records, including underlying and up to 20 contributory causes of death. The causes of death are coded centrally at Statistics Sweden according to the international (English) version of the ICD used at time of death. The CDR has had complete national coverage since 1961.
Register-based dementia diagnoses were based on comprehensive definitions that included all primary and contributory diagnoses in the NPR and all underlying and contributory causes of death in the CDR. ICD codes used to detect AD were 304, 305 (ICD-7); 290 (ICD-8); 290.0, 290.1, 331.0 (ICD-9); F00, F00.0, F00.1, F00.2, F00.9, G30, G30.0, G30.1, G30.8, G30.9 (ICD-10). ICD codes used to detect VaD were 306 (ICD-7); 293.0, 293.1 (ICD-8); 290.4 (ICD-9); F01, F01.0, F01.1, F01.2, F01.3, F01.8, F01.9 (ICD-10). Additional ICD codes used to detect OD were 290.8 290.9, 294.1, 331.1, 331.2, 331.9 (ICD-9) and F02, F02.0, F02.1, F02.2, F02.3, F02.4, F02.8, F03, F03.9, F05.1, G31.1, G31.8A (ICD-10).
Statistical analyses
Dementia cases in HARMONY and baseline examination of KP (1987–1989) and SNAC-K (2001–2004) were considered as prevalent cases. Incident cases were identified using follow-up examinations for KP (1991–1998) and SNAC-K (2004–2010), and the entire follow-up time for SATSA, OCTO-twin, and GENDER. Cases identified as prevalent were analyzed for ability to detect cases, and incident cases were separately analyzed for time of onset to detection.
The ability of the registers to detect dementia cases was determined by sensitivity, calculated as the number of demented individuals identified in the registers divided by the number of dementia cases identified in the population-based studies. The ability of the register to detect non-cases was determined by specificity, computed as the number of non-demented individuals identified in the registers divided by the number of non-demented individuals identified in the population-based studies. In addition, we have estimated the probability of positive register-based cases being confirmed by the population-based studies, the positive predictive value (PPV), given by the number of dementia cases identified in the population-based studies divided by the dementia cases recorded in the registers. Clopper-Pearson exact confidence limits for proportions [21] were used to construct 95% confidence intervals (CI) for the estimates.
For the NPR, the sensitivity was estimated for register-based diagnoses between 1964 and the last available follow-up from the register, which was in 2000 for KP, and in 2012 for SNAC-K and the National Swedish samples. The specificity and PPV for the NPR were estimated for register-based diagnoses between 1964 and the cognitive screening date for each participant. To determine true positive and false positive cases in an unbiased way, the date of cognitive screening was used as the date on which the register diagnosis was determined to be true or false. The sensitivity of the CDR was estimated among deaths occurring between the last cognitive screening and the last available follow-up from the register in 2008 for KP, 2011 for SNAC-K, and 2012 for the National Swedish samples. Specificity and PPV for the CDR were estimated among study participants who died within one year from the last cognitive screening in order to limit possible misclassification due to non-dementia cases developing dementia between screening and death.
Incident dementia cases were followed from the year of diagnosis of dementia until first hospitalization with a reported dementia diagnosis (first detection in NPR), last available date in NPR, or date of death, whichever came first. Using Laplace regression, we evaluated the time to detection of dementia cases in NPR. The time to detection for patients with a dementia diagnosis in the NPR that preceded the dementia diagnosis in the population-based studies was considered as zero (n = 69). Using the Laplace regression, we examined whether sex, age at diagnosis, and educational attainment of the dementia cases affected the time to detection. Age at diagnosis and educational attainment were evaluated as binary variables. The cut-off for the age at diagnosis was chosen as the median value (85 years), the cut-off for educational attainment was chosen as compulsory school versus any higher education.
Statistical analyses were performed using Stata 14.2 (StataCorp, College Station, Texas).
RESULTS
Descriptive characteristics of study populations are reported in Table 1. Of the total sample of 19,035 individuals, 945 prevalent dementia cases were found in the national and regional (Stockholm) samples: 494 cases in HARMONY, 211 in KP, and 240 in SNAC-K. During the follow up, 809 incident cases were identified: 260 cases in the SATSA/OCTO-Twin/GENDER studies, 389 in KP, and 160 in SNAC-K. AD accounts for 67.2% of cases.
Table 1
Descriptive characteristics of the study populations
| Population based studies | |||||
| All (N = 19,035) | KP 1987–1998 (N = 1,692) | Harmony 1998–2002 (N = 12,120) | SATSA, OCTO-Twin, GENDER 1986–2010 (N = 1,913) | SNAC-K 2001–2010 (N = 3,310) | |
| Sex | |||||
| Men | 7734 (40.6) | 402 (23.8) | 5377 (44.4) | 786 (41.1) | 1169 (35.3) |
| Women | 11301 (59.4) | 1290 (76.2) | 6743 (55.6) | 1127 (58.9) | 2141 (64.7) |
| Age (mean, SD)1 | 74.7 (7.8) | 81.5 (4.9) | 73.9 (6.6) | 72.4 (10.7) | 74.5 (11.1) |
| Educational attainment2 | |||||
| Compulsory school | 9560 (51.0) | 889 (52.9) | 6874 (57.6) | 1278 (68.9) | 519 (15.8) |
| Any higher education | 9193 (49.0) | 789 (47.0) | 5065 (42.4) | 577 (31.1) | 2762 (84.2) |
| Dementia status | |||||
| Dementia -free participants | 17281 (90.8) | 1092 (64.5) | 11626 (95.9) | 1653 (86.4) | 2910 (87.9) |
| Prevalent dementia cases | 945 (5.0) | 211 (12.5) | 495 (4.1) | –3 | 240 (7.3) |
| Incident dementia cases | 809 (4.2) | 389 (23.0) | –3 | 260 (13.6) | 160 (4.8) |
| Men | 449 (25.6) | 106 (17.7) | 168 (34.0) | 100 (38.5) | 75 (18.7) |
| Women | 1305 (74.4) | 494 (82.3) | 326 (66.0) | 160 (61.5) | 325 (81.3) |
| AD | 1178 (67.2) | 429 (71.5) | 316 (64.0) | 123 (47.3) | 310 (77.5) |
| VaD | 264 (15.0) | 14 (2.3) | 121 (24.5) | 85 (32.7) | 44 (11.0) |
| OD | 312 (17.8) | 157 (26.2) | 57 (11.5) | 52 (20.0) | 46 (11.5) |
Unless otherwise specified the number reported in table are frequency and the percentage. KP, Kungsholmen Project; HARMONY, Study of Dementia in Swedish Twins; SATSA, Swedish Adoption/Twin Study of Aging; OCTO-Twin; Origins of Variance among Oldest-Old; GENDER, Study of Gender Differences in Health Behaviors and Health among Elderly; SNAC-K, Swedish National study on Aging and Care in Kungsholmen; SD, standard deviation; AD, Alzheimer’s disease; VaD, vascular dementia; OD, other type of dementia. 1Refers to age at first screening; 2Missing data for 287 participants; 3Data excluded.
Sensitivity of register-based dementia diagnoses
Overall, less than half of the prevalent dementia cases were ever detected by the NPR (47%) or CDR (44%) (Table 2). Out of the 945 prevalent dementia cases, 355 were never detected by any register, 266 were detected by both the NPR and CDR, 181 were detected by NPR only and 143 cases were detected by CDR only. In both registers, the sensitivity was higher in women than in men and decreased with increasing age. Moreover, AD patients had much higher probability to be identified as AD than VaD or OD patients as their respective subtypes in both registers. Approximately half of the AD (52.1%, 95% CI: 48.1–56) and OD (45.6%, 95% CI: 37.9–53.4) prevalent cases were detected as demented by the NPR. Less than 30% of the VaD prevalent cases were detected as demented by the NPR (28.8%, 95% CI: 21.6–36.8). Of the 447 prevalent dementia cases detected by NPR over the whole follow-up (48 years for HARMONY and SNAC-K and 36 years for KP), dementia was the main cause of hospitalization for 54.1% (n = 242). Out of the detected cases (n = 945), 174 (38.9%) were detected before the clinical diagnosis in the population based studies.
Table 2
Sensitivity of dementia detection in the National Patient and Cause of Death Registers
| No of cases | National Patient Register1 | Cause of Death Register2 | |||
| TP (n) | Sensitivity (95% CI) | TP (n) | Sensitivity3 (95% CI) | ||
| Prevalent cases | 945 | 447 | 47.3 (44.1–50.5) | 408 | 44.1 (40.9–47.4) |
| Men | 244 | 112 | 45.9 (39.5–52.4) | 94 | 39.2 (33.0–45.7) |
| Women | 701 | 335 | 47.8 (44.0–51.6) | 314 | 45.8 (42.1–49.7) |
| ≤69 y | 24 | 15 | 62.5 (40.6–81.2) | 18 | 81.8 (59.7–94.8) |
| 70–79 y | 225 | 114 | 50.7 (43.9–57.4) | 101 | 47.0 (40.2–53.9) |
| 80–89 y | 443 | 207 | 46.7 (42.0–51.5) | 198 | 45.5 (40.8–89.1) |
| ≥90 y | 253 | 111 | 43.9 (37.7–50.2) | 91 | 36.0 (30.1–42.2) |
| AD | 630 | 205 | 32.5 (28.9–36.4) | 236 | 38.3 (34.5–42.3) |
| VaD | 146 | 17 | 11.6 (6.9–18.0) | 8 | 5.6 (2.5–10.7) |
| OD | 169 | 42 | 24.9 (18.5–32.1) | 5 | 3.0 (1.0–6.9) |
Age refers to the age at dementia diagnosis or last screening in the population based studies. 1Follow-up from 1964 to 2012 for HARMONY, SNAC-K, SATSA, OCTO-Twin, and GENDER, and 2000 for KP. 2Follow-up from screening to 2015 for HARMONY, SATSA, OCTO-Twin, and GENDER, 2011 for SNAC-K, 2008 for KP. 3The denominator was the total number of dementia cases diagnosed in the population based studies that were deceased. TP, true positive; CI, confidence interval; AD, Alzheimer’s diseases; VaD, vascular dementia; OD, other type of dementia.
Of all prevalent dementia cases, 553 (58.5%) were hospitalized on one or more occasion after they received their dementia diagnosis, and the main cause of hospitalization was dementia only for 26.6% (n = 147). For 258 (46.7%) of the hospitalized cases, their existing dementia diagnosis was never recorded despite the fact they were hospitalized on average on 3.3 (standard deviation, SD 4.0) occasions. We examined the primary cause of hospitalization for these cases and found that the most common causes were circulatory diseases (ICD-10 I-codes) and external causes (ICD-10 T/S-codes), accounting for 23.5% and 13.8% of the hospitalizations respectively. There were only small differences in main cause of hospitalization between the prevalent dementia cases who were and were not detected, with the possible exception of circulatory disease which was slightly more common among the undetected cases where it accounted for 23.5% of hospitalizations, versus 15.5% of hospitalizations among the detected cases.
Restricting the register-based definition of dementia to main cause of hospitalization or underlying cause of death reduced the sensitivity to 32.6% (95% CI: 29.2–36.1) in the NPR and 27.2% (95% CI 24.1–30.3) in the CDR. Restricting the register-based definition of AD or VaD to main cause of hospitalization or underlying cause of death reduced the sensitivity to 21.9% (95% CI 19.0–25.1) and 4.9% (95% CI 3.4–6.8) in the NPR and 22.1% (95% CI 19.2–25.4) and 5.0% (95% CI 3.4–6.7) in the CDR.
Specificity of register-based dementia diagnoses
In all, there were 16,144 individuals evaluated as cognitively intact at baseline in the population-based studies (HARMONY and baseline assessment of KP and SNAC-K). Among them 40 individuals were given a diagnosis of dementia in NPR before the screening date in the population based studies. This resulted in a specificity of 99.8% (95% CI 99.7–99.8). Specificity for the CDR was 99.0% (95% CI 97.4–99.7). Similar specificity was found across sex, age, and dementia type (Supplementary Table 1).
Positive predictive value of register-based dementia diagnoses
Register-based dementia diagnoses were confirmed by the population-based studies to a large degree. Of the 214 dementia cases detected in the NPR between 1964 and the cognitive screening date in the population-based studies, 174 were given a diagnosis of dementia by the population based studies, giving a PPV of 81.3% (Table 3). In total, there were 85 dementia cases recorded as dead in the CDR. PPV for dementia diagnoses in the CDR was 99.0%, calculated based on 624 participants who died within one year after being examined in the national and regional studies (Table 3).
Table 3
Positive Predictive value with 95% confidence interval of dementia diagnoses in the National Patient and Cause of Death Registers
| National Patient Register1 | Cause of Death Register2 | |||||
| FP (n) | TP (n) | PPV (95% CI) | FP (n) | TP (n) | PPV (95% CI) | |
| Prevalent cases | 40 | 174 | 81.3 (75.4–86.3) | 4 | 81 | 99.0 (97.4–99.7) |
| Men | 12 | 41 | 77.4 (63.8–87.7) | 1 | 16 | 94.1 (71.3–99.9) |
| Women | 28 | 133 | 82.6 (75.9–88.1) | 3 | 65 | 95.6 (87.6–99.1) |
| ≤69 y | 16 | 18 | 52.9 (35.1–70.2) | 1 | 4 | 80.0 (28.3–99.5) |
| 70–79 y | 15 | 71 | 82.6 (72.9–89.9) | 0 | 10 | 100 (69.2–100) |
| 80–89 y | 8 | 74 | 90.2 (81.7–95.7) | 3 | 41 | 93.2 (81.3–98.6) |
| ≥90 y | 1 | 11 | 91.7 (61.5–99.8) | 0 | 26 | 100 (86.8–100) |
| AD | 56 | 73 | 56.6 (47.6–65.3) | 23 | 44 | 65.7 (53.1–76.8) |
| VaD | 20 | 11 | 35.5 (19.2–54.6) | 1 | 1 | 50.0 (1.26–98.7) |
| OD | 58 | 11 | 15.9 (8.24–26.7) | 14 | 2 | 12.5 (1.55–38.3) |
Age refers to age at first register detection in National Patient Register and age at death in Cause of Death Register. 1Follow-up from 1964 to screening; 2Follow-up for one year from screening. FP, false positive; TP, true positive; PPV, positive predictive value; CI, confidence interval; AD, Alzheimer’s diseases; VaD, vascular dementia.
The PPV for register-based AD and VaD diagnoses was much lower than for any dementia diagnosis. This was to a large degree due to misclassification of other differential dementia diagnoses. Indeed, more than half of the false positive NPR AD cases (n = 56) were found by the population study to have VaD (n = 8) or OD (n = 25) diagnoses. Similarly, the 23 AD false negatives identified in CDR, 10 were diagnosed as VaD, and 9 as OD in the population based studies. The PPV for VaD in NPR was 35.5% as most of them (14 cases out of 20) received an AD diagnosis in the population-based studies. When restricting the register-based definition of AD diagnosis to main cause of hospitalization only in NPR or underlying cause of death only in CDR, the PPV slightly improved, although not significantly, to 60% (95% CI: 48.4–68.9) in NPR and to 67% (95% CI: 50.7–83.1) in CDR. Restricting the register-based definition of VaD diagnosis to main cause of hospitalization only in NPR did not improve the PPV.
Time from onset of dementia to detection in NPR
Overall, 809 incident cases were diagnosed in the population-based studies. Mean age at onset was 85.7 years (SD 5.7, range 63.0–104.1). Out of the incident cases, 653 (80.7%) died by the end of the study observation, and 50% of them died within 4.1 years (95% CI: 3.9–4.4) after diagnosis of dementia.
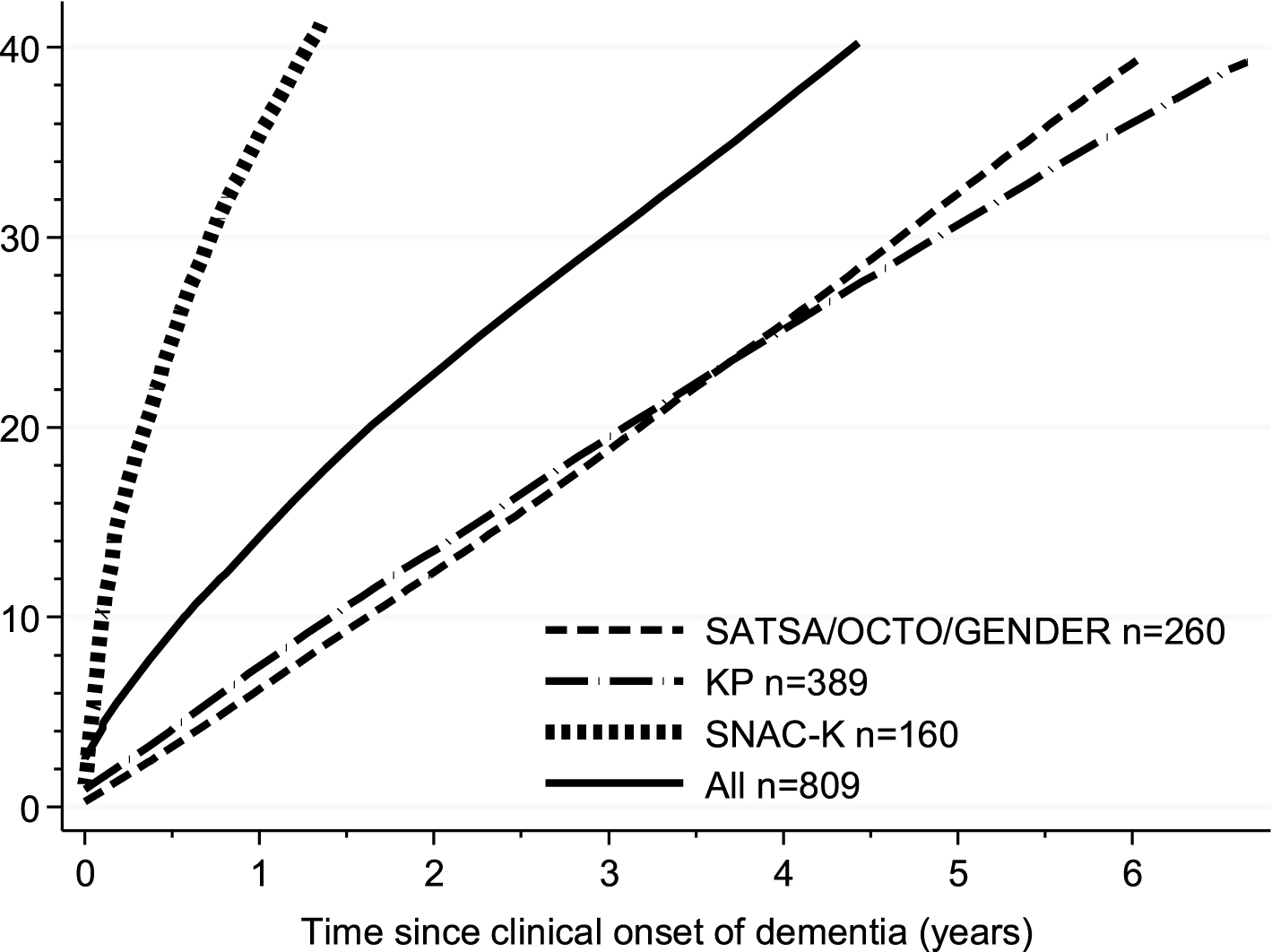
In total, 325 out of the 809 cases were detected in NPR, representing a sensitivity of 40.2% with an average gap of 5.5 years (95% CI: 5.1–5.8, estimates adjusted for sex, education, and age of dementia onset) between date of clinical diagnosis by the population-based study and date of discharge associated with a dementia diagnosis in the NPR. Sixty-nine cases (8%) were given a dementia diagnosis in NPR before the clinical diagnosis of dementia in the population based studies. Among those people, 28 received a diagnosis less than 1 year before the clinical diagnosis of dementia in the population based studies (average age at diagnosis 86.2±4.4). The remaining 41 received the diagnosis of dementia between one and six years before the clinical diagnosis of dementia in the population based studies, two of them received the dementia diagnosis 11 and 18 years before, respectively (average age at diagnosis 84.2±7.3). For the following analyses, we set to 0.0 the time to diagnosis for those people. Figure 1 shows the cumulative proportion of incident dementia cases detected in NPR for all percentiles from the 1st to the 40th, adjusting for age of dementia onset, sex, and education. There was no significant difference in time to detection according to sex (40th percentile difference = –0.8 years, 95% CI: –1.6–0.1, women versus men) or age of dementia onset (40th percentile difference = 0.4 years, 95% CI: –0.4–1.2, for people aged 85 years or older versus younger). Individuals with high education had a significantly shorter time from clinical diagnosis to registry detection compared to those with low education (40th percentile difference = –1.0 year, 95% CI: –1.8––0.2). The time to detection of dementia diagnosis in NPR was much longer in KP (40th percentile difference = 3.6 years, 95% CI: 2.5–4.7) and SATSA, OCTO-Twin, and GENDER (40th percentile difference = 3.5 years, 95% CI: 2.7–4.3) as compared with the SNAC-K (Fig. 1).
Fig.1
Cumulative proportion of being detected in the National Patient Register (NPR) after clinical diagnosis in the population based studies (incident cases). Curves were calculated by estimating a Laplace regression model for all percentiles from the 1st to the 40th, adjusting for age of dementia onset, sex, and education. KP, Kungsholmen Project; SATSA, Swedish Adoption/Twin Study of Aging; OCTO-Twin; Origins of Variance among Oldest-Old; GENDER, Study of Gender Differences in Health Behaviors and Health among Elderly; SNAC-K, Swedish National study on Aging and Care in Kungsholmen.
Sensitivity, specificity, and PPV of register-based dementia diagnoses were estimated considering each specific population-based study separately and are reported in Supplementary Table 2.
DISCUSSION
The main finding of this study is that national population-based health registers in Sweden are overall reliable and specific in detecting dementia cases; however, the sensitivity is low.
The NPR and CDR underestimated the occurrence of dementia, AD, and VaD. The sensitivities for dementia diagnosis were 62.7%, approximately 50% for AD and less than 10% for VaD. Dementia is not a disease primarily treated in hospital and we do not expect many cases to be hospitalized due to dementia, which is reflected in the low estimates of sensitivity. However, we found that when dementia cases were hospitalized for other reasons, the dementia diagnosis was only included on hospital records for about half of all cases. Furthermore, we found that dementia may be underreported to a larger degree when hospitalization is due to circulatory diseases, as indicated by the analysis of primary causes of hospitalization among the undetected dementia cases and the remarkably low sensitivity of VaD cases. Comparable values based on similar assumptions have been reported based on the Finnish Inpatient Discharge Registry [3] but there is a divergence between most of our findings and the previous Swedish reports [1, 2]. For instance, estimated sensitivity of CDR was considerably higher in the present study than previously reported [2]. This difference may be due to a difference in choice of denominator in the calculation; we used all dementia cases who had died whereas the previous study used all dementia cases including those still alive. Moreover, the sensitivity in NPR reported by Dahl and colleagues was much lower (26%) based on 498 people aged 70–81 years old. For a similar age group we have estimated a sensitivity of 51% [1].
Although the ability of the registers to detect dementia cases was moderate, the ability to detect non-dementia cases was near perfect (99%). When registers indicate that there is a dementia diagnosis, there are very few instances in which the clinicians determined the person not to be demented. Indeed, PPVs were close to 90%. The PPV improved with increasing age at diagnosis. However, misclassification between dementia subtypes is quite common, especially in NPR as indicated by the low PPVs of AD and VaD diagnoses. Nevertheless, misclassification was less common when AD was the primary cause of hospitalization or death, and thus PPV of register-based AD diagnoses may be increased by restricting the case definition to only primary diagnoses in NPR and underlying cause of death in CDR, although this comes at a cost of reduced sensitivity. The PPV of register-based VaD diagnoses did not improve when restricting the register-based definition. The poor PPV of the register-based VaD diagnoses may reflect the short time in which VaD has been included in ICD classifications, and lack of widely accepted criteria for diagnosing VaD. Based on these results it is not advisable to study VaD when health registers are the only source of diagnostic information. Our results are in agreement with previous Swedish and Finnish studies concerning the high specificity of register-based dementia diagnoses [1–3].
Using data available from longitudinal studies (KP, SNAC-K, SATSA, OCTO-Twin, and GENDER) we estimated that the information of dementia diagnosis appears in the NPR on average 5.5 years after the actual diagnosis is made. We did not find any difference in time to detection according to sex and age of dementia onset. However, individuals with high education had a significantly shorter time (1.0 year) from clinical diagnosis to registry detection compared to those with only compulsory school (average detection time 3.8 years). Data also showed a significantly reduced time to detection of dementia diagnosis in NPR over the last decades. Indeed, the dementia diagnoses made in SNAC-K (diagnosis period 2004–2010) were detected in NPR approximately three years earlier than those made in SATSA, OCTO-Twin, and GENDER (diagnosis period 1984–2005), and in KP (diagnosis period 1991–1998). Most likely the observed improvements in the reduced time to detection of dementia diagnosis in the registry is due to some combination of factors: the promising indication of decreasing incidence of dementia, major modification in the ICD criteria, increasing dementia awareness, and the introduction of several new drugs in dementia treatment.
Strengths of these analyses include large population-based studies with a long follow-up time. Study samples included people living both at home and in institutions. Moreover, we used an alternative method (Laplace regression) to evaluate the time-to-event outcomes instead of the traditional approaches (i.e., Cox proportional hazard model). This approach allowed us to examine the timing of the event rather than the risk. This is one of the few validation studies about dementia registration in the NPR and CDR and is therefore of great value for further research on the prognosis of dementia [19]. A potential source of bias may have been introduced in estimating the sensitivity for register-based diagnosis since participants were followed for different lengths of time (i.e., participants followed for a longer period have more chance to be detected in the NPR). The present analyses used data from regional and national Swedish samples, therefore results may not be equally generalizable to other countries.
In conclusion, the high specificity (or PPV) but low sensitivity indicates that the quality of dementia diagnoses in the national health registers can be used in epidemiological studies, but that a high proportion of the cases will be misclassified as non-cases. Misclassification of the outcome can introduce bias into epidemiological studies if it is related to the study exposure [22]. However, independent non-differential disease misclassification with perfect specificity will not introduce bias in the estimation of the risk ratio [22]. Moreover, if service planners relying only on register-based diagnoses of dementia to project care needs they might fail to capture all individuals with dementia and therefore might underestimate the population burden of the disease. It should be recognized that no single source alone can be assumed to be completely accurate; population-based studies with in-person assessment protocols are also subject to inaccuracies due to refusal or to attrition during follow-up or death, especially when elderly people are involved. In order to minimize any missed cases, it would be sensible to collect data from multiple data sources.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Health and Social Affairs, Sweden, the participating County Councils and Municipalities; the Swedish Research Council; the Swedish Research Council for Health, Working Life and Welfare; the Gun and Bertil Stohnes foundation; the Konung Gustaf Vs och Drottning Victorias Frimurarestiftelse foundation; the Loo and Hans Ostermans foundation, and grant R01 AG08724 from the U.S. National Institutes of Health. Many thanks also to the invaluable contributions by the study participants and data collection staff.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-0572r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170572.
REFERENCES
[1] | Dahl A , Berg S , Nilsson SE ((2007) ) Identification of dementia in epidemiological research: A study on the usefulness of various data sources. Aging Clin Exp Res 19: , 381–389. |
[2] | Jin YP , Gatz M , Johansson B , Pedersen NL ((2004) ) Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology 63: , 739–741. |
[3] | Solomon A , Ngandu T , Soininen H , Hallikainen MM , Kivipelto M , Laatikainen T ((2014) ) Validity of dementia and Alzheimer’s disease diagnoses in Finnish national registers. Alzheimers Dement 10: , 303–309. |
[4] | Perera G , Stewart R , Higginson IJ , Sleeman KE ((2016) ) Reporting of clinically diagnosed dementia on death certificates: Retrospective cohort study. Age Ageing 45: , 668–673. |
[5] | Gatz M , Fratiglioni L , Johansson B , Berg S , Mortimer JA , Reynolds CA , Fiske A , Pedersen NL ((2005) ) Complete ascertainment of dementia in the Swedish Twin Registry: The HARMONY study. Neurobiol Aging 26: , 439–447. |
[6] | Morris JC , Heyman A , Mohs RC , Hughes JP , van Belle G , Fillenbaum G , Mellits ED , Clark C ((1989) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39: , 1159–1165. |
[7] | American Psychiatric Association ((1994) ) Diagnostic and statistical manual of mental disorders, 4th ed. American Psychiatric Association, Washington, DC. |
[8] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[9] | Roman GC , Tatemichi TK , Erkinjuntti T , Cummings JL , Masdeu JC , Garcia JH , Amaducci L , Orgogozo JM , Brun A , Hofman A , et al. ((1993) ) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43: , 250–260. |
[10] | Pedersen NL , Friberg L , Floderus-Myrhed B , McClearn GE , Plomin R ((1984) ) Swedish early separated twins: Identification and characterization. Acta Genet Med Gemellol (Roma) 33: , 243–250. |
[11] | Pedersen NL , McClearn GE , Plomin R , Nesselroade JR , Berg S , DeFaire U ((1991) ) The Swedish Adoption Twin Study of Aging: An update. Acta Genet Med Gemellol (Roma) 40: , 7–20. |
[12] | McClearn GE , Johansson B , Berg S , Pedersen NL , Ahern F , Petrill SA , Plomin R ((1997) ) Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science 276: , 1560–1563. |
[13] | Gold CH , Malmberg B , McClearn GE , Pedersen NL , Berg S ((2002) ) Gender and health: A study of older unlike-sex twins. J Gerontol B Psychol Sci Soc Sci 57: , S168–176. |
[14] | American Psychiatric Association ((1987) ) Diagnostic and Statistical Manual of Mantal Disorders, 3rd ed. American Psychiatric Association, Washington, DC. |
[15] | Fratiglioni L , Viitanen M , Backman L , Sandman PO , Winblad B ((1992) ) Occurrence of dementia in advanced age: The study design of the Kungsholmen Project. Neuroepidemiology 11: (Suppl 1), 29–36. |
[16] | Lagergren M , Fratiglioni L , Hallberg IR , Berglund J , Elmstahl S , Hagberg B , Holst G , Rennemark M , Sjolund BM , Thorslund M , Wiberg I , Winblad B , Wimo A ((2004) ) A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 16: , 158–168. |
[17] | Ludvigsson JF , Otterblad-Olausson P , Pettersson BU , Ekbom A ((2009) ) The Swedish personal identity number: Possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 24: , 659–667. |
[18] | National Board of Health and Welfare. The National Patient Register. http://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish, Accessed September 2011. |
[19] | Ludvigsson JF , Andersson E , Ekbom A , Feychting M , Kim J-L , Reuterwall C , Heurgren M , Olausson PO ((2011) ) External review and validation of the Swedish national inpatient register. BMC Public Health 11: , 450. |
[20] | National Board of Health and Welfare. The Cause of Death Register. http://www.socialstyrelsen.se/register/dodsorsaksregistret# (in Swedish), Accessed September 2011. |
[21] | Clopper CJ , Pearson ES ((1934) ) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26: , 404–413. |
[22] | Rothman KJ , Greenland S , Lash TL ((2008) ) Modern epidemiology. Lippincott Williams & Wilkins. |



