Culture-Fair Cognitive Screening Tools for Assessment of Cognitive Impairment: A Systematic Review
Abstract
Background:
Cognitive screening tools are important in the detection of dementia, including Alzheimer’s disease; however, they may contain cultural biases.
Objective:
This review examines culture-fair cognitive screening tools and evaluates their screening accuracy, strengths, and limitations.
Methods:
Medline, Embase, PsychINFO and CINAHL were searched. The protocol was registered on PROSPERO (CRD42021288776). Included studies used a culture-fair tool to assess cognition in older adults from varying ethnicities. Narrative synthesis was conducted.
Results:
28 studies were included assessing eleven different tools. The Rowland Universal Dementia Assessment Scale (RUDAS) was as accurate as the Mini-Mental State Examination (MMSE) (AUC 0.62-0.93), with a similar sensitivity (52–94%) and better specificity (70–98%), and the Multicultural Cognitive Examination (MCE) had improved screening accuracy (AUC 0.99) compared to RUDAS (AUC 0.92). The Visual Cognitive Assessment Test (VCAT) was equivalent to MMSE (AUC 0.84–0.91). The Kimberley Indigenous Cognitive Assessment tool (KICA) had AUC of 0.93–0.95; sensitivity of 90.6%, specificity 92.6%.
Conclusions:
The RUDAS, KICA and VCAT were superior to MMSE for screening dementia in ethnic minorities. Other tools also showed good screening accuracy. Further research should be done to validate tools in different populations.
INTRODUCTION
Dementia affects approximately 500,000 people in the UK with profound socioeconomic implications; the care of people living with dementia is estimated to cost up to £14.93 billion annually, exceeding the cost of stroke, heart disease, and cancer combined [1]. While the number of people living with dementia in the UK is expected to double by 2050, prevalence among ethnic minority communities is expected to increase sevenfold [2]. Commonly used cognitive assessment tools include the Addenbrooke’s Cognitive Examination (ACE III), Mini-Mental State Examination (MMSE), and the Montreal Cognitive Assessment (MoCA), which have been shown to be helpful in aiding diagnosis of dementia [3]. However, these tools were created for “Western” populations and a multitude of studies have demonstrated that performance is significantly influenced by age, educational status, ethnicity, and language [3–5], thus resulting in poor test accuracy for non-Caucasian patients [6]. Comprehensive and accurate cognitive assessment is very important in the workup for dementia. For example, it can inform referral to other members of the multi-disciplinary team such as speech and language, or occupational therapy, monitor longitudinally cognitive changes and guide pharmacological and social interventions. Under-diagnosis of dementia can have negative implications including inadequate support and delayed use of anti-dementia drugs, which may reduce the efficacy even during the earliest stages of Alzheimer’s disease [7]. There are also negative implications of over-diagnosis, which can lead to unnecessary anxiety, and implications for driving, work, and insurance. Moreover, the accurate identification of mild cognitive impairment is essential to the advancement of preventive and therapeutic research.
Attempts to translate or adapt commonly used cognitive tests have been explored, however these still pose issues [8]. For example, repetition of the English idiom “no ifs, ands, or buts” used in the MMSE does not maintain the same level of linguistic difficulty, test of fluency or cultural relevance upon translation. Ethnic disparities in performance during neuropsychological testing have long been recognized, regardless of socioeconomic status or level of education [9]. Such findings are attributed to an over-reliance of these tools on cultural norms and language [10]. A variety of cognitive assessments have been developed for specific populations. However, at present there is no universal gold standard tool for the diagnosis of cognitive impairment in ethnic minority groups. A culture-free test are test which is devoid of all culture-related content, which can be very difficult to achieve. In contrast, a culture fair test is a test designed to reduce the influence of cultural elements and therefore provide an accurate assessment without favoring any one culture [11].
Thus far, there has not been an up-to-date review comparing the variety of available culture-fair cognitive screening tools. This systematic review aims to determine the commonly used culture-fair cognitive screening tools and evaluate their test accuracy, strengths, and limitations.
MATERIALS AND METHODS
Search
This review was reported in accordance with the PRISMA guidance for reporting systematic reviews and a PRISMA checklist is included in the Supplementary Material. Medline, Embase, PsychINFO, and CINAHL databases were searched from inception using the search strategy presented in the Supplementary Material. The search strategy was developed in conjunction with a librarian at the Leicestershire Partnership Trust (LH). The search was completed in November 2021 and updated in July 2023. No additional studies were found in the second search in July 2023. Included articles were published in any year and there was no restriction on time. Reference lists of included articles and citation indices were screened for additional relevant material. Only adult human studies where patient data could be successfully extracted were considered. Included studies had to use a cognitive screening tool, however, were not limited to a specific cognitive test and included neuropsychological test batteries. Abstracts, conference papers, posters, translated or adapted tools, adapted for specific populations, or studies reporting on less than five subjects were excluded. The review protocol was registered on PROSPERO prior to commencement of the review (CRD42021288776). The PRISMA flow diagram is presented in Fig. 1, to outline the papers identified through the database searches.
Fig. 1
Prisma flow diagram.
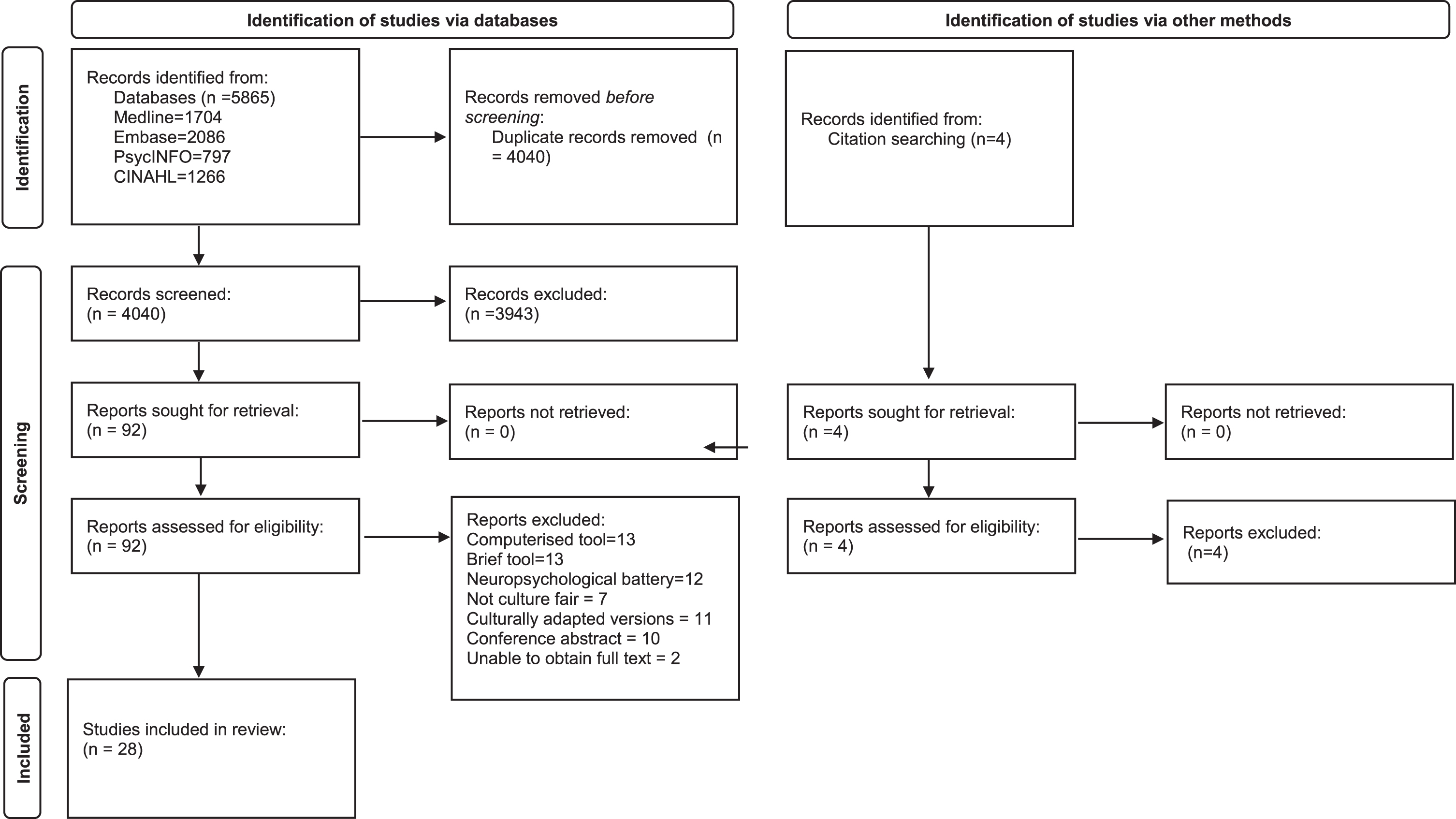
Study selection
Included studies were not limited by study design, with inclusion of randomized controlled trials, non-controlled trials, case control, and cross-sectional studies. Systematic and narrative reviews were excluded, however, analyzed as sources for potential references. We included studies which used a culture-fair or culture-free cognitive tool to assess cognition in healthy adults or older adults with dementia (over 65 years old), from varying ethnicities. Studies were excluded if they used translated versions of widely used validated tools, cognitive tests which were not specified to be culture-free or culture-fair, tools which were too time consuming in a clinical setting such as outpatient clinics (for example those taking over an hour), namely neuropsychological batteries, and tools which were too brief and not designed for outpatient settings, for example tools designed for quick assessment at the bedside or in emergency departments, or included a small number of participants (<10). Studies were screened on title and abstract and then by full text by two independent reviewers (TC, SS). Disagreements were resolved internally, with an option of a third reviewer (LB).
Data extraction, quality assessment, and data analysis
Data were extracted into Microsoft Excel by one reviewer (TC), and a second reviewer independently checked 20% (SS). No discrepancies in numerical data were found. Quality assessments were independently conducted by two reviewers (TC, SS), using the QADAS II tool [12]. Disagreements were internally resolved between the two reviewers. The only group with adequate data for a meta-analysis were studies examining the Rowland Universal Dementia Assessment Scale (RUDAS), and this has published recently [13]. There was insufficient data for a further meta-analysis and so a narrative synthesis was conducted of all included studies.
RESULTS
The search strategy identified 5,865 articles, with 4,040 remaining after duplicates were removed (Fig. 1). An additional four studies were found through manual search of the references. A total of 92 full texts were identified based on initial screening of abstracts and 28 studies were included in the review. As described in Table 1, studies took place in multiple countries including Singapore, Malaysia, Indonesia, Philippines, Netherlands, Tunisia, Germany, Belgium, Denmark, Sweden, Norway, Greece, India, Taiwan, Israel, Japan, USA, Nigeria, Jamaica, Canada, Latin America, Chine, Africa, Australia, Spain, and Tanzania. There were 18 test accuracy studies, seven cross sectional studies, one normative data study and one longitudinal study. Six studies were multicenter. The studies ranged between local community, general practice, outpatient memory clinic, geriatric rehabilitation, and inpatient settings. Mean age of participants ranged between 65–80 years old.
Table 1
Study characteristics of included studies
| Author, Year | Country | Tool Used | Type of study | Reference Test | Number of participants | Number with dementia | Number controls | Mean age dementia | Mean age controls | Sex | Education mean age | Setting | Results |
| Low et al., 2020 [6] | Singapore | VCAT | Diagnostic accuracy | MMSE | 471 | 121 | 233 HC 117 MCI | 70.25 | HC 61.47 (7.19) MCI 63.28 (9.05) | F=285 M = 186 | AD 8.39 (5.09) MCI 11.48 (4.54) HC 12.36 (3.76) | Outpatient memory clinic | The VCAT AUC = 0.84 (95% CI = 0.79–0.89) MMSE AUC = 0.76 (95% CI = 0.70–0.82) MoCA AUC = 0.81 (95% CI = 0.76–0.87). The optimal cut off was≤24 with a specificity of 0.71and a sensitivity of 0.75. |
| Lim et al., 2018 [29] | Singapore, Malaysia, Indonesia and Philippines | VCAT | Multicenter Diagnostic accuracy | None | 284 | 120 | 164 | 69.38 (9.51) | 66.88 (8.09) | F=216 M = 146 | AD 11.39 (3.84) HC 11.60 (3.75) | Memory clinic | MoCA AUC = 0.91 (95% CI 0.88–0.95) VCAT AUC = 0.91, (95% CI = 0.87–0.94) |
| Goudsmit et al., 2017 [30] | Netherlands | CCD | Diagnostic accuracy | None | 108 | 54 | 54 | 77 y (total) | - | F=60 M = 48 | - | GP | ROC analysis, CCD has high predictive validity for dementia (sensitivity = 85%, specificity = 89%). AUC per test, ranged from 0.85 (sun-moon test part A) to 0.95 (objects test, part b). |
| Bellaj et al., 2017 [31] | Tunisia | DSB-100 | Diagnostic accuracy | None | 201 | 42 | 159 | 69.38 (7.75) | 67.8 (7.25) | F=108 M = 93 | AD 5.93 (6.84) HC 5.47 (6.64) | - | Test-retest correlation coefficients were high for both patients (r = 0.81, p < 0.001) and healthy matched controls (r = 0.87, p < 0.001) |
| Nielsen et al., 2019 [19] | Germany, Belgium, Denmark, Sweden, Norway, Greece | MCE | Multicenter Diagnostic accuracy | RUDAS | 189 | 66 | 123 | 76.7 (6.8) | 74.9 (6.9) | F=107 M = 82 | Dementia 7.6 (5.8) Control 8.8 (5.9) | Outpatient memory clinics and Community | MCE AUC = 0.99 RUDAS alone AUC = 0.92 |
| Chopra et al., 2018 [32] | India | NEST | Cross sectional study | HMSE | 406 | 180 | 226 | - | - | F=104 M = 302 | - | - | NEST optimal cut off, through ROC analysis, was three or more errors, sensitivity 94%, specificity 60%. |
| Thajeb et al., 2006 [33] | Taiwan | MMT | Cross-sectional study | None | 161 | 61 vascular dementia | 50 Indonesian 50 Taiwanese/ Chinese | - | - | F=51 M = 49 | - | Outpatients | MMT scores with vascular dementia significantly lower than healthy controls (p < 0.001). MMT lower in healthy subjects with low level of education, regardless of ethnic background (p < 0.001). Cut off of 33/55, sensitivity (98%) and positive predictive value 94%. |
| Ritchie et al., 1989 [34] | Israel | Iowa screening test | Diagnostic accuracy | None | 78 | 32 | 46 | - | - | - | - | - | Iowa test sensitivity = 85%, specificity = 66% in Israeli sample. American sample sensitivity = 85%, specificity = 91% |
| Wolfe et al., 1992 [35] | Japan | CCCE | Diagnostic accuracy | None | 185 | 108 | 77 | 71.4 (8.7) | 68.4 (8.9) | F=102 M = 83 | - | Inpatients and outpatients | CCCE sensitivity = 88%, specificity = 97% |
| Kua et al., 1992 [36] | Singapore | ECAQ | Diagnostic accuracy | Kahn’s mental status questionnaire | 105 | - | - | 72 | - | F=63 M = 42 | - | Community | ECAQ has similar sensitivity (0.85) and higher specificity (0.91) to Kahn’s mental status questionnaire. |
| Hall et al., 2000 [22] | USA, Nigeria, Jamaica and Canada | CSI-D | Multicenter Diagnostic accuracy | None | 5,237 | – | 5,237 | 74 (7) | – | F=3,297 M = 1,940 | USA 9.6 (3.1) Nigeria 0.8 (2.3) Jamaica 5.2 (2.1) Crees reserve 2.2 (2.7) Winnipeg 9.2 (4.1) | In all sites, other than Jamaica, correlation coefficients show cognitive scores were correlated with years of education. CSI-D produces better sensitivity and specificity for dementia, however, can be affected by education (9% of variance). | |
| Prince et al., 2011 [23] | Latin America, India and China | BRIEF CSI-D | Multicenter Cross sectional study | None | 15,022 | – | – | – | – | – | – | – | The AUC for brief CSI-D range = 0.88-0.92 across countries. The optimal cut off points were the same for all regions, five or less to favor specificity or six or less to favor sensitivity. |
| Author, Year | Country | Tool Used | Type of study | Reference Test | Number of participants | Number with dementia | Number controls | Mean age dementia | Mean age controls | Sex | Education mean age | Setting | Results |
| Prince et al., 2003 [21] | India, China, Africa, Latin America | CSI-D | Multicenter cross sectional study | None | 2,885 | 729 | 2,156 | – | – | – | – | Community | Sensitivity India = 96%, China = 90%, Latin America = 90%, Nigeria = 100%. |
| Nielsen et al., 2019 [19] | Germany, Belgium, Denmark, Sweden, Norway, Greece | RUDAS | Multicenter cross sectional, diagnostic accuracy | 421 | 80 | 361 | 75 (8.8) | 69.4 (9.7) | F=159 M = 121 | AD 7.6 (5.6) HC 8.1 (5.7 | Community and memory clinics | RUDAS whole sample AUC = 0.93 AUC mild dementia = 0.89 AUC native-born=0.91 AUC immigrant born = 0.95 | |
| Celik et al., 2021 [38] | Germany | RUDAS | Cross-sectional study | MMSE | 65 | 45 | 20 | Native born Turkish 70.33 (7.73) Turkish immigrant 71.62 (7.41) | 74.7 (7.5) | F=45 M = 20 | Dementia Turkish 8.13 (4.11) Turkish immigrant 7.05 (4.44) Controls 11 (4.09) | Outpatient memory clinics | RUDAS score for native born Turkish = 9.63, Turkish immigrant = 20.62 and German = 20.75, p = 0.584. MMSE scores for Turkish native = 18.79, Turkish immigrant = 17.90, German = 21.60, p = 0.019. Performance of MMSE was significantly affected by education in the Turkish immigrant group (rs = 0.75, p < 0.001); however, RUDAS was not (rs = 0.15, p = 0.526) |
| Nielsen et al., 2013 [20] | Denmark | RUDAS | Diagnostic accuracy | MMSE | 137 | 73 | 64 | 77 (71.5-81) | 61 (50.5-70) | F=65 M = 72 | Dementia 9.3 (3.6) Other patients 10.4 (3.7) | Outpatient memory clinics | RUDAS AUC = 0.84 MMSE AUC = 0.84 at a cut off of < 24/30 for RUDAS (sensitivity = 69%, specificity = 80%) and < 25/30 for MMSE (sensitivity = 76%, specificity = 83%). |
| Emerson et al., 2019 [18] | Australia | RUDAS | Longitudinal study | MoCA | 102 | - | - | - | - | - | - | Geriatric rehabilitation | RUDAS at a cut-off of < 23/30), sensitivity = 52%, specificity = 0.70. MoCA at cut-off<18/30, sensitivity = 57%, specificity = 69%. MoCA AUC = 0.69 RUDAS AUC = 0.62. |
| Mateos-Alvarez et al., 2017 [14] | Spain | RUDAS | Cross sectional study | MMSE | 97 | 35 | 62 | 80.2 (5.6) | 76.5 (5.6) | F=74 M = 23 | AD 4 (11.4) HC 11 (17.7) | Outpatients | RUDAS AUC = 0.90 (95% CI 0.84-0.96) MMSE was 0.89 (95% CI 0.82-0.95). Optimal cut off for RUDAS = 21/22, sensitivity = 72%, specificity = 70%. Optimal cut of for MMSE = 16/17, sensitivity = 85%, specificity = 77% for ethnic minority population |
| Nielsen et al., 2012 [34] | Denmark | RUDAS | Cross sectional | MMSE | 76 | - | 76 | - | 61.6 (7.3) | F=43 M = 34 | 3.9 (3.9) Years of schooling | Community | RUDAS mean score = 26.8 (SD 2.4) MMSE mean score = 23.7 (SD4.3), with lower levels of acculturation. RUDAS scores were significantly correlated by age (rho=-0.355, p = 0.002), years of schooling (rho = 0.420, p < 0.001) and acculturation score (rho = 0.382, p = 0.001) and not by gender. MMSE scores were affected by age, years of schooling, acculturation score and gender. Years of schooling affected scores to a greater degree for MMSE (44% of the variance) compared to RUDAS (16% of the variance). |
| Basic et al., 2009 [15] | Australia | RUDAS | Diagnostic accuracy | MMSE, GPCOG | 151 | 58 | HC 60 MCI 33 | Range 59.7-93.3 | Range HC 45.6-89.8 MCI 65.1-96.5 | F=104 M = 47 | AD 7 (6-8) MCI 9 (5-10.5 HC 9 (5-12) | Outpatient memory clinics and community | RUDAS is at least as accurate as the MMSE and GPCGOG, RUDAS is not influenced by culture. The RUDAS AUC = 0.94 (0.88-0.97), MMSE = 0.93 (0.87-0.97) GPCOG = 0.95 (0.89-0.98), p > 0.05. For the cut-off of < 23/30, RUDAS sensitivity = 87%, specificity = 90% |
| Author, Year | Country | Tool Used | Type of study | Reference Test | Number of participants | Number with dementia | Number controls | Mean age dementia | Mean age controls | Sex | Education mean age | Setting | Results |
| Iype et al., 2006 [17] | India | RUDAS | Diagnostic accuracy | MMSE | 108 | 58 | 50 | 65.ta07 (11.3) | 65.1 (10.9) | - | AD 5.59 (4.07) HC 5) | Outpatient dementia clinics and caregivers | RUDAS sensitivity = 88%. Specificity = 76% MMSE sensitivity = 90%, Specificity = 48% p < 0.001. There were positive correlations with years of formal education of 0.45 for RUDAS (p < 0.001) and 0.64 (p < 0.001) for MMSE. |
| Rowland et al., 2006 [16] | Australia | RUDAS | Diagnostic accuracy | MMSE | 111 | 63 | HC 48 MCI 18 | 77.7 (8.6) | 81.5 (7.5) | F=80 M = 31 | AD 6 (1-9) HC 6.5 (4-9.5) | Referrals to aged care team | RUDAS AUC = 0.92 (95% CI 0.85-0.96), MMSE AUC.91, (95% CI 0.84-0.95), and cut offs were RUDAS < 23 and MMSE < 25. |
| Storey et al., 2004 [37] | Australia | RUDAS | Diagnostic accuracy | None | 90 | 45 | 45 | 81.4 (8.1) | 78.1 (8.4) | F=70 M = 20 | - | Community | RUDAS AUC = 0.95 (95% CI 0.88-0.98) with an optimal cut off of 23 and sensitivity of 89%, specificity of 98%. |
| Radford et al., 2015 [27] | Australia | RUDAS | Diagnostic accuracy | MMSE mKICA | 235 | 11 MCI 19 | 111 | 68.96 (7.16) MCI 68.5 (6.94) | 64.96 (5.16) | F=100 M = 135 | 8.04 (3.44), MCI 8.77 (2.83) HC 10.01 (2.75) | Community | The MMSE is an effective cognitive screening tool in urban Aboriginal populations. The mKICA is a good alternative when illiteracy, language or cultural considerations deem it appropriate. The RUDAS has adequate validity in this population. There was no significant difference in AUCs between the three tests. At the standard cutoff points (of 24, 34, and 23 respectively), MMSE AUC = 0.94 MMSE = 0.93 RUDAS = 0.89 |
| Lo Giudice et al., 2006 [28] | Australia | KICA | Diagnostic accuracy | None | 70 | 27 | HC 32 MCI 11 | 70.7 (8.1) | HC 73.6 (8.2) MCI 70.9 (13.5) | F=40 M = 30 | No schooling dementia 19 (70.4%), HC 18 (56.3%), MCI 6 (54.5%) | Community | KICA AUC = 0.95 (95% CI 0.87-1.00). At a cut off of 31/32 sensitivity = 90.6%, specificity = 92.6% Three items of KICA-COG (pension week, recall and free recall with discriminant factor coefficients of 0.34, 0.51, and 0.71) were able to successfully classify 85.7% of participants as dementia or no cognitive impairment. |
| Collingwood et al., 2014 [26] | Tanzania | IDEA and IDEA-IADL | Diagnostic accuracy | None | 417 | 130 | 387 | F=253 M = 164 | Community across 6 Tanzanian villages | The combined IDEA and IDEA-IADL questionnaire AUC = 0.94 (0.90–0.98), IDEA alone AUC = 0.85 (0.78–0.92) IDEA-IADL alone AUC = 0.90 (0.84–0.91). Scores of < 7 indicated probable dementia and 8-9 possible dementia. | |||
| Gray et al., 2014 [24] | Tanzania | IDEA | Diagnostic accuracy | CSI-D | 60 | - | - | - | - | - | - | 70 years and older resident in 6 randomly selected villages | 5 item IDEA AUC = 0.871 (0.77-0.98) with a cutoff of < 7 out of 12, sensitivity = 91.7%, specificity = 61.7%. 7 item AUC = 0.786 (0.648-0.925), with a cut off of < 6 out of 9, sensitivity 91.7%, specificity 38.3% |
| Author, Year | Country | Tool Used | Type of study | Reference Test | Number of participants | Number with dementia | Number controls | Mean age dementia | Mean age controls | Sex | Education mean age | Setting | Results |
| Gray et al., 2021 [25] | Tanzania | IDEA | Normative data | None | 4,128 | Tanzania 105 | F=2,400 M = 1,728 | 1,515 with no formal education, 1,180 with 1–4 years primary school, 1,211 5–7 years of primary school, 230 secondary school or higher | Community across 12 villages of Tanzania and Nigeria | The 50th decile values for IDEA were 13 (60–64 y) vs. 8/9 (above 85 y), 10–11 uneducated vs. 13 primary educated, and 11/12 in females vs. 13 in males. The normative values for 10-word list delayed recall and categorical verbal fluency varied with education [i.e., delayed recall mean 2.8 [standard deviation (SD) 1.7] uneducated vs. 4.2 (SD 1.2) secondary educated; verbal fluency mean 9.2 (SD 4.8) uneducated vs. 12.2 (SD 4.3) secondary educated], substantially lower than published high-income country values. |
VCAT, Visual Cognitive Assessment Test; MMSE, Mini-Mental State Examination; HC, healthy controls; MCI, mild cognitive impairment; F, female; M, male; AD, Alzheimer’s disease; AUC, area under the curve; MoCA, Montreal Cognitive Assessment; CCD, Cross-Cultural Dementia Screening; ROC, receiver operating characteristic curve; DSB-100, Development of the Dementia Screening Battery-100; MCE, Multicultural Cognitive Examination; RUDAS, Rowland Universal Dementia Assessment Scale; NEST, Neuropsychological Evaluation Screening tool; HMSE, Hindi Mental State Examination; MMT, Modified Mini Mental Test; CCCE, Cross-cultural cognitive examination; ECAQ, Elderly Cognitive Assessment Questionnaire; CSI-D, Community Screening Instrument for Dementia; GPCOG, General Practitioner Assessment of Cognition; KICA/mKICA, Kimberley Indigenous Cognitive Assessment Tool/modified Kimberley Indigenous Cognitive Assessment; IDEA, Identification of Dementia in Elderly Africans.
Eleven studies assessed RUDAS as the primary cognitive tool, of which nine studies used MMSE or MoCA as reference tests. RUDAS was found to be as accurate as the MMSE [14–16], with a similar sensitivity (range: 52–94%) and better specificity (range: 70% –98%) [17]. Area under the curve (AUC) values reported ranged between 0.62 [18] to 0.93 [19]. The majority of studies showed that RUDAS scores were found to be uninfluenced by education [16], language [16, 20], and sex [16]; however, three studies identified that RUDAS scores were influenced by education, as highlighted in Table 2 [14, 17, 19]. Nielsen et al. (2013) established it to be superior to MMSE, for use in an older multicultural population [20].
Table 2
Details including strengths and limitations of included cognitive screening tools
| Type of tool | Time to administer | Number of items | Cognition testing | Limitations | Strengths |
| RUDAS | 7–20 min | Six items | •Body orientation •Praxis •Drawing •Judgment •Memory •Language | •May be affected by education, may need to be education-adjusted [16, 17, 19] | •Requires minimal training [19] •Relatively unaffected by gender, cultural background, and language use [39] •Well accepted by interviewees [16] •Easily translated into other languages without change in format [37] •Assesses impairment in frontal lobe and executive functioning [17] |
| VCAT | 15 min [29] | Five items [6] | •Memory •executive function •visuospatial function •attention •semantic knowledge | •Language neutral without need for translation [29] •Can identify early cognitive impairment [29] •Interviewees found the instructions easy to understand [29]. | |
| CCD | 20 min | Three items | •Memory •Mental speed and inhibition •Mental speed and divided attention [30] | •Currently available in six languages •Requires well trained examiners [30] | •Can be administered without the experimenter speaking patient’s language. •minimal verbal response only behavioral responses such as pointing [30] |
| DSB-100 | 15-20 min [31] | Ten subtests [31] | •Memory •Executive functions •Praxia •Language •Attention •Visuospatial functions [31] | •Scores impacted by education and literacy levels [31] | •Easily adaptable to western and non-western cultures •Allows the clinician to differentiate between dementia subtypes [31]. |
| CSI-D/ Brief CSI-D | 30 min [22] | Six domains [22] | •Memory •Abstract thinking •Judgement •Higher cortical functioning •Personality changes •Functioning at work and in social relationships [22] | •The total score requires information from close relative, who must therefore be reliable to avoid skewed scores [22] •Brief CSI-D needs further validation in primary care setting [16] | •Includes interview with the patient and close relative which provides insight into daily functioning of the individual [22]. •The brief CSI-D can be administered in 5 min [16]. |
| MCE | 15-20 min [19] | Four domains [19] | •Incorporates RUDAS and expands on: •Memory •Verbal fluency •Visuospatial function [19] | •Clinical utility not yet validated [19] | •Interpreter not necessary to administer test •Applied in over 20 languages without change of content •No specialized training required to administer test [19] |
| NEST | 5 min [32] | Six domains [32] | •Immediate recall •Verbal fluency •Abstraction •Executive functioning •Visual-constructional ability •Delayed recall [32] | •Requires basic training •Intended as a screening tool not diagnosis [32] | •Easily translated into various languages •Requires minimal time to administer [32] |
| MMT | 16 min [33] | Five subsets [33] | •Orientation •Attention, right–left discrimination, speech, and calculation •Immediate recall, and recent and remote memory retrieval •Praxis •Visuospatial orientation, agnosia, hemianopsia, and visual hemineglect [33] | •Only trialed for screening patients with vascular dementia •Scores impacted by education level [33] | •Design is short and simple to administer •Scores unaffected by ethnicity or sex [33] |
| Iowa Screening test | 15 min [34] | Three subsets [34] | •Temporal orientation •Controlled oral word association •Visual retention test [34] | •Screening tool not diagnostic test [34] •Interpreter may be required for administration [34] | •Provides insight into functional ability [34] •Brief to administer [34] •Acceptable to patients [34] |
| CCCE | 25 min [35] | Eight domains [35] | •Attention/ concentration •Orientation •Verbal memory •Visual memory •Visuo-constructional abilities •Language •Psychomotor speed •Abstract reasoning/ problem solving [35] | •Training required for administration of tool [35] | •Two elements to the test with a screening tool and diagnostic component. •Includes non-performance information such as behavioral observations, psychiatric observations, and activities of daily living [35] |
| ECAQ | Less than 10 min [36] | Ten items assessing three domains [36] | •Cognitive function •Memory •Orientation and information [36] | •Quick screening tool not diagnostic test [36] | •Appropriate tool for elderly patients, removing generational biases [36]Type of tool |
| KICA | 25–30 min | Three sections, 16 questions assessing six domains [27, 28] | •Orientation •free and cued recall •language •verbal fluency •copying sequence pattern •ideational praxis [27, 28] | •Does not assess executive functioning. •Not widely validated [27, 28] | •Similar accuracy to MMSE •Designed for people of all educational abilities (however not specifically covaried for) [27, 28] |
| IDEA | 5 min | Six domains | •Abstraction •Orientation •Long-term memory •Categorical verbal fluency •Verbal delayed recall •Visuospatial construction [24, 25] | •Not widely validated [24, 25] | •No items require reading, writing, drawing or calculation [24, 25] |
RUDAS, Rowland Universal Dementia Assessment Scale; VCAT, Visual Cognitive Assessment Test; CCD, Cross-Cultural Dementia Screening; DSB-100, Development of the Dementia Screening Battery-100; CSI-D, Community Screening Instrument for Dementia; MCE, Multicultural Cognitive Examination; NEST, Neuropsychological Evaluation Screening tool; MMT, Modified Mini Mental Test; CCCE, Cross-cultural cognitive examination; ECAQ, Elderly Cognitive Assessment Questionnaire; KICA, Kimberley Indigenous Cognitive Assessment Tool; IDEA, Identification of Dementia in Elderly Africans.
The remaining seventeen studies analyzed an array of other cognitive tests; three studies used the Community Screening Instrument for Dementia (CSI-D) [21–23], three studies used the Identification of Dementia in Elderly Africans (IDEA) [24–26], two studies for the Kimberley Indigenous Cognitive Assessment tool (KICA) [27, 28], two studies used the Visual Cognitive Assessment Test (VCAT) [6, 29], and there was one study each for the Cross-Cultural Dementia Screening (CCD) [30], Dementia screening battery (DSB-100) [31], Multicultural Cognitive Examination (MCE) [20], Neuropsychological Evaluation Screening tool (NEST) [32], Modified Mini Mental Test (MMT) [33], Iowa screening test [34], Cross-cultural cognitive examination (CCCE) [35], and the Elderly Cognitive Assessment Questionnaire (ECAQ) [36]. Sensitivity testing demonstrated NEST had better sensitivity compared to the Hindi Mental State Examination (HMSE) at all educational levels (94.8%) [32]. CSI-D had good sensitivity across different countries and the brief version of the full CSI-D shared favorable culture-fair screening properties of the full assessment [21]. The Iowa test, ECAQ and CCCE showed similar sensitivity of 85%, 85.3% and 88% respectively [34–36]. In terms of specificity of dementia identification, Iowa test had 66%, CCCE showed 97.4% and ECAQ 91.5% [34–36]. VCAT had good construct validity and good internal consistency [6] and was not affected by age, years of education, race, or employment, whereas MoCA was [29]. Receiver operating characteristic (ROC) analysis found VCAT to be equivalent or more effective to the MMSE and MoCA (AUC 0.84–0.91) [6, 29] and CCD to have high predictive validity for dementia (AUC 0.85–0.95, sensitivity 85%, specificity 89%) [30]. CCD showed no differences between the ethnic groups after adjusting for age and education [30]. MCE had significantly improved test accuracy (AUC 0.99) compared with using the RUDAS alone (AUC 0.92) [19]. The KICA showed good test accuracy based upon AUC scores of 0.93 and 0.95 [27, 28] with a sensitivity of 90.6% and specificity 92.6% [28]. The IDEA had an AUC of 0.78–0.87 [24, 26] which was improved in combination with the IDEA-IADL to 0.94 [26]. The MMT was useful in specifically testing for vascular dementia, however, was affected by educational level [30] and DSB-100 was valid in a Tunisian elderly population [31]. As detailed in Table 2, the DSB-100 was found to be affected by education level. The CSI-D, brief-CSI-D, CCD, and VCAT were found to have little effect of educational level. The RUDAS, VCAT, and Iowa screening test were all found to be acceptable to patients. The Iowa screening test, NEST, and ECAQ were all quick to administer (under 15 min). Many studies assessed the accuracy of tools at different cut off points [6, 14–16, 18, 20, 23, 24, 27, 28, 33]. Cut off for IDEA was quoted as < 7 out of 12 for the 5-item instrument, or < 6 out of 9 for the 7-item instrument [24]. Optimal cut off to detect cognitive impairment was≤24. Optimal cut off for the KICA was quoted as 31/32 (KICA) and 34 [27]. RUDAS cut offs varied from 21 [14–16, 18, 19, 27] to 24 [19]. Cut off for CSI-D was five or less to favor specificity or six or less to favor sensitivity [23] and for MMT was 33/55 [33]. Optimal cut off for NEST was three or more errors [32].
Risk of bias assessment
The majority of studies showed low risk of bias, yet there were a few studies deemed to be high risk on domains such as reference standards [30, 35], flow and timing [19, 22, 30, 34, 37], patient selection [14, 23], and Index test [33, 34].
DISCUSSION
Our systematic review highlights that there have been multiple attempts across various countries to create a culture-fair cognitive assessment tool. The need for such a tool has been widely recognized and there have been numerous studies highlighting the shortcomings of the currently used cognitive assessment tools in screening for cognitive impairment in multicultural populations [3–5]. We propose the universal use of cognitive assessment tools free of cultural bias as a practical approach for memory services in response to the ever-growing diversity of the UK population. Our review examined 28 studies identifying eleven different culture-fair cognitive assessment tools, all of which show promising results in studies in their country of development. The RUDAS, VCAT, KICA, and CCD were found to have preferable specificity and sensitivity to the MMSE when used in ethnic minorities.
A strength of the review is the inclusion of studies assessing patients from multiple different countries, and both native and migrant patients in those countries. Including studies from a variety of countries and multi-country studies increases the likelihood of generalizability of the results across the world. However, there are multiple countries which have not been studied, including the UK, which brings scope for further research.
The RUDAS and KICA have been recognized as promising options to reduce cultural bias in ethnic minorities [13]. Both have been compared to the MMSE and shown superior or comparable diagnostic accuracy, and less effect of both education and culture. One study compared the modified KICA and RUDAS together, and showed the modified KICA had greater diagnostic accuracy (94%) compared to the RUDAS (89%) [27]. The diagnostic accuracy of the RUDAS is increasingly well documented as a cross-cultural cognitive test [40]. One meta-analysis that included 26 studies found the RUDAS to have a comparable diagnostic accuracy across a multitude of sociocultural settings, with limited impact from educational, linguistic, or cultural biases [13]. However, despite being deemed culture-fair, both have cultural adaptations available. For example, for the RUDAS, in both the Peruvian and Spanish validation study [14, 41], the word tea in the supermarket list in the memory item was changed to coffee, and in the Thai validation study, animal fluency was substituted with fruit fluency to reduce the impact of formal education on the language item [40]. Although these may appear to be minor changes, the fact that cultural adaptations are required suggests that the tool is not entirely culture-fair, as without the adaptations the results may be skewed. KICA has been incorporated into Australia’s dementia services following recognition of its’ superior sensitivity and specificity for the diagnosis of cognitive impairment in Indigenous communities where illiteracy, language, or cultural barriers may impede assessment [27]. The KICA has been adapted for Canadian, Persian, and Brazilian populations, as discussed in the review by Mukaetova-Ladinska et al. [42]. Neither the KICA nor RUDAS have been validated for use in the UK memory services as of yet. Furthermore, it is important to note that our review included participants aged 65 and over. In a study of 70 UK participants, in the younger persons memory service the RUDAS was found to have modest accuracy (AUC = 0.70 (0.57–0.82), p = 0.002, sensitivity 71.7%, specificity 58%) and in the younger adults was found to be influenced by educational and ethnic background, which is in direct contrast to results in the older people as stated above [43]. Further research should be done in validating culture-fair tools for diagnosing dementia in the younger population.
The review has highlighted the challenges in separating the effects of culture and education in cognitive tools, and many patients may be affected by both cultural and educational biases. The RUDAS, MMT, and DSB-100 were found to be affected by educational level to varying degrees and may require adjustment for educational qualifications. However, regarding the RUDAS, the effect of education was still much less than the effect on the MMSE [17, 44] and some studies did not find it to be affected by educational level at all. Educational level was found to have little impact on the CSI-D, brief-CSI-D, CCD and VCAT [16, 22, 29, 30].
The VCAT provides an option which is neutral to both language and education [29]. It is a 30-item tool and includes testing of executive functioning which is very useful, and something not all cognitive tools cover, including the widely used MMSE. The items are visual based and therefore may be an extremely useful tool across cultures, removing the barriers of language [29].
An interesting finding from the review was the study testing the IDEA-IADL, which combines the cognitive assessment with an assessment of instrumental activities of daily living [26]. These are complex activities which are generally affected earlier in cognitive impairment, and therefore can aid in the holistic diagnosis of dementia. It is well known that diagnosis of dementia does not rely on cognitive screens alone, and an assessment of functioning is required. Activity of Daily Living (ADL) questionnaires can be a useful tool, however the authors highlighted that the commonly used ADL tools, such as the Lawton’s ADL tool, were developed in high income countries and therefore may also be subject to cultural biases [24, 25]. As seen above, AUC curve was 0.896 for diagnosing dementia when using the IDEA-IADL alone and 0.94 when used with in combination with the IDEA [24, 25]. This could indicate areas for further research, to create tools which can be easily administered, avoid cultural biases, and can be used to assess both cognitive abilities and instrumental activities of daily living.
A major hurdle identified through this review for many of the cognitive assessment tools is the lack of validation worldwide and in varying populations. Many countries, including the UK now have a very multicultural elderly population, and therefore having a tool suitable for one ethnic population is of minimal use. Therefore, many of the studies will need to be validated across more countries.
Limitations
Despite having 28 studies included in the review, the number of studies examining each tool was limited, other than for the RUDAS (n = 11). There were three studies examining the CSI-D [21, 22], two studies for the KICA [27, 28], two studies examining the VCAT [6, 29], and one study for each remaining cognitive assessment tool [19, 24–26, 28, 30–36]. The heterogeneity of studies and limited number of studies for each assessment tool meant that we were unable to perform a meta-analysis and hampered direct comparisons between tools.
One of the areas of heterogeneity of studies are the varying ethnic groups assessed. It may be difficult to compare results of various culture fair tools based upon which groups were studied, and this could affect the AUC results given. Future research is needed to evaluate these tools in different populations. It would also be useful for all tools to be directly compared to cognitive assessment tools widely used such as the MMSE, to compare screening accuracy for cognitive impairments among ethnic minorities.
Although majority of studies showed mostly low risk of bias, there were a few studies deemed to be high risk on domains such as reference standards [30, 35], flow and timing [20, 30, 34, 36, 37] patient selection [14, 23] and index test [34, 30]. A potential reason for this could be that some studies were published more than two decades ago, when reporting standards were lower. However, it is a strength of the review that most studies showed mainly a low risk of bias.
Furthermore, this review excluded culture-fair computerized and digital tools. Computerized tools are increasingly being utilized in clinical practice [45], and may be useful in remote assessments, which have become increasingly popular, especially after the COVID-19 pandemic. Some cognitive assessment tools have been created as purely computerized tools, which are culture-fair [45]. An example of the digital tool which was excluded from this review is the MemTax Memory Test, which is a digital cognitive screening instrument that takes approximately 90 s to complete [46]. It consists of culture-fair images and is available in 120 languages. It has been shown to be able to detect both mild cognitive impairment and AD with better accuracy compared to the MoCA (AUC = 0.799 compared to AUC = 0.767) [47]. These were excluded as there are limitations in facilities to administer computerized and digital tools to all patients, and this may not be generalizable to all services worldwide. However, it would be beneficial for a further review to summarize the available computerized and digital tools and their efficacy, as this may be a useful tool in future practice.
Conclusion
The review has identified various cognitive assessment tools which are all good options for supporting a diagnosis of dementia in patients of any ethnic background. The RUDAS, KICA, CCD and VCAT were comparable to MMSE in terms of specificity and sensitivity. The RUDAS, CSI-D, brief-CSI-D, CCD, and VCAT were minimally influenced by educational level. The VCAT is especially useful in non-verbal patients or patients who struggle with language and education. These tools should be considered in memory clinic settings, to reduce inaccuracy in diagnosis due to cultural biases, especially in ethnically diverse patient populations. Further research is needed to evaluate computerized cognitive assessment tools, and validation of cognitive tools in varying populations, including the UK population. Further research is also needed to create a tool which does not need to be adapted to varying cultures, and could be used across any culture without adaptation, which some of the tools above still require.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230194.
REFERENCES
[1] | Lowin A , Knapp M , McCrone P ((2001) ) Alzheimer’s disease in the UK:Comparative evidence on cost of illness and volume of healthservices research funding., Int J Geriatr Psychiatry 16: , 1143–1148. |
[2] | All-Party Parliamentary Group on Dementia (2013) Dementia does not discriminate: The experiences of black, Asian and minority ethnic communities. All-Party Parliamentary Group on Dementia, London. |
[3] | Mirza N , Panagioti M , Waheed MW , Waheed W ((2017) ) Reporting ofthe translation and cultural adaptation procedures of theAddenbrooke’s Cognitive Examination version III (ACE-III) and itspredecessors: A systematic review., BMC Med Res Methodol 17: , 141. |
[4] | O’Driscoll C , Shaikh M ((2017) ) Cross-cultural applicability of theMontreal Cognitive Assessment (MoCA): A systematic review. JAlzheimers Dis 58: , 789–801. |
[5] | Milani A , Marsiske M , Striley CW ((2018) ) Sensitivity and specificityof the Montreal Cognitive Assessment among minority populations. Alzheimers Dement 14: , 956–957. |
[6] | Low A , Lim L , Wong B , Silva E , Ng K , Kandiah N ((2020) ) Constructvalidity of the Visual Cognitive Assessment Test (VCAT)— across-cultural language-neutral cognitive screening tool. IntPsychogeriatr 32: , 141–149. |
[7] | Winblad B , Wimo A , Engedal K , Soininen H , Verhey F , Waldemar G , Wetterholm AL , Haglund A , Zhang R , Schindler R ((2006) ) 3-year studyof donepezil therapy in Alzheimer’s disease: Effects of early andcontinuous therapy. Dement Geriatr Cogn Disord 21: , 353–363. |
[8] | Steis MR , Schrauf RW ((2009) ) A review of translations and adaptationsof the Mini-Mental State Examination in languages other than Englishand Spanish. Gerontol Nurs 2: , 214–224. |
[9] | Manly JJ , Espino DV ((2004) ) Cultural influences on dementiarecognition and management. Clin Geriatr Med 20: , 93–119. |
[10] | Gibbons LE , van Belle G , Yang M , Gill C , Brayne C , Huppert FA , Paykel E , Larson E ((2002) ) Cross-cultural comparison of the Mini-Mental State Examination in United Kingdom and United Statesparticipants with Alzheimer’s disease. Int J GeriatrPsychiatry 17: , 723–728. |
[11] | Melikyan ZA , Agranovich AV , Puente AE (2019) Fairness in psychological testing. In Handbook of Psychological Assessment, Goldstein G, Allen DN, DeLuca J, eds. Elsevier Academic Press, pp. 551-572. |
[12] | Whiting PF , Rutjes AW , Westwood ME , Mallett S , Deeks JJ , Reitsma JB , Leeflang MM , Sterne JA , Bossuyt PM ((2011) ) QUADAS-2 Group. QUADAS-2:A revised tool for the quality assessment of diagnostic accuracystudies. Ann Intern Med 155: , 529–536. |
[13] | Nielsen T , Jørgensen K ((2020) ) Cross-cultural dementia screeningusing the Rowland Universal Dementia Assessment Scale: A systematicreview and meta-analysis. Int Psychogeriatr 32: , 1031–1044. |
[14] | Mateos-Álvarez R , Ramos-Ríos R , López-MoríñigoJD ((2017) ) Comparative analysis between the MMSE and the RUDAS fordementia screening in low educated people in a Spanishpsychogeriatric clinic., Eur J Psychiatry 31: , 119–126. |
[15] | Basic D , Khoo A , Conforti D , Rowland J , Vrantsidis F , LoGiudice D , Hill K , Harry J , Lucero K , Prowse R ((2009) ) Rowland UniversalDementia Assessment Scale, Mini-Mental State Examination and GeneralPractitioner Assessment of Cognition in a multicultural cohort ofcommunity-dwelling older persons with early dementia. AustPsychol 44: , 40–53. |
[16] | Rowland JT , Basic D , Storey JE , Conforti DA ((2006) ) The RowlandUniversal Dementia Assessment Scale (RUDAS) and the Folstein MMSE ina multicultural cohort of elderly persons., Int Psychogeriatr 18: , 111–120. |
[17] | Iype T , Ajitha BK , Antony P , Ajeeth NB , Job S , Shaji K ((2006) ) Usefulness of the Rowland Universal Dementia Assessment Scale inSouth India., J Neurol Neurosurg Psychiatry 77: , 513–514. |
[18] | Emerson A , Muruganantham P , Park MY , Pillay D , Vasan N , Park SJ , Van KL , Pampapathi P , Seow CS , Yau SM ((2019) ) Comparing the MontrealCognitive Assessment and Rowland Universal Dementia Assessment Scalein a multicultural rehabilitation setting., Intern Med J 49: , 1035–1040. |
[19] | Nielsen T , Segers K , Vanderaspoilden V , Beinhoff U , Minthon L , Pissiota A , Bekkhus-Wetterberg , Bjørkløf G , Tsolaki M , Gkioka M , Waldemar G ((2019) ) Validation of a brief Multicultural CognitiveExamination (MCE) for evaluation of dementia. IntPsychogeriatr 34: , 982–989. |
[20] | Nielsen TR , Andersen BB , Gottrup H , Lutzhoft JH , Hogh P , Waldemar G ((2013) ) Validation of the Rowland universal dementia assessment scalefor multicultural screening in Danish memory clinics., DementGeriatr Cogn Disord 36: , 354–362. |
[21] | Prince M , Acosta D , Chiu H , Scazufca M , Varghese M ((2003) ) Dementiadiagnosis in developing countries: A cross-cultural validationstudy. Lancet 361: , 909–917. |
[22] | Hall KS , Gao S , Emsley CL , Ogunniyi AO , Morgan O , Hendrie HC S ((2000) ) Community Screening Interview for Dementia (CSI ‘D’);performance in five disparate study sites. Int J GeriatrPsychiatry 15: , 521–531. |
[23] | Prince M , Acosta D , Ferri CP , Guerra M , Huang Y , Jacob H , LlibreRodrigueqz J , Salas A , Sosa L , Williams JD , Hall KS ((2011) ) A briefdementia screener suitable for use by non-specialists in resourcepoor settings-the cross-cultural derivation and validation of theBrief Community Screening Instrument for Dementia. Int JGeriatr Psychiatry 26: , 899–907. |
[24] | Gray WK , Paddick S , Walker RW ((2014) ) Development and validation of the Identification and Intervention for Dementia in Elderly Africans (IDEA) Study Dementia Screening Instrument., J Geriatr Psychiatry Neurol 27: , 110–118. |
[25] | Gray WK , Paddick S , Ogunniyi A , Olakehinde O , Dotchin C , Kissima J , Urasa S , Kisoli A , Rogathi J , Mushi D , Adebiyi A , Haule I , RobinsonL , Walker R ((2021) ) Population normative data for three cognitivescreening tools for older adults in sub-Saharan Africa. DementNeuropsychol 15: , 339–349. |
[26] | Collingwood C , Paddick S , Kisoli A , Dotchin CL , Gray WK , Mbowe G , Mkenda S , Urasa S , Mushi D , Chaote P , Walker RW ((2014) ) Developmentand community-based validation of the IDEA study InstrumentalActivities of Daily Living (IDEA-IADL) questionnaire. GlobalHealth Action 7: , 25988. |
[27] | Radford K , Mack HA , Draper B , Chalkley S , Delbaere K , Daylight G , Cumming RG , Bennett H , Broe GA ((2015) ) Comparison of three cognitivescreening tools in older urban and regional Aboriginal Australians. Dement Geriatr Cogn Disord 40: , 22–32. |
[28] | LoGiudice D , Smith K , Thomas J , Lautenschlager NT , Almeida OP , Atkinson D , Flicker L ((2006) ) Kimberley Indigenous CognitiveAssessment tool (KICA): Development of a cognitive assessment toolfor older indigenous Australians., Int Psychogeriatr 18: , 269–280. |
[29] | Lim L , Ng TP , Ong AP , Tan MP , Cenina AR , Gao Q , Ng A , Kandiah N ((2018) ) A novel language-neutral Visual Cognitive Assessment Test(VCAT): Validation in four Southeast Asian countries. Alzheimers Res Ther 10: , 6. |
[30] | Goudsmit M , Uysal-Bozkir O , Parlevliet JL , van Campen JPCM , de Rooij SE , Schmand B ((2017) ) The Cross-Cultural Dementia Screening (CCD): Anew neuropsychological screening instrument for dementia in elderlyimmigrants. J Clin Exp Neuropsychol 39: , 163–172. |
[31] | Bellaj T , Jemaa SB , Khelifa M , Djebara MB , Gouider R , Gall DL ((2017) ) The development of the Dementia Screening Battery-100: Instrumentpresentation, reliability, and construct validity., DementGeriatr Cogn Disord Extra 7: , 215–229. |
[32] | Chopra S , Kaur H , Pandey RM , Nehra A ((2018) ) Development ofneuropsychological evaluation screening tool: An education-freecognitive screening instrument. Neurol India 66: , 391–399. |
[33] | Thajeb P , Thajeb T , Dai D ((2006) ) Cross-cultural studies using aModified Mini Mental Test for healthy subjects and patients withvarious forms of vascular dementia., J Clin Neurosci 14: , 236–241. |
[34] | Ritchie KA , Hallerman EF ((1989) ) Cross-validation of a dementiascreening test in a heterogeneous population., Int J Epidemiol 18: , 717–719. |
[35] | Wolfe N , Imai Y , Otani C , Nagatani H , Hasegawa K , Sugimoto K , Tanaka Y , Kuroda Y , Glosser G , Albert ML ((1992) ) Criterion validity of thecross-cultural cognitive examination in Japan., J Gerontol 47: , 289–291. |
[36] | Kua EH , Ko SM ((1992) ) A questionnaire to screen for cognitiveimpairment among elderly people in developing countries. ActaPsychiatr Scand 85: , 119–122. |
[37] | Storey J , Rowland J , Conforti D , Dickson H ((2004) ) The RowlandUniversal Dementia Assessment Scale (RUDAS): A multiculturalcognitive assessment scale. Int Psychogeriatr 16: , 13–31. |
[38] | Celik S , Onur O , Yener G , Kessler J , Özbek Y , Meyer P , Frölich L , Teichmann B ((2022) ) Cross-cultural comparison of MMSEand RUDAS in German and Turkish patients with Alzheimer’s disease. Neuropsychology 36: , 195–205. |
[39] | Naqvi RM , Haider S , Tomlinson G , Alibhai S ((2015) ) Cognitiveassessments in multicultural populations using the Rowland UniversalDementia Assessment Scale: A systematic review and meta-analysis. Can Med Assoc J 187: , 169–175. |
[40] | Nielsen T , Segers K , Vanderaspoilden V , Bekkhus-Wetterberg P , Bjørkløf G , Beinhoff U , Waldemar G ((2019) ) Validation of theRowland Universal Dementia Assessment Scale (RUDAS) in amulticultural sample across five Western European countries:Diagnostic accuracy and normative data., Int Psychogeriatr 31: , 287–296. |
[41] | Custodio N , Montesinos R , Lira D , Herrera E , Chavez K , Reynoso-Guzman W , Caipa M , Cuenca-Alfaro J , Gamboa C , Metcalf T ((2019) ) Validation of the RUDAS in patients with a middle-leveleducation in Lima, Peru., Am J Alzheimers Dis Other Demen 34: , 513–522. |
[42] | Mukaetova-Ladinska EB , De Lillo C , Arshad Q , Subramaniam HE , Maltby J ((2022) ) Cognitive assessment of dementia: The need for an inclusivedesign tool. Curr Alzheimer Res 19: , 265–273. |
[43] | Mukaetova-Ladinska EB , Abdullah S , Critchfield M , Maltby J ((2022) ) Suspected dementia in young adults: Cognitive screening tools foruse in primary care. J Alzheimers Dis 86: , 333–341. |
[44] | Nielsen TR , Vogel A , Gade A , Waldemar G ((2012) ) Cognitive testing in non-demented Turkish immigrants–comparison of the RUDAS and theMMSE. Scand J Psychol 53: , 455–460. |
[45] | Kalafatis C , Modarres MH , Apostolou P , Marefat H , Khanbagi M , Karimi H , Vahabi Z , Aarsland D , Khaligh-Razavi SM ((2021) ) Validity andcultural generalisability of a 5-minute AI-based, computerisedcognitive assessment in mild cognitive impairment and Alzheimer’sdementia. Front Psychiatry 22: , 706695. |
[46] | Ashford JW , Clifford JO , Bergeron MF ((2023) ) Advancing screening forcognitive impairment: The MemTrax continuous recognition test. Aging (Albany NY) 15: , 5230–5231. |
[47] | Liu X , Chen X , Zhou X , Shang Y , Xu F , Zhang J , He J , Zhao F , Du B , Wang X , Zhang Q , Zhang W , Bergeron MF , Ding T , Ashford JW , Zhong L ((2021) ) Validity of the MemTrax Memory Test compared to the MontrealCognitive Assessment in the detection of mild cognitive impairmentand dementia due to Alzheimer’s disease in a Chinese cohort. J Alzheimers Dis 80: , 1257–1267. |




