Screening for Cognitive Impairment in Movement Disorders: Comparison of the Montreal Cognitive Assessment and Quick Mild Cognitive Impairment Screen in Parkinson’s Disease and Lewy Body Dementia
Abstract
Background:
The Montreal Cognitive Assessment (MoCA) is recommended by the Movement Disorder Society for cognitive testing in movement disorders including Parkinson’s disease (PD) and lewy body dementia. Few studies have compared cognitive screening instruments in these diseases, which overlap clinically.
Objective:
To compare the MoCA and Quick Mild Cognitive Impairment (Qmci) screen in this population.
Methods:
Patients attending memory and movement disorder clinics associated with a university hospital had the MoCA and Qmci screen performed and diagnostic accuracy compared with the area under the receiver operating characteristic curve (AUC). Duration and severity of movement disorders was assessed using the Unified PD Rating Scale (UPDRS).
Results:
In total, 133 assessments were available, median age 74±5. Median education was 11±4 years and 65% were male. Median total UPDRS score was 37±26. Median Qmci screen was 51±27, median MoCA was 19±10. There were statistically significant differences in test scores between those with subjective symptoms but normal cognition, mild cognitive impairment (MCI) and dementia (p < 0.001). The Qmci screen had significantly greater accuracy differentiating normal cognition from MCI versus the MoCA (AUC 0.90 versus 0.72, p = 0.01). Both instruments had similar accuracy in identifying cognitive impairment and separating MCI from dementia. The median administration time for the Qmci screen and MoCA were 5.19 and 9.24 minutes (p < 0.001), respectively.
Conclusions:
Both the MoCA and Qmci screen have good to excellent accuracy in a population with movement disorders experiencing cognitive symptoms. The Qmci screen was significantly more accurate for those with early symptoms and had a shorter administration time.
INTRODUCTION
The prevalence of movement disorders is increasing in tandem with population aging [1]. Approximately 28% of adults aged over 50 years can be classified as having a movement disorder, most commonly a tremor [2]. One of the most prevalent movement disorders worldwide is Parkinson’s disease (PD); the number of persons with PD is expected to double by 2030 [3] with an increasing incidence and burden associated with this condition in most regions and countries globally [4]. As cognitive impairment (CI) is common in PD, particularly as the disease advances, this will result in an overall increase in the number of patients reporting cognitive symptoms associated with PD. Many of these will be older patients. Prevalence rates of PD-related CI approach 80% [5], while the rate of progression to Parkinson’s disease dementia (PDD) is approximately 10% per year [6]. Mean duration from onset of PD symptoms to dementia is estimated at 10 years [7]. Many patients with PD have CI from the time of diagnosis (approximately 15–20%), though the prevalence in prodromal disease is less clear [8]. Lewy body dementia (LBD) is one of the most common dementia subtypes and is also associated with the development of a movement disorder [9]. Similar to PD, LBD is a dementia associated with parkinsonism and Lewy body formation and it likewise increases in prevalence with age, representing approximately 5% of dementia cases in older populations [9, 10]. As with CI in PD, it is likely under-reported [10].
Mild cognitive impairment (MCI) affects at least one-third (27%–42.5%) of people with PD [11–13] and is associated with a greater chance of developing dementia, especially if present when patients are first diagnosed [14]. Single domain, non-amnestic is the most common subtype [12]. The diagnosis of PD-MCI is challenging with uncertainty surrounding the duration of follow-up and number of repeated cognitive tests required to make the diagnosis [15]. Reflecting this, some guidelines including the Movement Disorder Society Task Force Guidelines [11], suggest that up to a minimum of 10 repeat assessments are required to diagnose and subtype PD-MCI [15]. PD-MCI features exaggerated attention and executive function deficits [16]. LBD is also associated with an MCI syndrome referred to as LBD-MCI, which, similar to PD-MCI, is characterized by executive, visuospatial, and attentional deficits [17, 18]. CI in persons with movement disorders impacts upon life expectancy [19], quality of life [20], healthcare costs [21] and activities of daily living (ADLs), even among those without dementia, i.e., at prodromal stages [20].
Early diagnosis of CI is important to facilitate prompt treatment, identify reversible or compounding factors and plan for the future [22]. Monitoring change over time is important in this context and relies on the use of accurate short cognitive screening instruments (CSIs) [23]. Repeated neuropsychological testing [14] is recommended to increase prognostic accuracy, particularly conversion from MCI syndromes to clinical dementia. The progression from PD-MCI and LBD-MCI to dementia is particularly challenging to diagnose. An ideal short CSI in this setting would be reliable, brief, sensitive to early change, have normative data available, cover core cognitive domains relevant to these conditions (i.e., attention-working memory, memory, executive functioning, visuospatial skills, and language) and be largely immune to the effects of motor limitations [23, 24]. Such CSIs would also ideally correlate well with functional measures and emerging biomarkers [23].
At present, the Movement Disorder Society recommends the use of the Montreal Cognitive Assessment (MoCA) [25] for cognitive testing in movement disorders including PD and LBD [26–28]. The reason why the MoCA is well suited for use in this population is because it lacks ceiling effects and is weighted towards domains such as executive and visuospatial functioning and less towards orientation and language, which are relatively well preserved in PD [29]. Other widely-used CSIs may be less suitable. For example, the Mini-Mental State Examination (MMSE), one of the most established and widely-used CSIs, is still commonly used for these patients [28, 30]. However, the MMSE, because of its low ceiling effects and selected cognitive domains, may miss early cognitive deficits in these conditions [29] and is recommended for use only with this caveat [28]. This may result in failed opportunities to initiate early appropriate treatment and discontinue inappropriate medications like anti-cholinergics (medications that bind to muscarinic receptors and block acetylcholine neurotransmission), which is particularly important in diseases associated with a cholinergic deficit such as Alzheimer’s disease, PDD and LBD [31]. Despite the increasing use of the MoCA, questions remain about its accuracy; studies suggest that up to one-quarter of patients with PD with normal MoCA scores report functional cognitive difficulties, while three-quarters with low MoCA scores report none [32]. On this basis, other tests with a greater emphasis on visio-cognitive impairment, may be preferable[33].
Despite the advantages of the MoCA and its extensive validation [34], some challenges remain with its use in persons with movement disorders and other populations such as in primary care and community-based memory clinics (where access to neuropsychology may be limited) including elements of redundancy, a relatively long administration time for a CSI (10–12 min), its known floor effects, its low specificity and a high false positive rate in older adults, particularly at its recommended cut-off score (≥26) [35–39]. Given this, we sought to investigate if the Quick Mild Cognitive Impairment (Qmci) screen [40], which has yet to be validated in patients with movement disorders and cognitive symptoms, is accurate in detecting cognitive impairment in this population and how it compares to the MoCA.
MATERIALS AND METHODS
Patients
This is a secondary analysis of a cross-sectional study that was conducted parallel to a larger study evaluating the Qmci screen in an Irish clinic population. The methods have been reported elsewhere [38] but in summary, a consecutive sample of patients with cognitive symptoms attending either a university hospital movement disorder clinic or a geriatric medicine memory clinic in Cork City, Ireland between January 2013 to December 2014, were included. Patients were classified as having normal cognition (subjective symptoms only), MCI or dementia by a consultant physician, specialized in the diagnosis and management of cognitive disorders. A diagnosis of dementia (PDD or LBD) was based on Diagnostic and Statistical Manual of Mental Disorders, (5th edition) criteria [41]. A diagnosis of PD-MCI was made using level I diagnostic criteria according to the Movement Disorder Society Task Force Guidelines [12], defined as recent, subjective but corroborated memory loss without obvious loss of social or occupational function, with evidence of objective deficits on a global cognitive scale: the Unified Parkinson’s Disease Rating Scale (UPDRS) part I, in those with established PD. LBD-MCI was diagnosed using the third report of the LBD Consortium [42]. Patients were excluded if they were aged < 40 years of age, if they had other movement disorders such as essential tremor (n = 3), if they were unable to communicate verbally in English, if they had depression (as defined by a Geriatric Depression Scale-short [GDS-SF] form with a cut-off ≥7 points to increase specificity [43]), or if a reliable collateral was not available but required. The GDS-SF, scored out of 15 points, is validated in older adults with PD [44] and has been used in LBD [45], again taking a higher cut-off score than in Alzheimer’s disease [46].
Data collection
Patients underwent comprehensive clinical assessment and were screened for cognitive impairment. This assessment included a full history, physical examination, laboratory testing and neuroimaging. A short neuropsychological battery including the Standardized MMSE [47] and two informant-rated assessments, the AD8 questionnaire [48] and IQCODE Short Form [49] were conducted by a consultant geriatrician, blind to the results of the CSIs, to inform the clinical diagnosis. The MoCA and Qmci screen were scored in random order, by a trained rater, prior to and independent of the clinical assessment. The presence and severity of any extrapyramidal symptoms were graded using the UPDRS performed by an Advanced Nurse Practitioner in movement disorders (MJF). The study adhered to the tenets of the Declaration of Helsinki and ethical approval was obtained from the Clinical Research Ethics Committee of the Cork Teaching Hospitals (reference number: ECM 4 (aa) 03/04/12) and patients provided informed consent. Assent was obtained from individuals who were felt to lack capacity.
Measures
Montreal cognitive assessment
The MoCA has seven subtests covering five cognitive domains; specifically, it includes visuospatial, attention, processing speed, language, memory, and cognitive control scored out of 30 points with lower points indicating cognitive impairment. A cut-off of < 26/30 is suggested for use in routine practice [25], although lower cut-offs have been suggested. Lower scores imply more impaired cognition.
Quick mild cognitive impairment screen
The Qmci screen is a brief and accurate CSI for MCI and has been studied in persons attending memory clinics but not in those with PD or LBD [38]. The Qmci screen has six subtests covering five cognitive domains; specifically, it includes five orientation items, five registration items, a clock drawing test, a delayed recall question, verbal fluency (e.g., animals named in 1 min) and a test of logical memory (immediate verbal recall of a short story) [50]. It takes 3–5 min to complete [50]. The optimal Qmci screen cut-off score for cognitive impairment (MCI or dementia) is < 62/100 [38]. Again, lower scores suggest greater cognitive impairment.
Unified Parkinson’s disease rating scale
The severity of PD was determined using the UPDRS [51]. The UPDRS total score consists of the sum of parts I (mentation, behavior, and mood), II (ADLs) and III (motor examination), with scores ranging from 0 (not affected) to 176 (most severely affected). A score of one or more on item one (intellectual impairment) of part I was taken as supportive of CI (PD-MCI or PDD).
Analysis
Data were analyzed using R version 4.2.2 (2022-10-31) -“Innocent and Trusting” (R Core Team, 2022). All significance tests were two sided, and a p-value of < 0.05 was considered statistically significant. Most data were non-normally distributed and analyzed with non-parametric tests. Spearman’s rho (r) measured rank correlation. The Chi Squared test assessed differences between the distributions of categorical variables. The Mann-Whitney U test and the Kruskal-Wallis test was used to examine differences between non-parametric continuous variables. Diagnostic accuracy was determined from analysis of the Area Under the Curve (AUC) generated by receiver operating characteristics (ROC) curves. These were compared using the compared using the DeLong method [52] Sensitivity and specificity were determined and optimal cut-offs calculated based on Youden’s Index [53].
RESULTS
In all, 133 patient assessments were available and included. In addition to those excluded with other movement disorders, one duplicate was removed. The characteristics of patients according to their diagnostic category are presented in Table 1. The median age of the sample was 74 years, interquartile range (IQR)±5. Most, 65%, were male. The median number of years in education was 11 IQR±4 years. The majority of patients were diagnosed with parkinsonism with 109 (82%) having idiopathic PD and 11 (8%) having vascular-type parkinsonism. The remaining 10% (n = 13) had a diagnosis of LBD. Most patients had either dementia (43%) or MCI (32%). The median time between diagnosis and the assessment was 7 IQR±7 years and the median UPDRS (total score) was 37 IQR±26. While patients with normal cognition were statistically younger (p = 0.02), there was no clinically meaningful difference in age according to cognitive status. There was no statistically significant difference in sex, years of education or time since diagnosis between the diagnostic categories. The median SMMSE score was 26/30 (IQR±6) for the total sample.
Table 1
Comparison of characteristics of patients with normal cognition, mild cognitive impairment (MCI) and dementia
| Variable | Total(Q3-Q1 =±IQR) | Normal(n = 34, 25%) (Q3-Q1 =±IQR) | MCI(n = 42, 32%) (Q3-Q1 =±IQR) | Dementia(n = 57, 43%) (Q3-Q1 =±IQR) | P = x |
| Age (Median &IQR) | 74 (79–72 =±5) | 73 (74–72 =±2) | 75 (79–70 =±9) | 76 (81–72 =±9) | 0.02* |
| Education (Median &IQR) | 11 (14–10 =±4) | 14 (19–14 =±5) | 13 (14–10 =±4) | 11 (12–10 =±2) | 0.28 |
| Years since Diagnosis (Median &IQR) | 7 (10–3 =±7) | 3 (7–2 =±5) | 7.5 (11–5 =±6) | 6 (11–3 =±8) | 0.10 |
| Gender (% male) | 65% | 65% | 71% | 61% | 0.58 |
| UPDRS – Total (Median &IQR) | 37 (50–24 =±26) | 36 (50–22 =±28) | 37 (45–24 =±21) | 38 (54–32 =±22) | 0.51 |
| UPDRS-Cog (Median &IQR) | 3 (5–2 =±3) | 1.5 (4.5–1 = 3.5) | 3 (5–2 =±3) | 4 (5–3 =±2) | 0.06 |
| SMMSE (Median &IQR) | 26 (28–22 =±6) | 28 (30–27 =±3) | 27 (29–26 =±3) | 22 (25–19 =±6) | < 0.001* |
| MoCA (Median &IQR) | 19 (23–13 =±10) | 25 (26–23 =±3) | 22 (24–18 =±6) | 13 (16–10 =±6) | < 0.001* |
| Qmci screen (Median &IQR) | 51 (63–36 =±27) | 70 (73–65 =±8) | 55 (59–53 =±6) | 36 (44–29 =±15) | < 0.001* |
IQR, Interquartile range. *Statistically significant. UPDRS, Unified Parkinson’s Disease Rating Scale; SMMSE, Standardized Mini-Mental State Examination; Qmci screen, Quick Mild Cognitive Impairment Screen; MoCA, Montreal Cognitive Assessment.
A strong, positive correlation was seen between both CSIs, r = 0.87, p < 0.001. The median MoCA score for all patients was 19/30 (IQR±10) with statistically significant differences between the diagnostic groupings (p < 0.001). The median Qmci screen score was 51/100 (IQR±17) and again there was a statistically significant gradient in median values between persons with normal cognition, MCI and dementia (p < 0.001). The median administration time for the Qmci screen was 5.19 IQR±1.4 minutes compared to 9.24 IQR±1.3 minutes for the MoCA, a statistically significant difference, p < 0.001.
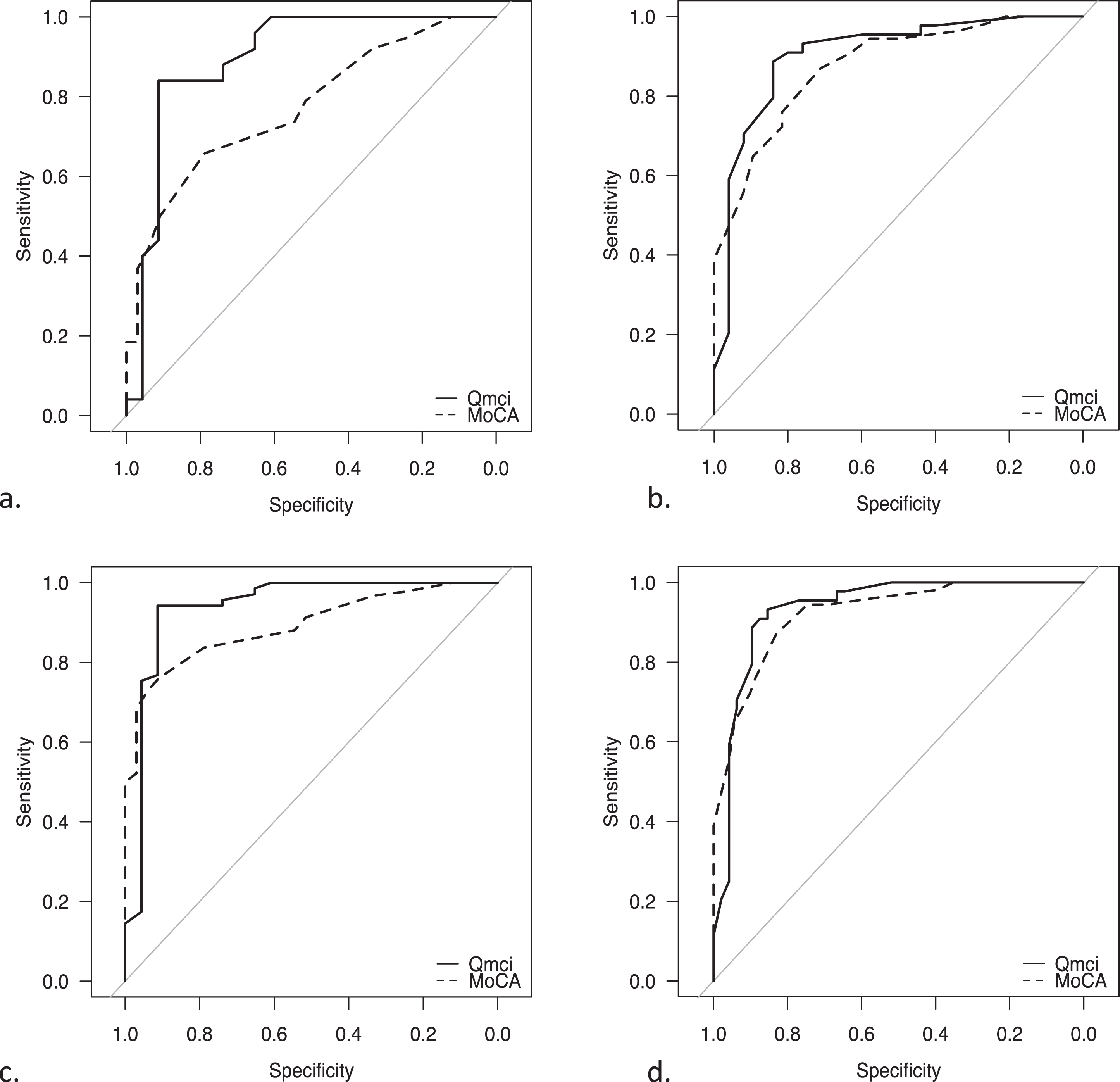
Table 2 shows the optimal cut-off scores with sensitivity and specificity values according to Youden’s Index for both CSIs for different comparisons. The diagnostic accuracy based on AUC are also provided and presented visually as ROC curves in Fig. 1. The Qmci screen had excellent accuracy, which was statistically significantly greater for separating patients with normal from those with MCI compared to the MoCA (AUC 0.90 versus 0.72, p = 0.01). The Qmci screen at a cut-off of < 60/100 had a sensitivity of 84% and specificity of 91%, while the MoCA’s optimal cut-off for MCI < 23/30 with lower sensitivity (66%) and specificity (79%) in this sample. Both instruments had similar accuracy in identifying cognitive impairment (either MCI or dementia) from normal and for differentiating MCI from dementia.
Table 2
Sensitivity, Specificity, and diagnostic accuracy for each cognitive screening instrument based upon area under the receiver operating characteristic curve (AUC) with 95% confidence intervals, for the optimal cut-off scores for cognitive impairment
| Figure | Diagnostic | MoCA | Qmci screen | Comparison | ||||||
| category | Youden’s Index (Optimal cut-off) | Sensitivity | Specificity | AUC (95% CIs) | Youden’s Index (Optimal cut-off) | Sensitivity | Specificity | AUC (95% CIs) | AUC comparison p = x | |
| a | MCI from Normal Cognition | 0.45 (< 23/30) | 0.66 | 0.79 | 0.72 (0.67-0.88) | 0.75 (< 60/100) | 0.84 | 0.91 | 0.90 (0.80-0.99) | p = 0.014 |
| b | MCI and Dementia | 0.58 (< 19/30) | 0.87 | 0.71 | 0.92 (0.81-0.95) | 0.71 (< 49/100) | 0.87 | 0.84 | 0.89 (0.82-0.99) | p = 0.397 |
| c | Normal from Cognitive Impairment (MCI and Dementia) | 0.67 (< 22/30) | 0.76 | 0.91 | 0.89 (0.84-0.95) | 0.85 (< 60/100) | 0.94 | 0.91 | 0.94 (0.87-0.99) | p = 0.143 |
| d | Dementia | 0.7 (< 19/30) | 0.87 | 0.83 | 0.95 (0.88-0.97) | 0.78 (< 53/100) | 0.93 | 0.85 | 0.93 (0.88-0.99) | p = 0.315 |
Qmci screen, Quick Mild Cognitive Impairment Screen; MoCA, Montreal Cognitive Assessment.
Fig. 1
Receiver Operating Characteristic curves demonstrating the accuracy of the Quick Mild Cognitive Impairment (Qmci) screen and Montreal Cognitive Assessment (MoCA) in differentiating patients with movement disorders experiencing cognitive symptoms (Parkinson’s disease and Lewy Body Dementia) in separating (a) mild cognitive impairment (MCI) from normal cognition, (b) MCI and dementia, (c) normal from cognitive impairment (MCI and dementia), (d) Dementia from everything else.
DISCUSSION
This study compares the diagnostic accuracy of two short CSIs, the MoCA and the Qmci screen, in their ability to differentiate those with subjective cognitive symptoms but normal cognition from those with CI due to movement disorders (Parkinsonism and LBD). The results show that the Qmci screen had statistically significantly greater accuracy in separating normal cognition from MCI than the MoCA but equivalence for other comparisons. This is reflected by a greater and better balance between sensitivity and specificity. The Qmci screen also had a significantly shorter administration time, approximately half that of the MoCA, another key feature of an ideal CSI for this population [23].
This middle-aged and older sample was predominantly comprised of patients with idiopathic PD and despite a moderate median UPDRS (total) scores, a large proportion (75%) of this sample had CI. Given that the median time since diagnosis in this study to the date of the assessment was seven years and this is likely an under-estimation, this likely explains the high prevalence of CI found in this sample. Evidence suggests that the mean time from PD onset to PDD is approximately 10 years [54]. While this study included middle-aged adults > 40 years, the median age of the sample was much older (median age 74 years±5), likely reflecting the service (geriatrician-led clinics). Most patients, 65%, were male, consistent with the higher prevalence of movement disorders and in particular PD, among male patients [55].
The optimal cut-off for the Qmci screen for differentiating CI (MCI and dementia) and MCI from normal cognition at < 60/100 in this sample is similar to that of the established cut-off found in an Irish sample attending a memory clinic (mainly with Alzheimer’s disease related cognitive decline) [38]. It is also similar to that found in a large pooled analysis of patients in Canada [56]. It also highlights that the traditional MoCA cut-off of < 26/30 [25] is unsuitable for classifying older patients in this population. Instead, a lower cut-off of < 23/30, produced a good sensitivity of 76% and excellent specificity of 91% for CI (MCI and dementia). This lower value has been found in other studies [57] including other language versions of the MoCA and populations with lower levels of educational attainment [58].
Limitations
This study has a number of limitations. As this study was a secondary analysis of an existing database (gathered approximately ten years ago), rather than a primary data collection and thus lacks a priori power calculation, limits the available sample. This small sample size hence likely under-powers the study, limiting the inferences that can be drawn. The timing of data collection, however, is unlikely to have affected the results. This study mainly included those with idiopathic PD, who represented the majority of the attendees. This reduces the generalizability of the study for other movement disorders especially LBD. Further, the population was largely comprised of older adults, reducing the generalizability of the findings, particularly to patients attending neurology clinics. This said, movement disorders are predominantly conditions of older people [5, 6]. In addition, the study was conducted at a single center, with a homogenous population, further reducing generalizability. Another limitation is the potential for misclassification. The diagnosis of PD-MCI remains contentious with much variation in how ADLs are evaluated across studies, a challenge that is compounded by difficulty in distinguishing cognitive and motor effects of PD [59]. In this study the diagnosis was made independently and clinically by physician experts in cognition and movement disorders in conjunction with the cognitive component of the UPDRS and an established battery of cognitive assessments. While this approach is not a gold standard, it is widely used and accepted in clinical research. Furthermore, the diagnosis of cognitive impairment was sometimes based on a single cognitive assessment, which may have reduced accuracy. That said, it is argued that where clinical findings are robust, more detailed neuropsychological assessment may not be required [15]. Nevertheless, there is potential for misclassification bias. As is common to such studies, the prevalence of cognitive impairment was high in this sample (75%), potentially resulting in in spectrumbias.
Further research comparing the Qmci screen and MoCA in a larger and adequately powered sample and to other cognitive scales, as recommended by the Movement Disorder Society [28] such as the Mattis Dementia Rating Scale Second Edition [60] and the Parkinson’s Disease-Cognitive Rating Scale [61], is now required.
In conclusion, the Qmci screen is a valid measure of CI in a population with movement disorders and compares favorably with the widely-used MoCA. Indeed, it has a significantly shorter administration time and greater accuracy in separating patients with PD and LBD related MCI from those reporting subjective cognitive symptoms but with normal cognition. Given the recognized challenges of using the MoCA in this sample, in particular its floor and ceiling effects in some populations and especially those with lower levels of education [62], the Qmci screen may be the better CSI, albeit more research is required to investigate this further.
AUTHOR CONTRIBUTIONS
Rónán O’Caoimh (Conceptualization; Data curation; Formal analysis; Writing – original draft); Mary J. Foley (Investigation); Suzanne Timmons (Supervision); D. William Molloy (Supervision).
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Dr. Rónán O’Caoimh and Prof. D. William Molloy are co-copyright holders of the Quick Mild Cognitive Impairment Screen. The authors report no other conflict of interest.
DATA AVAILABILITY
Available on request.
REFERENCES
[1] | Tanner CM ((2013) ) A second honeymoon for Parkinson’s disease? NEJM 368: , 675–676. |
[2] | Wenning GK , Kiechl S , Seppi K , Müller J , Högl B , Saletu M , Rungger G , Gasperi A , Willeit J , Poewe W ((2005) ) Prevalence of movement disorders in men and women aged 50–89 years (Bruneck Study cohort): A population-based study. Lancet Neurol 4: , 815–820. |
[3] | Dorsey ER , Constantinescu R , Thompson JP , Biglan KM , Holloway RG , Kieburtz K , Marshall FJ , Ravina BM , Schifitto G , Siderowf A , Tanner CM ((2007) ) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68: , 384–386. |
[4] | Ou Z , Pan J , Tang S , Duan D , Yu D , Nong H , Wang Z ((2021) ) Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories From 1990 to 2019. Front Public Health 9: , 776847. |
[5] | Aarsland D , Andersen K , Larsen JP , Lolk A ((2003) ) Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch Neurol 60: , 387–392. |
[6] | Aarsland D , Kurz MW ((2010) ) The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol 20: , 633–639. |
[7] | Hely MA , Reid WG , Adena MA , Halliday GM , Morris JG ((2008) ) The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord 23: , 837–844. |
[8] | Fengler S , Liepelt-Scarfone I , Brockmann K , Schäffer E , Berg D , Kalbe E ((2017) ) Cognitive changes in prodromal Parkinson’s disease: A review. Mov Disord 32: , 1655–1666. |
[9] | Hogan DB , Fiest KM , Roberts JI , Maxwell CJ , Dykeman J , Pringsheim T , Steeves T , Smith EE , Pearson D , Jetté N ((2016) ) The prevalence and incidence of dementia with Lewy bodies: A systematic review. Can J Neurol Sci 43: , 83–95. |
[10] | Kane JPM , Surendranathan A , Bentley A , Barker SAH , Taylor JP , Thomas AJ , Allan LM , McNally RJ , James PW , McKeith IG , Burn DJ , O’Brien JT ((2018) ) Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 10: , 19. |
[11] | Litvan I , Aarsland D , Adler CH , Goldman JG , Kulisevsky J , Mollenhauer B , Rodriguez-Oroz MC , Tröster AI , Weintraub D ((2011) ) MDS Task Force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord 26: , 1814–1824. |
[12] | Litvan I , Goldman JG , Tröster AI , Schmand BA , Weintraub D , Petersen RC , Mollenhauer B , Adler CH , Marder K , Williams-Gray CH , Aarsland D , Kulisevsky J , Rodriguez-Oroz MC , Burn DJ , Barker RA , Emre M ((2012) ) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27: , 349–356. |
[13] | Yarnall AJ , Breen DP , Duncan GW , Khoo TK , Coleman SY , Firbank MJ , Nombela C , Winder-Rhodes S , Evans JR , Rowe JB , Mollenhauer B , Kruse N , Hudson G , Chinnery PF , O’Brien JT , Robbins TW , Wesnes K , Brooks DJ , Barker RA , Burn DJ ; ICICLE-PD Study Group ((2014) ) Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology 82: , 308–316. |
[14] | Pedersen K , Larsen J , Tysnes O , Alves G ((2013) ) Prognosis of mild cognitive impairment in early Parkinson disease: The Norwegian Park West Study. JAMA Neurol 70: , 580–586. |
[15] | Sitek EJ , Sławek J , Evans JJ ((2014) ) Mild cognitive impairment in Parkinson’s disease: How much testing is needed for correct diagnosis? Basal Ganglia 4: , 89–94. |
[16] | Petrova M , Raycheva M , Zhelev Y , Traykov L ((2010) ) Executive functions deficit in Parkinson’s disease with amnestic mild cognitive impairment. Am J Alzheimers Dis Other Demen 25: , 455–460. |
[17] | Ferman TJ , Smith GE , Kantarci K , Boeve BF , Pankratz VS , Dickson DW , Graff-Radford NR , Wszolek Z , Van Gerpen J , Uitti R , Pedraza O , Murray ME , Aakre J , Parisi J , Knopman DS , Petersen RC ((2013) ) Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology 81: , 2032–2038. |
[18] | Hemminghyth MS , Chwiszczuk LJ , Rongve A , Breitve MH ((2020) ) The cognitive profile of mild cognitive impairment due to dementia with Lewy bodies-an updated review. Front Aging Neurosci 12: , 597579. |
[19] | Keener AM , Paul KC , Folle A , Bronstein JM , Ritz B ((2018) ) Cognitive impairment and mortality in a population-based Parkinson’s disease cohort. J Parkinsons Dis 8: , 353–362. |
[20] | Leroi I , McDonald K , Pantula H , Harbishettar V ((2012) ) Cognitive impairment in Parkinson disease: Impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 25: , 208–214. |
[21] | Desai U , Chandler J , Kirson N , Georgieva M , Cheung HC , Westermeyer B , Lane H , Biglan H ((2022) ) Epidemiology and economic burden of Lewy body dementia in the United States. Curr Med Res Opin 38: , 1177–1188. |
[22] | Boise L , Camicioli R , Morgan DL , Rose JH , Congleton L ((1999) ) Diagnosing dementia: Perspectives of primary care physicians. Gerontologist 39: , 457–464. |
[23] | Burn D , Weintraub D , Ravina B , Litvan I ((2014) ) Cognition in movement disorders: Where can we hope to be in ten years? Mov Disord 29: , 704–711. |
[24] | Marras C , Tröster AI , Kulisevsky J , Stebbins GT ((2014) ) The tools of the trade: A state of the art “How to Assess Cognition” in the patient with Parkinson’s disease. Mov Disord 29: , 584–596. |
[25] | Nasreddine ZS , Phillips NA , Bedirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[26] | Emre M , Aarsland D , Brown R , Burn DJ , Duyckaerts C , Mizuno Y , Broe GA , Cummings J , Dickson DW , Gauthier S , Goldman J , Goetz C , Korczyn A , Lees A , Levy R , Litvan I , McKeith I , Olanow W , Poewe W , Quinn N , Sampaio C , Tolosa E , Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22: , 1689–1707. |
[27] | Barton B , Grabli D , Bernard B , Czernecki V , Goldman JG , Stebbins G , Dubois B , Goetz CG ((2012) ) Clinical validation of Movement Disorder Society-recommended diagnostic criteria for Parkinson’s disease with dementia. Mov Disord 27: , 248–253. |
[28] | Skorvanek M , Goldman JG , Jahanshahi M , Marras C , Rektorova I , Schmand B , van Duijn E , Goetz CG , Weintraub D , Stebbins GT , Martinez-Martin P ; members of the MDS Rating Scales Review Committee ((2018) ) Global scales for cognitive screening in Parkinson’s disease: Critique and recommendations. Mov Disord 33,: , 208–218. |
[29] | Zadikoff C , Fox SH , Tang-Wai DF , Thomsen T , de Bie RM , Wadia P , Miyasaki J , Duff-Canning S , Lang AE , Marras C ((2008) ) A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease.. Mov Disord 23: , 297–299. |
[30] | Burdick DJ , Cholerton B , Watson GS , Siderowf A , Trojanowski JQ , Weintraub D , Ritz B , Rhodes SL , Rausch R , Factor SA , Wood-Siverio C , Quinn JF , Chung KA , Srivatsal S , Edwards KL , Montine TJ , Zabetian CP , Leverenz JB ((2014) ) People with Parkinson’s disease and normal MMSE score have a broad range of cognitive performance. Mov Disord 29: , 1258–1264. |
[31] | López-Álvarez J , Sevilla-Llewellyn-Jones J , Agüera-Ortiz L ((2019) ) Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci 13: , 1309. |
[32] | Rosenblum S , Meyer S , Gemerman N , Mentzer L , Richardson A , Israeli-Korn S , Livneh V , Karmon TF , Nevo T , Yahalom G , Hassin-Baer S ((2020) ) The Montreal Cognitive Assessment: Is it suitable for identifying mild cognitive impairment in Parkinson’s disease? Mov Disord Clin Pract 7: , 648–655. |
[33] | Liebermann-Jordanidis H , Roheger M , Boosfeld L , Franklin J , Kalbe E ((2022) ) Which test is the best to assess visuo-cognitive impairment in patients with Parkinson’s disease with mild cognitive impairment and dementia? A systematic review and meta-analysis. J Parkinsons Dis 12: , 1749–1782. |
[34] | Chou KL , Amick MM , Brandt J , Camicioli R , Frei K , Gitelman D , Goldman J , Growdon J , Hurtig HI , Levin B , Litvan I , Marsh L , Simuni T , Tröster AI , Uc EY ; Parkinson Study Group Cognitive/Psychiatric Working Group ((2010) ) A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord 25: , 2501–2507. |
[35] | Ohta K , Takahashi K , Gotoh J , Yamaguchi K , Seki M , Nihei Y , Iwasawa S , Suzuki N ; Keio Parkinson’s Disease Database Collaborators ((2014) ) Screening for impaired cognitive domains in a large Parkinson’s disease population and its application to the diagnostic procedure for Parkinson’s disease dementia. Dement Geriatr Cogn Dis Extra 4: , 147–159. |
[36] | Davis DH , Creavin ST , Yip JL , Noel-Storr AH , Brayne C , Cullum S ((2021) ) Montreal Cognitive Assessment for the detection of dementia. Cochrane Database Syst Rev 7: , CD010775. |
[37] | Federico A , Tinazzi M , Tamburin S ((2018) ) MoCA for cognitive screening in Parkinson’s disease: Beware of floor effect. Mov Disord 33: , 499. |
[38] | O’Caoimh R , Timmons S , Molloy DW ((2016) ) Screening for mild cognitive impairment: Comparison of “MCI specific” screening instruments. J Alzheimers Dis 51: , 619–629. |
[39] | Clarnette R , O’Caoimh R , Antony DN , Svendrovski A , Molloy DW ((2017) ) Comparison of the Quick Mild Cognitive Impairment (Qmci) screen to the Montreal Cognitive Assessment (MoCA) in an Australian geriatrics clinic. Int J Geriatr Psychiatry 32: , 643–649. |
[40] | O’Caoimh R , Gao Y , McGlade C , Healy L , Gallagher P , Timmons S , Molloy DW ((2012) ) Comparison of the Quick Mild Cognitive Impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 41: , 624–629. |
[41] | Sachdev PS , Blacker D , Blazer DG , Ganguli M , Jeste DV , Paulsen JS , Petersen RC ((2014) ) Classifying neurocognitive disorders: The DSM-5 approach. Nat Rev Neurol 10: , 634–642. |
[42] | McKeith IG , Dickson DW , Lowe J , Emre M , O’Brien JT , Feldman H , Cummings J , Duda JE , Lippa C , Perry EK , Aarsland D , Arai H , Ballard CG , Boeve B , Burn DJ , Costa D , Del Ser T , Dubois B , Galasko D , Gauthier S , Goetz CG , Gomez-Tortosa E , Halliday G , Hansen LA , Hardy J , Iwatsubo T , Kalaria RN , Kaufer D , Kenny RA , Korczyn A , Kosaka K , Lee VM , Lees A , Litvan I , Londos E , Lopez OL , Minoshima S , Mizuno Y , Molina JA , Mukaetova-Ladinska EB , Pasquier F , Perry RH , Schulz JB , Trojanowski JQ , Yamada M ; Consortium on DLB ((2005) ) Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 27: , 1863–1872. |
[43] | Marc LG , Raue PJ , Bruce ML ((2008) ) Screening performance of the Geriatric Depression Scale (GDS-15) in a diverse elderly home care population. Am J Geriatr Psychiatry 16: , 914–921. |
[44] | Weintraub D , Oehlberg KA , Katz IR , Stern MB ((2006) ) Test characteristics of the 15-Item Geriatric Depression Scale and Hamilton Depression Rating Scale in Parkinson disease. Am J Geriatr Psychiatry 14: , 169–175. |
[45] | Kao AW , Racine CA , Quitania LC , Kramer JH , Christine CW , Miller BL ((2009) ) Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy. Alzheimer Dis Assoc Disord 23: , 365. |
[46] | Yamane Y , Sakai K , Maeda K ((2011) ) Dementia with Lewy bodies is associated with higher scores on the Geriatric Depression Scale than is Alzheimer’s disease. Psychogeriatrics 11: , 157–165. |
[47] | Molloy DW , Standish TIM ((1997) ) A guide to the Standardized Mini-Mental State Examination. Int Psychogeriatr 9: , 87–94. |
[48] | Galvin JE , Rose CM , Powlishta KK , Coats MA , Muich SJ , Grant E , Miller JP , Storandt M , Morris JC ((2005) ) The AD8. A brief informant interview to detect dementia. Neurology 65: , 559–564. |
[49] | Jorm AF ((1994) ) A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychol Med 24: , 145–153. |
[50] | O’Caoimh R , Gao Y , Gallagher P , Eustace J , McGlade C , Molloy DW ((2013) ) Which part of the Quick mild cognitive impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing 42: , 324–330. |
[51] | Ebersbach G , Baas H , Csoti I , Müngersdorf M , Deuschl G ((2006) ) Scales in Parkinson’s disease. J Neurol 253: , 432–435. |
[52] | DeLong ER , DeLong DM , Clarke-Pearson DL ((1988) ) Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: , 837–845. |
[53] | Youden WJ ((1950) ) Index for rating diagnostic tests. Cancer 3: , 32–35. |
[54] | Aarsland D , Kvaløy JT , Andersen K , Larsen JP , Tang MX , Lolk A , Kragh-Sørensen P , Marder K ((2007) ) The effect of age of onset of PD on risk of dementia. J Neurology 254: , 38–45. |
[55] | Cerri S , Mus L , Blandini F ((2019) ) Parkinson’s disease in women and men: What’s the difference? J Parkinsons Dis 9: , 501–515. |
[56] | O’Caoimh R , Gao Y , Svendovski A , Gallagher P , Eustace J , Molloy DW ((2017) ) Comparing approaches to optimize cut-off scores for short cognitive screening instruments in mild cognitive impairment and dementia. J Alzheimers Dis 57: , 123–133. |
[57] | Marras C , Armstrong MJ , Meaney CA , Fox S , Rothberg B , Reginold W , Tang-Wai DF , Gill D , Eslinger PJ , Zadikoff C , Kennedy N , Marshall FJ , Mapstone M , Chou KL , Persad C , Litvan I , Mast BT , Gerstenecker AT , Weintraub S , Duff-Canning S ((2013) ) Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov Disord 28: , 626–633. |
[58] | Almeida KJ , Carvalho LCLS , Monteiro THOH , Gonçalves PCJ , Campos-Sousa RN ((2019) ) Cut-off points of the Portuguese version of the Montreal Cognitive Assessment for cognitive evaluation in Parkinson’s disease. Dement Neuropsychol 13: , 210–215. |
[59] | Goldman JG , Aggarwal NT , Schroeder CD ((2015) ) Mild cognitive impairment: An update in Parkinson’s disease and lessons learned from Alzheimer’s disease.. Neurodegener Dis Manag 5: , 425–443. |
[60] | Rosca EC , Simu M ((2020) ) Parkinson’s Disease-Cognitive Rating Scale for evaluating cognitive impairment in Parkinson’s disease: A systematic review. Brain Sci 10: , 588. |
[61] | Matteau E , Dupré N , Langlois M , Jean L , Thivierge S , Provencher P , Simard M ((2011) ) Mattis Dementia Rating Scale 2: Screening for MCI and dementia. Am J Alzheimers Dis Other Demen 26: , 389–98. |
[62] | Koshimoto BHB , Brandão PRP , Borges V , Ferraz HB , Schumacher-Schuh AF , Rieder CRM , Olchik MR , Mata IF , Tumas V , Santos-Lobato BL ((2023) ) Floor and ceiling effects on the Montreal Cognitive Assessment in patients with Parkinson’s disease in Brazil. Dement Neuropsychol 17: , e20230022. |




