Retinal and Cortical Visual Processing Dysfunction in a Case of Mild Cognitive Impairment with Lewy Bodies: A Case Report
Abstract
The prodromal stage of Lewy body dementia includes a mild cognitive impairment with visual processing and/or attention-executive deficits. A clinical presentation with progressive visual loss is indeed seldom reported and can be misleading with a posterior cortical atrophy disease. While the neurodegeneration at the occipital cortex can only partially explain the visual disturbances of Lewy body dementia, more recently a retinal dysfunction has been suggested by preliminary optical coherence tomography and autoptic findings. Herein, we present a case of a mild cognitive impairment with Lewy bodies, who presented initially with visual disturbances and signs of both retinal and cortical visual processing dysfunction. A complete neuropsychological, neurophysiological and brain imaging assessment highlighted a prominent ventral visual pathway involvement. This report provides first that the prodromal stage of Lewy body dementia can manifest as a primarily progressive visual loss, second that the involvement of visual pathway, particularly the ventral stream, can be detectable from the retinal to the cortical level.
INTRODUCTION
Lewy body dementia (LBD) is the second most common form of neurodegenerative dementia and together with Parkinson’s disease is included in the spectrum of α-synuclein-related diseases called Lewy body diseases [1]. Recently, the prodromal stage of LBD has gained a lot of attention. Recognizing the prodromal phase of LBD is challenging due to the heterogeneous clinical onset spectrum, including a mild cognitive impairment presentation predominantly with visual processing deficits and/or attention-executive deficits [2]. Although visual symptoms are frequently seen early in the disease, ranging from minor and complex visual hallucinations to impairments in complex visual tasks and visual fields deficits being reported as well, a clinical complaint of isolated visual loss is seldom reported at the disease onset [3]. A schematic interpretation of visual perception postulates two visual streams, namely ventral and dorsal streams. Visual deficits in LBD appear to be specifically associated with impaired processing in the ventral visual pathway, manifesting as more severe impairments in object recognition than space perception [4]. Interestingly, visual symptoms have been partly related to disorders of cortical visual processing [4–6], in addition to disruption of retinal structure and function [7–10]. The contributions of the different parts of the visual pathway still remain to be elucidated. Notably, the rapid progression of cognitive impairment in LBD may affect the assessment of neuropsychological tasks. Herein, we report a man with a prodromal LBD, who presented initially with visual disturbances and signs of both retinal and cortical visual processing dysfunction.
CASE PRESENTATION
We report the case of a 76-year-old man, right-handed, retired butcher with 5 years of education, complaining an insidious and slowly progressive deficit in distinguishing between an object and its background, lasting for two years. For example, he reported disturbances in distinguishing a clear glass on a tablecloth with a geometric pattern, and, while watching a football match, in correctly discriminating the player when wearing a T-shirt of the same color as a billboard in the background. He also referred difficulties in ambulation, particularly concerning the avoidance of obstacles, as well as in driving a car, but otherwise reported independence on daily life activities. Past medical history included hypercholesterolemia treated with statin, discontinued for unspecified reason, and laminectomy for lumbar spinal stenosis. There was no family history of neurodegenerative disease.
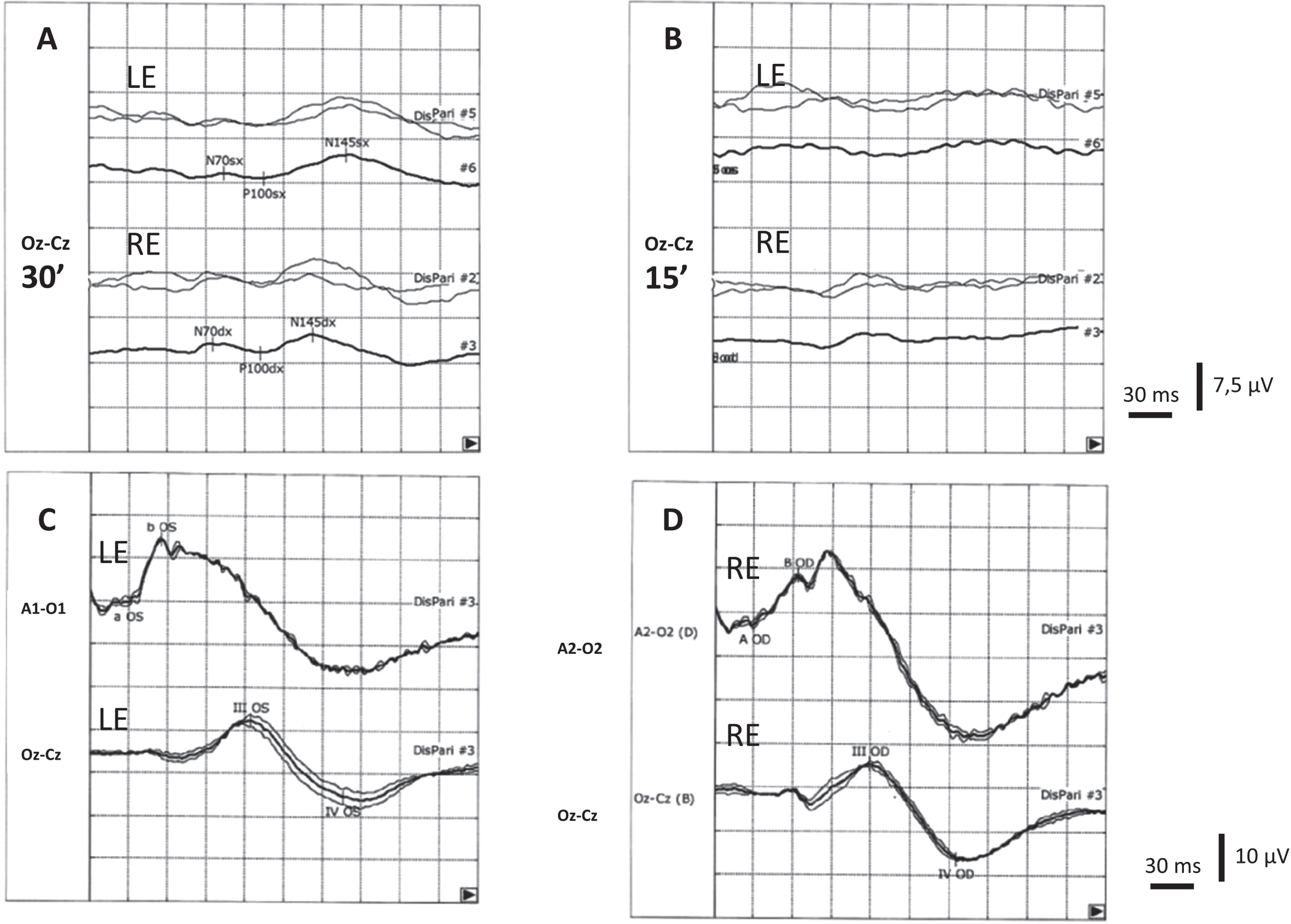
The ophthalmologic examination revealed dyslacrimia, blepharitis, and a very initial corticonuclear cataract. Visual acuity was 9/10 in both eyes. Formal automated visual field testing demonstrates bilateral enlargement of the blind spot and scotomas in all quadrants. Fundus examination showed a flat retina, optic disc drusen, and cellophane reflex at the macula. Tonometry readings were within the normal range. The optical coherence tomography (OCT) demonstrated a consistent loss of ganglion cell layer (GCL) in both eyes, and papilla deposits compatible with optic disc drusen. Pattern-reversal visual evoked potentials (VEPs) with 30’ checks revealed decreased amplitude and increased latency of P100 in both eyes, while VEPs with 15’ checks were found to be non-reproducible bilaterally. Flash electroretinogram showed normal external and intermediate retinal responses (Fig. 1).
Fig. 1
Visual evoked potentials (VEPs) performed by checkerboard pattern reversal stimuli system with 30’ (A) and 15’ (B) checks in our patient. All VEPs were recorded after monocular stimulation using Ag/AgCl skin electrodes placed accordingly to the 10–20 international system in Oz (active electrode) and Cz (reference electrode). Two series of 100 artifact-free responses were averaged for each check and each eye. The peak latency of the major positive wave (P100) was measured to the nearest millisecond. With 30’ checks the latency was 132 and 135 ms for right eye (RE) and left eye (LE) stimulation, respectively (upper normal limit: 118 ms), while the amplitude was 1.4μV and 0.8μV for right eye (RE) and left eye (LE) stimulation respectively (lower normal limit: 3μV). Cortical responses were non-recordable using the 15’ checks. Full-field electroretinogram (ERG) was recorded using flash stimulation of the LE (C) and RE (D) in mesopic condition by a Ag/AgCl a skin electrode placed over the ipsilateral ear lobe and reference electrode placed over the skin of the lower eyelid of the stimulated eye. Flash-induced VEPs (lower traces) were recorded by occipital surface electrode positioned as above said. III and IV waves were recordable after stimulation of both eyes.
Once an ophthalmological etiopathogenesis of the disorder was ruled out, the patient was referred to our memory clinic. In his recent history he emerged to be more irritable, sometimes even apathetic and some falls apparently due to motor impairment were reported. Notably, hyposmia and rapid eye movement sleep behavior disorder (RBD), without dysautonomia, had been present for about 5–6 years. Neurological examination revealed a mild akinetic-rigid parkinsonism (right greater than left), hypomimia, snout and glabellar tap signs, hypometric saccades, reduced pursuit gain, and partial vertical gaze palsy.
On formal neuropsychological assessment [11–26], he displayed normal performance in global cognitive functioning, as well as normal scores in memory and language tests (Table 1). Spontaneous speech was normal for articulation, fluency, and content. Impairments were found in executive functions, specifically in the Frontal Assessment Battery [20] and in attentional shifting measured with Trail Making Test (TMT B, B-A) [19]. Visuo-perceptual abilities were explored with Barletta and Rodolfi battery, tapping the different stages of visual perception [21]. In line with the reported disturbances in figure-background discrimination, the patient presented impairments in the X-O-N test. The test included a random pattern background with a fragmented shape, i.e., either X or O, superimposed in half of the trials and absent in the other half. The patient was required to indicate whether the shape was present or not. A normal performance was reported in the other visuo-perceptual tests. Specifically, the patient performed within normal range when asked to identify the object progressively presented with a decreasing level of degradation (Gollin test) and to identify a set of overlapping figures (Ghent overlapping figure test). Perceptual classification, evaluated asking him to match objects seen from different viewpoints (Perceptual constancy test), was preserved. Visuo-spatial abilities, evaluated with dot counting and position discrimination subtests of the Visual Object and Space Perception battery [22] were spared. Patient’s performance in the tests evaluating the presence of unilateral visual neglect, namely sentences reading and cancellation tests [23, 24], was also spared. Visuo-constructional and praxic abilities were overall preserved, although the presence of an uncertain ideomotor apraxia was encountered for left and right hands [26].
Table 1
Results of the neuropsychological tests
| Neuropsychological tests | max. score of the | Patient’s corrected score |
| test (cut off) | (equivalent score or normal/deficit) | |
| Global Cognitive Efficacy | ||
| MMSE [11] | 30 (< 22) | 26.7 (normal) |
| Memory | ||
| Digit span forward [12] | 9 (< 4.26) | 5.65 (3) |
| Rey list (immediate recall) [13] | 75 (≤28.53) | 29 (1) |
| Rey list (delayed recall) [13] | 15 (≤4.69) | 7.1 (2) |
| Short Story Recall (immediate recall) [14] | 8 (≤3.09) | 5.2 (2) |
| Short Story Recall (delayed recall) [14] | 8 (≤2.38) | 4 (2) |
| Naming Celebrities Test: naming score [15] | 78 (< 53) | 78 (4) |
| Naming Celebrities Test: semantic score [15] | 6 (< 0.5) | raw score: 4.67 (normal) |
| Language | ||
| Verbal Fluency, semantic [16] | (< 25) | 29 (4) |
| CaGi battery (naming task) [17] | 48 (≤41.48) | 47.27 (3) |
| CaGi battery (comprehension task) [17] | 48 (≤47.08) | 48 (4) |
| Executive functions | ||
| Verbal Fluency, phonemic [13] | (< 17.35) | 28.4 (3) |
| Raven’s colored matrices [13] | 36 (≤18.96) | 20.2 (1) |
| Attentional matrices [18] | 60 (≤30) | 39.25 (2) |
| TMT: part A (s) [19] | (> 93) | 52 (3) |
| TMT: part B (s) [19] | (> 282) | 364 (0) |
| TMT: part B-A (s) [19] | (> 186) | 312 (0) |
| Digit span backward [12] | 8 (< 2.65) | 4.77 (4) |
| FAB [20] | 18 (≤13.4) | 8.5 (0) |
| Visuo-perceptual abilities | ||
| X-O-N test [21] | 33 (< 29.03) | raw score: 29 (deficit) |
| Ghent overlapping figures [21] | 40 (< 36.61) | raw score: 37 (normal) |
| Gollin pictures test –short version [21] | 75 (< 58.56) | raw score: 59 (normal) |
| Perceptual constancy [21] | 40 (< 30.79) | raw score: 33 (normal) |
| Visuo-spatial abilities | ||
| VOSP: dot counting [22] | 10 (< 8) | raw score: 10 (normal) |
| VOSP: position discrimination [22] | 20 (< 18) | raw score: 20 (normal) |
| Sentence reading [23] | 6 (< 1) | raw score: 6 (normal) |
| Cancellation test: omissions [24] | 35 (≥5) | raw score: 3 (normal) |
| Cancellation test: right-left difference [24] | 15 (≥5) | raw score: 1 (normal) |
| Cancellation test: total false positive [24] | 280 (≥0) | raw score: 0 (normal) |
| Visuo-constructional and praxic abilities | ||
| Clock drawing test [25] | 10 (< 3) | raw score: 6 (normal) |
| Constructional apraxia [18] | 14 (≤7.75) | 10.75 (2) |
| Ideomotor apraxia:left upper limb [26] | 72 (< 52) | 60 (uncertain) |
| Ideomotor apraxia: right upper limb [26] | 72 (< 52) | 62 (uncertain) |
The table reports corrected scores for age and education and the equivalent scores, ranging from 0 to 4, with 0 indicating a pathological performance; for each test the maximum and the cut-off scores are reported; pathological scores are showed in bold. FAB, Frontal Assessment Battery; max, maximum score; MMSE, Mini-Mental State Examination; s, seconds; TMT, Trail Making test; VOSP, Visual Object and Space Perception.
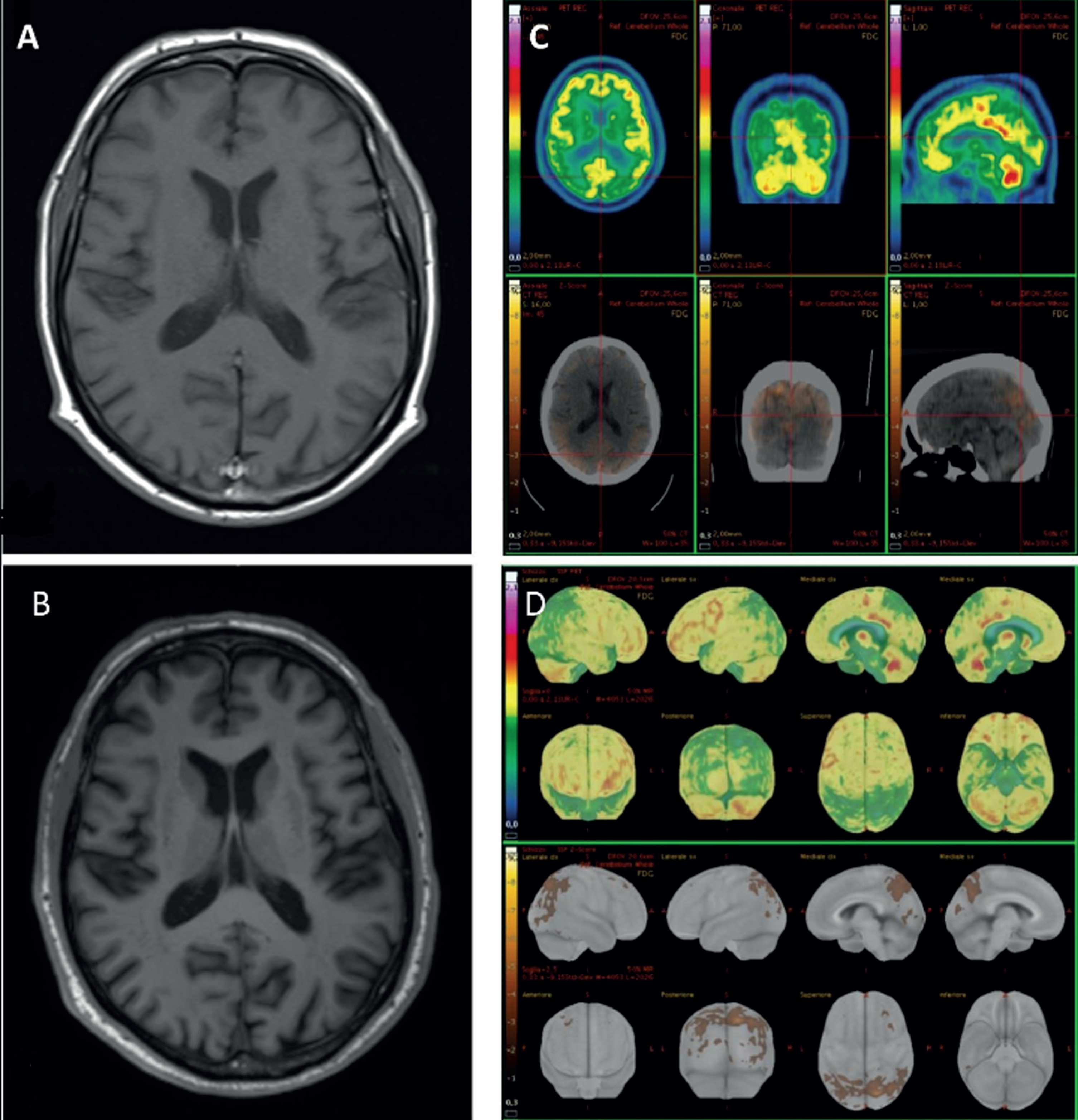
His brain magnetic resonance imaging (MRI) showed progressive moderate bilateral parieto-occipital atrophy (Fig. 2A, B), which corresponds to a fluorodeoxyglucose (FDG)-positron emission tomography (PET) hypometabolism pattern with a postero-anterior gradient, involving bilateral precuneus, superior parietal, and lateral occipital regions (Fig. 2C, D). Dat-SPECT showed a significant reduced DAT uptake in both caudate and putamen bilaterally. Cerebrospinal fluid (CSF) examination revealed normal pressure as well as normal chemical routine analyses and no cells, while biomarkers for Alzheimer’s disease pathology showed normal tau and p-tau levels (378, n.v. ≤404 and 51.40 pg/mL, n.v. ≤56.50, respectively), slightly reduced amyloid-β (Aβ)1–42 (546 pg/mL, n.v. ≥599), normal Aβ1–42/tau and Aβ1–42/p-tau ratios (1.44, n.v. ≥1.27 and 10.62, n.v. ≥8.10, respectively) and reduced Aβ1–42/Aβ1–40 ratio (0.064, n.v. ≥0.069).
Fig. 2
Magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG)-positron emission tomography (PET) images of our patient. MRI-3T T1w axial slice shows the worsening of parieto-occipital atrophy (A in 2021, B in 2022). FDG-PET axial, coronal, and sagittal slices with correspondent computed tomography slices (C). Z-score maps calculated from FDG-PET imagines exhibited a significant bilateral precuneus, parietal superior and occipital lateral hypometabolism (D).
DISCUSSION
Clinical and instrumental investigations were suggestive of a neurodegenerative disorder involving posterior cortical areas, presenting as progressive visual loss, which is the hallmark of posterior cortical atrophy (PCA) [27]. The diagnosis of PCA was then excluded on the basis of the coexistence of anamnestic RBD and mild parkinsonism, together with Dat-SPECT positivity, which moved towards a diagnosis of probable mild cognitive impairment with Lewy bodies (MCI-LB), according to the recent criteria of McKeith et al. 2020 [2]. In support of that, CSF biomarkers profile excluded an Alzheimer’s disease pathology, while an isolated slight decrease in CSF Aβ1–42 levels is a common finding of a LB pathology [28].
Our case of MCI-LB presenting initially with a particular complaint in distinguishing between an object and its background, shows at an extensive neuropsychological assessment a consistent selected deficit at a figure-background discrimination test, with the preservation of visuo-spatial abilities. At the instrumental investigations, he shows signs of both retinal and cortical visual processing dysfunctions. The retinal involvement in LBD is confirmed by histopathological findings of a preferential degeneration of neurons within inner retinal layers, that is the GCL, associated with anomalous α-synuclein deposits [7]. Consistent with these findings, several in vivo studies with OCT have demonstrated a GCL thinning and, what is more interesting, the selective macular damage in the most central and parafoveal region, which is the input of the ventral stream [8]. Specific visual outcomes have therefore been found to correlate with the parafoveal atrophy. These outcomes include worst low-contrast visual acuity, visual attention and processing speed, and visual perception. Interestingly, thickness measurements in the perifoveal region were not correlated with visual outcomes, a finding that further suggested the parafoveal thinning of GCL at the OCT as a potential biomarker for LBD [8]. Moving along the visual pathway, a delayed P100 latencies in VEPs has been observed in LBD and recently supposed to be linked with impairment of subcortical structures posterior to the lateral geniculate nucleus, in particular the pulvinar nucleus of the thalamus which seems implicated in visual attention modulation [6]. Evidence of a correlation between VEPs alterations and visual outcomes still lacks. Nevertheless, our finding of a greater alteration with 15’ checks, which activate more the central portions of the retina with greater discriminative capacity, compared to 30’ checks are in line with OCT findings and could indicate a predominantly involvement of the parvicellular rather than magnicellular system. This finding deserves further investigations to be confirmed in the view of a potential contribution of VEPs in the diagnosis of LBD. Lastly, considering the cortical level, LBD primarily affects associative visual areas, particularly extrastriatal occipital, inferior temporal, parietal and frontal areas, with a preservation of function in primary visual areas (V1), as demonstrated by morphological and functional neuroimaging studies as well as pathological evidence [4]. FDG-PET of our patient showed indeed a relative sparing of V1.
Conclusion
Altogether, our case supports a complex involvement of visual pathway disturbances in LBD, mainly affecting the ventral stream mediating object recognition, from retinal to cortical areas. This in contrast to PCA condition, which seems to affect all visual association areas and in particular the dorsal pathway mediating visuospatial processing. These alterations can be detected in the early stages of the disease, when distinguishing between PCA and LBD presentations with visual deficits might be particularly challenging. In this context, OCT and VEPs investigations, typically used to rule out peripheral causes of visual disturbances, could be combined with a comprehensive neuropsychological evaluation. This aims to characterize the nature of visual disturbances, discerning between visual disturbances of ventral or dorsal pathway involvement.
AUTHOR CONTRIBUTIONS
Giulia Perini (Conceptualization; Investigation; Writing – Original Draft); Matteo Cotta Ramusino (Investigation; Writing – Review & Editing); Francesca Conca (Methodology; Investigation); Giuseppe Cosentino (Methodology; Visualization); Lisa Maria Farina (Methodology; Visualization); Alfredo Costa (Investigation; Writing – Review & Editing); Elisabetta Farina (Conceptualization; Investigation; Writing – Review & Editing).
ACKNOWLEDGMENTS
The authors are grateful to Diego Franciotta and Elisabetta Zardini for CSF assay.
FUNDING
This work was supported by the Italian Ministry of Health “Ricerca Corrente 2022–2024” granted to IRCCS Mondino Foundation.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Upon reasonable request, the data described in this study are available from the corresponding author.
REFERENCES
[1] | Jellinger KA , Korczyn AD ((2018) ) Are dementia with Lewy bodies andParkinson’s disease dementia the same disease? BMC Med 16: , 34. |
[2] | McKeith IG , Ferman TJ , Thomas AJ , Blanc F , Boeve BF , Fujishiro H , Kantarci K , Muscio C , O’Brien JT , Postuma RB , Aarsland D , Ballard C , Bonanni L , Donaghy P , Emre M , Galvin JE , Galasko D , Goldman JG , Gomperts SN , Honig LS , Ikeda M , Leverenz JB , Lewis SJG , Marder KS , Masellis M , Salmon DP , Taylor JP , Tsuang DW , Walker Z , Tiraboschi P ((2020) ) Research criteria for the diagnosis of prodromal dementiawith Lewy bodies. Neurology 94: , 743–755. |
[3] | Armstrong RA ((2012) ) Visual signs and symptoms of dementia with Lewybodies. Clin Exp Optom 95: , 621–630. |
[4] | Metzler-Baddeley C , Baddeley RJ , Lovell PG , Laffan A , Jones RW ((2010) ) Visual impairments in dementia with lewy bodies and posteriorcortical atrophy. Neuropsychology 24: , 35–48. |
[5] | Taylor JP , Firbank MJ , He J , Barnett N , Pearce S , Livingstone A , Vuong Q , McKeith IG , O’Brien JT ((2012) ) Visual cortex in dementiawith Lewy bodies: Magnetic resonance imaging study. Br JPsychiatry 200: , 491–498. |
[6] | Carrarini C , Russo M , Pagliaccio G , Dono F , Franciotti R , Deluca G , Nanni S , Saracino A , Onofrj M , Bonanni L ((2021) ) Visual evokedpotential abnormalities in dementia with Lewy bodies. Neurophysiol Clin 51: , 425–431. |
[7] | Beach TG , Carewa J , Serranoa G , Adler CH , Holly SA , Suea LI , Sabbagha MN , Akiyamac H , Cuenca N ((2014) ) Phosphorylatedα-synuclein-immunoreactive retinal neuronal elements inParkinson’s disease subjects. Neurosci Lett 571: , 34–38. |
[8] | Murueta-Goyena A , del Pino R , Reyero P , Galdós M , Arana B , Lucas-Jiménez O , Acera M , Tijero B , Ibarretxe-Bilbao N , Ojeda N , Peña J , Cortés J , Gómez-Esteban JC , Gabilondo I ((2019) ) Parafoveal thinning of inner retina is associated with visualdysfunction in Lewy body diseases. Mov Disord 34: , 1315–1324. |
[9] | Devos D , Tir M , Maurage C , Waucquier N , Defebvre L , Defoort-Dhellemmes S , Destée A ((2005) ) ERG and anatomicalabnormalities suggesting retinopathy in dementia with Lewy bodies. Neurology 65: , 1107–1110. |
[10] | Archibald NK , Clarke MP , Mosimann UP , Burn DJ ((2009) ) The retina inParkinson’s disease. Brain 132: , 1128–1145. |
[11] | Magni E , Binetti G , Bianchetti A , Rozzini R , Trabucchi M ((1966) ) Mini-Mental State Examination: A normative study in Italian elderlypopulation. Eur J Neurol 3: , 198–202. |
[12] | Monaco M , Costa A , Caltagirone C , Carlesimo GA ((2013) ) Forward andbackward span for verbal and visuo-spatial data: Standardization andnormative data from an Italian adult population. Neurol Sci 34: , 749–754. |
[13] | Carlesimo GA , Caltagirone C , Gainotti G , Fadda L , Gallassi R , Lorusso S , Parnetti L ((1996) ) The mental deterioration battery:Normative data, diagnostic reliability and qualitative analyses ofcognitive impairment. Eur Neurol 36: , 378–384. |
[14] | Carlesimo GA , Buccione I , Fadda L , Graceffa A , Mauri M , Lorusso S , Caltagirone C ((2002) ) Normative data of two memory tasks: Short-storyrecall and Rey’s figure. Nuova Riv Neurol 12: , 1–13. |
[15] | Bizzozero I , Lucchelli F , Saetti MC , Spinnler H ((2007) ) “Whose faceis this?”: Italian norms of naming celebrities. Neurol Sci 28: , 315–322. |
[16] | Novelli G , Papagno C , Capitani E , Laiacona M , Vallar G , Cappa SF ((1986) ) Tre test clinici di ricerca e produzione lessicale. Taraturasu soggetti normali. Arch Psicol Neurol Psichiatr 4: , 477–506. |
[17] | Catricala E , Della Rosa PA , Ginex V , Mussetti Z , Plebani V , Cappa SF ((2013) ) An Italian battery for the assessment of semantic memorydisorders. Neurol Sci 34: , 985–993. |
[18] | Spinnler H , Tognoni G ((1987) ) Taratura e standardizzazione italianadi test neuropsicologici. Ital J Neurol Sci Suppl 8: , 1–120. |
[19] | Giovagnoli AR , Del Pesce M , Mascheroni S , Simoncelli M , Laiacona M , Capitani E ((1996) ) Trail making test: Normative values from 287normal adult controls. Ital J Neurol Sci 17: , 305–309. |
[20] | Appollonio I , Leone M , Isella V , Piamarta F , Consoli T , Villa ML , Forapani E , Russo A , Nichelli P ((2005) ) The frontal assessmentbattery (FAB): Normative values in an Italian population sample. Neurol Sci 26: , 108–116. |
[21] | Barletta-Rodolfi C , Gasparini F , Ghidoni E (2011) Kit del Neuropsicologo Italiano. Società Italiana di Neuropsicologia, Bologna. |
[22] | Warrington EK , James M (1991) The Visual Object and Space Perception Battery. Thames Valley Test Company, Bury St Edmunds, England. |
[23] | Pizzamiglio L , Judica A , Razzano C , Zoccolotti P ((1989) ) Toward acomprehensive diagnosis of visual-spatial disorders in unilateralbrain damaged patients. Eval Psicol 5: , 199–218. |
[24] | Vallar G , Rusconi ML , Fontana S , Musicco M ((1994) ) Tre test diesplorazione visuo-spaziale: Taratura su 212 soggetti normali. Arch Psicol Neurol Psichiatr 55: , 827–841. |
[25] | Mondini S , Mapelli D , Vestri A , Bisiacchi PS (2003) L’Esame Neuropsicologico Breve. Raffaello Cortina, Milano. |
[26] | De Renzi E , Motti F , Nichelli P ((1980) ) Imitating gestures. Aquantitative approach to ideomotor apraxia. Arch Neurol 37: , 6–10. |
[27] | Crutch SJ , Schott JM , Rabinovici GD , Murray M , Snowden JS , van derFlier WM , Dickerson BC , Vandenberghe R , Ahmed S , Bak TH , Boeve BF , Butler C , Cappa SF , Ceccaldi M , de Souza LC , Dubois B , Felician O , Galasko D , Graff-Radford J , Graff-Radford NR , Hof PR , Krolak-Salmon P , Lehmann M , Magnin E , Mendez MF , Nestor PJ , Onyike CU , Pelak VS , Pijnenburg Y , Primativo S , Rossor MN , Ryan NS , Scheltens P , Shakespeare TJ , Suárez González A , Tang-Wai DF , Yong KXX , Carrillo M , Fox NC Alzheimer’s Association ISTAART AtypicalAlzheimer’s Disease and Associated Syndromes Professional InterestArea ((2017) ) Consensus classification of posterior cortical atrophy. Alzheimers Dement 8: , 870–884. |
[28] | Gómez-Tortosa E , Gonzalo I , Fanjul S , Sainz MJ , Cantarero S , Cemillán C , Yébenes JG , del Ser T ((2003) ) Cerebrospinal fluidmarkers in dementia with Lewy bodies compared with Alzheimerdisease. Arch Neurol 60: , 1218–1222. |




