Association Between Oral Malodor and Dementia: An 11-Year Follow-Up Study in Japan
Abstract
Background:
As infrequent social interaction is a potential risk of dementia, oral malodor may increase the risk of dementia, including Alzheimer’s disease.
Objective:
This study investigated the association between malodor and dementia.
Methods:
We used the Japan Public Health Center-based Prospective Study data obtained at Yokote City. A total of 1,493 individuals aged 56 to 75 years underwent a dental examination and self-reported survey from May 2005 to January 2006. Follow-up for the onset of dementia was conducted using long-term care insurance data from 2006 to 2016. Hazard ratios of oral malodor on dementia were estimated by the Cox proportional hazards model. The inverse probability-weighted Cox model was used as a sensitivity analysis.
Results:
The study comprised 1493 participants (53.6% women) with a mean age of 65.6 (SD = 5.8) years old; at the end of the follow-up, 6.4% (n = 96) developed dementia, and the percentage was 20.7 in severe malodor group. Throughout 15274.133 person-years of follow-up, the average incidence rate for the onset of dementia per 1000 person-years was 6.29. The highest incidence rate was seen in participants with severe malodor (22.4 per 1000 person-years). After adjusting for confounders, compared to those with no malodor, there was a 3.8 (95% confidence interval: 1.5 to 9.4) times greater hazard of developing dementia in participants with severe malodor. The inverse probability weighted Cox model confirmed the same trend with an adjusted marginal hazard ratio of 4.4 (1.2 to 16.4).
CONCLUSIONS:
A significant association between oral malodor and the onset of dementia exists.
INTRODUCTION
Along with increasing global life expectancy, there has been a remarkable expansion of the world’s aging population [1]; consequently, dementia has become a growing concern worldwide. Globally, the number of people with dementia is estimated to triple from 57.4 (95% uncertainty interval of 50.4 to 65.1) million cases in 2019 to 152.8 (130.8 to 175.9) million cases in 2050 [2]. In addition, handling this complex syndrome requires substantial economic resources; a meta-analysis reported the annual expense of dementia per person in 2015 in European countries and the United States was € 32,506.73 and € 42,898.65, respectively [3]. People with all types of dementia, including Alzheimer’s disease (AD), required approximately 15.3 billion hours of care from 11 million caregivers around the world in 2020 [4]. Moreover, the prevalence of dementia among older people over 65 years old in Japan is expected to surpass 25% in the next 25 years [5].
Studies have indicated that oral health contributes to the risk of dementia. A systematic review revealed that tooth loss was shown to raise the risk of dementia and cognitive decline [6]. Another meta-analysis of 419 articles and 11 studies identified a link between a greater number of remaining teeth and a lower risk of dementia [7]. A recent meta-analysis also reported the association of periodontal disease with AD, the most common form of dementia [8].
The proposed mechanism, which elaborated the relationship between oral health and dementia, was largely based on animal experiments [9]. A previous systematic review highlighted the triggering biomedical mechanisms such as the mechanical, aggravation, and long-term inflammatory stress pathways [9]. The reduction in mastication stimuli decreasing the strength of neural pathway connections, tooth loss accelerating neurodegeneration, and inflammatory stress from periodontitis causing inflammatory cascade in the central nervous system are arguments used to explain the link between oral health and dementia.
In addition, the reduction in social contact resulting from poor oral health is considered a mechanism linking oral health and dementia because a low level of social communication is one of the 12 potentially modifiable risks of dementia [10]. Other studies reported that among older people, poor oral health was a predictor of becoming homebound, which reduces social interaction [11, 12]. Furthermore, one study using mediation analysis found that social factors were particularly effective at explaining the relationship between tooth loss and the onset of dementia [13]. From this perspective, oral malodor could increase the risk of dementia because it largely impacts social interactions [14].
Oral malodor, or bad breath, is a foul smell that emerges from the mouth or nose [15]. This unpleasant odor is primarily caused by the excessive amount of volatile sulfur compounds in exhaled air as a consequence of oral bacteria’s actions [16, 17]. Even though there are numerous causes for bad breath, the origin of 90% of the cases may arise from oral cavity issues such as inadequate oral hygiene, periodontal disease, tongue coating, and many other problems [18]. According to epidemiological research, the prevalence of oral malodor is thought to vary from 2.4% to 78% [17, 19], and the American Dental Association estimated that approximately 50% of adults in the United States possess foul breath [20]. This disease may negatively impact relationships with other people and create social and psychological difficulties for bad breath bearers [21]. Hence, oral malodor is increasingly acknowledged as a notable public health concern [15, 22]. No studies have investigated the relationship between oral malodor and dementia. Therefore, this cohort study aimed to evaluate the association between oral malodor and dementia.
METHODS
Data collection
The participants of the present study comprised a sub-cohort of individuals in the area of Yokote Public Health Center, which belonged to the Japan Public Health Center-based Prospective Study (JPHC Study) Cohort I. This Cohort I consisted of residents aged 40 to 59 years and aimed to prospectively monitor the morbidity and mortality of numerous diseases in the Japanese population [23]. The study was launched in 1990 and conducted in 5 public health center areas (Iwate, Akita, Nagano, Okinawa, and Tokyo). In this study, we used the JPHC study data obtained at the Yokote Public Health Center area in Akita Prefecture between 2005 and 2006 because it was the only location where the oral health survey was carried out. As such, the previously mentioned time frame served as the baseline for our analysis.
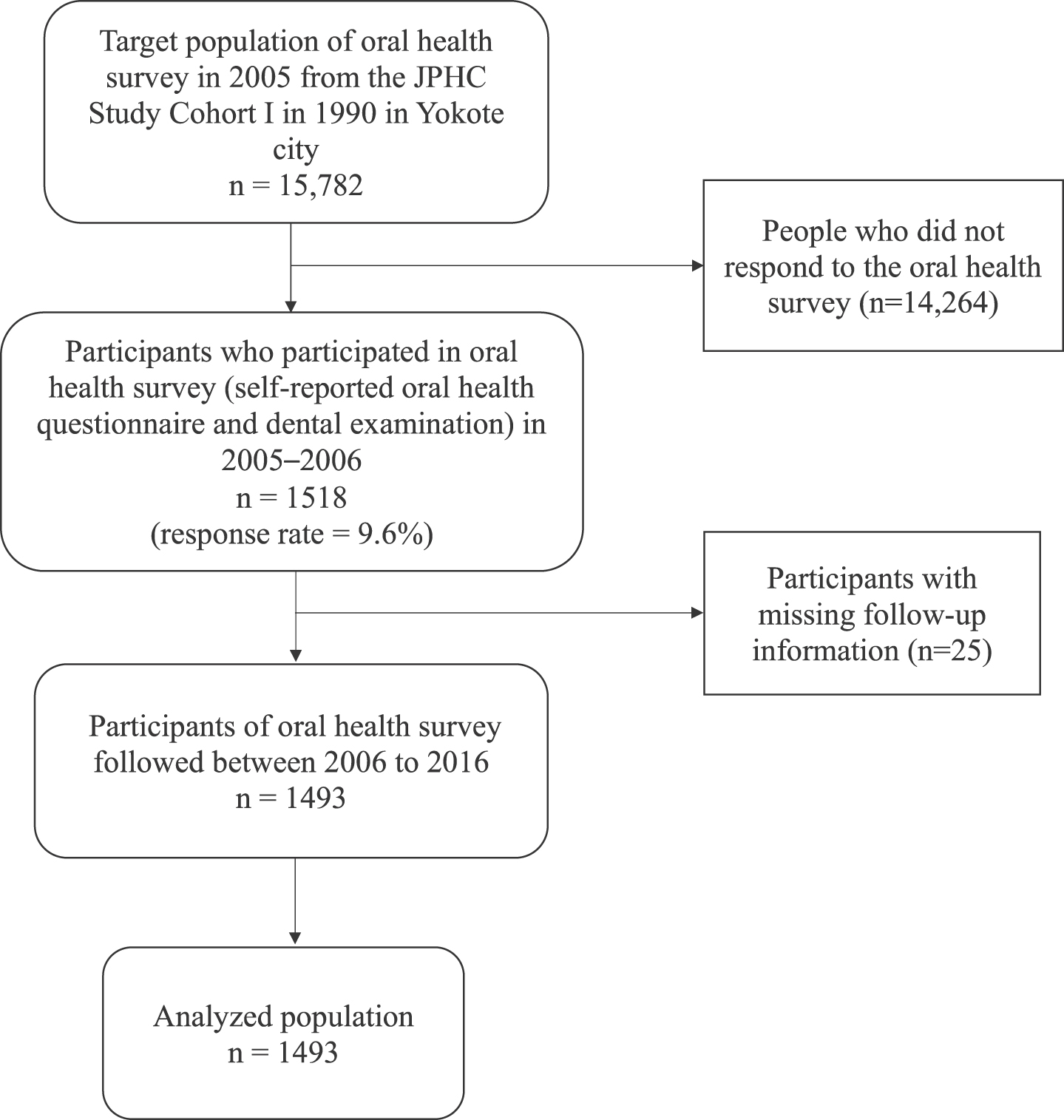
This oral health survey targeted 15,782 residents aged 56 to 75. The recruitment of participants for the survey was completed by sending out letters of invitation addressing the study’s protocol. In May 2005, self-reported oral health questionnaires were collected. From July 2005 to January 2006, 1518 adults who completed the previous questionnaires participated in the dental examination performed by clinical dentists. Participants were followed up from January 2006 to December 2016. Individuals with missing follow-up information were excluded from our analysis. Overall, data from 1,493 participants were included in the study. The flowchart of the participants is shown in Fig. 1.
Fig. 1
Flowchart of the study participants.
In this 11-year follow-up cohort study utilizing the data from the JPHC Study, all the procedures involving human subjects conformed with the Declaration of Helsinki in 1975. For the secondary data analysis, approval was obtained from the Ethics Committee of the National Cancer Center Japan (Approval No. 2015-085) and the Tokyo Medical and Dental University Ethical Committee, Japan (Approval No. D2019-070). Hence, participant consent was not required for this study. Prior to use, the data were anonymized.
Outcome variable
The outcome variable of this study was the onset of disabling dementia. The certified records from the national Long-term Care Insurance system were used to determine participants with disabling dementia.
The Long-term Care Insurance system is a government-mandated insurance program initiated by the Japanese Ministry of Health, Labor, and Welfare in 2000. Residents who are 65 years and older and people with disability between 40 and 64 years could request for “functionally disabled status” to obtain long-term care at the municipality level. The municipal government evaluated the application using two documents: a thorough evaluation of the applicant’s state of functional health and a written report from a primary care doctor providing that individual’s level of cognitive disability [24].
The cognitive disability severity scales were categorized into eight levels: level 0, I, IIa, IIb, IIIa, IIIb, IV, and Medical (M), ranging from no dementia to severe dementia with specialized medical care [24, 25]. Level 0 denoted no dementia, while level I indicated individuals exhibiting specific symptoms of dementia yet maintaining a high level of independence in both domestic and social aspects of daily life. Level IIa and IIb characterized dementia with mild communication difficulties but maintaining independence in daily living with minimal supervision. At levels IIIa and IIIb, individuals faced greater challenges in communication and required partial care. Level IV demonstrated severe dementia with significant communication difficulties and a need for complete care. Finally, level M specified those with additional psychiatric symptoms, behavioral issues, or serious medical conditions demanding specialized medical attention.
From that assessment process, disabling dementia was determined with the certification of any degree which required long-term care, and the physician’s written judgement in the following levels of cognitive disability severity (level IIa, IIb, IIIa, IIIb, IV, or M) [24, 25].
Furthermore, the dementia rating scale was shown to have a strong correlation (r = 0.74) with the results of the Mini-Mental State Examination, according to a clinically-based study in 2009 [26]. Besides the JPHC study, other studies, such as the Japan Gerontological Evaluation Study (JAGES) and other previous studies in Japan, also used the same standards to determine participants with disabling dementia [25, 27–30].
From January 1, 2006, the follow-up of participants continued until December 31, 2016. The end of the follow-up date was whichever came first of the following: the date the participant left the study, the date of death, the date at which dementia onset was determined, or the final day of the follow-up.
Main exposure: Oral malodor
We used the variables of oral malodor and oral health confounders from the 2005–2006 oral health survey. In this survey, 43 participating dentists performed standardized clinical oral examinations. Before the survey, a guidebook was distributed and verbal explanations were provided to the dentists as part of the training and calibration process [31]. The guidebook included the essential oral examination criteria based on the WHO’s handbook [32] and other examination criteria for oral malodor and gingival redness.
The main exposure variable of this study was oral malodor. Genuine oral malodor [33–35] was assessed through the organoleptic method by a dentist and classified as no, mild, or severe malodor. The following criteria were used to determine the condition during the oral examination with the mask removed. Mild oral malodor was described as an odor that can be eventually detected by bringing the nose close to the patient. Severe oral malodor was defined as an odor that can be clearly diagnosed at the face-to-face position when conducting the examination.
Confounders
Based on previous studies [10, 13], confounders in this study included demographic factors (age and sex), socioeconomic status (highest educational level), body mass index (BMI), health behaviors (frequency of alcohol consumption and smoking status), and comorbidities. We also used oral health confounders: gingival redness, the number of remaining teeth, and the frequency of tooth brushing. Gingival redness was used as a proxy for periodontal disease.
Information on these confounders was gathered from the self-reported survey carried out in 1990 for Cohort I of the JPHC Study [36]. The age of the participants in 2006 was used in this paper. The highest educational level was divided into three categories: above high school, high school, and junior high school. BMI was calculated using the formula weight (kg)/height (m)2 and categorized as either < 25 or≥25 kg/m2. The frequency of alcohol consumption was classified as rarely, a few times a week, and daily. Smoking status, extracted from the self-reported oral health questionnaire, was recorded as one of three categories: never, past, or current. We used a dichotomous variable for comorbidities that indicated the presence of a history of diabetes, stroke, hypertension, or myocardial infarction.
Gingival redness and the number of remaining teeth were obtained from the dental examinations conducted in dental clinics. The frequency of tooth brushing was obtained from the self-reported oral health questionnaire. Gingival redness was recorded as yes or no. The number of remaining teeth was categorized as≥28, 20–27, 10–19, or 0–9. The frequency of tooth brushing was split into three groups:≥3 times a day, 2 times a day, and≤1 time a day.
Statistical analysis
At first, we calculated the incidence rates of dementia per 1000 person-years by subgroups defined by oral malodor and the aforementioned confounders. Then, we calculated the hazard ratios (HRs) of oral malodor on dementia using the Cox proportional hazards model. We fitted both the univariable model and multivariable model with all previously listed confounders. From the multivariable model, we drew the adjusted survival curves predicted by the mean values of each confounder.
As a sensitivity analysis, we applied the inverse probability weighted (IPW) Cox model as an alternative to the multivariable Cox model [37, 38]. In this approach, the IPW Cox Model created a pseudo-population in which exposed or unexposed groups have similar baseline confounder distributions [39]. This method enabled the estimation of the marginal HR for the entire population without conditioning on the confounders [40]. In this method, the propensity scores of each malodor group were estimated through the multinomial logistic model with a general logit link function by a formula that depends on all the confounders. The estimated propensity scores were converted into inverse probability weights using the R package “WeightIt” with selected estimand of average treatment effect among the whole study population [41, 42]. The average treatment effect can be explained as the disparity if all the population members were exposed, as opposed to if they were all not exposed [43]. After that, we fitted the IPW Cox model using the weights to estimate the marginal HR [44] with the robust variance estimator for calculating confidence intervals (CIs) [38, 45].
The numbers and percentages of missing variables were demonstrated in Supplementary Table 1. To handle missing data, we assumed the “missing at random” mechanism and conducted multiple imputation to minimize bias [46]. Multiple imputation by chained equations was applied to create 20 imputed datasets. The variables used in the imputation were exposure, confounders, and outcome variables [47], including: oral malodor, age, sex, the number of remaining teeth, the frequency of tooth brushing, gingival redness, BMI, comorbidities, smoking status, the frequency of alcohol consumption, the highest educational level, and the onset of dementia. The average estimates were obtained using Rubin’s rule [48]. For another sensitivity analysis, we made analyses for those without any missing responses - complete case analysis.
Table 1
Baseline characteristics of participants, stratified by oral malodor after multiple imputation (n = 1,493)
| Characteristics | Oral malodor | |||
| None | Mild | Severe | Total | |
| (%) | (%) | (%) | (%) | |
| Total | 886 (100%) | 578 (100%) | 29 (100%) | 1493 (100%) |
| Age | ||||
| 55–59 | 174 (19.6%) | 112 (19.4%) | 6 (20.7%) | 292 (19.6%) |
| 60–64 | 229 (25.8%) | 134 (23.2%) | 4 (13.8%) | 367 (24.6%) |
| 65–69 | 214 (24.2%) | 144 (24.9%) | 6 (20.7%) | 364 (24.4%) |
| 70–75 | 269 (30.4%) | 188 (32.5%) | 13 (44.8%) | 470 (31.4%) |
| Sex | ||||
| Men | 370 (41.8%) | 307 (53.1%) | 16 (55.2%) | 693 (46.4%) |
| Women | 516 (58.2%) | 271 (46.9%) | 13 (44.8%) | 800 (53.6%) |
| Frequency of tooth brushing | ||||
| ≥3 times a day | 276 (31.2%) | 160 (27.7%) | 6 (1.0%) | 442 (29.6%) |
| 2 times a day | 413 (46.6%) | 244 (42.2%) | 12 (2.1%) | 669 (44.8%) |
| ≤1 time a day | 197 (22.2%) | 174 (30.1%) | 11 (1.9%) | 382 (25.6%) |
| Gingival redness | ||||
| No | 489 (55.2%) | 190 (32.9%) | 3 (10.3%) | 682 (45.7%) |
| Yes | 397 (44.8%) | 388 (67.1%) | 26 (89.7%) | 811 (54.3%) |
| Number of remaining teeth | ||||
| ≥28 | 130 (14.7%) | 89 (15.4%) | 8 (27.6%) | 227 (15.2%) |
| 20–27 | 409 (46.2%) | 252 (43.6%) | 11 (37.9%) | 672 (45.0%) |
| 10–19 | 175 (19.8%) | 137 (23.7%) | 9 (31.0%) | 321 (21.5%) |
| 0–9 | 172 (19.3%) | 100 (17.3%) | 1 (3.5%) | 273 (18.3%) |
| BMI | ||||
| <25 | 683 (77.1%) | 455 (78.7%) | 23 (79.3%) | 1161 (77.8%) |
| ≥25 | 203 (22.9%) | 123 (21.3%) | 6 (20.7%) | 332 (22.2%) |
| Comorbidities† | ||||
| No | 745 (84.1%) | 473 (81.8%) | 24 (4.2%) | 1242 (83.2%) |
| Yes | 141 (15.9%) | 105 (18.2%) | 5 (0.8%) | 251 (16.8%) |
| Smoking status | ||||
| Never | 639 (72.1%) | 366 (63.3%) | 20 (69.0%) | 1025 (68.7%) |
| Past | 163 (18.4%) | 118 (20.4%) | 4 (13.8%) | 284 (19.0%) |
| Current | 84 (9.5%) | 94 (16.3%) | 5 (17.2%) | 184 (12.3%) |
| Frequency of alcohol consumption | ||||
| Rarely | 407 (45.9%) | 226 (39.1%) | 10 (1.7%) | 643 (43.1%) |
| A few times a week | 264 (29.8%) | 157 (27.2%) | 8 (1.4%) | 429 (28.7%) |
| Daily | 215 (24.3%) | 195 (33.7%) | 11 (1.9%) | 421 (28.2%) |
| Highest educational level | ||||
| Above high school | 157 (17.7%) | 94 (16.3%) | 4 (0.7%) | 254 (17.0%) |
| High school | 453 (51.1%) | 279 (48.3%) | 13 (2.2%) | 746 (50.0%) |
| Junior high school | 276 (31.2%) | 205 (35.4%) | 12 (2.1%) | 493 (33.0%) |
| Onset of dementia | ||||
| No | 826 (93.2%) | 548 (94.8%) | 23 (79.3%) | 1397 (93.6%) |
| Yes | 60 (6.8%) | 30 (5.2%) | 6 (20.7%) | 96 (6.4%) |
†Comorbidities variable indicated the presence of a history of diabetes, stroke, hypertension, or myocardial infarction.
Besides, in order to evaluate the robustness of the causal relationship against unmeasured confounders, the E-values were computed for the estimated HRs [49, 50]. These values indicate the magnitude of the associations of an unmeasured confounder required to fully explain the relationship with oral malodor (i.e., prevalence ratios of the unmeasured confounder between oral malodor groups) and dementia (i.e., relative dementia risk between the presence and absence of the unmeasured confounder) [49, 50]:
The data were analyzed by the software Stata (version 17.0; Stata Corp LLC, College Station, TX, USA) for multiple imputation and unweighted Cox models, and R (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria) for the IPW Cox model. Two-sided P < 0.05 was considered statistically significant.
RESULTS
In total, 1,493 participants were included in the analyzed population. The mean age was 65.6 (SD = 5.8) years old, and 53.6% of the participants were women. Overall, 6.4% of participants experienced the onset of dementia during follow-up. During the 15274.133 person-years of follow-up (mean 7.54, median 8.23, maximum 10.99 years), the total incidence rate for the onset of dementia per 1000 person-years was 6.29.
The baseline characteristics of participants, stratified by oral malodor after multiple imputation, are shown in Table 1. Those with severe malodor tended to be older, men, and had poorer oral health conditions except for the number of remaining teeth. The percentages of the participants who experienced the onset of dementia among participants with no, mild, and severe malodor were 6.8%, 5.2%, and 20.7%, respectively.
The incidence rates of dementia per 1000 person-years stratified by the characteristics of the participants after multiple imputation are presented in Table 2. On average, the incidence rate of dementia was 6.29 per 1000 person-years. The highest incidence rate was observed among those with severe malodor (22.4 per 1000 person-years), and the rates among those with mild and no malodor were 5.1 and 6.6, respectively.
Table 2
Incidence rates of dementia per 1000 person-years stratified by the characteristics of the participants after multiple imputation (n = 1,493)
| Characteristics | Total No. of subjects | Onset of Dementia | Incidence rate per 1000 person-years |
| Total | 1493 (100%) | 96 (100%) | 6.29 |
| Age | |||
| 55–59 | 292 (19.6%) | 4 (4.2%) | 1.28 |
| 60–64 | 367 (24.6%) | 5 (5.2%) | 1.29 |
| 65–69 | 364 (24.4%) | 25 (26.0%) | 6.62 |
| 70–75 | 470 (31.4%) | 62 (64.6%) | 13.76 |
| Sex | |||
| Men | 693 (46.4%) | 37 (38.5%) | 5.33 |
| Women | 800 (53.6%) | 59 (61.5%) | 7.08 |
| Frequency of tooth brushing | |||
| ≥3 times a day | 442 (29.6%) | 32 (33.3%) | 6.95 |
| 2 times a day | 669 (44.8%) | 43 (44.8%) | 6.24 |
| ≤1 time a day | 382 (25.6%) | 21 (21.9%) | 5.56 |
| Gingival redness | |||
| No | 682 (45.7%) | 44 (45.8%) | 6.29 |
| Yes | 811 (54.3%) | 52 (54.2%) | 6.28 |
| Number of remaining teeth | |||
| ≥28 | 227 (15.2%) | 10 (10.4%) | 4.29 |
| 20–27 | 672 (45.0%) | 29 (30.2%) | 4.16 |
| 10–19 | 321 (21.5%) | 28 (29.2%) | 8.62 |
| 0–9 | 273 (18.3%) | 29 (30.2%) | 10.63 |
| BMI | |||
| <25 | 1161 (77.8%) | 76 (79.2%) | 6.36 |
| ≥25 | 332 (22.2%) | 20 (20.8%) | 6.02 |
| Comorbidities† | |||
| No | 1242 (83.2%) | 68 (70.8%) | 5.33 |
| Yes | 251 (16.8%) | 28 (29.2%) | 11.36 |
| Smoking status | |||
| Never | 1025 (68.7%) | 74 (77.1%) | 6.98 |
| Past | 285 (19.1%) | 15 (15.6%) | 5.32 |
| Current | 183 (12.2%) | 7 (7.3%) | 3.77 |
| Frequency of alcohol consumption | |||
| Rarely | 643 (43.1%) | 56 (58.3%) | 8.38 |
| A few times a week | 429 (28.7%) | 20 (20.8%) | 4.52 |
| Daily | 421 (28.2%) | 20 (20.9%) | 4.83 |
| Highest educational level | |||
| Above high school | 254 (17.0%) | 17 (17.7%) | 6.68 |
| High school | 746 (50.0%) | 42 (43.8%) | 5.38 |
| Junior high school | 493 (33.0%) | 37 (38.5%) | 7.46 |
| Oral malodor | |||
| None | 886 (59.3%) | 60 (62.5%) | 6.59 |
| Mild | 578 (38.8%) | 30 (31.2%) | 5.08 |
| Severe | 29 (1.9%) | 6 (6.3%) | 22.41 |
†Comorbidities variable indicated the presence of a history of diabetes, stroke, hypertension, or myocardial infarction.
The HRs of oral malodor on dementia using Cox models after multiple imputation are shown in Table 3. After adjusting for all confounders, compared to those with no malodor, those with severe malodor had a 3.8 (95% CI: 1.5 to 9.4) times significantly higher hazard for developing dementia.
Table 3
The hazard ratios of oral malodor on dementia using the univariable, multivariable-adjusted, and inverse probability weighted Cox proportional hazards models after multiple imputation (n = 1,493)
| Univariable model | Multivariable model† | Inverse probability weighted‡ | ||||
| Hazard | (95% Confidence | Hazard | (95% Confidence | Hazard | (95% Confidence | |
| Ratio | Interval) | Ratio | Interval) | Ratio | Interval) | |
| None | 1 | Reference | 1 | Reference | 1 | Reference |
| Mild | 0.77 | 0.5; 1.19 | 0.78 | 0.5; 1.23 | 0.78 | 0.50; 1.24 |
| Severe | 3.53 | 1.53; 8.18 | 3.80 | 1.54; 9.37 | 4.44 | 1.20; 16.44 |
†Multivariable Cox proportional hazards model and ‡the inverse probability weighted Cox proportional hazards model were estimated using all previously listed potential confounders: sex, age, number of remaining teeth, frequency of tooth brushing, gingival redness, BMI, comorbidities, smoking status, frequency of alcohol consumption, and highest educational level.
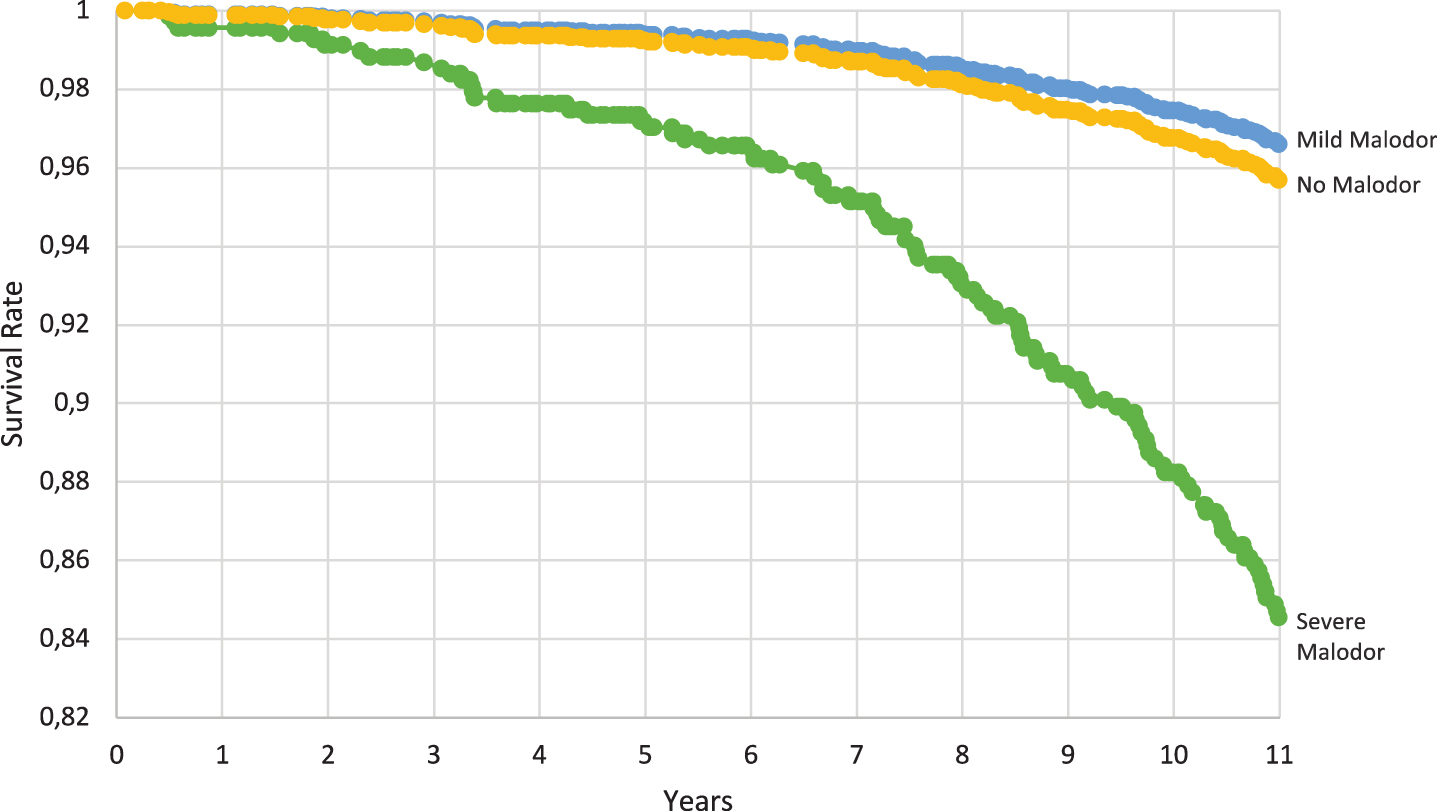
Figure 2 shows the adjusted survival curves for the probability of not developing dementia during 11 years of follow-up by oral malodor condition. Participants with severe malodor showed lower survival rates.
Fig. 2
Adjusted survival curves showing the probability of not developing dementia by oral malodor condition during 11 years of follow-up, estimated by the multivariable-adjusted Cox model fitted after multiple imputation (n = 1,493).
The marginal HRs of oral malodor on dementia obtained from IPW Cox model after multiple imputation are shown in Table 3. Compared to no malodor, the marginal HR for developing dementia was estimated to be 4.4 (95% CI: 1.2 to 16.4) in severe malodor.
Additionally, the E-values for the point estimate of the HR obtained from the multivariable Cox model and the HR obtained from the IPW Cox model were 7.1 (95% CI: 4.6 to 9.5) and 8.4 (95% CI: 6.7 to 10.0), respectively. These values suggested that the observed HR in the multivariable or IPW Cox model (HR is around 4) could be explained by an uncontrolled confounder that was 7–8 times more prevalent among severe oral malodor and associated with the onset of dementia by the relative risk of 7–8. However, less powerful confounders could not explain that.
Complete case analysis in SupplementaryTable 2 showed results similar to those described in Table 3.
DISCUSSION
To the best of our knowledge, this is the first study to evaluate the association between oral malodor and dementia. Within 11 years of the study, after adjusting for all confounders, those with severe malodor had a 3.8 (95% CI: 1.5 to 9.4) times substantially higher hazard of developing dementia than those with no malodor. The same trends were confirmed when we examined the association using a different analysis. In the confounder-adjusted IPW Cox Model, when all participants had severe malodor, these people might have 4.4 (95% CI: 1.2 to 16.4) times significantly higher hazard for developing dementia compared to when all the participants had no malodor. From the E-value, this association was considered to be robust.
Previous studies have also reported that people with poor oral health conditions may be at a higher risk of dementia. Another systematic review and meta-analysis of 21 observational studies concluded that tooth loss was a risk factor for dementia [51]. The findings from a recent meta-analysis showed that periodontitis was linked to cognitive impairment, and subjects with severe periodontitis had higher chances of developing dementia [52]. Our study provided additional knowledge about oral malodor and dementia. Even after considering the number of remaining teeth and gingival health, oral malodor was significantly and independently associated with the onset of dementia.
Concerning possible mechanisms, the association between oral malodor and dementia might be explained by the mediation of social isolation. Social isolation is a conceivable risk factor for dementia [10]. A meta-analysis of 19 longitudinal cohort studies demonstrated that people with less social participation had a higher risk of developing dementia [53]. Oral malodor affects social engagement. Self-perceived oral malodor is a potential obstacle to social interaction [54]. There is a correlation between self-perceived bad breath and objective malodor detection by organoleptic assessment [55]. Besides, people are less likely to communicate with individuals who have severe malodor. Bad breath is regarded as one of the least attractive features of social connections and may possess negative impacts on relations and psychological communications [56]. Patients with halitosis experienced a moderate level of social distancing [57]. Moreover, young people experiencing oral malodor reported worse depression, lower self-esteem, and isolation from society [58]. Hence, the association between oral malodor and dementia can be explained by the mediation of social isolation.
Aside from social isolation, arguably alternative pathways could be derived from periodontal disease or periodontal bacteria. A previous systematic review and meta-analysis reported an association between periodontitis and oral malodor [59]. Nonetheless, as this finding was drawn from cross-sectional studies, the temporality of the events was not clearly identified. Since periodontitis was recognized as a risk factor for dementia [60, 61], there is a possibility that oral malodor might eventually be associated with the onset of dementia. Besides, the presence of the periodontopathic bacterium Porphyromonas gingivalis could also be a potential threat. This type of pathogen could be detected on the upper surface of the tongue [62], contributing to the production of the offensive odor. The quantity of these bacteria found on the dorsal surface of the tongue in patients with periodontitis was substantially correlated with oral malodor [63]. Moreover, prior research has demonstrated the attention to the connection between Porphyromonas gingivalis and AD [64, 65]. This bacterium, along with its toxins, could cause persistent inflammation in the brain and destruction of neurons, thus worsening the pathology of AD [64]. Further studies are needed to verify these potential directions.
This study possesses several strengths. First, this is a longitudinal cohort study with a relatively long follow-up period of 11 years. Therefore, the studied period is sufficiently long to determine the temporal relationship between oral malodor and dementia. Second, our study focused on oral malodor as a new factor in relation to oral health and dementia. Oral malodor is associated with communication and social interactions. Social isolation is considered a potential risk factor for dementia [10]. The present result suggested the potential of oral health interventions as a means of mitigating the increased risk of dementia due to social isolation. Third, the oral malodor parameter in this study was measured in oral examinations conducted by dentists. Thus, it has better validity than self-reported indices.
Nevertheless, there are some limitations in this study. First, objective instruments such as Halimeter or OralChroma [66] were not used to determine oral malodor. However, organoleptic assessment by a qualified expert is regarded as the gold standard for identifying oral malodor [67]. The reliability of other detection methods is compared with this organoleptic assessment to confirm the disease. [67]. This method was proven to be more reliable than bad breath testing instruments [68]. For the quality assurance procedure, the dentists received the guidebooks and verbal explanations. However, clinical calibration was not conducted. This could cause measurement errors of oral malodor. If dentists tended to underestimate oral malodor status in people who were less likely to have dementia or overestimate oral malodor status in people who were more likely to have dementia, this measurement error might lead to an overestimation of results. However, there was no evidence that this had happened. If the direction of the measurement error were random, it would be expected to widen the confidence intervals of the estimates. Hence, the observed association in this study is considered to be robust. Second, we could not record the number of participants examined by each dentist. Third, some confounders, such as BMI, frequency of alcohol consumption, and comorbidities, were collected before the baseline time point of our analysis. Therefore, there might be some possible changes in these variables at the baseline. Fourth, considering the length of the follow-up period, we were not able to eliminate the possibility of reversal of causality. Fifth, we could not account for confounders or exposure status changing over time. Seventh, the number of incident cases of dementia was relatively small due to the relatively younger target population. However, reasonable associations were observed in our sample. For example, concerning age and incidence rate of dementia, there was a stable, increasing trend of incidence with age (Table 1). Finally, the number of people with severe malodor was relatively small. Thus, chance variation (random error) is possible in the association between malodor and dementia. We found a robust association between malodor and dementia with a relatively large E-value. However, further studies with a large sample of those with severe malodor are required. In conclusion, there is a significant association between oral malodor and the onset of dementia.
AUTHOR CONTRIBUTIONS
Duc Sy Minh Ho (Conceptualization; Formal analysis; Methodology; Software; Validation; Visualization; Writing – original draft; Writing – review & editing); Takashi Zaitsu, (Formal analysis; Funding acquisition; Methodology; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing); Hikaru Ihira (Data curation; Formal analysis; Investigation; Resources; Validation; Writing – original draft; Writing – review & editing); Masanori Iwasaki (Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing); Akihiro Yoshihara (Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing); Seitaro Suzuki (Formal analysis; Methodology; Validation; Writing – original draft; Writing – review & editing); Manami Inoue (Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing); Kazumasa Yamagishi (Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing); Nobufumi Yasuda (Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing); Jun Aida (Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing); Tomohiro Shinozaki (Formal analysis; Methodology; Software; Validation; Writing – original draft; Writing – review & editing); Atsushi Goto (Formal analysis; Methodology; Software; Validation; Writing – original draft; Writing – review & editing); Shoichiro Tsugane (Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing); Norie Sawada (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Writing – original draft; Writing – review & editing).
ACKNOWLEDGMENTS
The authors thank all staff members of the JPHC Study in each study area and central office for their extensive efforts in conducting the baseline survey and oral health study. Special thanks are given to Dr. Yoko Kawaguchi, who was affiliated with the Department of Oral Health Promotion, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan, for her research supervision at the time the oral health survey was conducted. The authors are also deeply grateful to Yokote City and Hiraka dental associations for their generous support.
FUNDING STATEMENT
This work was supported by Grants-in-Aid from the Ministry of Health, Labor and Welfare of Japan (Grant Number 19FA1018, 21FA1013, 23FA1022, 22FA1010, and 22FA0601), JSPS KAKENHI (Grant Number 23H03117, 19H03860, 22H03299, 21K10250, 21K19635, and 19H03860), National Cancer Center Research and Development Fund (23-A-31 (toku), 26-A-2, 29-A-4, 2020-J-4) (since 2011) and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
For information on how to submit an application to gain access to JPHC data, please follow the instructions at this website: https://epi.ncc.go.jp/en/jphc/805/8155.html.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-240015.
REFERENCES
[1] | Newgard CB , Sharpless NE ((2013) ) Coming of age: Molecular drivers of aging and therapeutic opportunities. J Clin Invest 123: , 946–950. |
[2] | Nichols E , Steinmetz JD , Vollset SE , Fukutaki K , Chalek J , Abd-Allah F , Abdoli A , Abualhasan A , Abu-Gharbieh E , Akram TT ((2022) ) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7: , e105–e125. |
[3] | Cantarero-Prieto D , Leon PL , Blazquez-Fernandez C , Juan PS , Cobo CS ((2020) ) The economic cost of dementia: A systematic review. Dementia (London) 19: , 2637–2657. |
[4] | Alzheimer’s Association ((2021) ) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17: , 327–406. |
[5] | Nakahori N , Sekine M , Yamada M , Tatsuse T , Kido H , Suzuki M ((2021) ) Future projections of the prevalence of dementia in Japan: Results from the Toyama Dementia Survey. BMC Geriatr 21: , 602. |
[6] | Cerutti-Kopplin D , Feine J , Padilha D , De Souza R , Ahmadi M , Rompré P , Booij L , Emami E ((2016) ) Tooth loss increases the risk of diminished cognitive function: A systematic review and meta-analysis. JDR Clin Transl Res 1: , 10–19. |
[7] | Oh B , Han DH , Han KT , Liu X , Ukken J , Chang C , Dounis K , Yoo JW ((2018) ) Association between residual teeth number in later life and incidence of dementia: A systematic review and meta-analysis. BMC Geriatr 18: , 48. |
[8] | Borsa L , Dubois M , Sacco G , Lupi L ((2021) ) Analysis the link between periodontal diseases and Alzheimer’s disease: A systematic review. Int J Environ Res Public Health 18: , 9312. |
[9] | Wang X , Hu J , Jiang Q ((2022) ) Tooth loss-associated mechanisms that negatively affect cognitive function: A systematic review of animal experiments based on occlusal support loss and cognitive changes. Front Neurosci 16: , 811335. |
[10] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[11] | Koyama S , Aida J , Kondo K , Yamamoto T , Saito M , Ohtsuka R , Nakade M , Osaka K ((2016) ) Does poor dental health predict becoming homebound among older Japanese? BMC Oral Health 16: , 51. |
[12] | Abbas H , Aida J , Kiuchi S , Kondo K , Osaka K ((2023) ) Oral status and homebound status: A 6-year bidirectional exploratory prospective cohort study. Oral Dis 29: , 1291–1298. |
[13] | Kiuchi S , Cooray U , Kusama T , Yamamoto T , Abbas H , Nakazawa N , Kondo K , Osaka K , Aida J ((2022) ) Oral status and dementia onset: Mediation of nutritional and social factors. J Dent Res 101: , 420–427. |
[14] | de Jongh A , van Wijk AJ , Horstman M , de Baat C ((2016) ) Self-perceived halitosis influences social interactions. BMC Oral Health 16: , 31. |
[15] | Rayman S , Almas K ((2008) ) Halitosis among racially diverse populations: An update. Int J Dent Hyg 6: , 2–7. |
[16] | Donaldson A , McKenzie D , Riggio M , Hodge P , Rolph H , Flanagan A , Bagg J ((2005) ) Microbiological culture analysis of the tongue anaerobic microflora in subjects with and without halitosis. Oral Dis 11: , 61–63. |
[17] | Loesche WJ , Kazor C ((2002) ) Microbiology and treatment of halitosis. Periodontol 2000 28: , 256–279. |
[18] | Aylıkcı BU , Çolak H ((2013) ) Halitosis: From diagnosis to management. J Nat Sci Biol Med 4: , 14. |
[19] | Lopes RG , de Godoy CHL , Deana AM , de Santi MESO , Prates RA , França CM , Fernandes KPS , Mesquita-Ferrari RA , Bussadori SK ((2014) ) Photodynamic therapy as a novel treatment for halitosis in adolescents: Study protocol for a randomized controlled trial. Trials 15: , 443. |
[20] | Vandekerckhove B , Bollen C (2009) Epidemiology in the general population, specific populations and in a multidisciplinary halitosis consultation. In Ademgeur, van Steenberghe D, ed. Prelum Uitgevers, Houten, The Netherlands, pp. 3-10. |
[21] | Tonzetich J ((1977) ) Production and origin of oral malodor: A review of mechanisms and methods of analysis. J Periodontol 48: , 13–20. |
[22] | Mogilnicka I , Bogucki P , Ufnal M ((2020) ) Microbiota and malodor—etiology and management. Int J Mol Sci 21: , 2886. |
[23] | Tsugane S , Sawada N ((2014) ) The JPHC study: Design and some findings on the typical Japanese diet. Jpn J Clin Oncol 44: , 777–782. |
[24] | Murai U , Sawada N , Charvat H , Inoue M , Yasuda N , Yamagishi K , Tsugane S , Group JS ((2022) ) Soy product intake and risk of incident disabling dementia: The JPHC Disabling Dementia Study. Eur J Nutr 61: , 4045–4057. |
[25] | Miyaguni Y , Tabuchi T , Aida J , Saito M , Tsuji T , Sasaki Y , Kondo K ((2021) ) Community social support and onset of dementia in older Japanese individuals: A multilevel analysis using the JAGES cohort data. BMJ Open 11: , e044631. |
[26] | Hisano S ((2009) ) The relationship between revised Hasegawa dementia scale (HDS-R), mini-mental state examination (MMSE) and bed-fast scale, dementia scale. Jpn J Geriatr Psychiatry 20: , 883–891. |
[27] | Ikeda A , Yamagishi K , Tanigawa T , Cui R , Yao M , Noda H , Umesawa M , Chei C , Yokota K , Shiina Y ((2008) ) Cigarette smoking and risk of disabling dementia in a Japanese rural community: A nested case-control study. Cerebrovasc Dis 25: , 324–331. |
[28] | Yamagishi K , Ikeda A , Chei C-L , Noda H , Umesawa M , Cui R , Muraki I , Ohira T , Imano H , Sankai T ((2017) ) Serum α-linolenic and other ω-3 fatty acids, and risk of disabling dementia: Community-based nested case–control study. Clin Nutr 36: , 793–797. |
[29] | Ihira H , Sawada N , Inoue M , Yasuda N , Yamagishi K , Charvat H , Iwasaki M , Tsugane S ((2022) ) Association Between physical activity and risk of disabling dementia in Japan. JAMA Netw Open 5: , e224590–e224590. |
[30] | Yamada K , Kubota Y , Tabuchi T , Shirai K , Iso H , Kondo N , Kondo K ((2019) ) A prospective study of knee pain, low back pain, and risk of dementia: The JAGES project. Sci Rep 9: , 10690. |
[31] | Ueno M , Ohara S , Inoue M , Tsugane S , Kawaguchi Y ((2013) ) Association between parity and dentition status among Japanese women: Japan public health center-based oral health study. BMC Public Health 13: , 993. |
[32] | WHO(1997) World Health Organization Oral Health Surveys-Basic Methods, World Health Organization, Geneva. |
[33] | Murata T , Yamaga T , Iida T , Miyazaki H , Yaegaki K ((2002) ) Classification and examination of halitosis. Int Dent J 52: , 181–186. |
[34] | Yaegaki K , Coil J ((2000) ) Genuine halitosis, pseudo-halitosis, and halitophobia: Classification, diagnosis, and treatment. Compend Contin Educ Dent 21: , 880-886–888. |
[35] | Chaudhary S , Singh A , Jaiswal R , Samadi FM , Nisha S ((2011) ) Halitosis: A review. Public Health Res Dev 2: , 17. |
[36] | Ueno M , Ohara S , Sawada N , Inoue M , Tsugane S , Kawaguchi Y ((2015) ) The association of active and secondhand smoking with oral health in adults: Japan public health center-based study. Tob Induc Dis 13: , 19. |
[37] | Higbee JD , Lefler JS , Burnett RT , Ezzati M , Marshall JD , Kim S-Y , Bechle M , Robinson AL , Pope CA III ((2020) ) Estimating long-term pollution exposure effects through inverse probability weighting methods with Cox proportional hazards models. Environ Epidemiol 4: , e085. |
[38] | Buchanan AL , Hudgens MG , Cole SR , Lau B , Adimora AA , Study WsIH ((2014) ) Worth the weight: Using inverse probability weighted Cox models in AIDS research. AIDS Res Hum Retroviruses 30: , 1170–1177 . |
[39] | Stürmer T , Rothman KJ , Glynn RJ ((2006) ) Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf 15: , 698–709. |
[40] | Kaufman JS ((2010) ) Marginalia: Comparing adjusted effect measures. Epidemiology 21: , 490–493. |
[41] | Greifer N ,Propensity Score Weighting Using Generalized Linear Models, https://ngreifer.github.io/WeightIt/reference/method_ps.html, Accessed May 17, 2023. |
[42] | McCaffrey DF , Griffin BA , Almirall D , Slaughter ME , Ramchand R , Burgette LF ((2013) ) A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 32: , 3388–3414. |
[43] | Naimi AI , Whitcomb BW ((2023) ) Defining and identifying average treatment effects. Am J Epidemiol 192: , 685–687. |
[44] | Haruka N , IPW and Cox Regression, https://dichika.hateblo.jp/entry/2021/06/30/155332, Accessed March 18, 2023. |
[45] | Lin DY , Wei L-J ((1989) ) The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84: , 1074–1078. |
[46] | Sterne JA , White IR , Carlin JB , Spratt M , Royston P , Kenward MG , Wood AM , Carpenter JR ((2009) ) Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338: , b2393. |
[47] | Woods AD , Gerasimova D , Van Dusen B , Nissen J , Bainter S , Uzdavines A , Davis-Kean PE , Halvorson M , King KM , Logan JA ((2021) ) Best practices for addressing missing data through multiple imputation. Infant Child Dev 33: , e2407. |
[48] | Rubin DB (2004) Multiple imputation for nonresponse in surveys, John Wiley & Sons. |
[49] | Haneuse S , VanderWeele TJ , Arterburn D ((2019) ) Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321: , 602–603. |
[50] | VanderWeele TJ , Ding P ((2017) ) Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med 167: , 268–274. |
[51] | Fang WL , Jiang MJ , Gu BB , Wei YM , Fan SN , Liao W , Zheng YQ , Liao SW , Xiong Y , Li Y , Xiao SH , Liu J ((2018) ) Tooth loss as a risk factor for dementia: Systematic review and meta-analysis of 21 observational studies. BMC Psychiatry 18: , 345. |
[52] | Guo H , Chang S , Pi X , Hua F , Jiang H , Liu C , Du M ((2021) ) The effect of periodontitis on dementia and cognitive impairment: A meta-analysis. Int J Environ Res Public Health 18: , 6823. |
[53] | Kuiper JS , Zuidersma M , Voshaar RCO , Zuidema SU , van den Heuvel ER , Stolk RP , Smidt N ((2015) ) Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22: , 39–57. |
[54] | De Jongh A , De Baat C , Horstman M , Van Wijk A ((2013) ) Self-perceived oral odour and social interaction. Ned Tijdschr Tandheelkd 120: , 194–198. |
[55] | Romano F , Pigella E , Guzzi N , Aimetti M ((2010) ) Patients’ self-assessment of oral malodour and its relationship with organoleptic scores and oral conditions. Int J Dent Hyg 8: , 41–46. |
[56] | De Jongh A , Van Wijk A , Horstman M , De Baat C ((2014) ) Attitudes towards individuals with halitosis: An online cross sectional survey of the Dutch general population. Br Dent J 216: , E8–E8. |
[57] | Azodo CC , Ogbebor OG ((2019) ) Social distance towards halitosis sufferers. Swiss Dent J 129: , 1026–1030. |
[58] | Briceag R , Caraiane A , Raftu G , Horhat RM , Bogdan I , Fericean RM , Shaaban L , Popa M , Bumbu BA , Bratu ML ((2023) ) Emotional and social impact of halitosis on adolescents and young adults: A systematic review. Medicina 59: , 564. |
[59] | Silva MF , Cademartori MG , Leite FR , Lopez R , Demarco FF , Nascimento GG ((2017) ) Is periodontitis associated with halitosis? A systematic review and meta-regression analysis. J Clin Periodontol 44: , 1003–1009. |
[60] | Gil-Montoya JA , Sanchez-Lara I , Carnero-Pardo C , Fornieles F , Montes J , Vilchez R , Burgos JS , Gonzalez-Moles MA , Barrios R , Bravo M ((2015) ) Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J Periodontol 86: , 244–253. |
[61] | Lee YT , Lee HC , Hu CJ , Huang LK , Chao SP , Lin CP , Su ECY , Lee YC , Chen CC ((2017) ) Periodontitis as a modifiable risk factor for dementia: A nationwide population-based cohort study. J Am Geriatr Soc 65: , 301–305. |
[62] | Faveri M , Feres M , Shibli JA , Hayacibara RF , Hayacibara MM , de Figueiredo LC ((2006) ) Microbiota of the dorsum of the tongue after plaque accumulation: An experimental study in humans. J Periodontol 77: , 1539–1546. |
[63] | Apatzidou A , Bakirtzoglou E , Vouros I , Karagiannis V , Papa A , Konstantinidis A ((2013) ) Association between oral malodour and periodontal disease-related parameters in the general population. Acta Odontol Scand 71: , 189–195. |
[64] | Matsushita K , Yamada-Furukawa M , Kurosawa M , Shikama Y ((2020) ) Periodontal disease and periodontal disease-related bacteria involved in the pathogenesis of Alzheimer’s disease. J Inflamm Res 13: , 275–283. |
[65] | Singhrao SK , Harding A , Poole S , Kesavalu L , Crean S ((2015) ) Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators inflamm 2015: , 137357. |
[66] | Tangerman A , Winkel E ((2008) ) The portable gas chromatograph OralChroma™: A method of choice to detect oral and extra-oral halitosis. J Breath Res 2: , 017010. |
[67] | Peres MA , Antunes JLF , Watt RG (2021) Oral epidemiology: A textbook on oral health conditions, research topics and methods, Springer. |
[68] | Dayma A , Jain M , Saxena V , Torwane N , Vishnu V , Khare A ((2020) ) Validation of organoleptics and instrumental measurement for halitosis among patient with malodour. J Dent Health Oral Disord Ther 11: , 6–10. |




