Early Postmenopausal Transdermal 17β-Estradiol Therapy and Amyloid-β Deposition
Abstract
Background: It remains controversial whether hormone therapy in recently postmenopausal women modifies the risk of Alzheimer’s disease (AD).
Objective: To investigate the effects of hormone therapy on amyloid-β deposition in recently postmenopausal women.
Methods: Participants within 5–36 months past menopause in the Kronos Early Estrogen Prevention Study, a randomized, double blinded placebo-controlled clinical trial, were randomized to: 1) 0.45 mg/day oral conjugated equine estrogens (CEE); 2) 50μg/day transdermal 17β-estradiol; or 3) placebo pills and patch for four years. Oral progesterone (200 mg/day) was given to active treatment groups for 12 days each month. 11C Pittsburgh compound B (PiB) PET imaging was performed in 68 of the 118 participants at Mayo Clinic approximately seven years post randomization and three years after stopping randomized treatment. PiB Standard unit value ratio (SUVR) was calculated.
Results: Women (age = 52–65) randomized to transdermal 17β-estradiol (n = 21) had lower PiB SUVR compared to placebo (n = 30) after adjusting for age [odds ratio (95% CI) = 0.31(0.11–0.83)]. In the APOE ɛ4 carriers, transdermal 17β-estradiol treated women (n = 10) had lower PiB SUVR compared to either placebo (n = 5) [odds ratio (95% CI) = 0.04(0.004–0.44)], or the oral CEE treated group (n = 3) [odds ratio (95% CI) = 0.01(0.0006–0.23)] after adjusting for age. Hormone therapy was not associated with PiB SUVR in the APOE ɛ4 non-carriers.
Conclusion: In this pilot study, transdermal 17β-estradiol therapy in recently postmenopausal women was associated with a reduced amyloid-β deposition, particularly in APOE ɛ4 carriers. This finding may have important implications for the prevention of AD in postmenopausal women, and needs to be confirmed in a larger sample.
INTRODUCTION
Hormone therapy consisting of conjugated equine estrogens (CEE) along with medroxyprogesterone acetate initiated in the late postmenopause stage (≥ 65 years) increased the risk of dementia in the Women’s Health Initiative Memory Study (WHIMS) [1]. However, there is controversy on whether estrogen with or without progesterone can preserve neurological function and decrease the risk of dementia when administered early in menopause, i.e., during a “window of opportunity” phase [2–8]. Although determining the effects of hormone treatment shortly after menopause on the risk of dementia would require decades of follow-up, non-invasive imaging markers of Alzheimer’s disease (AD) pathophysiology can potentially assess the efficacy of preventive interventions in the short term.
The Kronos Early Estrogen Prevention Study (KEEPS) was a multi-center, randomized, placebo-controlled, double-blinded trial of hormone treatment in recently menopausal women who were in good cardiovascular health. KEEPS tested the hypothesis that hormone therapies administered soon after the onset of menopause would slow progression of atherosclerosis; [9] however, no effect was observed on several imaging markers of progression of atherosclerosis during the four year trial [10], or cognitive function [11]. Although estrogens, in particular, are thought to modify the risk of AD, estrogen effects on amyloid-β (Aβ) pathology have not been investigated in a hormone treatment trial. Examining data obtained at one KEEPS enrollment site, we report the effects of two forms of hormone therapy, oral CEE and transdermal 17β estradiol therapy on Aβ deposition measured by Pittsburgh compound-B (PiB) PET.
METHODS
Participants
KEEPS (NCT00154180) was a multicenter, randomized, double blinded, placebo-controlled clinical trial in recently menopausal women (n = 727) that was conducted between 2006 and 2011. Participants enrolled in KEEPS were between 42 to 59 years of age, within 5 to 36 months past their last menses, and were in good cardiovascular health and did not have a history of hysterectomy or oophorectomy [9]. Estrogens were administered through two different routes: Oral or transdermal. Participants were randomized to either: 1) oral conjugated equine estrogen (CEE; Premarin, 0.45 mg/day); 2) transdermal 17β-estradiol (skin patch, Climara, 50μg/day); or 3) placebo pills and patch. Progesterone was given orally (Prometrium; micronized progesterone, 200 mg/day) for the first 12 days each month to both active treatment groups. Participants were treated for four years.
Neuroimaging for the current study was conducted from December 2012 through July 2014 and included the subsample of women who were enrolled in KEEPS at the Mayo Clinic, to investigate the effects of the KEEPS hormone treatments on Aβ deposition three years after the end of the trial. This study was approved by the Mayo Clinic Institutional Review Board and all subjects or appropriate surrogates provided informed consent for participation Exclusion criteria for the imaging studies were contraindications for safety and neurologic disorders such as brain tumors, multiple sclerosis, neurodevelopmental abnormalities, or treatments (e.g., systemic chemotherapy) that would affect the brain structure. Apolipoprotein E (APOE) genotyping was performed after randomization and clinical examinations were performed at the Mayo Clinic Specialized Center of Research on Sex Differences within three months of the imaging studies. The study was approved by the Institutional Review Board at Mayo Clinic and all participants gave informed consent.
Neuropsychological assessment and cognitive function
A confirmatory factor analysis was used to assess the underlying structure of baseline cognitive data from the KEEPS cognitive and affective study (n = 662), and to derive summary scores [12]. Using standard criteria for model fit, the cognitive variables were summarized with a general domain representing global cognitive function at baseline.
A battery of neuropsychological tests three years after the end of the hormone therapy phase were administered within three weeks of the neuroimaging examinations in the Research Psychometrics Resource Laboratory at Mayo Clinic’s Center for Translational Science Activities (CTSA) under the direction of a neuropsychologist (JP). Cognitive performance was investigated in four domains: 1) Learning & Memory from the California Verbal Learning Test (CVLT), New York University (NYU) Paragraphs, and Benton Visual Retention Test; 2) Auditory Attention & Working Memory from Wechsler Memory Scale-III Letter-Number Sequencing and Digit Span subtests; 3) Visual Attention & Perceptual Speed from Trail Making Test part A, Color and Word trials of the Stroop test, and Wechsler Adult Intelligence Scale-III Digit Symbol Coding subtest; 4) Speeded Language & Flexibility from phonemic (F, A, S) and category (animals, fruits, vegetables) verbal fluency, Trail Making Test part B, and Color-Word Interference trial of the Stroop.
MRI and PET imaging
MRI studies were performed on a single 1.5T system, with an 8-channel phased-array coil (GE Healthcare). A 3D high resolution MPRAGE acquisition with TR/TE/TI = 7/3/900 ms; flip angle 8 degrees; in plane resolution of 1.0 mm and a slice thickness of 1.2 mm was performed for anatomical segmentation and labeling of PiB PET scans.
PET images were acquired using a PET/CT scanner (DRX; GE Healthcare) operating in 3D mode. The participants were injected with 292–729 MBq [11C]PIB. A CT image was obtained for attenuation correction. After a 40-min uptake period, a 20-min PiB scan was obtained. The PiB- PET acquisition consisted of four 5-min dynamic frames, acquired from 40 to 60 min after injection. Standard corrections were applied. The pixel size for PET images was 1.0 mm and the slice thickness was 3.3 mm.
Analysis of PiB PET
PiB PET quantitative image analysis was performed using the fully automated image processing pipeline which has been described in detail elsewhere [13]. Briefly, the method includes gray matter (GM) sharpening of PET images using MRI and partial volume correction of cerebrospinal fluid and tissue compartments using Statistical Parametric Mapping unified segmentation algorithm [14]. PiB PET cortical ratio images were calculated by dividing each PiB PET GM voxel value by the median value in the cerebellar GM region in patient’s MRI space. PiB retention was calculated by the PiB Standard unit value ratio (SUVR), with the median values of the PiB PET GM ratio from the bilateral parietal, posterior cingulate, precuneus, temporal, prefrontal, orbitofrontal, anterior cingulate GM regions in the in-house modified anatomical labeling atlas.
Statistical analysis
Characteristics of participants were compared across the treatment and the placebo groups using Kruskal-Wallis tests or Fisher exact tests, as appropriate. We also compared the characteristics of the participants and non-participants. Cognitive test scores were compared across the treatment and the placebo groups using ANOVA and Tukey’s honest significant differences test for the post-hoc comparisons with adjustments. Age was tested for association with PiB SUVR using Spearman correlations. We performed the comparisons of PiB SUVR values across treatment and placebo groups, adjusting for age, using proportional odds logistic regressions [15]. This semiparametric model mitigates the effect of outliers while allowing for parametric effects of age and treatment, and simultaneously estimates the log (odds) of higher versus lower value of PiB SUVR, at all possible threshold values. Thus, we did not classify the participants into Aβ-positive and Aβ-negative categories based on PiB SUVR.
RESULTS
All women enrolled in KEEPS at the Mayo Clinic in Rochester, Minnesota (n = 118) were considered for participation in the current study. Six participants were excluded due to neurological disorders or MRI contraindications, forty women declined to participate in both MRI and/or PET studies, and four participants were lost to follow-up. Of the 112 eligible KEEPS participants, 68 women (61%) with median age of 60 (range, 52–65) participated in both the MRI and PET studies and were included in the analysis (Fig. 1). Participants included in the analysis did not differ from those who did not participate in the neuroimaging study on age (p = 0.09), education (p = 0.42), smoking status (p = 0.48), time past from menopause to randomization (p = 0.55), or APOE status (p = 0.47).
The time elapsed between last menses and randomization was on average ten months longer in the oral CEE (p = 0.05) and five months longer in the transdermal 17β-estradiol group compared to placebo (p = 0.04). The transdermal 17β-estradiol group had a higher proportion of APOE ɛ4 carriers (50%) than the oral CEE (18%; p = 0.08) and the placebo groups (18%; p = 0.03). All APOE ɛ4 carriers had the ɛ4/ɛ3 genotype. Three women declined APOE genetic testing (Table 1). All participants were cognitively normal on clinical examination and neuropsychological testing. There were no correlations between neuropsychometric test scores and the PiB SUVR values in the entire group as well as in the oral CEE, transdermal 17β-estradiol and placebo groups separately (p > 0.09). However, after adjusting for age, education, APOE ɛ4 status, and time from menopause to randomization, CVLT Total Score was lower in the oral CEE group compared to placebo on ANOVA and post hoc Tukey’s Honest Significant differences test (p = 0.03) (Table 2).
Because of a difference in the proportion of APOE ɛ4 carriers among treatment groups, and the potential impact of this variable on outcome, a stratified analysis in APOE ɛ4 carriers and non-carriers was conducted. There was a significant association of PiB SUVR with age in the whole group (r = 0.37; p = 0.002), in APOE ɛ4 carriers (r = 0.48; p = 0.046), and in APOE ɛ4 non-carriers (r = 0.43; p = 0.003). Therefore, all analyses were adjusted by age (Fig. 2).
The distribution of PiB SUVR varied by treatment group and by APOE ɛ4 carrier status (Fig. 3). Participants who were treated with 17β-estradiol were were more likely to have lower PiB SUVR compared to placebo after adjusting for age [odds ratio (95% CI) = 0.31 (0.11–0.83)]. By use of the proportional odds model, this odds ratio applies to any possible cut-point for PiB SUVR. In the APOE ɛ4 carriers (n = 18), transdermal 17β-estradiol treated participants were more likely to have lower PiB SUVR compared to placebo [odds ratio (95% CI) = 0.04 (0.004–0.44)], and compared to the oral CEE treated participants [odds ratio (95% CI) = 0.01 (0.0006–0.23)] after adjusting for age. Treatment with either oral CEE or transdermal 17β-estradiol was not associated with PiB SUVR in APOE ɛ4 non-carriers (n = 47) (Fig. 4).
DISCUSSION
This study of recently menopausal women who participated in a randomized controlled hormone therapy trial showed that Aβ deposition measured by PiB retention on PET was lower in women who received transdermal 17β-estradiol for four years compared to placebo. In contrast, oral CEE was not associated with a lower level of PiB retention. Although the oral CEE group performed worse on verbal learning and memory compared to placebo, this finding should be interpreted with caution because of the small sample size and because no correlation was found between PiB retention and cognitive test scores. Stratified analysis by APOE ɛ4 genotype showed that the lower PiB retention in the transdermal 17β-estradiol group was present only in the APOE ɛ4 carriers. Hormone therapy was not associated with PiB retention in APOE ɛ4 non-carriers.
A precipitous decline in endogenous estrogens with menopause is thought to be a major driver of AD risk in women. Hence, hormone therapy with estrogens offers the possibility for preventing or delaying the onset of AD in aging women [6, 8, 16, 17]. Observational studies suggest that estrogen treatment, when administered to recently menopausal women, protects from age-associated cognitive decline and dementia [5, 17–25]. KEEPS was a randomized, placebo- controlled hormone therapy trial designed to test for intervention during the period of rapid estrogen depletion in recently menopausal women. Thus, KEEPS is ideally positioned to investigate the effects of hormone therapy on prevention of AD-related pathology during this “window of opportunity”.
PiB retention on PET imaging is a quantitative measure of Aβ deposition [26]. High Aβ deposition measured on PET imaging or via cerebrospinal fluid is considered to be the earliest biomarker change observed during the preclinical stages of AD [27, 28]. Thus, PiB retention on PET is an appropriate biomarker to investigate whether hormone therapy influences Aβ deposition specifically during the early menopausal years when the effect of Aβ deposition on cognitive function is not yet manifested. We observed no differences in cognitive function among the 17β-estradiol and placebo groups. However, a randomized controlled trial of oral 17β-estradiol in older women (age: 61–87) found less decline in short-delayed verbal recall compared to placebo [29]. Hence, the effects of lower levels of Aβ deposition in the transdermal 17β-estradiol group on cognitive function may be apparent later in life.
Carriers of the APOE ɛ4 allele are at an increased risk of AD dementia; moreover the risk may be higher in women than in men [30, 31]. APOE ɛ4 carriers have increased Aβ deposition at an earlier age than APOE ɛ4 non-carriers, and this difference is more pronounced in women than in men [32, 33]. Thus, women who are APOE ɛ4 carriers are at a higher risk for AD-related pathology and may benefit most from preventive interventions at an early age. In the current study, we found that postmenopausal transdermal 17β-estradiol is associated with lower levels of Aβ deposition compared to placebo particularly among women who are APOE ɛ4 carriers. We interpret this finding in two possible ways.
The first possible interpretation is that APOE status modifies the effect of transdermal 17β-estradiol on Aβ deposition as a pharmacogenetic effect. This interpretation is consistent with observations where APOE also modulates the effect of transdermal 17β-estradiol therapy on Aβ deposition in live mice, [34] and in cultured adult mouse cortical neurons [35]. APOE ɛ4 status appears to modify the effects of hormone therapy on cognitive function and dementia also in humans; however, the findings are conflicting [36–38]. In one observational study, APOE ɛ4 positive women opting to use hormone therapy had lower risk of dementia, however, the forms of hormone therapy were not specified [36]. On the contrary, APOE ɛ4 positive women had more cognitive decline than APOE ɛ4 negative women if they used hormone therapy (primarily with oral CEE) in two other observational studies [37, 38]. Similarly, we did not find an association of oral CEE therapy with Aβ deposition compared to placebo. In fact, APOE ɛ4 carriers treated with oral CEE showed higher levels of Aβ deposition than APOE ɛ4 carriers treated with transdermal 17β-estradiol. However because of the low number of APOE ɛ4 carriers in the CEE group, this finding needs to be interpreted with caution. In WHIMS, oral CEE therapy along with medroxyprogesterone acetate, initiated in older women (age≥65) increased the risk of dementia and brain atrophy, which persisted into older ages [1, 2, 39, 40]; however, the APOE ɛ4 status of women in WHIMS was not reported. Because CEE increases serum levels of estrone and of sulfonated conjugates more than transdermal 17β-estradiol, it is possible that the various circulating estrogens would have different efficacy in binding and activation of estrogen receptor mediated events such as the deposition of Aβ [41]. Further work is needed to understand how higher doses of oral CEE (e.g., 0.625 mg/day as used in the Women’s Health Initiative), may increase the circulating levels of 17β-estradiol to those comparable to the transdermal 17β- estradiol treatment group.
A second possible interpretation of the finding is that the APOE ɛ4 non-carriers included in our study were too young to show hormone therapy effects on Aβ deposition. Participants recruited to the PET study three years after KEEPS were at a median age of 60 with a range of 52 to 65. In the population-based Mayo Clinic Study of Aging, the estimated age at which 10% of the population had high levels of Aβ deposition was 57 years for APOE ɛ4 carriers and 64 years for APOE ɛ4 non-carriers [42]. Thus, it may be too early to detect transdermal 17β-estradiol effects on Aβ deposition in APOE ɛ4 non-carriers in the current study. Further follow-up of the cohort is planned to determine whether transdermal 17β-estradiol therapy in recently menopausal women is associated with Aβ deposition in older age.
This study was conducted at a single KEEPS site; therefore, the sample size is limited. Our findings need to be confirmed in a larger sample perhaps by including all KEEPS sites. The participation rate (with the exclusions) for this multimodality imaging study is comparable to the imaging participation rate observed in other hormone therapy trials such as the WHIMS-MRI study [40]. A higher proportion of APOE ɛ4 carriers in the transdermal 17β-estradiol group is not surprising given the relatively small number of women included this pilot study. Randomization does not guarantee a balanced allocation across treatment groups when the numbers are small. Lower Aβ deposition in the transdermal 17β-estradiol group compared to placebo cannot be explained by the higher proportion of APOE ɛ4 carriers in the transdermal 17β-estradiol group than the placebo. In fact, the opposite would be expected, because Aβ deposition should be highest in a group with a higher proportion of APOE ɛ4 carriers. Although the study cohort was randomized to hormone therapies and placebo 7 years ago, cardiovascular risk factors and biomarkers remained comparable in the oral CEE, transdermal 17β-estradiol and placebo groups at 84 months (7 years) post-randomization. KEEPS was designed to include women who were in good cardiovascular and neurological health, therefore generalization of our findings to a broader population may be limited. Yet, in a homogenously healthy cohort of women, the potential effects of hormone therapy on Aβ deposition are not confounded by vascular disease and perhaps define a population who might benefit from the use of transdermal 17β-estradiol.
The consequences of Aβ deposition during early menopausal years are not fully understood, and effectiveness of early menopausal hormone therapy in preventing AD-related pathology in the long-term remains unclear. However, reducing Aβ deposition through Aβ-modifying therapies is a widely accepted strategy for preventing AD, and clinical trials are underway in cognitively normal individuals with high PiB retention, [43] and in APOE ɛ4 carriers [44]. The association of transdermal 17β-estradiol therapy in recently menopausal women with lower Aβ deposition has the potential to change the concepts for preventive interventions that drive the field, and may have a significant impact on women making the decision to use hormone therapy in the early postmenopausal years.
ACKNOWLEDGMENTS
This study is funded by the Aurora Foundation to the Kronos Longevity Research Institute, NIH (NS66147, AG029624 and AG44170). The authors thank Ms. Kim Jensen for consenting and scheduling of the participants. The authors do not have any conflicts of interest related to the submitted work. The funding sources had no role in study design, collection, analysis, interpretation, or decision to submit this paper. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0258r1).
REFERENCES
[1] | Shumaker SA , Legault C , Kuller L , Rapp SR , Thal L , Lane DS , Fillit H , Stefanick ML , Hendrix SL , Lewis CE , Masaki K , Coker LH ((2004) ) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291: , 2947–2958. |
[2] | Espeland MA , Shumaker SA , Leng I , Manson JE , Brown CM , LeBlanc ES , Vaughan L , Robinson J , Rapp SR , Goveas JS , Wactawski-Wende J , Stefanick ML , Li W , Resnick SM ((2013) ) Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med 173: , 1429–1436. |
[3] | LeBlanc ES , Janowsky J , Chan BK , Nelson HD ((2001) ) Hormone replacement therapy and cognition: Systematic review and meta-analysis. JAMA 285: , 1489–1499. |
[4] | Rocca WA , Grossardt BR , Shuster LT ((2011) ) Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Res 1379: , 188–198. |
[5] | Sherwin BB , Henry JF ((2008) ) Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Front Neuroendocrinol 29: , 88–113. |
[6] | Waring SC , Rocca WA , Petersen RC , O’Brien PC , Tangalos EG , Kokmen E ((1999) ) Postmenopausal estrogen replacement therapy and risk of AD: A population-based study. Neurology 52: , 965–970. |
[7] | Yaffe K , Sawaya G , Lieberburg I , Grady D ((1998) ) Estrogen therapy in postmenopausal women: Effects on cognitive function and dementia. JAMA 279: , 688–695. |
[8] | Zandi PP , Carlson MC , Plassman BL , Welsh-Bohmer KA , Mayer LS , Steffens DC , Breitner JC , Cache County Memory Study I ((2002) ) Hormone replacement therapy and incidence of Alzheimer disease in older women: The Cache County Study. JAMA 288: , 2123–2129. |
[9] | Harman SM , Brinton EA , Cedars M , Lobo R , Manson JE , Merriam GR , Miller VM , Naftolin F , Santoro N ((2005) ) KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 8: , 3–12. |
[10] | Harman SM , Black DM , Naftolin F , Brinton EA , Budoff MJ , Cedars MI , Hopkins PN , Lobo RA , Manson JE , Merriam GR , Miller VM , Neal-Perry G , Santoro N , Taylor HS , Vittinghoff E , Yan M , Hodis HN ((2014) ) Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann Intern Med 161: , 249–260. |
[11] | Gleason CE , Dowling NM , Wharton W , Manson JE , Miller VM , Atwood CS , Brinton EA , Cedars MI , Lobo RA , Merriam GR , Neal-Perry G , Santoro NF , Taylor HS , Black DM , Budoff MJ , Hodis HN , Naftolin F , Harman SM , Asthana S ((2015) ) Effects of hormone therapy on cognition and mood in recently postmenopausal women: Findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med 12: , e1001833; discussion e1001833. |
[12] | Dowling NM , Gleason CE , Manson JE , Hodis HN , Miller VM , Brinton EA , Neal-Perry G , Santoro MN , Cedars M , Lobo R , Merriam GR , Wharton W , Naftolin F , Taylor H , Harman SM , Asthana S ((2013) ) Characterization of vascular disease risk in postmenopausal women and its association with cognitive performance. PLoS One 8: , e68741. |
[13] | Jack CR Jr , Lowe VJ , Senjem ML , Weigand SD , Kemp BJ , Shiung MM , Knopman DS , Boeve BF , Klunk WE , Mathis CA , Petersen RC ((2008) ) 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131: , 665–680. |
[14] | Ashburner J , Friston KJ ((2005) ) Unified segmentation. Neuroimage 26: , 839–851. |
[15] | McCullagh P ((1980) ) Regression models for ordinal data (with discussion). J R Stat Soc Series B Stat Methodol 42: , 109–142. |
[16] | Paganini-Hill A , Henderson VW ((1994) ) Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 140: , 256–261. |
[17] | Rocca WA , Grossardt BR , Shuster LT ((2014) ) Oophorectomy, estrogen, and dementia: A 2014 update. Mol Cell Endocrinol 389: , 7–12. |
[18] | Resnick SM , Henderson VW ((2002) ) Hormone therapy and risk of Alzheimer disease: A critical time. JAMA 288: , 2170–2172. |
[19] | Brinton RD , Yao J , Yin F , Mack WJ , Cadenas E ((2015) ) Perimenopause as a neurological transition state. Nat Rev Endocrinol 11: , 393–405. |
[20] | Brinton RD ((2009) ) Estrogen-induced plasticity from cells to circuits: Predictions for cognitive function. Trends Pharmacol Sci 30: , 212–222. |
[21] | Henderson VW , Benke KS , Green RC , Cupples LA , Farrer LA ((2005) ) Postmenopausal hormone therapy and Alzheimer’s disease risk: Interaction with age. J Neurol Neurosurg Psychiatry 76: , 103–105. |
[22] | Maki PM ((2013) ) Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause 20: , 695–709. |
[23] | Shao H , Breitner JC , Whitmer RA , Wang J , Hayden K , Wengreen H , Corcoran C , Tschanz J , Norton M , Munger R , Welsh-Bohmer K , Zandi PP ((2012) ) Hormone therapy and Alzheimer disease dementia: New findings from the Cache County Study. Neurology 79: , 1846–1852. |
[24] | Whitmer RA , Quesenberry CP , Zhou J , Yaffe K ((2011) ) Timing of hormone therapy and dementia: The critical window theory revisited. Ann Neurol 69: , 163–169. |
[25] | Zhao L , Yao J , Mao Z , Chen S , Wang Y , Brinton RD ((2011) ) 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: Relevance to Alzheimer’s prevention. Neurobiol Aging 32: , 1949–1963. |
[26] | Ikonomovic MD , Klunk WE , Abrahamson EE , Mathis CA , Price JC , Tsopelas ND , Lopresti BJ , Ziolko S , Bi W , Paljug WR , Debnath ML , Hope CE , Isanski BA , Hamilton RL , DeKosky ST ((2008) ) Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 131: , 1630–1645. |
[27] | Sperling RA , Aisen PS , Beckett LA , Bennett DA , Craft S , Fagan AM , Iwatsubo T , Jack CR Jr , Kaye J , Montine TJ , Park DC , Reiman EM , Rowe CC , Siemers E , Stern Y , Yaffe K , Carrillo MC , Thies B , Morrison-Bogorad M , Wagster MV , Phelps CH ((2011) ) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 280–292. |
[28] | Jack CR Jr , Knopman DS , Jagust WJ , Shaw LM , Aisen PS , Weiner MW , Petersen RC , Trojanowski JQ ((2010) ) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: , 119–128. |
[29] | Tierney MC , Oh P , Moineddin R , Greenblatt EM , Snow WG , Fisher RH , Iazzetta J , Hyslop PS , MacLusky NJ ((2009) ) A randomized double-blind trial of the effects of hormone therapy on delayed verbal recall in older women. Psychoneuroendocrinology 34: , 1065–1074. |
[30] | Payami H , Zareparsi S , Montee KR , Sexton GJ , Kaye JA , Bird TD , Yu CE , Wijsman EM , Heston LL , Litt M , Schellenberg GD ((1996) ) Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: A possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 58: , 803–811. |
[31] | Rocca WA , Mielke MM , Vemuri P , Miller VM ((2014) ) Sex and gender differences in the causes of dementia: A narrative review. Maturitas 79: , 196–201. |
[32] | Altmann A , Tian L , Henderson VW , Greicius MD ((2014) ) Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol 75: , 563–573. |
[33] | Corder EH , Ghebremedhin E , Taylor MG , Thal DR , Ohm TG , Braak H ((2004) ) The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: Modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci 1019: , 24–28. |
[34] | Kunzler J , Youmans KL , Yu C , Ladu MJ , Tai LM ((2014) ) APOE modulates the effect of estrogen therapy on Abeta accumulation EFAD-Tg mice. Neurosci Lett 560: , 131–136. |
[35] | Nathan BP , Barsukova AG , Shen F , McAsey M , Struble RG ((2004) ) Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology 145: , 3065–3073. |
[36] | Ryan J , Carriere I , Scali J , Dartigues JF , Tzourio C , Poncet M , Ritchie K , Ancelin ML ((2009) ) Characteristics of hormone therapy, cognitive function, and dementia: The prospective 3C Study. Neurology 73: , 1729–1737. |
[37] | Kang JH , Grodstein F ((2012) ) Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging 33: , 1129–1137. |
[38] | Yaffe K , Haan M , Byers A , Tangen C , Kuller L ((2000) ) Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology 54: , 1949–1954. |
[39] | Espeland MA , Tindle HA , Bushnell CA , Jaramillo SA , Kuller LH , Margolis KL , Mysiw WJ , Maldjian JA , Melhem ER , Resnick SM ((2009) ) Brain volumes, cognitive impairment, and conjugated equine estrogens. J Gerontol A Biol Sci Med Sci 64: , 1243–1250. |
[40] | Coker LH , Hogan PE , Bryan NR , Kuller LH , Margolis KL , Bettermann K , Wallace RB , Lao Z , Freeman R , Stefanick ML , Shumaker SA ((2009) ) Postmenopausal hormone therapy and subclinical cerebrovascular disease: The WHIMS-MRI Study. Neurology 72: , 125–134. |
[41] | Jaffe AB , Toran-Allerand CD , Greengard P , Gandy SE ((1994) ) Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem 269: , 13065–13068. |
[42] | Jack CR Jr , Wiste HJ , Weigand SD , Knopman DS , Vemuri P , Mielke MM , Lowe V , Senjem ML , Gunter JL , Machulda MM , Gregg BE , Pankratz VS , Rocca WA , Petersen RC ((2015) ) Age, sex, and APOE epsilon4 effects on memory, brain structure, and beta-amyloid across the adult life span. JAMA Neurol 72: , 511–519. |
[43] | Sperling RA , Rentz DM , Johnson KA , Karlawish J , Donohue M , Salmon DP , Aisen P ((2014) ) The A4 study: Stopping AD before symptoms begin?. Sci Transl Med 6: , 228fs213. |
[44] | Reiman EM , Langbaum JB , Fleisher AS , Caselli RJ , Chen K , Ayutyanont N , Quiroz YT , Kosik KS , Lopera F , Tariot PN ((2011) ) Alzheimer’s Prevention Initiative: A plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 26: (Suppl 3), 321–329. |
Figures and Tables
Fig.1
Participation flowchart: * There were six exclusions: One woman with an MRI incompatible implant (oral CEE group); one woman with posterior fossa developmental abnormality and hydrocephalus, one woman who developed breast cancer and underwent systemic chemotherapy (transdermal 17β-estradiol group); two women with multiple sclerosis and one woman with a benign brain tumor (placebo group).
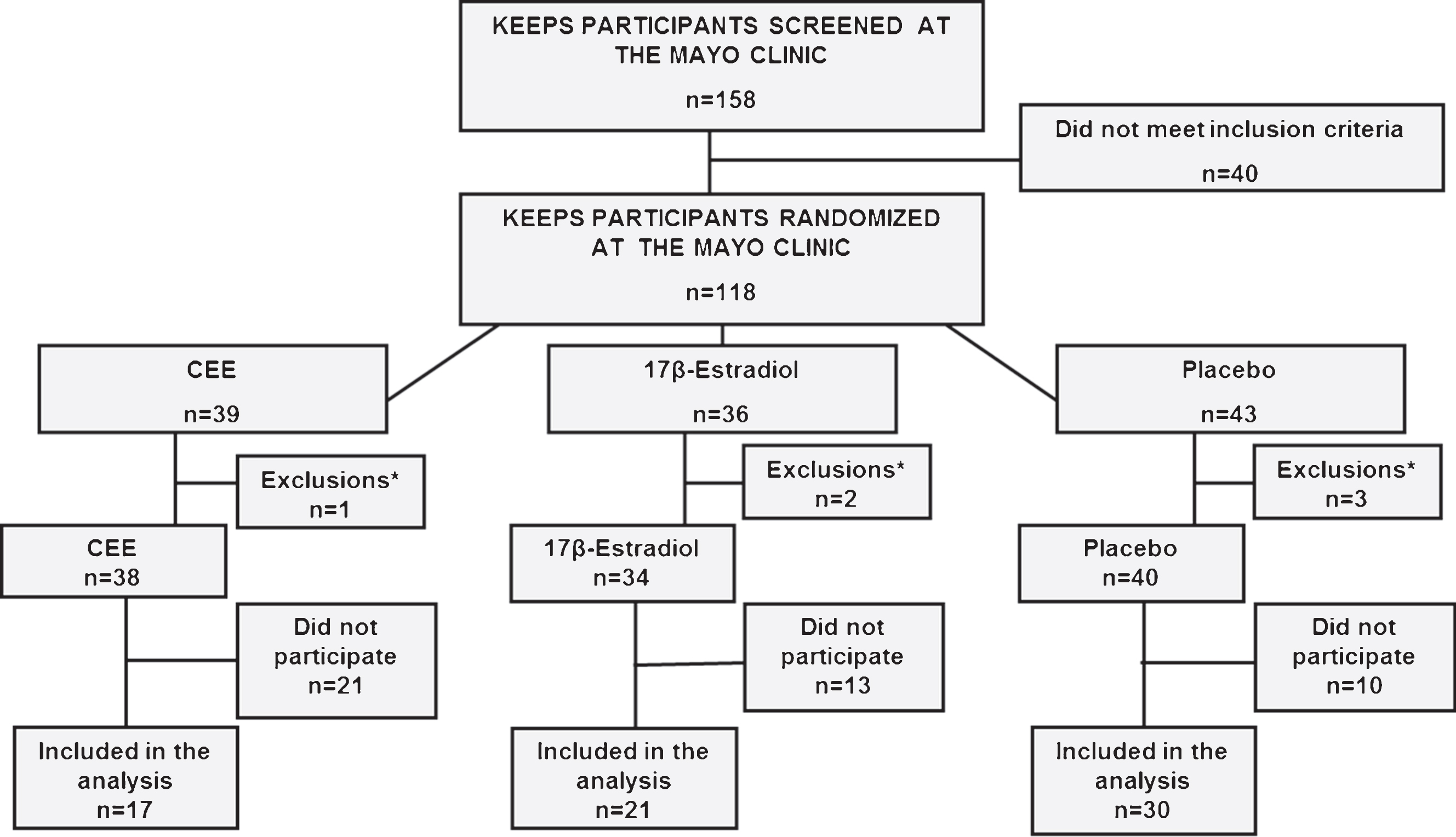
Fig.2
Associations of PiB SUVR with age in the whole group of participants, in APOE ɛ4 carriers, and in APOE ɛ4 non-carriers.
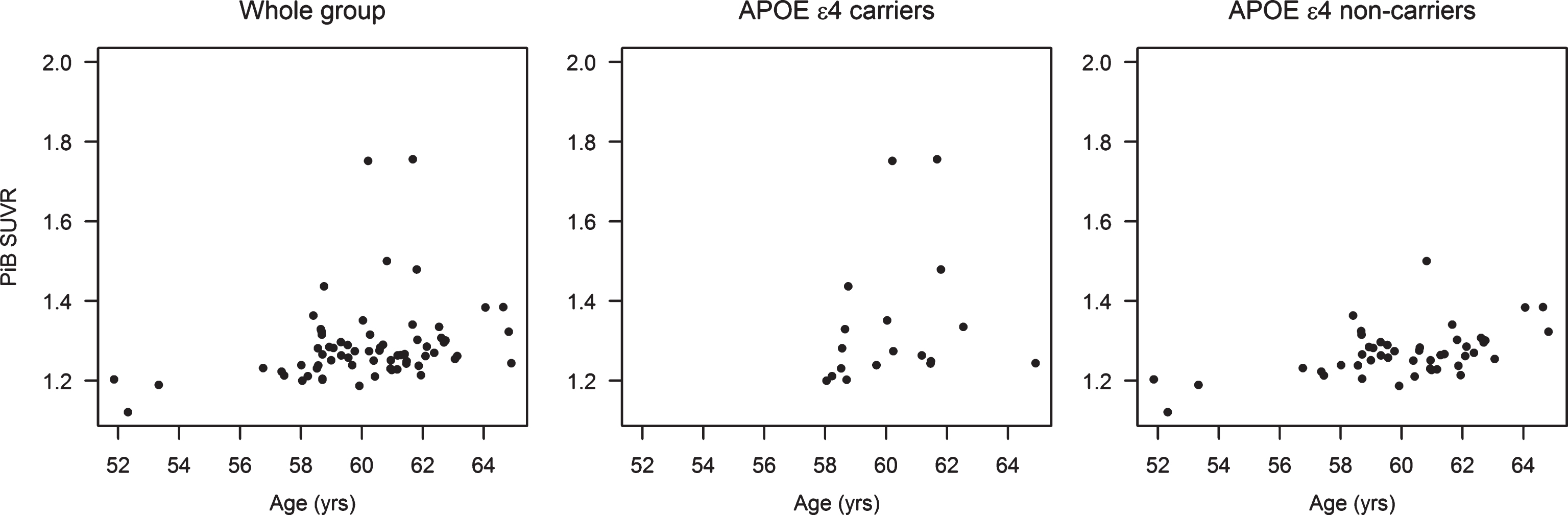
Fig.3
PiB SUVR in the oral CEE, transdermal 17β-estradiol, and the placebo groups in the whole group of participants, in APOE ɛ4 carriers, and in APOE ɛ4 non-carriers.
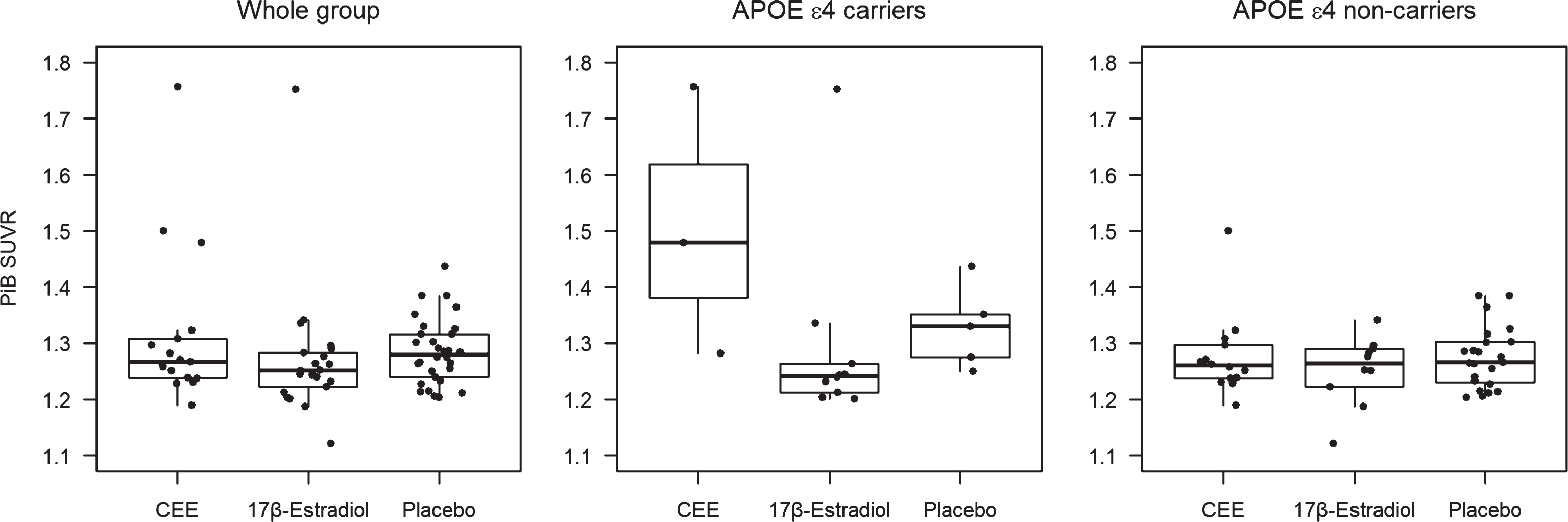
Fig.4
Odds ratios for PiB SUVR from proportional odds logistic regression models and 95% Wald confidence limits comparing PiB SUVR in oral CEE, transdermal 17β-estradiol, and the placebo groups in the whole group of participants, in APOE ɛ4 carriers, and in APOE ɛ4 non-carriers after adjusting for age. The odds ratio axis is logarithmic to accommodate the entire range of 95% Wald confidence limits.
Table 1
Characteristics of the participants by treatment status
| Characteristica | CEE (N = 17) | 17β-Estradiol (N = 21) | Placebo (N = 30) | pb |
| Age, year at baseline | 54 (46, 58) | 53 (45, 58) | 53 (45, 58) | 0.47 |
| Age, year at the PET scan | 61 (53, 65) | 60 (52, 65) | 60 (52, 65) | 0.48 |
| Education, n (%) | 0.65 | |||
| High school or less | 1 (7) | 1 (5) | 3 (10) | |
| Some college / College graduate | 12 (80) | 12 (63) | 17 (57) | |
| Some graduate / Graduate | 2 (13) | 6 (32) | 10 (33) | |
| Smoking status, n (%) | 0.33 | |||
| Non-smoker | 10 (71) | 8 (50) | 20 (71) | |
| Smoker | 4 (29) | 8 (50) | 8 (29) | |
| Time past menopause to randomization (months) | 23 (7, 35)* | 18 (7, 36)* | 13 (5, 36) | 0.045 |
| APOE carrier, n (%) | 3 (18) | 10 (50)* | 5 (18) | 0.04 |
| Migraines, n (%) | 1 (6) | 0 (0) | 3 (10) | 0.36 |
| Global Cognition at baseline | –0.12 (–1.79, 1.67) | 0.38 (–1.84, 1.15) | 0.08 (–1.06, 1.83) | 0.23 |
| Mean systolic blood pressure, mm Hg at baseline | 121 (96, 146) | 114 (88, 149) | 124 (96, 152) | 0.13 |
| Mean systolic blood pressure, mm Hg at the PET scan | 88 (77, 116) | 84 (68, 104) | 93 (68, 116) | 0.32 |
| Mean diastolic blood pressure, mm Hg at baseline | 78 (66, 91) | 72 (60, 87) | 76 (60, 88) | 0.10 |
| Mean diastolic blood pressure, mm Hg at the PET scan | 123 (95, 156) | 128 (94, 149) | 128 (97, 149) | 0.89 |
| Body mass index, kg/m2 at baseline | 26 (20, 36) | 25 (18, 34) | 26 (19, 33) | 0.44 |
| Body mass index, kg/m2 at the PET scan | 77 (62, 96) | 76 (60, 88) | 79 (60, 93) | 0.81 |
| Coronary arterial calcification present, n (%) at baseline | 0 (0) | 2 (10) | 4 (13) | 0.41 |
| Coronary arterial calcification present, n (%) at the PET scan | 1 (6) | 3 (14) | 4 (13) | 0.79 |
| Carotid intima-media thickness at baseline | 0.69 (0.55, 0.80) | 0.64 (0.56, 0.85) | 0.66 (0.57, 0.87) | 0.91 |
| Carotid intima-media thickness at the PET scan | 0.73 (0.61, 0.88) | 0.74 (0.56, 0.99) | 0.73 (0.58, 1.01) | 0.73 |
| Low-density lipoprotein, mg/dL at baseline | 121 (79, 163) | 117 (64, 172) | 114 (53, 178) | 0.71 |
| Low-density lipoprotein, mg/dL at the PET scan | 124 (91, 191) | 117 (66, 181) | 120 (66, 166) | 0.89 |
| High-density lipoprotein, mg/dL at baseline | 70 (45, 84) | 70 (54, 89) | 68 (50, 122) | 0.80 |
| High-density lipoprotein, mg/dL at the PET scan | 64 (41, 98) | 64 (39, 92) | 58 (43, 131) | 0.44 |
| Triglycerides, mg/dL at baseline | 68 (29, 229) | 83 (33, 226) | 72 (27, 233) | 0.47 |
| Triglycerides, mg/dL at the PET scan | 100 (62, 230) | 83 (52, 204) | 94 (59, 336) | 0.60 |
| Fasting Blood Glucose, mg/dL at baseline | 76 (65, 100) | 78 (67, 94) | 78 (68, 94) | 0.38 |
| Fasting Blood Glucose, mg/dL at the PET scan | 96 (88, 113) | 93 (82, 108) | 94 (75, 126) | 0.59 |
aUnless otherwise indicated, data are given as the median (range). bp-values are assessed using Kruskal Wallis and Fisher’s Exact Tests. *Pairwise comparison to placebo p < 0.05. Abbreviations: CEE: Conjugated equine estrogen; APOE: Apolipoprotein E
Table 2
Cognitive Test Scores at the time of PiB PET imaging
| Cognitive scoresa | Oral CEE (N = 17) | Transdermal 17β-Estradiol (N = 21) | Placebo (N = 30) | p-valuesb |
| NYU Paragraph Immediate Recall Total Score | 25 (16, 33) | 26 (15, 39) | 24 (17, 40) | 0.86 |
| NYU Delayed Recall Total Score | 16 (5, 24) | 14 (7, 23) | 14 (9, 32) | 0.88 |
| CVLT-II Trials 1–3 Total Score | 29 (16, 36) | 33 (25, 42) | 31 (14, 43) | 0.03* |
| CVLT-II Trial Short Delay Free Recall score | 10 (3, 16) | 12 (7, 16) | 11 (4, 16) | 0.06 |
| CVLT-II Trial Long Delay Free Recall score | 9 (3, 15) | 11 (6, 15) | 10 (4, 16) | 0.19 |
| WMS-III Digit Span Total Score | 15 (10, 22) | 18 (10, 26) | 17 (8, 26) | 0.20 |
| WMS-III Letter Number Sequencing Trial Total Score | 10 (6, 14) | 11 (6, 15) | 10 (7, 17) | 0.50 |
| Trail Making Test A (Time to complete in seconds) | 24 (15, 44) | 23 (15, 43) | 24 (15, 39) | 0.89 |
| Trail Making Test B (Time to complete in seconds) | 56 (33, 83) | 59 (35, 249) | 57 (33, 135) | 0.85 |
| Phonemic Fluency (F,A,S) Total Score | 44 (27, 69) | 43 (19, 59) | 46 (22, 77) | 0.40 |
| Semantic Fluency (animals, fruits, vegetables) Total Score | 56 (30, 77) | 55 (38, 68) | 52 (36, 71) | 0.35 |
| Stroop Trial Word | 99 (69, 136) | 105 (70, 120) | 100 (69, 140) | 0.95 |
| Stroop Trial Color | 78 (61, 96) | 74 (60, 110) | 75 (58, 101) | 0.55 |
| Stroop Trial Color-Word | 43 (18, 58) | 44 (31, 61) | 46 (21, 78) | 0.78 |
| Digit Symbol Total Score | 82 (57, 93) | 83 (61, 108) | 82 (64, 103) | 0.09 |
aData shown are median (range) of raw scores. bp-values were assessed using Analysis of Variance adjusting for age at PiB PET, levels of education, time from menopause to randomization (months) and APOE ɛ4 carrier status. *Tukey Honest significant differences test for post hoc comparisons: Oral CEE versus placebo (p = 0.03); CEE versus transdermal17β-estradiol (p = 0.08); transdermal17β-estradiol versus placebo (p = 0.98).




