Long Objective Sleep Duration is a Marker of Cognitive Impairment in Older Adults: Findings from the Cretan Aging Cohort
Abstract
We examined associations between objective sleep duration and cognitive status in older adults initially categorized as cognitively non-impaired (CNI, n = 57) or diagnosed with mild cognitive impairment (MCI, n = 53). On follow-up, 8 years later, all participants underwent neuropsychiatric/neuropsychological evaluation and 7-day 24-h actigraphy. On re-assessment 62.7% of participants were cognitively declined. Patients who developed dementia had significantly longer night total sleep time (TST) than persons with MCI who, in turn, had longer night TST than CNI participants. Objective long sleep duration is a marker of worse cognitive status in elderly with MCI/dementia and this association is very strong in older adults.
INTRODUCTION
The demographic shift towards an older population is a defining characteristic of modern Western societies. By 2050, estimates suggest that more than 20% of the population will be in the 65 or older age group [1]. Human aging is inevitably associated with a decline in physiological reserves and increased inflammation known as “inflammaging”, which in turn leads to various chronic diseases, such as cardiovascular disease, dementia, and increased mortality [2].
Dementia is a highly prevalent syndrome characterized by the loss in cognitive functioning that exceeds the natural aging process [3, 4], impacting one’s capacity for independent daily activities [5]. Mild cognitive impairment (MCI) is considered as a transitional phase between normal cognitive aging and dementia [6]. While MCI does not significantly impede daily activities and independence of older adults, approximately 40% of individuals with MCI will eventually develop dementia [7].
The risk of dementia and cognitive impairment varies greatly within a uniform age group, underscoring the importance of accurate predictors to characterize the progression of dementia [8]. There is evidence to suggest that dementia may be influenced by a range of modifiable factors, including obesity, sleep duration and quality, levels of physical activity, diet, social interactions, and the presence of co-morbidities [9]. Given that approximately 40% of dementia risk factors are modifiable, there is a strong potential for prevention [10].
Importantly, a significant association has been observed between poor sleep and cognitive impairment in elderly individuals [11]. Evidence further indicates that sleep complaints serve as powerful predictors of excessive daytime cognitive fatigue, which has detrimental effects on multiple aspects of cognition [12]. Nonetheless, there are gaps in the current body of literature exploring associations between sleep and cognition, as most of the studies conducted in older adults rely on self-reported sleep data [13–15], which may differ from objective sleep measures [16]. Moreover, previous research on cognitive function and sleep mainly studied cognitively healthy older individuals [17] and did not confirm these findings in people with MCI. A number of studies examining the associations between multiple facets of objective sleep measures and cognitive performance in these patients have yielded inconclusive results [18–25], probably due to variations in methodological approaches, such as differences in age range, sample sizes, and the specific cognitive domains assessed [26]. Therefore, the long-term impact of sleep on cognitive impairment yet remains unclear. The scarcity of relevant literature is particularly evident in Mediterranean countries like Greece. With a Greek population where over 20% are aged 60 years or older [27], it is estimated that 5% of individuals 65 and above are affected by dementia [28]. Our previous findings from the Cretan Aging Cohort study (CAC) indicate that, even though sleep duration is similar between cognitively non-impaired (CNI) elders and patients with MCI, long sleep duration is linked to greater disease severity in patients with MCI and dementia [29]. Furthermore, longitudinal findings suggest that prolonged sleep and/or poor sleep quality in elderly predicts poor memory outcomes in this population [30]. To our knowledge, up to now no studies have examined the associations between cognitive status and objective sleep in older adults with or without cognitive deficits. To fill in the gap in the existing literature, the objective of the current study was to investigate the association between sleep duration and cognitive status in adults in a more advanced age (≥68 years old) in a community-dwelling sub-sample of the CAC, both in CNI and in those with MCI who were followed-up 8 years after the initial assessment. We hypothesize that objective long sleep is associated with worse cognitive status in older adults.
MATERIALS AND METHODS
Study design
In this cross-sectional study, we analyzed, a subset of 110 participants from the CAC (Phase III), a large cohort study conducted on the island of Crete, Greece, who had undergone follow-up within a period of 7–9 years from the initial evaluation (phase I & II). All participants underwent comprehensive neuropsychological and neuropsychiatric assessment, along with actigraphy recording during baseline (Phase I and II) and follow-up (Phase III). The current study protocol (Phase III) was approved by the Ethics Committee of the University of Crete (approval number: 61/9-3-2020).
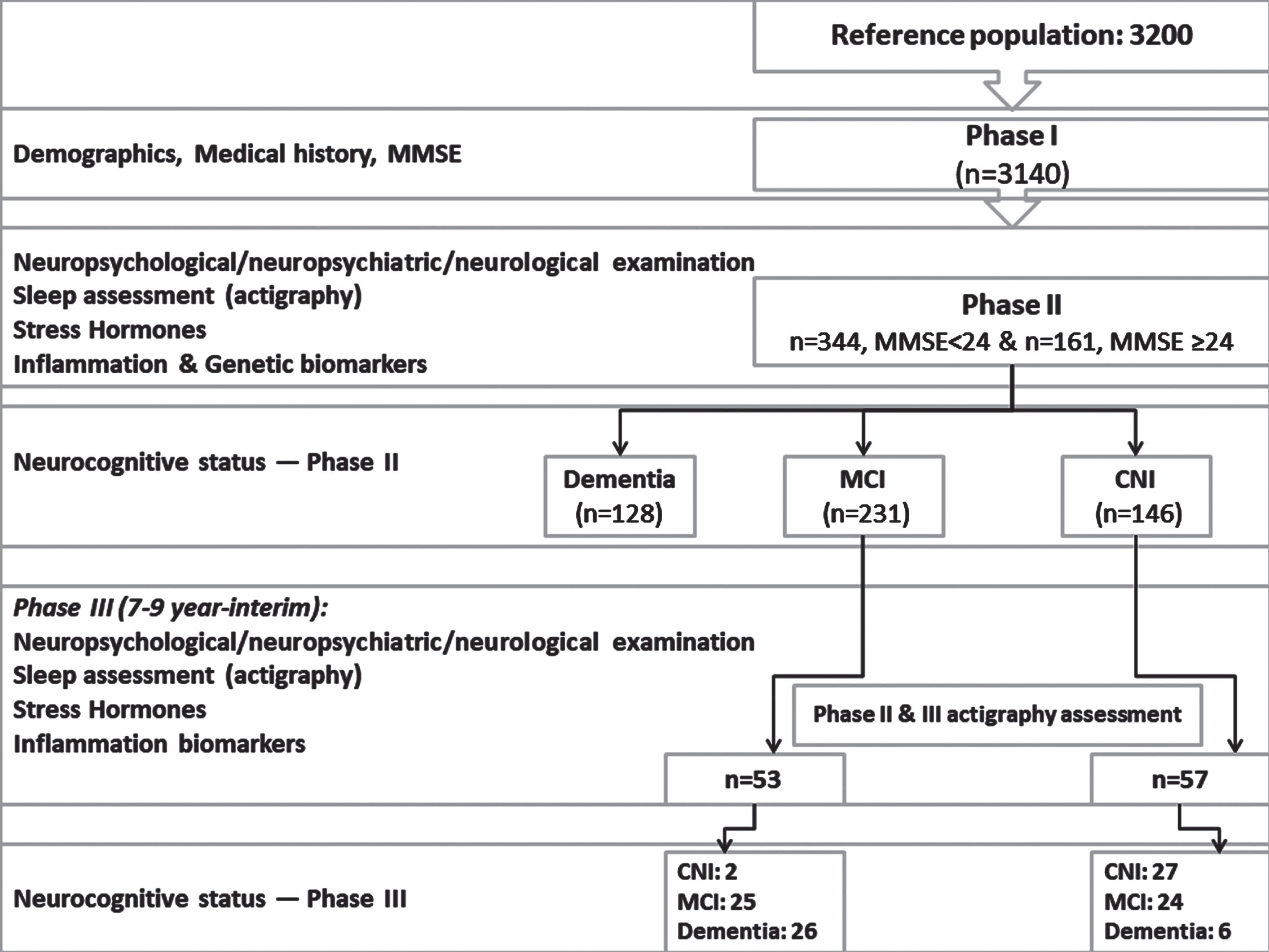
A comprehensive overview of Phases I, II, and III can be found in previous publications [29, 31, 32] (Fig. 1 and Supplementary Material).
Fig. 1
Flow diagram depicting the study protocol (Phase I, II, and III). Clinical categorization of the current sample (n = 110) in Phase II and III is also provided.
Sociodemographic and medical information
Comprehensive records were maintained for anthropometric measurements, comorbidities, use of SSRIs, benzodiazepines, and other anxiolytics. Presence of at least one insomnia-type symptom (i.e., difficulty initiating/maintaining sleep and/or early morning awakening) and sleep apnea was estimated based on the Penn State Sleep Questionnaire [33].
Objective sleep measures
Objective sleep variables were estimated by analyzing consecutive 7-days actigraphy recording at follow-up [30, 32]. The sleep variables estimated were night sleep efficiency (SE), night wake after sleep onset time (WASO), night and 24-h total sleep time (TST) and finally, night and 24-h time spent in bed (TiB). Following our previous publication [34], we categorized the sample into quartiles. We then further divided it into two groups: the long sleep duration group, consisting of the top 25% of individuals with sleep times above the median, and the normal sleep duration group, consisting of the remaining 75% in the lower half.
Statistical analysis
Statistical analysis was conducted using SPSS 29 (IBM SPSS, Version 29.0, Armonk, NY: IBM Corp). Continuous variables at follow-up are depicted as means and standard deviations, while categorical variables are displayed as frequencies and percentages. To analyze group differences in demographic characteristics, we employed a two-tailed t-test for continuous variables and the Chi-square test for categorical variables. The three groups were compared on sleep indices at follow-up using Analysis of Covariance (ANCOVA) controlling for gender, age, education, depression, and psychotropic medications. We followed the standard step-wise approach in order to control for type I error, which involved performing omnibus tests evaluated at p = 0.008 to control for the number of ANOVAs (k = 6). Only significant omnibus ANOVAs were followed up by pairwise comparisons which were in turn evaluated at p = 0.05/3 = 0.017 to control for family-wise error rate.
RESULTS
As previously described in detail [32], during Phase III in total, 103 participants were deceased, 56 could not be located and 63 refused to participate in the study. A total of 155 individuals completed the evaluation in phase III (response rate 55%). Among those a sub-sample of 110 participants (77.3% women) with complete sleep data were included in the current analysis.
The participants were initially classified (Phase I and II) as CNI (n = 57) or MCI (n = 53), with a mean baseline age of 72.7 years. The patients were followed up 7–9 years later, with an average age at follow-up of 80.3 (SD = 6.6) years. Upon re-evaluation, cognitive decline was observed in 62.7% of the participants. Specifically, 29 (26%) individuals were diagnosed with CNI, 49 (45%) with MCI, and 32 (29%) with dementia (probable Alzheimer’s disease except for two patients who were diagnosed with probable Lewy body dementia) (Fig. 1). In the MCI group, 11 patients met criteria for the pure amnestic type (22.4%), 21 for the amnestic-multidomain type (42.9%) and 17 (34.7%) for the non-amnestic MCI type. The demographic, clinical data, and sleep metrics at follow-up for each diagnostic group are presented in Table 1. Patients with dementia were significantly older followed by those in the MCI group who were in turn older than the CNI group. MCI and dementia groups had comparable education attainment, which was significantly lower than the CNI group. The three groups were comparable on gender, physical comorbidities and frequency of insomnia-type symptoms (p > 0.1) although, as expected, patients with dementia were more likely to receive psychotropic medications than the CNI group (p = 0.02).
Table 1
Sociodemographic, clinical characteristics, and sleep indices of the three groups at Phase III (follow-up)
| A. CNI (n = 29) | B. MCI (n = 49) | C. Dementia (n = 32) | A vs C | A vs B | B vs C | |
| Demographics | ||||||
| Age (y) | 76.8 (6.0) | 79.7 (6.2) | 85.0 (5.5) | 0.04 | <0.001 | <0.001 |
| Education (y) | 6.4 (3.5) | 4.6 (2.9) | 4.4 (3.1) | 0.01 | 0.03 | 0.8 |
| Females (%) | 72.4 | 83.7 | 71.9 | 0.2 | 0.8 | 0.1 |
| Psychotropic Medications (%)1 | 48.3 | 46.9 | 71.4 | 0.9 | 0.08 | 0.02 |
| ≥5 Physical Comorbidities (%) | 20.7 | 22.4 | 19.0 | 0.9 | 0.9 | 0.7 |
| Depression Diagnosis (%) | 20.7 | 40.8 | 52.9 | 0.09 | 0.01 | 0.3 |
| Objective sleep indices | ||||||
| Sleep efficiency | 86±5 | 86±6 | 86±7 | 0.9 | 0.4 | 0.8 |
| Night TST (min) | 415±58 | 451±68 | 503±93 | <0.001 | 0.01 | 0.03 |
| 24-h TST (min) | 435±61 | 469±78 | 522±106 | 0.001 | 0.02 | 0.06 |
| Night TiB (min) | 482±58 | 522±72 | 580±82 | <0.001 | 0.006 | 0.01 |
| 24-h TiB (min) | 506±60 | 545±83 | 608±94 | <0.001 | 0.02 | 0.03 |
| WASO (min) | 61±22 | 65±31 | 73±38 | 0.1 | 0.8 | 0.5 |
| Subjective sleep complaints | ||||||
| ≥2 insomnia symptoms (%) | 27.6 | 28.6 | 23.1 | 0.9 | 0.7 | 0.6 |
Data are presented as mean values±SD unless otherwise indicated, adjusted for age, education, gender, and psychotropic medications. 1SSRIs, anxiolytics, benzodiazepines. TST, Total Sleep Time; TiB, Time in Bed; WASO, Wake after sleep onset time.
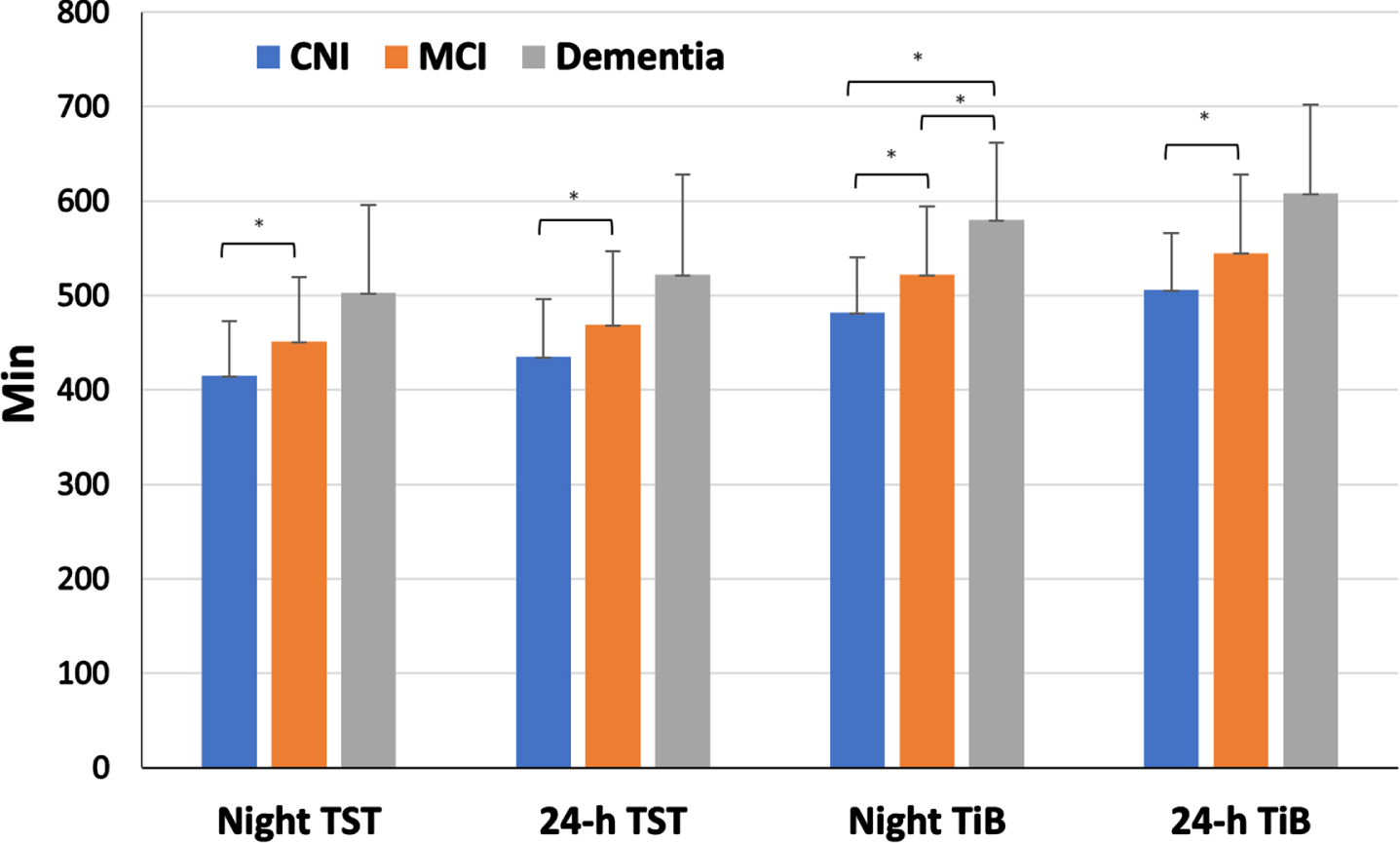
One-way ANCOVAs revealed significant group differences on all sleep duration and time in bed indices (Night TST: p = 0.001, 24-h TST: p = 0.006, Night TiB: p < 0.001, 24-h TiB: p = 0.001, adjusting for age, education, gender, and psychotropic medications (Fig. 2). As shown in Table 1, individuals with dementia had significantly prolonged, adjusted night total sleep time (503±93 min) in comparison to those with MCI (451±68 min, p = 0.03), who, in turn, had longer night TST than CNI participants (415±58 min, p = 0.01). Similar trends were found for 24 h sleep and time in bed times. In addition, there was a significantly higher prevalence of long night sleep duration in the dementia (67.7%) as compared to the MCI (36.7%, p = 0.01) and CNI groups (10.3%, p < 0.001). Long night sleep duration was also more frequent in the MCI as compared to the CNI group (p = 0.016).
Fig. 2
Average sleep duration values across clinical groups. *corresponds to statistically significant differences between groups.
DISCUSSION
The current study aimed to investigate the potential correlation between objective sleep duration and cognitive status in older adults initially diagnosed with CNI and MCI, after a follow-up period of 8 years. The main findings of our study indicated that among older adults, patients with dementia had significantly longer night TST than persons with MCI who in turn had longer night TST than cognitively non-impaired participants. In addition, the prevalence of long sleep duration during the night was significantly higher among dementia patients and individuals with MCI as compared to CNI individuals in this age group.
The novel finding of this study is the robust and consistent over time negative correlation between objective long sleep duration and cognitive performance in elderly with or without cognitive impairment. Our study confirms and expands prior evidence, indicating that long objective sleep duration is linked to cognitive impairment among elderly with dementia and MCI. Studies in the past have reported prolonged sleep among patients with dementia with sleep duration becoming longer in more severe stages of the disease [29, 35, 36, 37]. However, previous literature examining objective sleep duration in MCI patients and its associations with cognitive performance is very limited. While earlier studies in small samples have yielded inconsistent findings, a previous cross-sectional study from the CAC found a correlation between long sleep duration and worse cognitive performance among elderly individuals with MCI [29]. Interestingly, in that study, mean sleep duration was similar between MCI patients and cognitively intact elders. The current study replicated associations between sleep and cognitive status in participants older than 75 years old. Additionally, these associations became more robust since, in this more advanced age group, sleep duration was significantly longer in MCI compared to cognitively intact elderly. Finally, based on our findings, it appears that objective long sleep is an index of cognitive function consistent over a rather long-term period. Some, but not all, longitudinal studies have also confirmed the role of objective sleep as a predictor of cognitive decline among elderly. Findings from a large community-based study among older women revealed that objective long sleep at baseline predicted cognitive decline at follow-up [24], while other studies failed to find such associations [38, 39]. Also, a recent study from our group confirmed that prolonged sleep and/or poor sleep quality predict unfavorable long-term memory outcomes in old participants from the CAC after an 8-year follow-up [30].
There are a number of potential explanations for the relationship between long sleep duration and the risk of dementia and MCI. To begin with, there is strong evidence linking sleep disturbances to amyloid-β (Aβ) deposition, tau pathology, and APOE ɛ4 allele genotype, all of which have the potential to be biomarkers for dementia [40–42]. Interestingly, research conducted on older adults without cognitive impairments demonstrated that sleep disruptions influenced the relationship between Aβ and cognitive function, potentially due to disruptions in non-rapid eye movement (NREM) slow wave sleep [43–45]. On the other hand, disturbance in NREM sleep may also lead to increased accumulation of Aβ, and the deposition of Aβ in the cortex has been linked to memory consolidation deficits [46]. Additionally, there appears to be an association between chronic, low-grade inflammation characterized by higher levels of interleukin (IL)-6 and C-reactive protein (CRP) and an increased likelihood of developing all-cause dementia [47, 48]. Considering the results of Irwin et al. meta-analysis, which showed that longer sleep duration is associated with higher levels of CRP and IL-6, it reinforces the potential association between long sleep duration, increased inflammation, and dementia also previously confirmed in elderly with or without cognitive decline from our sample [34, 49, 50]. Finally, individuals who consistently sleep for longer periods may have other underlying health conditions, such as depression and sleep apnea, that could potentially contribute to cognitive deficits. Despite adjusting for multiple co-morbidities, including depression and sleep apnea in our analysis, there is a possibility that other diseases or under-recognized health-related conditions were not accounted for.
The outcomes of the current study shed light on the significant association between sleep duration and cognitive performance. A prolonged duration of sleep in older adults could potentially serve as an early warning sign for cognitive decline, such as the development of MCI and later dementia. Therefore, based on our findings, in elderly with prolonged sleep, prevention of controlling modifiable factors associated with cognitive decline should be considered. Also, based on the association between inflammation and cognitive performance, treatments targeting inflammatory pathways, as well as interventions known to decrease inflammatory levels, such as diet, and physical activity, may be useful [50]. Finally, since long sleep is associated with worse cognitive performance, restricted use of sedative psychotropics during the pre-clinical or early stage of the disease as well as throughout its progression, is suggested. At the same time, addressing key factors that may negatively impact sleep, such as multiple co-morbidities, emotional or anxiety disorders, chronic pain, and sleep-related breathing disorders is crucial.
The strengths of the study include a well-characterized sample based on thorough neuropsychiatric/neuropsychological evaluation, objective sleep assessment for a rather long period (7–24-h actigraphy), and naturalistic design. Limitations of the study that should be pointed out are the rather small sample size, its cross-sectional design, use of actigraphy and not PSG to assess objective sleep, and a rather low response rate compared to the previous evaluation related to the advanced age of our participants.
In conclusion, our study confirms and expands previous findings that objective long sleep duration is a marker of worse cognitive status in elderly with or without cognitive decline in a dose-response manner and that this association is more evident in older adults. Therefore, in elderly who sleep long, early interventions targeting on potential modifiable factors, may improve or delay cognitive decline.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
National Strategic Reference Framework (NSRF)-Research Funding Program: THALES entitled “UOC-Multidisciplinary network for the study of Alzheimer’s Disease” Grant Code: MIS 377299.
HELLENIC FOUNDATION FOR RESEARCH AND INNOVATION (HFRI)- Research Funding Program: ELIDEK entitled “Sleep Apnea (OSA) and poor sleep as Risk Factors for decreased cognitive performance in patients with Mild Cognitive Impairment: the Cretan Aging Cohort (CAC)”, Grant Code: HFRl-FM17-4397.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230203.
REFERENCES
[1] | Merlino G , Piani A , Gigli GL , Cancelli I , Rinaldi A , Baroselli A , Serafini A , Zanchettin B , Valente M ((2010) ) Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Med 11: , 372–327. |
[2] | Troen B ((2003) ) The Biology of aging. Mt Sinai J Med 70: , 3–22. |
[3] | Shin JH ((2022) ) Dementia Epidemiology Fact Sheet 2022. Ann Rehabil Med 46: , 53–59. |
[4] | World Health Organization. Geneva, Switzerland: World Health Organization; 2021. Fact sheets of dementia [Internet] [cited 2022 Apr 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia. Posted 15 March 2023. Accessed 14 December 2023. |
[5] | Am Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5) Washington, DC: Am Psychiatric Association. |
[6] | Knopman DS , Beiser A , Machulda MM , Fields J , Roberts RO , Pankratz VS , Aakre J , Cha RH , Rocca WA , Mielke MM , Boeve BF , Devine S , Ivnik RJ , Au R , Auerbach S , Wolf PA , Seshadri S , Petersen RC ((2015) ) Spectrum of cognition short of dementia: Framingham heart study and Mayo clinic study of aging. Neurology 85: , 1712–1721. |
[7] | Mitchell AJ , Shiri-Feshki M ((2009) ) Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand 119: , 252–265. |
[8] | Basu T , Sehar U , Malhotra K , Culberson J , Khan H , Morton H , Orlov E , Brownell M , Reddy PH ((2023) ) Healthy brain aging and delayed dementia in Texas rural elderly. Ageing Res Rev 91: , 102047. |
[9] | Yannakoulia M , Kontogianni M , Scarmeas N ((2015) ) Cognitive health and Mediterranean diet: Just diet or lifestyle pattern? Ageing Res Rev 20: , 74–78. |
[10] | De Looze C , Feeney J , Seeher KM , Amuthavalli Thiyagarajan J , Diaz T , Kenny RA ((2023) ) Assessing cognitive function in longitudinal studies of ageing worldwide: Some practical considerations. Age Ageing 52: (Suppl 4), iv13–iv25. |
[11] | Chiu HY , Lai FC , Chen PY , Tsai PS ((2016) ) Differences Between Men and Women Aged 65 and Older in the Relationship Between Self-Reported Sleep and Cognitive Impairment: A Nationwide Survey in Taiwan. J Am Geriatr Soc 64: , 2051–2058. |
[12] | Mohan J , Xiaofan G , Yingxian S ((2017) ) Association between sleep time and depression: A cross-sectional study from countries in rural Northeastern China. J Int Med Res 45: , 984–992. |
[13] | Kyle SD , Sexton CE , Feige B , Luik AI , Lane J , Saxena R , Anderson SG , Bechtold DA , Dixon W , Little MA , Ray D , Riemann D , Espie CA , Rutter MK , Spiegelhalder K ((2017) ) Sleep and cognitive performance: Cross-sectional associations in the UK Biobank. Sleep Med 38: , 85–91. |
[14] | Lo JC , Groeger JA , Cheng GH , Dijk DJ , Chee MW ((2016) ) Self-reported sleep duration and cognitive performance in older adults: A systematic review and meta-analysis. Sleep Med 17: , 87–98. |
[15] | Wild CJ , Nichols ES , Battista ME , Stojanoski B , Owen AM ((2018) ) Dissociable effects of self-reported daily sleep duration on high-level cognitive abilities. Sleep 41,: , zsy182. |
[16] | Thurman SM , Wasylyshyn N , Roy H , Lieberman G , Garcia JO , Asturias A , Okafor GN , Elliott JC , Giesbrecht B , Grafton ST , Mednick SC , Vettel JM ((2018) ) Individual differences in compliance and agreement for sleep logs and wrist actigraphy: A longitudinal study of naturalistic sleep in healthy adults. PLoS One 13: , e0191883. |
[17] | Qin S , Leong RLF , Ong JL , Chee MWL ((2023) ) Associations between objectively measured sleep parameters and cognition in healthy older adults: A meta-analysis. Sleep Med Rev 67: , 101734. |
[18] | Maestri M , Carnicelli L , Tognoni G , Di Coscio E , Giorgi FS , Volpi L , Economou NT , Ktonas P , Ferri R , Bonuccelli U , Bonanni E ((2015) ) Non-rapid eye movement sleep instability in mild cognitive impairment: A pilot study. Sleep Med 16: , 1139–1145. |
[19] | Wei R , Ganglberger W , Sun H , Hadar P , Gollub RL , Pieper S , Billot B , Au R , Iglesias JE , Cash SS , Kim S , Shin C , Westover MB , Thomas RJ ((2023) ) Linking brain structure, cognition, and sleep: Insights from clinical data. Sleep 47: (2), zsad294. |
[20] | Boa Sorte Silva NC , Falck RS , Chan PCY , Tai D , Backhouse D , Stein R , Liu-Ambrose T ((2022) ) The association of sleep and cortical thickness in mild cognitive impairment. Exp Gerontol 167: , 111923. |
[21] | Guarnieri B , Maestri M , Cucchiara F , Lo Gerfo A , Schirru A , Arnaldi D , Mattioli P , Nobili F , Lombardi G , Cerroni G , Bartoli A , Manni R , Sinforiani E , Terzaghi M , Arena MG , Silvestri R , La Morgia C , Di Perri MC , Franzoni F , Tognoni G , Mancuso M , Sorbi S , Bonuccelli U , Siciliano G , Faraguna U , Bonanni E ((2020) ) Multicenter study on sleep and circadian alterations as objective markers of mild cognitive impairment and Alzheimer’s disease reveals sex differences. J Alzheimers Dis 78: , 1707–1719. |
[22] | Hita-Yañez E , Atienza M , Cantero JL ((2013) ) Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep 36: , 1327–1334. |
[23] | Kim S , Lee J , Lee D , Jhoo J , Woo J ((2011) ) Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry 19: , 374–381. |
[24] | Spira AP , Stone KL , Redline S , Ensrud KE , Ancoli-Israel S , Cauley JA , Yaffe K ((2017) ) Actigraphic sleep duration and fragmentation in older women: Associations with performance across cognitive domains. Sleep 40: , zsx073. |
[25] | Hayes TL , Riley T , Mattek N , Pavel M , Kaye JA ((2014) ) Sleep habits in mild cognitive impairment. Alzheimer Dis Assoc Disord 28: , 145–150. |
[26] | Dzierzewski JM , Perez E , Ravyts SG , Dautovich N ((2022) ) Sleep and cognition: A narrative review focused on older adults. Sleep Med Clin 17: , 205–222. |
[27] | Statista (2023) Greece: Age distribution from 2012 to 2022. Available at: https://www.statista.com/statistics/276391/age-distribution-in-greece/ (accessed 13 December 2023). |
[28] | Kosmidis MH , Vlachos GS , Anastasiou CA , Yannakoulia M , Dardiotis E , Hadjigeorgiou G , Sakka P , Ntanasi E , Scarmeas N ((2018) ) Dementia Prevalence in Greece: The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD). Alzheimer Dis Assoc Disord 32,: , 232–239. |
[29] | Basta M , Simos P , Vgontzas A , Koutentaki E , Tziraki S , Zaganas I , Panagiotakis S , Kapetanaki S , Fountoulakis N , Lionis C ((2019) ) Associations between sleep duration and cognitive impairment in mild cognitive impairment. J Sleep Res 28: , e12864. |
[30] | Skourti E , Simos P , Zampetakis A , Koutentaki E , Zaganas I , Alexopoulou C , Vgontzas A , Basta M ((2023) ) Long-term associations between objective sleep quality and quantity and verbal memory performance in normal cognition and mild cognitive impairment. Front Neurosci 17: , 1265016. |
[31] | Zaganas IV , Simos P , Basta M , Kapetanaki S , Panagiotakis S , Koutentaki I , Fountoulakis N , Bertsias A , Duijker G , Tziraki C , Scarmeas N , Plaitakis A , Boumpas D , Lionis C , Vgontzas AN ((2019) ) The Cretan Aging Cohort: Cohort description and burden of dementia and mild cognitive impairment. Am J Alzheimers Dis Other Demen 34: , 23–33. |
[32] | Basta M , Skourti E , Alexopoulou C , Zampetakis A , Ganiaris A , Aligizaki M , Simos P , Vgontzas AN ((2023) ) Cretan Aging Cohort-Phase III: Methodology and descriptive characteristics of a long-term longitudinal study on predictors of cognitive decline in non-demented elderly from Crete, Greece. Healthcare (Basel) 11: , 703. |
[33] | Basta M , Simos P , Bertsias A , Duijker G , Zaganas I , Koutentaki E , Anastasaki M , Mavroidis G , Kalomoiri G , Panagiotakis S , Lionis C , Vgontzas A ((2018) ) Association between insomnia symptoms and cognitive impairment in the Cretan Aging Cohort. Eur Geriatr Med 9: , 697–706. |
[34] | Basta M , Belogianni C , Yannakoulia M , Zaganas I , Panagiotakis S , Simos P , Vgontzas AN ((2022) ) Poor diet, long sleep, and lack of physical activity are associated with inflammation among non-demented community-dwelling elderly. Healthcare (Basel) 10,: , 143. |
[35] | Vitiello MV , Prinz PN , Williams DE , Frommlet MS , Ries RK ((1990) ) Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol 45: , M131–M138. |
[36] | Prinz PN , Peskind ER , Vitaliano PP , Raskind MA , Eisdorfer C , Zemcuznikov N , Gerber CJ ((1982) ) Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc 30: , 86–93. |
[37] | Prinz PN , Vitaliano PP , Vitiello MV , Bokan J , Raskind M , Peskind E , Gerber C ((1982) ) Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging 3: , 361–70. |
[38] | Blackwell T , Yaffe K , Laffan A , Ancoli-Israel S , Redline S , Ensrud KE , Song Y , Stone KL ; Osteoporotic Fractures in Men (MrOS) Study Group ((2014) ) Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: The MrOS sleep study. Sleep 37: , 655–663. |
[39] | Diem SJ , Blackwell TL , Stone KL , Yaffe K , Tranah G , Cauley JA , Ancoli-Israel S , Redline S , Spira AP , Hillier TA , Ensrud KE ((2016) ) Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry 24: , 248–258. |
[40] | Park JE , Gunasekaran TI , Cho YH , Choi SM , Song MK , Cho SH , Kim J , Song HC , Choi KY , Lee JJ , Park ZY , Song WK , Jeong HS , Lee KH , Lee JS , Kim BC ((2022) ) Diagnostic blood biomarkers in Alzheimer’s disease. Biomedicines 10: , 169. |
[41] | Mander BA , Winer JR , Jagust WJ , Walker MP ((2016) ) Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci 39: , 552–566. |
[42] | Basta M , Zaganas I , Simos P , Koutentaki E , Dimovasili C , Mathioudakis L , Bourbouli M , Panagiotakis S , Kapetanaki S , Vgontzas A ((2021) ) Apolipoprotein E ɛ4 (APOE ɛ4) allele is associated with long sleep duration among elderly with cognitive impairment. J Alzheimers Dis 79: , 763–771. |
[43] | Wilckens KA , Tudorascu DL , Snitz BE , Price JC , Aizenstein HJ , Lopez OL , Erickson KI , Lopresti BJ , Laymon CM , Minhas D , Mathis CA , Buysse DJ , Klunk WE , Cohen AD ((2018) ) Sleep moderates the relationship between amyloid beta and memory recall. Neurobiol Aging 71: , 142–148. |
[44] | You JC , Jones E , Cross DE , Lyon AC , Kang H , Newberg AB , Lippa CF ((2019) ) Association of β-amyloid burden with sleep dysfunction and cognitive impairment in elderly individuals with cognitive disorders. JAMA Netw Open 2: , e1913383. |
[45] | Winer JR , Mander BA , Helfrich RF , Maass A , Harrison TM , Baker SL , Knight RT , Jagust WJ , Walker MP ((2019) ) Sleep as a potential biomarker of tau and beta-amyloid burden in the human brain. . J Neurosci 39: , 6315–6324. |
[46] | Mander BA , Marks SM , Vogel JW , Rao V , Lu B , Saletin JM , Ancoli-Israel S , Jagust WJ , Walker MP ((2015) ) β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18: , 1051–1057. |
[47] | Lai KSP , Liu CS , Rau A , Lanctôt KL , Köhler CA , Pakosh M , Carvalho AF , Herrmann N ((2017) ) Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry 88: , 876–882. |
[48] | Darweesh SKL , Wolters FJ , Ikram MA , de Wolf F , Bos D , Hofman A ((2018) ) Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimers Dement 14: , 1450–1459. |
[49] | Irwin MR , Olmstead R , Carroll JE ((2016) ) Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 80: , 40e52. |
[50] | Basta M , Koutentaki E , Vgontzas A , Zaganas I , Vogiatzi E , Gouna G , Bourbouli M , Panagiotakis S , Kapetanaki S , Fernandez-Mendoza J , Simos P ((2020) ) Objective daytime napping is associated with disease severity and inflammation in patients with mild to moderate dementia. J Alzheimers Dis 74: , 803–815. |




