Association of Cerebral Hypoperfusion and Poor Collaterals with Cognitive Impairment in Patients with Severe Vertebrobasilar Artery Stenosis
Abstract
Background:
Effect of stenosis of vertebrobasilar artery (VBA) on cognitive function is elusive.
Objective:
To investigate association of cerebral hypoperfusion and poor collaterals with vascular cognitive impairment (VCI) in severe VBA stenosis patients.
Methods:
We consecutively enrolled patients with severe VBA stenosis confirmed by digital subtraction angiography who underwent computed tomographic perfusion (CTP) and cognitive assessments. Patients were divided into poor or good collaterals groups according to the collateral circulation status, and were grouped into different perfusion groups according to CTP. Cognitive function was measured by Montreal Cognitive Assessment (MoCA), Clock Drawing Test, Stroop Color Word Test, Trail Making Test, Digital Span Test, Auditory Verbal Learning Test, and Boston Naming Test scales. The association of cerebral perfusion and collaterals with VCI were explored.
Results:
Among 88 eligible patients, VCI occurred in 51 (57.9%) patients experienced. Poor collateral was present in 73 (83.0%) patients, and hypoperfusion in 64 (72.7%). Compared with normal perfusion patients, the odds ratio with 95% confidence interval for VCI was 12.5 (3.7–42.4) for overall hypoperfusion, 31.0 (7.1–135.5) for multiple site hypoperfusion, 3.3 (1.0–10.5) for poor collaterals, and 0.1 (0–0.6) for presence of posterior communicating artery (PcoA) compensated for posterior cerebral artery (PCA) and basilar artery (BA). Additionally, decreased scores of cognitive function tests occurred in patients with decompensated perfusion or poor collaterals.
Conclusions:
Hypoperfusion and poor collaterals were positively associated with cognitive impairment in patients with severe VBA. However, PcoA compensated for the PCA and BA had a protective role in cognitive impairment development.
INTRODUCTION
As the population ages, cognitive impairment is becoming more common and has become one of the main causes of disability and death in the elderly [1]. Alzheimer’s disease is the major cause of cognitive impairment. It is characterized by amyloid plaque deposits from amyloid-β protein aggregation and neurofibrillary tangles from tau protein hyperphosphorylation [2]. Vascular cognitive impairment (VCI), as the second most common type of cognitive impairment after Alzheimer’s disease, is defined as a syndrome ranging from mild cognitive impairment to dementia brought on by dominant and recessive cerebrovascular diseases [3]. It has turned into a hotspot for research because of the characteristics of intervention and its high prevalence. Cerebrovascular stenosis is established a risk factor for VCI [4]. the most studies showed that anterior cerebral artery stenosis was associated with VCI, which may result in a deterioration in patients’ executive function, short-term memory, verbal fluency, spatial structure, and attention [5, 6]. The plausible mechanism is that decreased cerebral blood perfusion leads to neuronal dysfunction [7]. A study with very small sample size demonstrated that approximate half of elderly patients with vertebrobasilar artery (VBA) stenosis might have VCI [8]. However, there was little data on elucidating effect of stenosis of posterior circulation or VBA on cognitive function in patients with severe VBA stenosis. We hypothesized that severe VBA stenosis had a detrimental role in cognitive impairment development in transient ischemic attack (TIA) or stroke patients. Therefore, the aim of the current study was to evaluate impact of VCI in patients with severe VBA stenosis on cognitive function in TIA or stroke patients using neuropsychological assessments, cerebral perfusion, and collateral circulation.
METHODS
Study population
This was a retrospective study. From July 2022 to May 2023, we consecutively recruited a total of 170 patients with severe VBA stenosis confirmed by digital subtraction angiography (DSA) who underwent computed tomographic perfusion (CTP) and cognitive assessments. The study inclusion criteria were as follows: 1) Age ≥18 years; 2) Symptomatic TIA or ischemic stroke due to 70%–99% arterial stenosis in the VBA confirmed by DSA; 3) With no or ≤50% stenosis in anterior circulation artery. Patients with following conditions were excluded: 1) Prior cerebral infarction, Alzheimer’s disease, epilepsy, severe leukoaraiosis (Fazekas grade≥2), trauma, tumors, infections, metabolic disorders, hydrocephalus and hypothyroidism, which may affect cognitive function; 2) mental illnesses including depression, anxiety or schizophrenia; 3) History of endovascular interventional therapy; 4) History of alcohol or drug abuse; 5) A specific condition that affects cognition; 6) Refusal to cooperate with the examination. Baseline clinical characteristics, imaging data and cognitions scales were collected.
The present study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University. All the enrolled patients or their legal representatives provided written informed consent.
Demographic data
Demographic information collected included: 1) Gender, age, and years of education; 2) History of TIA or ischemic stroke, National Institutes of Health Stroke Scale (NHISS), modified Rankin Scale (mRS); 3) Vascular risk factors included hypertension, diabetes, hyperlipidemia, coronary heart disease and smoking; 4) Evaluation of anxious or depressive situations using the Hamilton Rating Scale for Depression (HAMD) and Hamilton Rating Scale for Anxiety (HAMA).
Computed tomographic perfusion (CTP)
CTP was performed using a SIEMENS 128-slice Dual Source CT Scanner. A double-tube high-pressure syringe was used to inject iopromide (370 mgI/ml) intravenously at a speed of 8 ml/s. After a 4-s delay, volume shuttle scanning was performed with a scanning range of approximately 0–110 mm, layer thickness of 12 mm, layer spacing of 0.75 mm, tube current of 200 mA, and tube voltage of 80 kV. We scanned each layer forty times. After scanning was completed, we sent the volume data to a workstation for post-processing (Neuschsoft Medical Company, Shenyang, China), where it was processed to produce perfusion images of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time-to-peak (TTP).
Digital subtraction angiography (DSA)
DSA was performed within 1 week to 1 month after TIA or stroke under local anesthesia and multi-parameter monitoring. The modified Seldinger technique was used to puncture the femoral artery, and a 5F arterial sheath was inserted. Subsequently, a 5F single curved catheter guided by a Loach guide wire was used for cerebral arteriography. Additionally, we recorded the collateral compensation pathways, including: 1) Posterior communicating artery (PcoA) compensated the posterior cerebral artery (PCA); 2) PcoA compensated both PCA and basilar artery (BA); 3) Anastomotic branches between cerebellar arteries and their branches, including the anastomotic branches formed among posterior inferior cerebellar artery, superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), and the anastomotic branches formed between AICA and BA; 4) Other compensatory pathways contained anastomotic branches formed between the anterior choroidal artery and posterior choroid artery, anastomotic branches among anterior spinal artery, vertebral artery, and BA.
DSA is the gold standard for assessing collateral circulation. According to the ASITN/SIR scale [9], which is established by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology, the degree of collateral circulation is evaluated as follows: Grade 0: No collateral blood flow to the ischemic area; Grade 1: Only slow collateral flow to the ischemic peripheral area with persistent perfusion defects; Grade 2: Rapid collateral blood flow to the ischemic peripheral area with persistent perfusion defects; Grade 3: Slow but complete blood flow to the ischemic area can be seen in the late venous period; Grade 4: Blood flow can be perfused to the whole ischemic area quickly and completely through the retrograde reperfusion. Grades 0–2 are defined as poor collaterals, while Grades 3–4 are defined as good collaterals. Two qualified neurointerventionists evaluated the radiographs and the degree of collateral circulation.
Cognitive function assessment
The patient cognitive function was evaluated within 1 week to 1 month after TIA or stroke using the following tests: Montreal Cognitive Assessment (MoCA), Clock Drawing Test (CDT), Stroop Color Word Test (SCWT), Trail Making Test (TMT), Digital Span Test (DST), Auditory Verbal Learning Test (AVLT), and Boston Naming Test (BNT). Cognitive impairment was defined as MoCA score <26 points (the cognitive assessment was adjusted for the educational attainment, one additional point was given to those patients with less than 12 years of education) [10]. For cognitive domain assessment, CDT was used for evaluating visuospatial and executive function, SCWT and TMT-B mainly for executive function, DST and TMT-A mainly for attention, AVLT for memory, BNT for naming ability[11, 12]. The cognitive assessment for all patients was performed by the same well-trained neurologist blind to the neuroradiological status in a quiet room. All patients underwent a separate, calm cognitive evaluation conducted by the same skilled neurologist.
Statistical analysis
Normally distributed continuous variables were expressed as mean±standard deviation (SD) and analyzed using the t-test or one-way analysis of variance for difference in cognitive function between groups. Data that did not conform to the normal distribution were represented by the median and interquartile range (IQR) and analyzed using the Mann-Whitney U test. Categorical variables are shown as frequencies and percentages and analyzed using the chi-square test or Fisher’s exact test. Multivariate logistic regression was performed to Logistic regression analysis was used to analyze the association of cerebral hypoperfusion and poor collaterals with cognitive impairment using the odds ratio (OR) and 95% confidence interval (CI) adjusting risk factors including age, sex, smoking, hypertension, diabetes mellitus, hyperlipidemia, and coronary artery disease. SPSS 20.0 software was utilized for statistical analysis. All statistical analyses were two-sided and the level of significance was α = 0.05.
RESULTS
Baseline characteristics
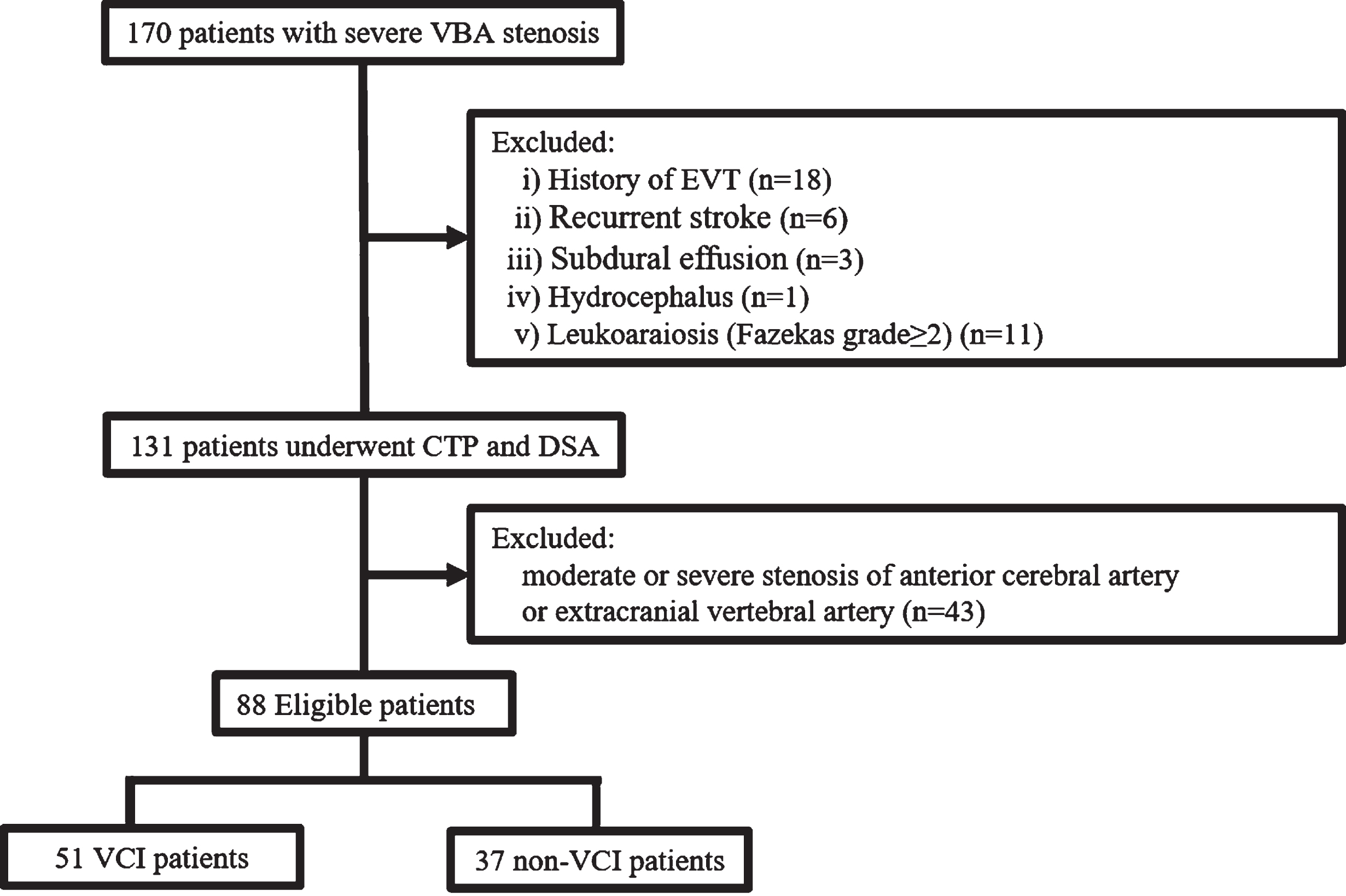
Of the 170 patients, eligible 88 were finally analyzed after excluding 18 due to a history of endovascular interventional therapy, 6 due to recurrent stroke, 3 due to subdural effusion, 1 due to hydrocephalus, 11 due to leukoaraiosis (Fazekas grade≥2), 43 patients due to comorbid moderate to severe stenosis in anterior cerebral artery or extracranial vertebral artery (Fig. 1).
Fig. 1
Flow chart for patient selection. VBA, vertebrobasilar artery; CTP, computed tomographic perfusion; DSA, digital subtraction angiography; EVT, endovascular interventional therapy.
The average age of patients was 61.7±9.5 years old, with 73 (83.0%) being men. There were 73 (83.0%) patients with poor collaterals, 64 (72.7%) patients with hypoperfusion, and 51 (57.9%) patients with abnormal MoCA scores.
Cognitive impairment risk factors in severe VBA stenosis patients
Based on MoCA scores, patients were divided into two groups: the VCI group with MoCA < 26 (51 patients) and the non-VCI group with MoCA≥26 (37 patients). There were no significant differences between the two groups in age, sex, years of education, TIA or stroke, NHISS or mRS scores, vascular risk factors, HAMA or HAMD scores and whether patients underwent thrombolysis (Table 1).
Table 1
Baseline characteristics of patients with vascular cognitive impairment or not
| Overall (n = 88) | VCI (n = 51) | Non-VCI (n = 37) | p | |
| Age, mean±SD | 61.7±9.5 | 61.8±9.6 | 61.6±9.3 | 0.894 |
| Male, n (%) | 73 (83.0) | 42 (82.4) | 31 (83.8) | 0.692 |
| Education, mean±SD | 9.4±3.1 | 9.0±3.2 | 9.9±2.9 | 0.218 |
| NHISS, median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.959 |
| mRS, mean±SD | 0.7±0.9 | 0.7±0.9 | 0.7±0.9 | 0.925 |
| HAMA, mean±SD | 1.3±2.0 | 1.6±2.1 | 0.9±1.7 | 0.076 |
| HAMD, mean±SD | 1.4±1.9 | 1.7±2.1 | 1.1±1.7 | 0.147 |
| Stroke risk factors, n (%) | ||||
| Smoking | 47 (53.4) | 26 (51.0) | 21 (56.8) | 0.667 |
| Hypertension | 69 (78.4) | 38 (74.5) | 31 (83.8) | 0.432 |
| Diabetes | 33 (37.5) | 17 (33.3) | 16 (43.2) | 0.379 |
| Hyperlipidemia | 18 (20.5) | 11 (21.6) | 7 (18.9) | 0.564 |
| Coronary artery disease | 13 (14.8) | 8 (15.7) | 5 (13.5) | >0.999 |
| Qualified events, n (%) | >0.999 | |||
| TIA | 50 (56.8) | 29 (56.9) | 21 (56.8) | |
| Stroke | 38 (43.2) | 22 (43.1) | 16 (43.2) | |
| Underwent thrombolysis | 8 (11%) | 2 (3.9) | 6 (16.2) | 0.065 |
| CTP, n (%) | ||||
| CT hypoperfusion | 64 (72.7) | 46 (90.2) | 18 (48.6) | <0.001 |
| Hypoperfusion site | <0.001 | |||
| No hypoperfusion | 25 (28.4) | 5 (9.8) | 20 (54.1) | |
| Temporoparietal-Occipital lobe | 14 (15.9) | 9 (17.6) | 5 (13.5) | |
| Cerebellum | 12 (13.6) | 7 (13.7) | 5 (13.5) | |
| Brain stem | 2 (2.2) | 0 | 2 (5.4) | |
| Multiple sites | 35 (39.8) | 30 (58.8) | 5 (13.5) | |
| Collateral circulation, n (%) | ||||
| Poor collaterals | 73 (83.0) | 45 (88.2) | 26 (70.3) | 0.040 |
| Collateral compensation pathways | ||||
| PcoA compensated PCA | 26 (29.5) | 13 (25.5) | 13 (35.1) | 0.353 |
| PcoA compensated PCA and BA | 7 (8.0) | 1 (2.0) | 6 (16.2) | 0.039 |
| Cerebellar arteries and branches | 38 (43.2) | 24 (47.1) | 14 (37.8) | 0.513 |
| Other pathways | 1 (1.1) | 1 (2.0) | 0 | >0.999 |
VCI, vascular cognitive impairment; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; HAMD, Hamilton Rating Scale for Depression; HAMA, Hamilton Rating Scale for Anxious; TIA, transient ischemic attack; CTP, computed tomographic perfusion.
In the univariate analysis, the following blood flow parameters were positively associated with cognitive impairment in patients with severe VBA stenosis: CT hypoperfusion (p < 0.001), temporoparietal-occipital lobe hypoperfusion (p = 0.008), cerebellum hypoperfusion (p = 0.025), multiple sites hypoperfusion (p < 0.001), and poor collaterals (p = 0.041). However, the presence of PcoA compensating PCA and BA (p = 0.040) was negatively associated with cognitive impairment in patients with severe VBA stenosis. In multivariable analysis, as compared with patients with normal cognition, ORs with 95% CI for VCI was 12.5 (3.7–42.4) for overall hypoperfusion, 7.9 (1.7–37.5) for temporoparietal-occipital lobe hypoperfusion, 6.0 (1.2–29.4) for cerebellum hypoperfusion, 31.0 (7.1–135.5) for multiple site hypoperfusion, 3.3 (1.0–10.5) for poor collaterals, and 0.1 (0–0.6) for presence of PcoA compensated for PCA and BA (Table 2).
Table 2
Association of cerebral perfusion and collateral circulation with vascular cognitive impairment in patients with VBA stenosis
| VCI (n = 51) | Crude | Adjusted† | |||
| OR (95% CI) | p | OR (95% CI) | p | ||
| CTP, n (%) | |||||
| CT hypoperfusion | 46 (90.2) | 9.7 (3.2–29.9) | <0.001 | 12.5 (3.7–42.4) | <0.001 |
| Hypoperfusion site | |||||
| No hypoperfusion | 5 (9.8) | Ref | Ref | ||
| Temporoparietal-Occipital lobe | 9 (17.6) | 7.2 (1.7–31.3) | 0.008 | 7.9 (1.7–37.5) | 0.009 |
| Cerebellum | 7 (13.7) | 5.6 (1.2–25.3) | 0.025 | 6.0 (1.2–29.4) | 0.026 |
| Brain stem | 0 | NA | 0.999 | NA | 0.999 |
| Multiple sites | 30 (58.8) | 24.0 (6.1–93.8) | <0.001 | 31.0 (7.1–135.5) | <0.001 |
| Collateral circulation, n (%) | |||||
| Poor collaterals | 45 (88.2) | 3.2 (1.1–9.6) | 0.041 | 3.3 (1.0–10.5) | 0.046 |
| PcoA compensated PCA and BA | 1 (2.0) | 0.1 (0–0.9) | 0.040 | 0.1 (0–0.6) | 0.015 |
VBA, vertebrobasilar artery; VCI, vascular cognitive impairment. CTP, computed tomographic perfusion. †Adjusted for age, sex, smoking, hypertension, diabetes mellitus, hyperlipidemia and coronary artery disease.
Profile of cognitive function based on cerebral perfusion status
Based on the results of dynamic CTP imaging and the state of local microcirculation in the VBA area, the qualitative evaluation method was described detailed in other studies [13, 14]. Three categories of cerebral perfusion were established: 25 patients in normal perfusion (NP) group (normal or prolonged TTP, normal MTT, CBV, and CBF), 31 patients in compensatory perfusion (CP) group (prolonged TTP and MTT, normal CBF, normal or elevated CBV), and 32 patients in decompensated perfusion (DP) group (prolonged TTP and MTT, decreased CBF, normal or decreased CBV). As compared to those in NP and CP groups, patients in DP group had lower scores on the MoCA (p < 0.001), CDT (p = 0.011), SCWT-C test (p = 0.023), DST (p < 0.001), AVLT (p = 0.002), and BNT (p = 0.042), which involved the executive function, attention, memory and naming ability disorders (Table 3).
Table 3
Cognitive characteristics of VBA stenosis patients with bad perfusion or not
| Cognitive Scale | CT perfusion | |||
| Normal (n = 25) | Compensatory (n = 31) | Decompensated (n = 32) | p | |
| MoCA | 25.2±3.2 | 23.0±3.4 | 20.4±4.8 | <0.001 |
| CDT | 3.5±0.9 | 3.2±0.7 | 2.6±1.3 | 0.011 |
| SCWT-A, s | 21.2±2.3 | 22.4±3.5 | 26.2±12.9 | 0.101 |
| SCWT-B, s | 48.1±15.1 | 48.8±14.9 | 61.6±28.4 | 0.184 |
| SCWT-C, s | 87.7±16.6 | 94.6±30.6 | 121.5±51.7 | 0.023 |
| TMT-A, s | 48.8±17.5 | 48.9±19.2 | 53.8±30.0 | 0.941 |
| TMT-B, s | 100.3±15.9 | 118.8±51.2 | 143.8±74.0 | 0.149 |
| DST | 8.9±1.7 | 8.6±1.8 | 6.8±1.9 | <0.001 |
| AVLT | 6.8±1.5 | 6.3±2.1 | 4.8±2.6 | 0.002 |
| BNT | 26.8±2.5 | 26.7±3.5 | 25.1±3.5 | 0.042 |
VBA, vertebrobasilar artery; MoCA, Montreal Cognitive Assessment; CDT, Clock Drawing Test; SCWT, Stroop Color Word Test; TMT, Trail Making Test; DST, Digital Span Test; AVLT, Auditory Verbal Learning Test; BNT, Boston Naming Test.
Profile of cognitive function based on collateral circulation status
Participants were classified into two groups using ASTIN/SIR: 71 patients had the poor collaterals (ASTIN: 0–2 degree) and 17 had the good collaterals (ASTIN: 3–4 degree). As compared to those with good collaterals, patients with poor collaterals had lower scores on the MoCA (p < 0.001), SCWT-C (p = 0.007), TMT-B (p < 0.001), and AVLT (p = 0.050), which involved executive function and memory impairment (Table 4).
Table 4
Cognitive characteristics of VBA stenosis patients with good collaterals or not
| Cognitive Scale | Poor collaterals (n = 71) | Good collaterals (n = 17) | p |
| MoCA | 22.1±4.5 | 25.2±2.4 | <0.001 |
| CDT | 3.0±1.2 | 3.2±1.0 | 0.663 |
| SCWT-A, s | 23.8±9.2 | 22.1±2.6 | 0.475 |
| SCWT-B, s | 54.9±22.9 | 46.3±13.6 | 0.146 |
| SCWT-C, s | 105.5±43.3 | 89.5±10.5 | 0.007 |
| TMT-A, s | 52.4±24.2 | 43.2±17.3 | 0.077 |
| TMT-B, s | 128.5±61.5 | 97.9±16.2 | <0.001 |
| DST | 7.9±2.2 | 8.4±1.1 | 0.179 |
| AVLT | 5.6±2.2 | 6.9±2.6 | 0.050 |
| BNT | 26.0±3.5 | 26.8±2.1 | 0.372 |
MoCA, Montreal Cognitive Assessment; CDT, Clock Drawing Test; SCWT, Stroop Color Word Test; TMT, Trail Making Test; DST, Digital Span Test; AVLT, Auditory Verbal Learning Test; BNT, Boston Naming Test.
DISCUSSION
The present study indicated that individuals with severe VBA stenosis experienced declines across various cognitive domains, including memory, executive function, naming skills, and attention. In patients with severe VBA stenosis, CT hypoperfusion, temporoparietal-occipital lobe hypoperfusion, cerebellum hypoperfusion, multiple sites hypoperfusion, and poor collaterals were risk factors for VCI, while the presence of the PcoA compensating the PCA and BA was a protective factor for VCI. Patients with hypoperfusion were more likely to experience worse executive function, attention, memory, and naming skills. Those with poor collaterals were more prone to memory loss and executive dysfunction.
Previous studies have indicated that vascular dementia is traditionally thought to be the second most common cause of dementia all over the world (comprising approximately 15% to 20% of clinically diagnosed dementia cases in North America and Europe and closer to 30% in Asia and some developing countries) [15]. In the present study focused on VCI (including mild cognitive impairment and dementia), approximate 58.0% of patients with severe VBA stenosis had VCI, which was consistent with findings from the previous study (53.8%) [16].These results indicated that severe VBA stenosis is not only an important risk factor for ischemic cerebrovascular diseases, but is also closely related to VCI.
Our study showed that patients with CT hypoperfusion in the VBA blood supply area responsible for a high percentage of VCI, which affected multiple cognitive domains including executive function, memory, attention, and naming skill. The plausible mechanism for the phenomenon was described as follows. Firstly, it is generally believed that executive function depends on the frontal cortex. However, previous research has shown that multiple regions outside the frontal lobe are involved in executive function [17], which suggested that cognitive functional area would not follow functional specialization theory strictly. Secondly, the vascular territory of the VBA appears linked to various cognitive functions. For instance, damage to the cerebellar posterior lobe may result in cerebellar cognitive affective syndrome, characterized by reduced executive function, impaired visuospatial skills, and linguistic dysfunction [18]. Medial temporal lobe damage can lead to sudden memory loss and long-term cognitive decline due to its involvement in memory regulation [19]. Isolated brainstem infarction may impair language and visuospatial skills, affecting crucial pathways linking the limbic system and frontal lobes [20], or alter serotonin levels, impacting the prefrontal cortex and impairing executive function, attention, and memory [21]. Additionally, executive function decline is considered an early indicator of cognitive impairment that can develop quietly before interfering with everyday living [22]. The parietal lobe, with extensive connections to the dorsal prefrontal and medial temporal lobes, is crucial for visuospatial function, attention, and computing skills [23]. The occipital lobe, besides its role in visuospatial disruption, is integral to language processing [24].
This current study revealed higher cognitive domain impairment in the DP group, compared to the NP and CP groups, which consistent with a 6.8 times greater risk of cognitive impairment in decompensated perfusion patients in the previous research [14]. Severe cerebrovascular stenosis at the macro level induces cerebral hypoperfusion, leading to decreased reactivity, reserve capacity, hemodynamic changes, and micro-embolus formation [25, 26]. Collateral circulation initially compensates, but when ischemia surpasses compensatory capacity, decompensated perfusion occurs, causing cognitive impairment. At the micro level, brain decompensated perfusion impacts molecular pathways, particularly in the hippocampus, triggering neuroinflammation, apoptosis, and blood-brain barrier injury, contributing to VCI [27]. Chronic cerebral hypoperfusion activates absent in melanoma 2 (AIM2) inflammasome and interleukin-1-β/-18, promoting cell apoptosis, neuron loss, and further contributing to cognitive impairment and memory decline [28]. Notably, interleukin-1-β’s involvement in inflammasome construction suggests a significant role in VCI [29]. Recent research highlights mitochondrial autophagy dysfunction as a key factor in persistent hypoperfusion leading to VCI [30].
We demonstrated a heightened incidence of VCI in patients with poor collaterals, identified as a cognitive risk factor. Inconsistencies in previous VCI research on collateral circulation may stem from variations in measurement techniques. Zavoreo et al. [31] noted that secondary collateral compensation correlated with a higher VCI incidence but better visuospatial and executive function, while Sussman et al. [32] identified loss of collateral circulation as an independent VCI risk factor. The researchers proposed that secondary collateral presence often indicates a more severe hemodynamic abnormality, potentially explaining these findings.
We also found that, in severe VBA stenosis patients, PCoA compensating for PCA and BA served as a protective cognitive factor. Contrary to Sussman et al.’s [32] findings, our research revealed that the presence of PCoA compensating for PCA alone did not protect cognition, but compensation for both PCA and BA was protective. This underscores the importance of the top of BA in preserving cognitive function, where anatomically, branch vessels (SCA, PCA, and BA) supply critical brain regions. PCoA compensating for both PCA and BA not only suggests better collateral compensatory capacity but also aligns with a previous study [33] associating poor blood flow filling in the upper BA with dysfunction and adverse prognosis after 90 days, indicating a higher likelihood of cognitive impairment.
This study utilized cognitive scales to categorize cognition into various domains. The SCWT, a widely used scale for executive function, comprises three versions: SCWT-A for visual search speed, SCWT-B for working memory and visual search speed, and SCWT-C for working memory, conflict monitoring, and visual search speed—SCWT-C being the best reflection of executive function [11]. The TMT, another executive function measure, has TMT-A reflecting attention and information processing, while TMT-B mainly reflects executive function [34]. Patients with poor collaterals exhibited lower SCWT-C and TMT-B scores, indicating impaired executive function, consistent with prior findings [35]. Furthermore, poor collateral circulation correlated with lower AVLT scores, suggesting memory impairment, possibly due to reduced blood supply to the medial temporal lobe [36].
The study had several limitations that need to be acknowledged. Firstly, as our analysis was based on cross-sectional data, we were unable to determine causality between cerebral hypoperfusion and poor collaterals and VCI. Secondly, the study included a small sample size, necessitating larger, multi-center research for validation. Our findings may not be generalizable to other ethnicities or races since our study was conducted solely on participants from a single research institute. Thirdly, the detailed lipid profile and the use of statins may influence stroke outcomes [37], the lack of this observation may be a limitation of the present study. And the retrospective nature of this study limited assessing if there was an impact of the cognitive impairment in daily activities, which needs further study. In summary, severe VBA stenosis is linked to a high incidence of VCI, affecting various cognitive domains including executive function, memory, attention, and naming skills. Identified risk factors for VCI include CT hypoperfusion, multiple sites hypoperfusion, and poor collaterals, while PCoA compensating for PCA and BA is a potential protective factor for VCI. These findings aid early VCI identification by clinicians. The study contributes to understanding cognitive impairment from vascular stenosis and lays the groundwork for assessing the impact of endovascular treatment on cognitive performance.
AUTHOR CONTRIBUTIONS
Weiyi Zhang (Data curation; Writing – original draft); Weilun Fu (Data curation); Yumei Zhang (Writing – review & editing).
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This study was supported by the National Natural Science Foundation of China (No. 81972144, No. 82372555), and National Key Research and Development Program of China (No. 2018YFC2002300, No. 2018YFC2002302).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Khan S , Barve KH , Kumar MS ((2020) ) Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease, Curr Neuropharmacol 18: , 1106–1125. |
[2] | Paasila PJ , Davies DS , Kril JJ , Goldsbury C , Sutherland GT ((2019) ) The relationship between the morphological subtypes of microglia and Alzheimer’s disease neuropathology, Brain Pathol 29: , 726–740. |
[3] | Hachinski V , Iadecola C , Petersen RC , Breteler MM , Nyenhuis DL , Black SE , Powers WJ , DeCarli C , Merino JG , Kalaria RN , Vinters HV , Holtzman DM , Rosenberg GA , Wallin A , Dichgans M , Marler JR , Leblanc GG ((2006) ) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards, Stroke 37: , 2220–2241. |
[4] | Jia J , Zhou A , Wei C , Jia X , Wang F , Li F , Wu X , Mok V , Gauthier S , Tang M , Chu L , Zhou Y , Zhou C , Cui Y , Wang Q , Wang W , Yin P , Hu N , Zuo X , Song H , Qin W , Wu L , Li D , Jia L , Song J , Han Y , Xing Y , Yang P , Li Y , Qiao Y , Tang Y , Lv J , Dong X ((2014) ) The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese, Alzheimers Dement 10: , 439–447. |
[5] | Buratti L , Balucani C , Viticchi G , Falsetti L , Altamura C , Avitabile E , Provinciali L , Vernieri F , Silvestrini M ((2014) ) Cognitive deterioration in bilateral asymptomatic severe carotid stenosis, Stroke 45: , 2072–2077. |
[6] | Duering M , Gonik M , Malik R , Zieren N , Reyes S , Jouvent E , Hervé D , Gschwendtner A , Opherk C , Chabriat H , Dichgans M ((2013) ) Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment, Neuroimage 66: , 177–183. |
[7] | Xie B , Liu Y , Wu D , Li G , Chen T , Xiao S , Yang J , Li J , Li X ((2021) ) Effects of site, cerebral perfusion and degree of cerebral artery stenosis on cognitive function, Neuroreport 32: , 252–258. |
[8] | Yan Y , Liang L , Yuan Y , Chen T , Shen Y , Zhong C ((2014) ) Influence of stent-assisted angioplasty on cognitive function and affective disorder in elderly patients with symptomatic vertebrobasilar artery stenosis, Med Sci Monit 20: , 1129–1136. |
[9] | Liebeskind DS , Sanossian N ((2012) ) How well do blood flow imaging and collaterals on angiography predict brain at risk? Neurology 79: (13 Suppl 1), S105–S109. |
[10] | Potocnik J , Ovcar Stante K , Rakusa M ((2020) ) The validity of the Montreal cognitive assessment (MoCA) for the screening of vascular cognitive impairment after ischemic stroke, Acta Neurol Belg 120: , 681–685. |
[11] | Periáñez JA , Lubrini G , García-Gutiérrez A , Ríos-Lago M ((2021) ) Construct validity of the Stroop Color-Word Test: Influence of speed of visual search, verbal fluency, working memory, cognitive flexibility, and conflict monitoring, Arch Clin Neuropsychol 36: , 99–111. |
[12] | Harvey PD ((2019) ) Domains of cognition and their assessment, Dialogues Clin Neurosci 21: , 227–237. |
[13] | Campanholo KR , Conforto AB , Rimkus CM , Miotto EC ((2015) ) Cognitive and functional impairment in stroke survivors with basilar artery occlusive disease, Behav Neurol 2015: , 971514. |
[14] | Deng Y , Wang L , Sun X , Liu L , Zhu M , Wang C , Sui B , Shen M , Gu W , Mo D , Ma N , Song L , Li X , Huo X , Miao Z , Chen D , Gao F ((2018) ) Association between cerebral hypoperfusion and cognitive impairment in patients with chronic vertebra-basilar stenosis, Front Psychiatry 9: , 455. |
[15] | Chang Wong E , Chang Chui H ((2022) ) Vascular cognitive impairment and dementia, Continuum (Minneap Minn) 28: , 750–780. |
[16] | Yan Y , Liang L , Yuan Y , Chen T , Shen Y , Zhong C ((2014) ) Influence of stent-assisted angioplasty on cognitive function and affective disorder in elderly patients with symptomatic vertebrobasilar artery stenosis, Med Sci Monit 20: , 1129–1136. |
[17] | Hoshi H , Kobayashi M , Hirata Y , Fukasawa K , Ichikawa S , Shigihara Y ((2023) ) Decreased beta-band activity in left supramarginal gyrus reflects cognitive decline: Evidence from a large clinical dataset in patients with dementia, Hum Brain Mapp 44: , 6214–6226. |
[18] | Argyropoulos GPD , van Dun K , Adamaszek M , Leggio M , Manto M , Masciullo M , Molinari M , Stoodley CJ , Van Overwalle F , Ivry RB , Schmahmann JD ((2020) ) The cerebellar cognitive affective/schmahmann syndrome: A task force paper, Cerebellum 19: , 102–125. |
[19] | Salami A , Wåhlin A , Kaboodvand N , Lundquist A , Nyberg L ((2016) ) Longitudinal evidence for dissociation of anterior and posterior MTL resting-state connectivity in aging: Links to perfusion and memory, Cereb Cortex 26: , 3953–3963. |
[20] | Garrard P , Bradshaw D , Jäger HR , Thompson AJ , Losseff N , Playford D ((2002) ) Cognitive dysfunction after isolated brain stem insult. An underdiagnosed cause of long term morbidity, J Neurol Neurosurg Psychiatry 73: , 191–194. |
[21] | Robbins TW ((2000) ) From arousal to cognition: The integrative position of the prefrontal cortex, Prog Brain Res 126: , 469–483. |
[22] | Zhao JH , Tian XJ , Liu YX , Yuan B , Zhai KH , Wang CW , Yue JY , Zhang LJ , Li Q , Yan HQ , Li GM , Ji SB ((2013) ) Executive dysfunction in patients with cerebral hypoperfusion after cerebral angiostenosis/occlusion, Neurol Med Chir (Tokyo) 53: , 141–147. |
[23] | Sack AT ((2009) ) Parietal cortex and spatial cognition, Behav Brain Res 202: , 153–161. |
[24] | O’Hare AE , Dutton GN , Green D , Coull R ((1998) ) Evolution of a form of pure alexia without agraphia in a child sustaining occipital lobe infarction at 2 1/2 years, Dev Med Child Neurol 40: , 417–420. |
[25] | Ahn J , Kim M ((2023) ) Effects of aerobic exercise on global cognitive function and sleep in older adults with mild cognitive impairment: A systematic review and meta-analysis, Geriatr Nurs 51: , 9–16. |
[26] | Nation DA , Sweeney MD , Montagne A , Sagare AP , D’Orazio LM , Pachicano M , Sepehrband F , Nelson AR , Buennagel DP , Harrington MG , Benzinger TLS , Fagan AM , Ringman JM , Schneider LS , Morris JC , Chui HC , Law M , Toga AW , Zlokovic BV ((2019) ) Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction, Nat Med 25: , 270–276. |
[27] | Tian Z , Ji X , Liu J ((2022) ) Neuroinflammation in vascular cognitive impairment and dementia: Current evidence, advances, and prospects, Int J Mol Sci 23: , 6224. |
[28] | Poh L , Fann DY , Wong P , Lim HM , Foo SL , Kang SW , Rajeev V , Selvaraji S , Iyer VR , Parathy N , Khan MB , Hess DC , Jo DG , Drummond GR , Sobey CG , Lai MKP , Chen CL , Lim LHK , Arumugam TV ((2021) ) AIM2 inflammasome mediates hallmark neuropathological alterations and cognitive impairment in a mouse model of vascular dementia, Mol Psychiatry 26: , 4544–4560. |
[29] | Zhou Y , Zhang J , Wang L , Chen Y , Wan Y , He Y , Jiang L , Ma J , Liao R , Zhang X , Shi L , Qin Z , Zhou Y , Chen Z , Hu W ((2017) ) Interleukin-1β impedes oligodendrocyte progenitor cell recruitment and white matter repair following chronic cerebral hypoperfusion, Brain Behav Immun 60: , 93–105. |
[30] | Zhou K , Chen J , Wu J , Wu Q , Jia C , Xu YXZ , Chen L , Tu W , Yang G , Kong J , Kou J , Jiang S ((2019) ) Atractylenolide III ameliorates cerebral ischemic injury and neuroinflammation associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial fission in microglia, Phytomedicine 59: , 152922. |
[31] | Zavoreo I , Bašić Kes V , Lisak M , Maršić N , Ciliga D , Trošt Bobić T ((2013) ) Cognitive decline and cerebral vasoreactivity in asymptomatic patients with severe internal carotid artery stenosis, Acta Neurol Belg 113: , 453–458. |
[32] | Sussman ES , Kellner CP , Mergeche JL , Bruce SS , McDowell MM , Heyer EJ , Connolly ES ((2014) ) Radiographic absence of the posterior communicating arteries and the prediction of cognitive dysfunction after carotid endarterectomy, J Neurosurg 121: , 593–598. |
[33] | Gao F , Tong X , Sun X , Miao Z ((2021) ) A new angiographic collateral grading system for acute basilar artery occlusion treated with endovascular therapy, Transl Stroke Res 12: , 559–568. |
[34] | Du M , Andersen SL , Cosentino S , Boudreau RM , Perls TT , Sebastiani P ((2022) ) Digitally generated Trail Making Test data: Analysis using hidden Markov modeling, Alzheimers Dement (Amst) 14: , e12292. |
[35] | Wei W , Yi X , Ruan J , Duan X , Luo H , Lv Z ((2019) ) Influence of collateral circulation on cerebral blood flow and frontal lobe cognitive function in patients with severe internal carotid artery stenosis, BMC Neurol 19: , 151. |
[36] | Xu Y , Chen K , Zhao Q , Li F , Guo Q ((2020) ) Short-term delayed recall of auditory verbal learning test provides equivalent value to long-term delayed recall in predicting MCI clinical outcomes: A longitudinal follow-up study, Appl Neuropsychol Adult 27: , 73–81. |
[37] | Vitturi BK , Gagliardi RJ ((2022) ) The prognostic significance of the lipid profile after an ischemic stroke, Neurol Res 44: , 139–145. |




