RPTOR Is an Alzheimer’s Disease Susceptibility Gene Associated with the Risk Factors Body Mass Index and Infectious Encephalitis
Abstract
Background:
In comparison to persons who did not have viral encephalitis, people with viral encephalitis had a later-life risk of Alzheimer’s disease (AD) that was 31 times higher. In a previous study, we were able to confirm the association of viral encephalitis with AD and suggest that West Nile Virus infection is a significant AD risk factor. A genome wide association study (GWAS) with UK Biobank data revealed that the gene RAR Related Orphan Receptor B (RORB) is significantly associated with viral encephalitis.
Objective:
To use data from the 8 PheWeb datasets to try to identify genes other than RORB that might be involved in both infectious encephalitis and AD.
Methods:
PheWeb includes data from UKBB and 5 other databanks. We used UK Biobank data to examine gene expression and phenotypic expression.
Results:
PheWeb identified additional genes associated with both infectious encephalitis and AD. RPTOR, a gene associated with the mTOR pathway, emerges as significant. Analyses of UK Biobank data reveal the impact of RPTOR on AD risk, with carriers of the minor allele A exhibiting decreased prevalence in subjects under age 55. Further analysis demonstrates that RPTOR genotypes influence body mass index (BMI) in subjects of all ages, with carriers of the minor allele A having lower BMI. Logistic regression analyses confirm the association between reduced BMI and increased AD risk, along with the established factor of age.
Conclusions:
RPTOR may represent an AD gene, though mTOR’s role in AD and BMI is complex. Nevertheless, RPTOR and mTOR could represent potential therapeutic targets for AD.
INTRODUCTION
An increased chance of developing Alzheimer’s disease (AD) or Parkinson’s disease later in life has been linked to infections with the influenza virus and other common viruses, according to an examination of about 450,000 electronic health records. One of the strongest correlations was found between viral encephalitis, an uncommon brain inflammation that can be brought on by a variety of viruses, and AD. In comparison to those who did not have encephalitis, those with encephalitis had a later-life risk of AD that was roughly 31 times higher [1, 2].
West Nile virus (WNV) is the main mosquito-borne disease in the continental United States. The most typical way for WNV to spread to humans is by a mosquito bite. The mosquito season, which begins in the summer and lasts until the fall, is when WNV cases develop. The majority of WNV carriers have no symptoms. One in five infected individuals have fever and other symptoms. One in 150 infected individuals develop encephalitis [3].
In a previous study, we were able to confirm the association of viral encephalitis with AD and suggest that WNV infection is a significant AD risk factor. A genome wide association study (GWAS) with UK Biobank data revealed that the gene RAR Related Orphan Receptor B (RORB) is significantly associated with viral encephalitis. [4]. RORB is an AD vulnerability factor [5] and an epilepsy gene [6]. Results in Finngen indicate that epilepsy increases risk of viral encephalitis, and thus AD, with hazards ratio 4.92 [7].
Some mutations and polymorphisms have been identified to increase the risk of acute infection-induced encephalitis. For instance, rare variants of the SCN1A and RANBP2 genes, thermolabile CPT2 variants, and HLA genotypes are some of the genetic factors that have been associated with encephalitis [8].
In the current study, we used data from PheWeb [9] to identify genes other than RORB that might be involved in both infectious encephalitis and AD. We did so because in our previous study of UK Biobank data, we had only 18 cases of viral encephalitis. We used UK Biobank data in the current study to examine gene expression and phenotypic expression.
Mammalian Target of Rapamycin, or mTOR, is a protein kinase that plays a crucial role in the regulation of cell growth, proliferation, and survival. It is involved in cellular processes, including protein synthesis, autophagy, and metabolism. Dysregulation of mTOR signaling has been implicated in the pathogenesis of several diseases, including neurodegenerative disorders and AD. The RPTOR gene (Regulatory-Associated Protein Of MTOR) is part of the mTOR pathway [10].
RPTOR is associated with body mass index (BMI) and obesity [11–13]. AD is associated with low BMI in old age [14]. Because of these associations and association with RPTOR snp rs12940622, which we studied here, we prioritized BMI as an outcome.
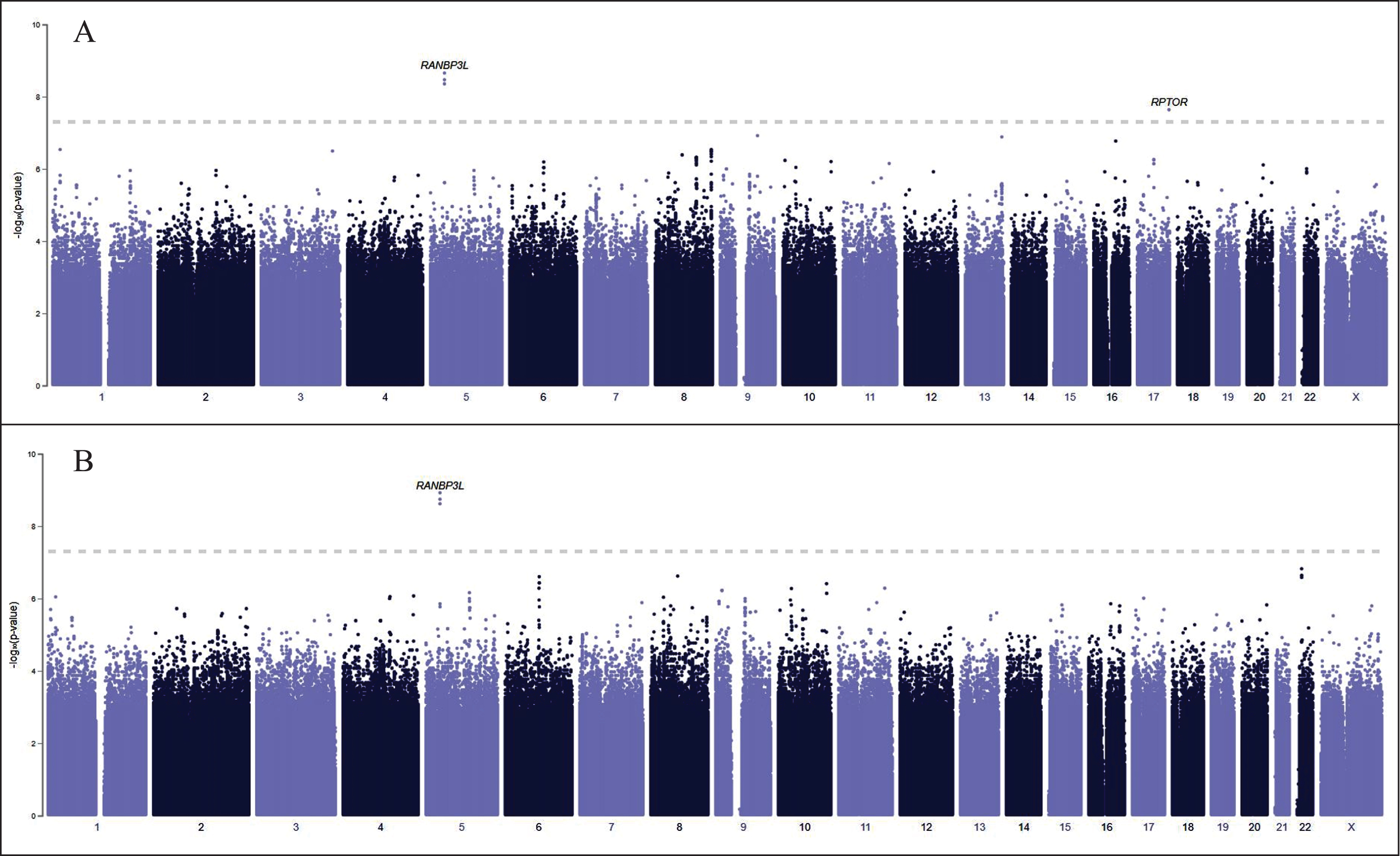
Fig. 1
PheWeb GWAS results for 134 cases of encephalitis (A) and 114 cases of non-infectious encephalitis (B). RANBP3 L and RPTOR are significantly associated with encephalitis (A). Most encephalitis cases that have been diagnosed in the United States are infectious, caused by enteroviruses, arboviruses (including the West Nile Virus), and herpes simplex virus types 1 and 2. We have excluded RANBP3 L (B), associated with non-infectious encephalitis, from our analysis.
METHODS
PheWeb is a user-friendly, open-source web application for sharing, exploring, and visualizing GWAS results [9]. PheWeb includes findings from GWAS of 1,542 broad phenome wide association studies (PheWAS) codes collected from electronic health records for over 51.8 million imputed variations in 51,583 individuals of European ancestry from the Michigan Genomics Initiative, a biobank of patients at Michigan Medicine. The PheWAS R package (https://github.com/PheWAS/PheWAS) is used to extract diagnosis codes from patient electronic health records and generate phenotypes. The TOPMed reference panel is used to impute genetic data in Michigan Genomics Initiative participants to provide association testing at high-quality genetic variants for each phenotype. Pheome-wide association analysis of individual genetic variants and interactive study of individual GWAS are made possible via the PheWeb interface. The gene RPTOR emerged in the GWAS (see results below).
To examine RPTOR in relation to AD and BMI we used UK Biobank data. The UK Biobank (UKBB) is a large prospective observational study comprising approximately 500,000 men and women (N = 229,134 men, N = 273,402 women), more than 90% white, aged 40–69 years at enrollment. Our UK Biobank project: 57245, Lehrer and Rheinstein. We analyzed the RPTOR snp rs12940622, an intron variant, G > A, minor allele (A) frequency 0.43.
Data processing was performed on Minerva, a Linux mainframe with Centos 7.6, at the Icahn School of Medicine at Mount Sinai. We used PLINK, a whole-genome association analysis toolset, to analyze the UKB chromosome files [15] and the UK Biobank Data Parser (ukbb parser), a python-based package that allows easy interfacing with the large UK Biobank dataset [16]. PheWeb uses LocusZoom for the Manhattan and q-q plots and calculation of lambda, the genetic control inflation factor [17].
RESULTS
Figure 1A displays PheWeb GWAS results for 134 cases of encephalitis and 114 cases of non-infectious encephalitis (Fig. 1B). RANBP3 L and RPTOR are significantly associated with encephalitis, but RANBP3 L is associated with non-infectious encephalitis. Most encephalitis cases that have been diagnosed in the United States are infectious, caused by enteroviruses, arboviruses (including the WNV), and herpes simplex virus types 1 and 2, which are spread from infected animals to humans through the bite of an infected tick, mosquito, or other bloodsucking parasite [18]. We have excluded RANBP3 L from our analysis to eliminate cases of non-infectious encephalitis.
Figure 2A shows the QQ plot of p values from encephalitis data (Fig. 1A). Note that the leftmost p-values observed follow a uniform distribution (lower segment of line) but those that are in linkage disequilibrium with causal polymorphisms produce significant p-values (right segment of line). The genomic control inflation factor lambda, calculation based on the 50th percentile (median), is 0.205. Values up to 1.1 are generally considered acceptable for GWAS and suggest no systematic biases. Figure 2B is the QQ plot of p values from non-infectious encephalitis data (Fig. 1B, lambda 0.188).
Fig. 2
A) QQ plot of p values from encephalitis data (Fig. 1A). Note that the leftmost p-values observed follow a uniform distribution (lower segment of line) but those that are in linkage disequilibrium with causal polymorphisms produce significant p-values (right segment of line). The genomic control inflation factor lambda, calculation based on the 50th percentile (median), is 0.205. Values up to 1.1 are generally considered acceptable for GWAS and suggest no systematic biases. B) QQ plot of p values from non-infectious encephalitis data (Fig. 1B, lambda 0.188).
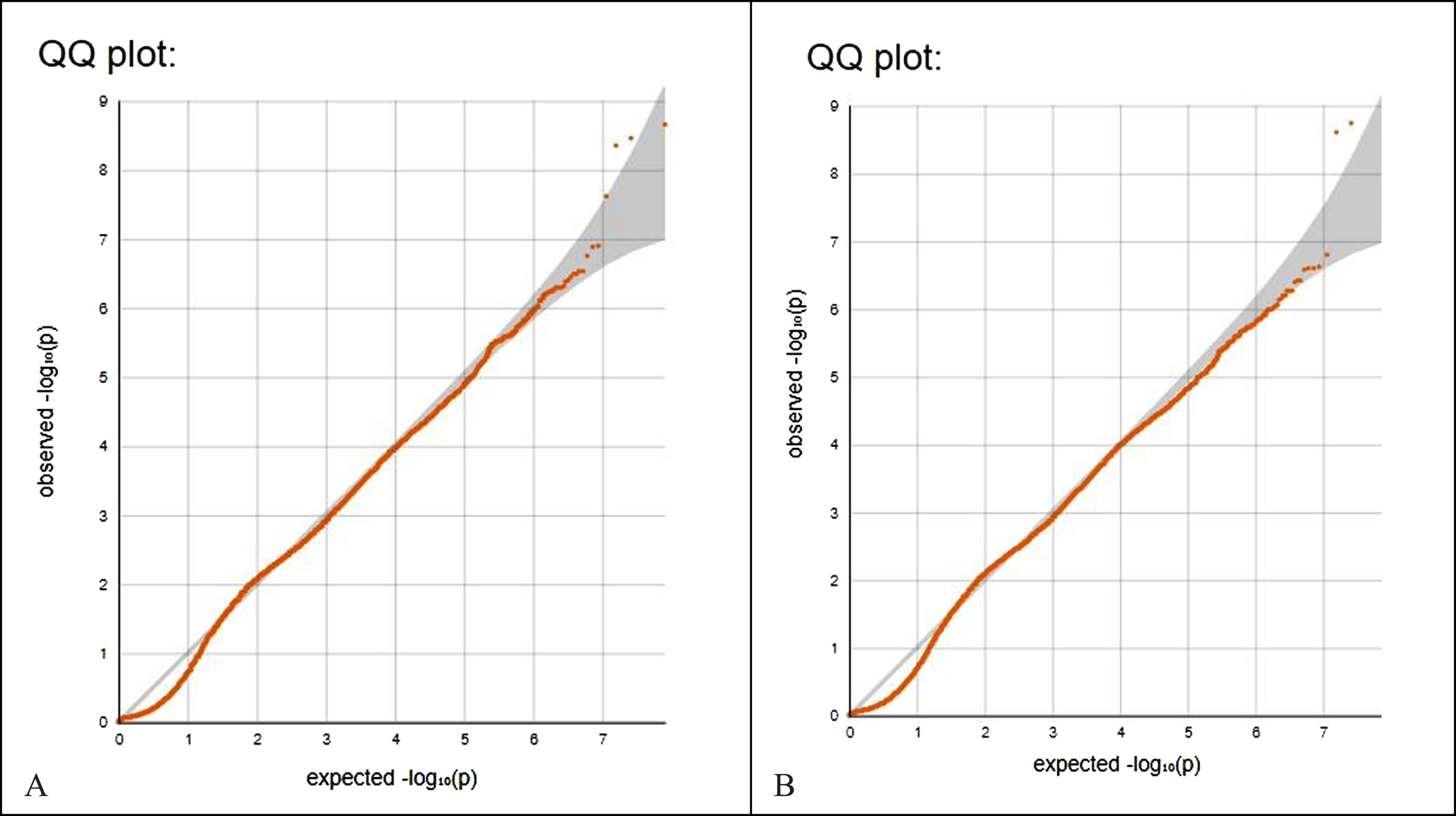
Table 1 shows RPTOR snp rs12940622 genotype versus AD in 187,297 subjects age < 55. Note that subjects homozygous for the major allele G had increased prevalence of AD, while subjects heterozygous or homozygous for the minor allele A had decreased prevalence of AD (p = 0.05, two tail Fisher exact test).
Table 1
RPTOR genotype versus AD in 187,297 subjects age < 55. Note that subjects homozygous for the major allele G had increased prevalence of AD, while subjects heterozygous or homozygous for the minor allele A had decreased prevalence of AD (p = 0.05, two tail Fisher exact test)
| no AD | AD | Total | ||
| GG | Count | 59,478 | 17 | 59,495 |
| % within ALZ | 31.80% | 47.20% | 31.80% | |
| GA + AA | Count | 127,783 | 19 | 127,802 |
| % within ALZ | 68.20% | 52.80% | 68.20% | |
| total | Count | 187,261 | 36 | 187,297 |
| % within ALZ | 100.00% | 100.00% | 100.00% |
Table 2 shows logistic regression with 95% confidence intervals, AD in subjects < age years dependent variable, sex, age, and RPTOR genotype independent variables. Increasing age was significantly associated with more AD (odds ratio [O.R.] 1.240). Subjects heterozygous or homozygous for the RPTOR minor allele A had decreased risk of AD (O.R. 0.517).
Table 2
Logistic regression with 95% confidence intervals, AD in subjects < 55 years dependent variable, sex, age, and RPTOR genotype independent variables, L.B., lower bound; O.R., odds ratio; U.B., upper bound. Increasing age was significantly associated with more AD (O.R. 1.240). Subjects heterozygous or homozygous for the RPTOR minor allele A had decreased risk of AD (O.R. 0.517)
| 95% L.B. | O.R. | 95% U.B. | p | |
| Sex | 0.743 | 1.430 | 2.753 | 0.284 |
| Age | 1.120 | 1.240 | 1.373 | <0.001 |
| RPTOR | 0.269 | 0.517 | 0.996 | 0.048 |
RPTOR genotypes had a significant effect on BMI. 153,249 subjects homozygous for major allele G had higher BMI 27.4702±4.81742 (mean±SD). 330,564 subjects heterozygous or homozygous for the minor allele A had lower BMI 27.3953±4.77236. This difference was significant (p < 0.001, two tailed t test).
Table 3 shows logistic regression with 95% confidence intervals, AD in subjects all ages dependent variable, sex, age, and BMI independent variables. Reduced BMI was significantly associated with more AD (O.R. 0.982). Increasing age was significantly associated with more AD (O.R. 1.213). Male sex was significantly associated with a higher odds ratio of AD (O.R. 1.198).
Table 3
Logistic regression with 95% confidence intervals, AD in subjects all ages dependent variable, sex, age, and BMI independent variables. Reduced BMI was significantly associated with more AD (O.R. 0.982). Increasing age was significantly associated with more AD (O.R. 1.213). Male sex was significantly associated with more AD (O.R. 1.198)
| 95% L.B. | O.R. | 95% U.B. | p | |
| Sex | 1.053 | 1.198 | 1.364 | 0.006 |
| Age | 1.196 | 1.213 | 1.230 | <0.001 |
| BMI | 0.967 | 0.982 | 0.998 | 0.020 |
DISCUSSION
The RPTOR gene is associated with the mTOR pathway [10]. mTOR is a protein kinase that plays a crucial role in the regulation of cell growth, proliferation, and survival. It is involved in cellular processes, including protein synthesis, autophagy, and metabolism. Dysregulation of mTOR signaling has been implicated in the pathogenesis of several diseases, including neurodegenerative disorders and AD.
AD is a neurodegenerative disorder that is characterized by the accumulation of amyloid-β and tau proteins in the brain [19]. mTOR is a central regulator of protein synthesis in neurons, and its signaling has been linked to neurodegenerative diseases such as AD [20]. In AD, the inhibition of mTOR by rapamycin ameliorates the synthesis of amyloid-β and tau protein and improves learning and memory abilities [20]. Autophagy is a cellular recycling process that is required to prevent the buildup of misfolded protein aggregates that contribute to the development of neurodegenerative diseases [21]. In the central nervous system, autophagy and its regulation by mTOR are critical for maintaining neuronal functions such as synaptic remodeling and plasticity associated with long-term memory formation [20]. Synaptic plasticity is the ability of synapses to strengthen or weaken over time in response to changes in their activity, and it is essential for learning and memory [20]. Neuronal function is the ability of neurons to communicate with each other and is critical for the proper functioning of the nervous system [20]. Therefore, mTOR-regulated autophagy plays an important role in the synaptic plasticity-related proteins alterations and the decline of cognitive function in AD [20].
The mTOR pathway can affect encephalitis susceptibility. Viral infections can activate, reduce, or even suppress the mTOR pathway. In addition, viruses have evolved various mechanisms to attack and co-opt the mTOR pathway to make the host cell a hospitable environment for replication [22]. We are unable to determine whether the specific variant, the RPTOR snp rs12940622, is associated with encephalitis. However, the GWAS data we present indicate that RPTOR is associated with encephalitis.
The RPTOR gene snp rs12940622 is related to BMI [11–13]. BMI is a measure of body mass based on an individual’s height and weight. It is widely used as a screening tool to categorize individuals into different weight categories, such as underweight, normal weight, overweight, and obesity. Increase in body weight often results from obesity (increased adipose tissue mass) but also from, for example, muscle mass. We were able to confirm the RPTOR-BMI relationship in UKBB data above, although the effect size was small [23]. There were no other hits in the data we examined.
While there is evidence that RPTOR can influence susceptibility to obesity, multiple genes are likely involved, and the interplay between genetic and environmental factors is complex. Numerous genetic variants have been associated with obesity-related traits, but the specific contribution of each gene and how they interact is still an area of active research [24].
Studies of the association between BMI and AD have yielded mixed findings [25]. There may be a link between being underweight or having low BMI and an increased risk of developing AD [26]. Malnutrition or other factors related to low BMI may influence cognitive function negatively. Obesity is a well-known risk factor for various health conditions, including cardiovascular diseases and type 2 diabetes. A possible link may exist between obesity and an increased risk of developing AD [25]. Indeed, both low and high BMI may be associated with an increased risk of AD, indicating a potential U-shaped relationship, the obesity paradox: obesity in midlife, low BMI in old age [14]. But any relationship between BMI and AD is complex, and additional factors such as age, genetics, and overall health contribute to the risk.
Infectious encephalitis disrupts the blood-brain barrier [27, 28] and derangements of the blood-brain barrier are an important part of AD [29]; however, our study is limited by inability to directly evaluate effects on the blood-brain barrier. Confirmatory studies with mRNA expression of the RPTOR snp rs12940622 variant or tissue/blood level of RPTOR protein expression would support findings presented here. A Mendelian randomization study to examine the causal relationship between encephalitis and AD would be important in future studies.
Most studies report that AD is more common in women; however, in the UKBB, AD is more common in men, which has been reported previously. In another study of UK Biobank data, men had a higher risk of all-cause, young-onset and late-onset dementias than women [30].
In conclusion, mTOR and RPTOR may represent potential therapeutic targets for AD, due to their involvement in various cellular processes implicated in AD. Modulating RPTOR and mTOR activity may have the potential to regulate protein synthesis, enhance autophagy, and improve neuronal function in people with AD. The role of RPTOR and mTOR in AD is complex, and both excessive and insufficient mTOR activity may have negative effects. Balancing mTOR activity to achieve therapeutic benefits without causing harmful side effects will be a challenge.
AUTHOR CONTRIBUTIONS
Steven Lehrer (Conceptualization; Data curation; Writing - Original Draft); Peter H. Rheinstein, MD, JD, MS (Conceptualization).
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai. Research reported in this paper was also supported by the Office of Research Infrastructure of the National Institutes of Health under award numbers S10OD018522 and S10OD026880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data sources described in the article are publicly available or can be accessed after approved application to UK Biobank.
REFERENCES
[1] | Levine KS , Leonard HL , Blauwendraat C , Iwaki H , Johnson N , Bandres-Ciga S , Ferrucci L , Faghri F , Singleton AB , Nalls MA ((2023) ) Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 111: , 1086–1093e1082. |
[2] | Kozlov M ((2023) ) Massive health-record review links viral illnesses to brain disease. Nature 614: , 18–19. |
[3] | Petersen LR , Roehrig JT , Hughes JM ((2002) ) West Nile virus encephalitis. N Engl J Med 347: , 1225–1226. |
[4] | Lehrer S , Rheinstein PH ((2023) ) RORB, an Alzheimer’s disease susceptibility gene, is associated with viral encephalitis, an Alzheimer’s disease risk factor. Clin Neurol Neurosurg 233: , 107984. |
[5] | Leng K , Li E , Eser R , Piergies A , Sit R , Tan M , Neff N , Li SH , Rodriguez RD , Suemoto CK , Leite REP , Ehrenberg AJ , Pasqualucci CA , Seeley WW , Spina S , Heinsen H , Grinberg LT , Kampmann M ((2021) ) Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat Neurosci 24: , 276–287. |
[6] | Rudolf G , Lesca G , Mehrjouy MM , Labalme A , Salmi M , Bache I , Bruneau N , Pendziwiat M , Fluss J , De Bellescize J ((2016) ) Loss of function of the retinoid-related nuclear receptor (RORB) gene and epilepsy. Eur J Hum Genet 24: , 1761–1770. |
[7] | Kurki MI , Karjalainen J , Palta P , Sipila TP , Kristiansson K , Donner KM , Reeve MP , Laivuori H , Aavikko M , Kaunisto MA , Loukola A , Lahtela E , Mattsson H , Laiho P , Della Briotta Parolo P , Lehisto AA , Kanai M , Mars N , Ramo J , Kiiskinen T , Heyne HO , Veerapen K , Rueger S , Lemmela S , Zhou W , Ruotsalainen S , Parn K , Hiekkalinna T , Koskelainen S , Paajanen T , Llorens V , Gracia-Tabuenca J , Siirtola H , Reis K , Elnahas AG , Sun B , Foley CN , Aalto-Setala K , Alasoo K , Arvas M , Auro K , Biswas S , Bizaki-Vallaskangas A , Carpen O , Chen CY , Dada OA , Ding Z , Ehm MG , Eklund K , Farkkila M , Finucane H , Ganna A , Ghazal A , Graham RR , Green EM , Hakanen A , Hautalahti M , Hedman AK , Hiltunen M , Hinttala R , Hovatta I , Hu X , Huertas-Vazquez A , Huilaja L , Hunkapiller J , Jacob H , Jensen JN , Joensuu H , John S , Julkunen V , Jung M , Junttila J , Kaarniranta K , Kahonen M , Kajanne R , Kallio L , Kalviainen R , Kaprio J , FinnGen , Kerimov N , Kettunen J , Kilpelainen E , Kilpi T , Klinger K , Kosma VM , Kuopio T , Kurra V , Laisk T , Laukkanen J , Lawless N , Liu A , Longerich S , Magi R , Makela J , Makitie A , Malarstig A , Mannermaa A , Maranville J , Matakidou A , Meretoja T , Mozaffari SV , Niemi MEK , Niemi M , Niiranen T , CJ OD , Obeidat ME , Okafo G , Ollila HM , Palomaki A , Palotie T , Partanen J , Paul DS , Pelkonen M , Pendergrass RK , Petrovski S , Pitkaranta A , Platt A , Pulford D , Punkka E , Pussinen P , Raghavan N , Rahimov F , Rajpal D , Renaud NA , Riley-Gillis B , Rodosthenous R , Saarentaus E , Salminen A , Salminen E , Salomaa V , Schleutker J , Serpi R , Shen HY , Siegel R , Silander K , Siltanen S , Soini S , Soininen H , Sul JH , Tachmazidou I , Tasanen K , Tienari P , Toppila-Salmi S , Tukiainen T , Tuomi T , Turunen JA , Ulirsch JC , Vaura F , Virolainen P , Waring J , Waterworth D , Yang R , Nelis M , Reigo A , Metspalu A , Milani L , Esko T , Fox C , Havulinna AS , Perola M , Ripatti S , Jalanko A , Laitinen T , Makela TP , Plenge R , McCarthy M , Runz H , Daly MJ , Palotie A ((2023) ) FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613: , 508–518. |
[8] | Mizuguchi M , Shibata A , Kasai M , Hoshino A ((2023) ) Genetic and environmental risk factors of acute infection-triggered encephalopathy. Front Neurosci 17: , 1119708. |
[9] | Gagliano Taliun SA , VandeHaar P , Boughton AP , Welch RP , Taliun D , Schmidt EM , Zhou W , Nielsen JB , Willer CJ , Lee S ((2020) ) Exploring and visualizing large-scale genetic associations by using PheWeb. Nat Genet 52: , 550–552. |
[10] | Passtoors WM , Beekman M , Deelen J , van der Breggen R , Maier AB , Guigas B , Derhovanessian E , van Heemst D , de Craen AJ , Gunn DA ((2013) ) Gene expression analysis of mTOR pathway: Association with human longevity. Aging Cell 12: , 24–31. |
[11] | Morris BJ , Carnes BA , Chen R , Donlon TA , He Q , Grove JS , Masaki KH , Elliott A , Willcox DC , Allsopp R ((2015) ) Genetic variation in the raptor gene is associated with overweight but not hypertension in American men of Japanese ancestry. Am J Hypertens 28: , 508–517. |
[12] | Nakayama K , Miyashita H , Iwamoto S ((2014) ) Seasonal effects of the UCP3 and the RPTOR gene polymorphisms on obesity traits in Japanese adults. J Physiol Anthropol 33: , 1–5. |
[13] | Trifonova E , Popovich A , Makeeva O , Minaycheva L , Bocharova A , Vagaitseva K , Stepanov V ((2021) ) Replicative association analysis of genetic markers of obesity in the Russian population. Russ J Genet 57: , 620–625. |
[14] | Pegueroles J , Jiménez A , Vilaplana E , Montal V , Carmona-Iragui M , Pané A , Alcolea D , Videla L , Casajoana A , Clarimón J ((2018) ) Obesity and Alzheimer’s disease, does the obesity paradoxreally exist? A magnetic resonance imaging study. Oncotarget 9: , 34691. |
[15] | Chang CC , Chow CC , Tellier LC , Vattikuti S , Purcell SM , Lee JJ ((2015) ) Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4: , 7. |
[16] | Zhu A , Salminen LE , Thompson PM , Jahanshad N (2019) The UK Biobank Data Parser: A tool with built in and customizable filters for brain studies. Organization for Human Brain Mapping, Rome, Italy, June 9-13, 2019. |
[17] | Pruim RJ , Welch RP , Sanna S , Teslovich TM , Chines PS , Gliedt TP , Boehnke M , Abecasis GR , Willer CJ ((2010) ) LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 26: , 2336–2337. |
[18] | Solomon T ((2004) ) Flavivirus encephalitis. N Engl J Med 351: , 370–378. |
[19] | Uddin MS , Rahman MA , Kabir MT , Behl T , Mathew B , Perveen A , Barreto GE , Bin-Jumah MN , Abdel-Daim MM , Ashraf GM ((2020) ) Multifarious rolesof mTOR signaling in cognitive aging and cerebrovascular dysfunctionof Alzheimer’s disease. IUBMB Life 72: , 1843–1855. |
[20] | Friedman LG , Qureshi YH , Yu WH ((2015) ) Promoting autophagic clearance: Viable therapeutic targets in Alzheimer’s disease. Neurotherapeutics 12: , 94–108. |
[21] | Gao S , Zhang S , Zhou H , Tao X , Ni Y , Pei D , Kang S , Yan W , Lu J ((2021) ) Role of mTOR-regulated autophagy in synaptic plasticity related proteins downregulation and the reference memory deficits induced by anesthesia/surgery in aged mice. Front Aging Neurosci 13: , 628541. |
[22] | Le Sage V , Cinti A , Amorim R , Mouland AJ ((2016) ) Adapting the stress response: Viral subversion of the mTOR signaling pathway. Viruses 8: , 152. |
[23] | Sullivan GM , Feinn R ((2012) ) Using effect size-or why the p value is not enough. J Grad Med Educ 4: , 279–282. |
[24] | Herrera BM , Lindgren CM ((2010) ) The genetics of obesity. Curr Diabetes Rep 10: , 498–505. |
[25] | Alford S , Patel D , Perakakis N , Mantzoros C ((2018) ) Obesity as a risk factor for Alzheimer’s disease: Weighing the evidence. Obes Rev 19: , 269–280. |
[26] | Xu W , Tan L , Wang H-F , Jiang T , Tan M-S , Tan L , Zhao Q-F , Li J-Q , Wang J , Yu J-T ((2015) ) Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 86: , 1299–1306. |
[27] | Li F , Wang Y , Yu L , Cao S , Wang K , Yuan J , Wang C , Wang K , Cui M , Fu ZF ((2015) ) Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol 89: , 5602–5614. |
[28] | Liu H , Qiu K , He Q , Lei Q , Lu W ((2019) ) Mechanisms ofblood-brain barrier disruption in herpes simplex encephalitis. J Neuroimmune Pharmacol 14: , 157–172. |
[29] | Zenaro E , Piacentino G , Constantin G ((2017) ) The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 107: , 41–56. |
[30] | Shang X , Roccati E , Zhu Z , Kiburg K , Wang W , Huang Y , Zhang X , Zhang X , Liu J , Tang S , Hu Y , Ge Z , Yu H , He M ((2023) ) Leading mediators of sex differences in the incidence of dementia in community-dwelling adults in the UK Biobank: A retrospective cohort study. Alzheimers Res Ther 15: , 7. |




