Cognitive Impairment in the Post-Acute Phases of COVID-19 and Mechanisms: An Introduction and Narrative Review
Abstract
Cognitive impairment is a primary manifestation of neurological symptoms associated with COVID-19 and may occur after disease resolution. Although cognitive impairment has been extensively reported in the literature, its duration and rate of remission remain controversial. This study discusses the various factors that influence cognitive impairment, including demographic characteristics, genetics, as well as disease course and severity. Furthermore, imaging and laboratory data have suggested various associations with cognitive impairment, most notably changes in EEG patterns, PET imaging, and serum markers. Some findings suggest similarities and potential links between COVID-related cognitive impairment and Alzheimer’s disease. Moreover, this study reviews the various mechanisms proposed to explain the development of cognitive impairment in COVID-19, including cytokine storm, damage to the blood-brain barrier, compromise of small vessel integrity, hypoxic conditions, and immune dysregulation.
INTRODUCTION
The COVID-19 pandemic has placed a tremendous strain on healthcare systems globally and has had a significant socioeconomic impact. In addition to pulmonary symptoms, COVID-19 patients exhibit neurological symptoms during the acute phase and following disease resolution [1, 2]. As time has progressed, the chronic effects of COVID-19 have garnered increasing attention, with cognitive function being a particularly significant one [1, 2]. In light of the potential long-term impact and harm caused by COVID-19, it is imperative to investigate the lasting cognitive function changes associated with infection. This will aid in assessing its impact on society and individuals, as well as exploring means of prevention and treatment.
Numerous studies have detailed the chronic effects of COVID-19, and terms such as “Long COVID” and “Post-acute Sequelae of COVID-19” (PASC) have been proposed to summarize and describe the range of chronic symptoms including cognitive dysfunction and other symptoms [3, 4]. Long COVID is currently defined as a multi-system disease, usually characterized by symptoms lasting more than four weeks after initial infection [5, 6]. As one of the symptoms of Long COVID, post-acute phase cognitive impairment refers to a patient’s cognitive decline that lasts more than 4 weeks after acute infection. Also various studies have investigated the mechanisms leading to cognitive decline, and it is currently believed that the pathological mechanisms causing cognitive decline may involve direct invasion of virus, cytokine storms, disruption to the integrity of the blood-brain barrier, hypercoagulability, hypoxia and immune dysregulation [1, 5, 7, 8].
However, in this area have indicated convoluted and conflicting outcomes. The mechanisms also remain uncertain, and there is an overlap and divergence between the different doctrines.
The review aims to offer a realistic assessment of cognitive impairment caused by COVID-19 by comparing findings from various studies in a way that combines existing data. At a mechanistic level, this study aims to present a comprehensive perspective for future researchers by summarizing the hypotheses and corresponding evidence. Based on the available literature, some neurocognitive symptoms of Long COVID appear to deteriorate over time and tend to persist for extended periods. Full recovery is rare for long-term COVID-19 patients; however, a considerable portion of the symptoms will progressively decrease during the months succeeding the disease [6]. The long-term prognosis remains uncertain. Cognitive dysfunction is one of the persistent symptoms of COVID-19 and exhibits traits similar to those seen in other neurodegenerative diseases [5, 9]. Currently, there are no effective treatments for long-term COVID-19. It is anticipated that the numerous neuropsychiatric disorders associated with COVID-19 will continue to pose a challenge in the aftermath of the epidemic. Furthermore, it is expected that the incidence of cognitive impairment and neurodegenerative diseases will rise in the population. At the mechanistic level, it is unlikely that direct viral invasion occurs. However, various related mechanisms to neurodegenerative disorders have been analyzed and acknowledged morefrequently.
METHODS
This review is a narrative review. We conducted a search of PubMed up to 11 November 2023. The following key words (MeSH or in the title/abstract) were searched: ((‘neurologic manifestations’[MeSH Terms] OR ‘neurological’[Title/Abstract] OR ‘cognition disorders’[MeSH Terms] OR ‘cognition’[Title/Abstract] OR ‘cognitive impairment’[Title/Abstract] AND ‘long COVID’[Title/Abstract] OR ‘post-COVID-19’[Title/Abstract] OR ‘post-COVID’[Title/Abstract] OR ‘post-acute COVID-19 syndrome’[Title/Abstract])). We conducted a search of the reference lists of relevant articles and manually searched for the most up-to-date and relevant research available.
COVID-19 RELATED COGNITIVE CHANGES: PREVALENCE AND MANIFESTATIONS
Cognitive changes in post-acute COVID-19
Long COVID comprises ongoing symptomatic COVID-19 and post-COVID-19 syndrome. Ongoing symptomatic COVID-19 presents as signs and symptoms of COVID-19 lasting from 4 to 12 weeks [4]. Post-COVID-19 syndrome is characterized by signs and symptoms that develop during or after an infection consistent with COVID-19 and continue for more than 12 weeks and are not explained by any other diagnosis [6]. Cognitive impairment is a common symptom of neurological post-COVID-19 syndrome [10]. Previous studies reported a high prevalence of cognitive decline, whether self-reported or clinically determined, in patients who recovered from COVID-19 [11, 12].
Long-term cognitive dysfunction is one of the most common impairments of Long COVID, affecting 17% to 28% of people [13]. A previous meta-analysis reported that 38.3% of individuals who recovered from COVID-19 self-reported cognitive deficits [11]. In addition, 22% of individuals presented with cognitive impairment 12 or more weeks following COVID-19 diagnosis, with more than 20% of patients noted to exhibit cognitive impairment with persistent symptoms of fatigue. Furthermore, EEG studies showed that 53% of patients exhibited impairment in at least one cognitive area two months after COVID-19 resolution [14, 15]. Post-COVID-19 syndrome includes a range of neuropsychiatric symptoms encompassing cognitive decline and is more common in women, older people, patients who suffered severe acute COVID-19, and patients with pre-existing comorbidities [15] (Table 1).
Table 1
Prevalence of cognitive impairment in different studies.
| Study | Prevalence | Factors Associated with cognitive impairment |
| Möller et al., 2023 [13] | 17%–28% | Prolonged cognitive dysfunction |
| Grover et al., 2021 [11] | 38.3% | Self-reported cognitive deficits |
| Ceban et al., 2022 [15] | 22% | Presented with cognitive impairment 12 or more weeks following COVID-19 diagnosis |
| Ceban et al., 2022 [15] | 20% | Fatigue |
| Cecchetti et al., 2022 [14] | 53% | EEG showed impairment in at least one cognitive area two months after COVID-19 resolution |
COVID-19-related cognitive impairment can be divided into two categories based on the presence or absence of structural brain lesions [16]. According to a previous meta-analysis, most patients with COVID-19 were reported to suffer functional deficits in executive function, memory, and attention. In addition, COVID-19 patients suffered deficits in learning, inhibitory control, speech fluency, and processing speed [17]. A study enrolling patients who recovered from COVID-19 reported deficits in working memory (55%), attention shifting (47%), distraction (46%), and processing speed (40%) [18].
Importantly, studies have reported correlations between the severity of cognitive impairment and illness severity. A cohort study evaluating cognitive deficits in patients who suffered moderate-to-severe COVID-19 infection 10–35 days after discharge showed that patients who required oxygen administration but did not receive ICU care had lower scores in verbal memory, visual memory, attention, working memory, complex working memory, processing speed, and overall cognition than patients who received ICU care [19, 20]. Similarly, another study reported that post-ICU patients performed worse on global cognitive functioning than patients who did not receive ICU care [21].
Furthermore, a previous study showed that cognitive impairment was associated with the severity of respiratory symptoms, the degree of pulmonary function, and d-dimer levels [19].
The significant variability in cognitive impairment in post-COVID-19 could be due to differences in cognitive function screening indicators and the demographic characteristics of study participants. A previous study revealed that young patients or patients with mild illness showed more diverse cognitive deficits. In contrast, patients with severe illnesses or older patients tended to have some of their cognitive domains spared. Similarly, a comparison of two groups of similarly-aged patients revealed significant variabilities in cognitive impairment [22]. Furthermore, a study enrolling adolescents with COVID-19 revealed no significant changes in cognitive function in asymptomatic patients, with only the executive function being affected compared with healthy controls. Furthermore, hospitalized patients exhibited greater impairment in cognitive and executive functions, with more significant impairment in working memory than non-hospitalized patients [23].
Overall, cognitive impairment is widespread and varied in post-acute COVID-19 patients. Impairments including verbal memory, visual memory, attention, working memory, complex working memory, processing speed and overall cognition have been reported. And its severity and presentation may be related to a range of factors, including the patient’s age and severity of disease.
ELECTROPHYSIOLOGICAL FINDINGS
A previous study exploring quantitative electroencephalogram (QEEG) in COVID-19 patients in the post-acute phase showed increased frequency of theta and alpha waves and sensorimotor rhythm (SMR) in the right hemisphere relative to the left. Meanwhile, significantly increased Beta2 waves in both hemispheres and increased Beta1 waves in the left hemisphere, and decreased SMR. Similarly, other studies have reported changes in theta and alpha waves, including studies investigating cognitive impairment in non-COVID-19 patients, suggesting that these QEEG changes are associated with cognitive impairment. Furthermore, signs of mental fatigue have been shown in QEEG changes. A follow-up study enrolling COVID-19 patients up to over ten months after hospital discharge revealed increased current density and connectivity in the delta band in areas associated with altered executive function and increased white matter hyperintensity (WMH) load associated with verbal memory deficits. In addition, the cognitive impairment and delta band EEG connectivity decreased over time [24, 25].
IMAGING
Imaging modalities are frequently employed in cognitive impairment. A longitudinal analysis of MRI data obtained from 401 patients before and after COVID-19 infection and 384 controls revealed a greater reduction in gray matter thickness and tissue contrast in the prefrontal cortex, parahippocampal gyrus, and regions associated with olfactory cortical functions among infected patients, this change occurs between 1 and 13 months after infection. Furthermore, COVID-19 is linked to the development of AD and other forms of dementia [26]. A previous study suggested that COVID-19 could induce an AD-like pathology by accelerating amyloid deposition [27]. Positive correlations have also been found between the degree of decline in cognitive function and the degree of cortical involvement in prefrontal and temporal gyrus, insula, posterior cingulate gyrus, parahippocampal gyrus, and parietal regions in patients with COVID-19 after the acute phase [28]. Given those studies have identified specific brain regions where reductions in GMV have been shown to correlate negatively with levels of inflammatory factors in the cerebrospinal fluid, structural changes in the brain after COVID-19 may be related to inflammation [28, 29].
Although the interpretation of post-COVID-19 PET imaging data remains controversial, significant differences exist between imaging findings of post-COVID-19 symptoms and COVID-19 encephalopathy, suggesting that post-COVID-19 syndrome could have different mechanisms with encephalopathy [26, 30].
LABORATORY ANALYSES IN POST-COVID-19 COGNITIVE IMPAIRMENT
Neurological damage and neurodegeneration caused by COVID-19 could be responsible for cognitive impairment. A previous study profiling transcriptomes of frontal cortex and choroidal samples from eight patients with severe COVID-19, revealed no molecular traces of SAR-CoV-2 in the brain. However, broad cellular pertubations were observed indicating that barrier cells on the choroidal plexus sense and relay inflammation into the brain. Furthermore, the study showed infiltration of peripheral T cells into the brain parenchyma. In addition, the researchers identified subpopulations of microglia and astrocytes associated with COVID-19 that share features with pathological cell states previously reported in human neurodegenerative diseases. Moreover, a pattern of dysfunction in neurons was reported in snRNA-seq studies of autism and associated with cognitive deficits [31]. In addition, a previous study showed a mutualistic relationship between COVID-19-associated and AD-associated neuroinflammation, and abnormal expression of AD biomarkers in the cerebrospinal fluid and serum of COVID-19 patients [32]. These findings suggest a molecular mechanism associated with long-term cognitive impairment in COVID-19 and mechanistic similarities and cross-talk between COVID-19 and AD-associated neuroinflammation.
A previous study also showed that plasma biomarkers of AD, including plasma neurofilament light chain (pNfL, a biomarker of nerve axon injury), and glial fibrillary acidic protein (pGFAP) were associated with COVID-19-related cognitive impairment. In addition, patients with severe infection were shown to have higher plasma levels of SARS-CoV-2 Nucleocapsid antigen (pNAg) even three weeks after the onset of symptoms, suggesting that the disease could have prolonged antigenic stimulation [33].
In addition, Frontera et al. explored the correlation between serum total tau (t-tau), phosphorylated tau-181 (p-tau181), ubiquitin carboxy-terminal hydrolase L1 (UCHL1), and amyloid-β (Aβ), and different levels of cognitive impairment in non-COVID-19 patients. They showed a correlation between these biomarkers and disease severity. In addition, they showed a significant correlation between neurodegenerative biomarkers and the inflammatory marker of d-dimer, which could explain the link between cognitive impairment and inflammation or hypoxia [34].
However, the mechanisms underlying COVID-19-related cognitive impairment are still unknown. A study exploring the relationship between AD-like signals and inflammation or hypoxia showed increased oxidative stress and cytokine expression in the brain tissue of COVID-19 patients [35].
Overall, there was a more frequent positive relationship between indicators of neuroinflammation and cognitive impairment. These findings provide a basis for linking inflammatory hypoxia, cognitive impairment, and neurodegeneration in COVID-19.
PROGNOSIS
A previous study analyzed the prognosis of patients with COVID-19-related neurological impairment, including cognitive impairment [36]. Both clinical and animal studies have shown that post-COVID-19-related neurological symptoms are associated with mortality and long-term neuropsychiatric impairment [37]. Furthermore, delirium in the acute phase could affect cognitive function. Moreover, acute delirium is associated with an increased risk of long-term cognitive impairment [9]. Another study reported that delirium was an independent predictor of long-term cognitive impairment [38–41]. However, studies have shown that patients with mild COVID-19 are only at risk for a small number of health problems, including cognitive impairment and weakness, and most of these resolve within a year. This may suggest a milder effect of mild COVID on neurological and cognitive function [2].
Molecular imaging techniques such as PET and SPECT suggested reversibility of brain lesions in severe cases of COVID-19. For example, case series of patients with COVID-19-related encephalopathy (acute to subacute phase) and systematic studies have consistently shown that neocortical dysfunction of the frontal lobe was reversible within six months in most cases [30].
Cognitive impairment and remission of long-term symptoms after COVID-19 remain rather complicated. Despite differences in specific time points and disease severity, most studies show commonalities, such as a significant proportion of patients experiencing persistent cognitive impairment at least a month after COVID-19 infection.
Studies have reported remission of cognitive impairment and worsening of cognitive impairment. In most studies, remission was reported to occur from 10-12 months after COVID-19 [14, 15, 42]. Several studies have shown the persistence of inflammation and neurodegeneration, indicating that cognitive impairment persists over time [15, 34, 43]. A follow-up study of post-infection symptoms in 31,486 patients found that 6% did not recover and 42% only recovered partially [44]. Furthermore, another study reported that only 0.9% of patients still showed symptoms at 12 months after recovery from the acute phase [45].
A follow-up study conducted at 6 months and 12 months after hospital discharge showed various changes in cognitive impairment over time, including no change in cognitive function, late-onset cognitive impairment (no cognitive decline on the first assessment), early onset cognitive impairment (no cognitive decline on the second assessment), and progressive cognitive impairment (cognitive decline in both assessments) [43].
In summary, long-term problems other than self-reported cognitive impairment are less common in mildly ill COVID-19 patients, and persistent cognitive impairment is more common in severely ill and older patients. Symptoms resolve over time in most patients, but some patients have incomplete remission after 12 months of acute infection. And the timing and extent of the onset of remission has varied widely between studies.
RISK FACTORS OF COGNITIVE CHANGES IN COVID-19
The factors influencing COVID-19-related cognitive impairment and the resulting changes can provide clues for the pathological processes and mechanisms underlying the cognitive impairment.
A previous study exploring post-COVID-19 Syndrome (PCS), including cognitive impairment and other neurological symptoms, such as fatigue, reported that older age, severe acute illness, and pre-existing comorbidities were all associated with a higher incidence of PCS symptoms [15].
Increased age is often associated with increased disease severity and cognitive impairment. A meta-analysis of 14 studies showed that increased age was associated with a higher incidence of PCS symptoms or a lower quality of life [15]. Furthermore, a previous study showed that every 1-year increase in age was associated with a -0.064 change in total MoCA score, indicating a link between age and cognitive impairment [19]. The association between age and cognitive impairment could be due to an inflammatory state hastening the onset of neurodegenerative diseases.
Underlying diseases and education could influence cognitive impairments following COVID-19. A previous study enrolling older patients with a post-infection showed that advanced low education and underlying diseases were related to cognitive impairments after COVID-19 [9]. Several studies have shown that comorbidities such as cerebrovascular diseases, diabetes mellitus, and chronic obstructive pulmonary disease can aggravate COVID-19 symptoms, thus influencing disease severity [46]. Chronic obstructive pulmonary disease (COPD) was also reported to be a risk factor for developing cognitive impairment and decline of cognitive function [9].
Dementia is a significant risk factor for COVID-19, with dementia patients with COVID pneumonia showing nearly twice higher mortality rates than those without dementia [47]. Evidence also suggests that AD is a potential risk factor for COVID-19 severity and patients with AD are more likely to get COVID-19 infection [48].
The immune response and excessive inflammation during COVID-19 infection may hasten neurodegeneration, increasing the risk of neuropathological consequences. Furthermore, pre-existing AD may exacerbate COVID-19 pathological changes by eliciting a strong immune response [49]. Moreover, AD-related genes, such as APOE ɛ4, are associated with the risk and severity of COVID infection [50].
The severity of symptoms and whether patients received hospitalization or ICU care have been reported to influence COVID-19-related cognitive impairment. Patients with severe COVID-19 in the acute phase show more severe cognitive function impairment [9, 15, 17, 21, 39, 43]. Hospitalization and the need for ICU care are frequently studied as criteria for infection severity. Previous studies reported that patients requiring hospitalization or ICU care had more severe cognitive impairment [21]. The need for ICU care during the acute phase of COVID-19 is considered one of the main factors influencing impairment in executive function [14].
Several studies have also investigated which specific factors in the exacerbation of COVID-19 infection contribute to cognitive decline. Cognitive impairment in COVID-19 often occurs after acute respiratory distress syndrome (ARDS). Furthermore, the negative impact of hypoxia on cognition has been demonstrated [51, 52]. Although several studies have investigated the relationship between cognitive impairment and pulmonary function, a previous meta-analysis showed mixing results [19]. It has also been reported that the relationship between ARDS and the degree of cognitive impairment is unclear [39]. In addition, some previous studies reported that spike proteins and nucleocapsid proteins during viral infection with coronavirus could affect host cell protein synthesis and the cellular senescence process. Furthermore, several studies have reported a correlation between elevated D-dimers and cognitive decline in COVID-19 patients [53, 54].
Moreover, different strains of the COVID-19 virus could influence COVID-19-related cognitive impairment. A previous study showed that the incidence of sequelae of the Omicron strain was 24–50% that of the Delta strain [55]. In addition, some neurological symptoms experienced during the acute phase of COVID-19, such as abnormal taste and smell, as well as memory vulnerability, could also be linked to cognitive decline following resolution of COVID-19. Verbal memory tests showed that patients with dysgeusia and hyposmia during acute illness improved much more slowly than patients without dysgeusia/hyposmia [14]. This finding may suggest a correlation between function loss and the mechanisms behind cognitive impairment. As mentioned earlier, delirium is an independent predictor of long-term cognitive impairment [39]. Individuals with acute delirium in COVID-19 could be more susceptible to developing long-term cognitive impairment [40, 41]. Researchers raised a possibility that PASC could be a post-infection autoimmune phenomenon because patients with post-neurological infection symptoms had a higher likelihood of autoimmune diseases prior to COVID-19 [33].
In conclusion, risk factors include demographic factors (age, race, genetics and underlying disease) and factors related to the COVID-19 infection (clinical symptoms, severity and treatments). Psychiatric symptoms in the post-infection phase may also be associated with cognitive impairment.
POSSIBLE PATHOPHYSIOLOGICAL MECHANISMS OF COVID-19-RELATED COGNITIVE IMPAIRMENT
Cognitive impairment due to COVID-19 infection could manifest in various ways. The main possible mechanisms include: neuroimmune-related mechanisms, inflammatory injury, autoimmune and neurodegeneration-related mechanisms, microvascular injury, and brain hypoxic injury as shown in Fig. 1.
Fig. 1
Possible pathophysiological mechanisms of cognitive impairment in COVID-19.
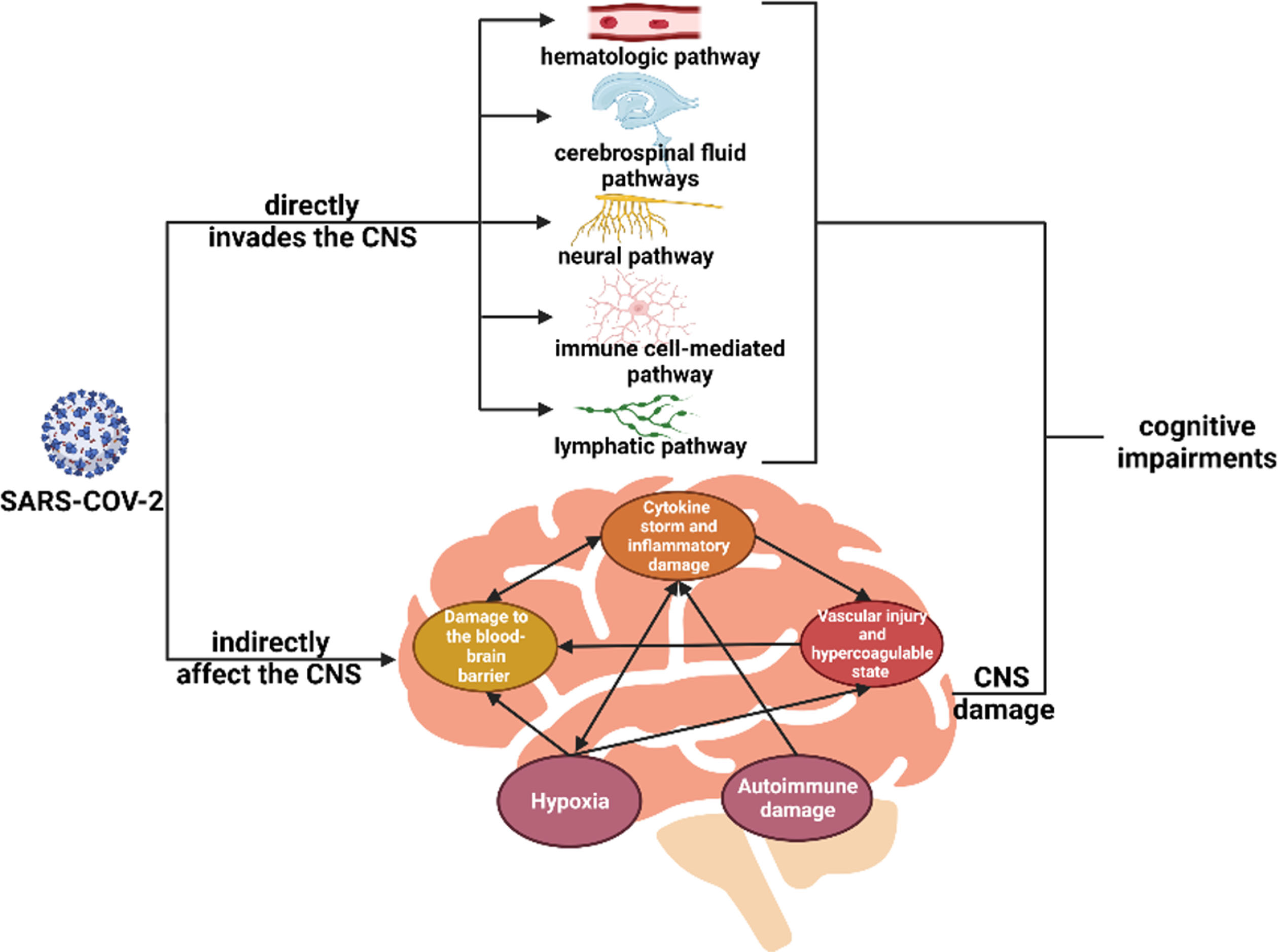
DIRECT INVASION OF THE NERVOUS SYSTEM BY CORONAVIRUS
Neuroinvasion is one of the widely discussed mechanisms behind the development of neurological symptoms in COVID-19. Previous studies have reported the discovery of virus-like particles and RNA in the central nervous system of COVID-19 patients [56, 57]. Viral particles are thought to invade the CNS through hematologic, neural, lymphatic, cerebrospinal fluid pathways, and the immune cell-mediated pathway [32]. In general, however, there is a lack of conclusive evidence of direct viral invasion.
VIRUS INDIRECTLY AFFECT THE CENTRAL NERVOUS SYSTEM
Several studies suggest that the mechanisms behind COVID-19-related cognitive impairment could be related to factors associated with viral infection in peripheral tissues and the cytokine storm.
CYTOKINE STORM AND INFLAMMATORY DAMAGE
Several cytokines have been detected in patients with severe COVID-19 infection, including IL-2, IL-6, IL-7, IL-1β, tumor necrosis factor-alpha (TNF-α), IFN-γ, interferon gamma-inducible protein 10 (IP-10), monocyte chemotactic protein 1 (MCP-1), granulocyte colony-stimulating factor (G-CSF), and other molecular and inflammatory markers associated with COVID-19 severity [8]. In critically ill patients, severe lymphopenia, pro-inflammatory T-cell hyperactivation, and a decrease in regulatory T-cells are also reported. The cytokine storm may affect macrophages, microglia, and astrocytes in the CNS, thus contributing to neuronal damage [31].
The effects of inflammatory processes on the central nervous system are closely linked to glial cells. It has been shown that during COVID-19 infection astrocytes are involved in the inflammatory process [58]. Changes in microglia and astrocytes have been reported in both animal experiments and autopsy reports of deceased cases. Microglia activation during inflammatory states leads to the release of various pro-inflammatory mediators, thus causing an overreaction. In addition, microglia activation and overreaction are associated with phagocytic hyperactivity, which may induce neurological damage such as neurodegeneration. Mice models of neuroinflammation were shown to have elevated levels of chemokines in cerebrospinal fluid and serum and activation of microglia in subcortical and hippocampal white matter regions. Furthermore, various genes associated with neuroinflammation were shown in a transcriptomic analysis of eight COVID-19 patients. A cluster of astrocytes was also shown to be associated with COVID-19 [31, 59].
While inflammatory changes in glial cells and other cellular components of CNS may be closely related to long-term cognitive impairment after COVID-19, several studies have demonstrated similarities between COVID-19-related neuroinflammation and neuroinflammation associated with neurodegenerative diseases such as AD [58]. Recent animal experiments have also validated the role of neuroinflammatory factors, particularly immune cells and cytokine factors, in aging and cognitive impairment. There is also a possibility of crossover between astrocyte and microglia activation during aging and systemic inflammation [60], which could provide some insights into the long-term cognitive impairment after COVID-19.
DAMAGE TO THE BLOOD-BRAIN BARRIER
The blood-brain barrier damage could be due to the direct invasion of the blood-brain barrier by SARS-CoV-2, a severe inflammatory state, or hypoxia [28]. The discovery of ACE2 receptors on microvascular endothelial cells of the human brain and the presence of SARS-CoV-2 particles in brain capillary endothelial cells explains the possibility that SARS-CoV-2 infection could directly damage vascular endothelial cells [61, 62]. Furthermore, pro-inflammatory cytokines such as IL-6 and TNF-α can reduce mRNA levels of Zonula Occluden (ZO-1) and promote its phosphorylation, an inflammatory-related mechanism that may compromise the integrity of the blood-brain barrier. In addition, blood-brain barrier components affected by inflammation could transmit inflammatory signals to the brain. Moreover, researchers have also identified endothelial cells expressing inflammasome-related mechanisms [63]. Impairment of the blood-brain barrier leads to increased permeability, which could lead to further inflammation and glial cell dysfunction [58].
VASCULAR INJURY AND HYPERCOAGULABLE STATE
Several studies and autopsies have reported intracranial thrombotic complications, small vessel leakage, and coagulation abnormalities in patients with COVID-19 [64, 34]. Small vessel injury and a hypercoagulable state of blood can contribute to CNS thrombosis, resulting in impaired CNS function. These injuries may be associated with both direct infectious damage and an inflammatory state. The damaging effect on the vascular endothelium during the infection process can have a substantial impact on coagulation. Cytokines play a role in the development of hypercoagulable state. A group of patients with COVID-19 and stroke from Wuhan, China showed elevated levels of IL-6, IL-8 and TNF-α. Both IL-8 and TNF-α have been reported to promote the release of vascular hemophilic factor, a marker of endothelial injury, and IL-6 inhibits the cleavage of vascular hemophilic factor and promotes platelet aggregation [65]. Other scholars reported that hypoxia may also contribute to these cerebrovascular events [65].
HYPOXIA
Hypoxia is a commonly discussed mechanism in COVID-19. It has been linked to the severity of post-COVID-19 symptoms, as mentioned earlier. Secondly, neuropathological findings indicate that microglia activation in COVID-19 resembles that observed in hypoxic patients. Moreover, there is significant evidence linking hypoxia to neurological damage, including cognitive impairment [66]. Additionally, COVID-19 can frequently induce hypoxic symptoms due to its impact on the respiratory system. Hypoxia can directly affect CNS function by influencing CNS energy metabolism, leading to hypoxic encephalopathy and causing a range of sequalae. Hypoxic changes in the CNS have been reported in autopsies from COVID-19 patients [67]. Peripheral infections and hypoxia can also affect the CNS and trigger inflammation in brain. For instance, hypoxia is thought to be a stressor that induces blood-brain barrier disruption, which promotes infiltration of peripheral immune cells and leakage of blood proteins (including cytokines) to the brain. The mechanism of hypoxia-induced blood-brain barrier disruption is well-documented. In the context of COVID-19, the presence of pulmonary lesions poses a risk of hypoxia to various tissues in the body. This hypoxia can trigger the release of systemic cytokines through HIF-related pathways[8]. Furthermore, HIF-1α stabilization in microvascular endothelial cells increased the transcription of VEGF and integrins to increase vascular permeability. Together, these mechanisms lead to the impairment of the blood-brain barrier and contribute to peripheral inflammation that affects the brain [68, 69].
AUTOIMMUNE DAMAGE
COVID-19 infection may cause autoimmune damage, which is reflected to some extent in several COVID-19-associated peripheral neurological disorders such as Guillain-Barré syndrome [70]. The idea that autoantibodies play a role in cognitive dysfunction has been supported by several studies [71]. Studies have shown reported the increased levels of autoantibodies in COVID-19 [5]. This autoimmune response may be due to molecular mimicry and other multiple mechanisms that cause autoimmune response, causing neurological damage. This mechanism can result in both peripheral neurological disorders and CNS effects. Research has demonstrated that SARS-CoV-2 can induce the production of anti-NMDA-R autoantibodies, leading to autoimmune encephalitis [72]. These findings suggest that antibody-mediated autoimmune attack plays a role in COVID-19-related neurological damage and may contribute to neurodegenerative changes in affected patients.
COMMONALITIES AMONG MECHANISMS
Taken together, it is evident that cognitive impairment in post-acute phase of COVID-19 is, to some extent, related to the inflammatory factors caused by the infection. Inflammatory damage is implicated in several processes, including damage to the blood-brain barrier and small vessel injury. Changes in inflammatory mediators exhibit similarities to certain other neurodegenerative diseases. Studies of structural changes in the brain and inflammatory factors have provided a basis for the role of inflammatory factors in this process.
CONCLUSION AND FUTURE PERSPECTIVES ON THE IMPACT OF COVID-19 IN COGNITIVE DISORDERS
As the COVID-19 epidemic fades, the focus of attention has shifted towards the post-COVID stage, which has emerged as a prominent topic of discussion. With increasing research on Long COVID, several concerning findings have been reported. Patients with Long COVID seldom experience full recovery, and the long-term prognosis of this condition remains uncertain. Cognitive impairment, as one of the symptoms of Long COVID, also exhibits persistent and delayed onset characteristics, and it has shown similar features as other neurodegenerative diseases [5, 35, 43]. Currently, there are no effective treatments for Long COVID. It is anticipated that the numerous neuropsychiatric disorders associated with COVID-19 will continue to be a challenge long after the epidemic. Furthermore, considering the potential mechanistic similarities with neurodegenerative diseases such as AD, there is an expected rise in the incidence of cognitive impairment and neurodegenerative disorders in the population. Consequently, there is a need for further investigation to improve our understanding of the mechanisms underlying this pathological process [73]. In addition, due to the mechanistic complexity, there may also be individual differences in the mechanisms that produce PASC in COVID-19 patients, and thus personalized treatment may be even more beneficial [3]. Follow-up studies, animal studies and follow-up of patients with neuroinflammatory markers as well as degenerative biomarkers is advocated using imaging techniques including MRI to help clinicians assess the long-term cognitive prognosis and guide the treatment of cognitive impairment.
AUTHOR CONTRIBUTIONS
Weiye Wang (Writing – original draft; Writing – review & editing); Guoping Peng (Writing – original draft; Writing – review & editing); Gang Wang (Writing – review & editing); Luming Leng (Writing – review & editing); Ruxin Cui (Writing – review & editing).
ACKNOWLEDGMENTS
This work was supported by Department of Neurology, the First Affiliated Hospital, Zhejiang University School of Medicine.
FUNDING
This work is financially supported by the Ministry of Science and Technology of the People’s Republic of China (2022YFC3602604) and the National Natural Science Foundation of China (82071182).
CONFLICT OF INTEREST
All authors declare that they have no competing interests.
REFERENCES
[1] | Dewanjee S , Vallamkondu J , Kalra RS , Puvvada N , Kandimalla R , Reddy PH ((2021) ) Emerging COVID-19 neurological manifestations: Present outlook and potential neurological challenges in COVID-19 pandemic. Mol Neurobiol 58: , 4694–4715. |
[2] | Mizrahi B , Sudry T , Flaks-Manov N , Yehezkelli Y , Kalkstein N , Akiva P , Ekka-Zohar A , Ben David SS , Lerner U , Bivas-Benita M , Greenfeld S ((2023) ) Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 380: , e072529. |
[3] | Proal AD , VanElzakker MB ((2021) ) Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12: , 698169. |
[4] | Mehandru S , Merad M ((2022) ) Pathological sequelae of long-haul COVID. Nat Immunol 23: , 194–202. |
[5] | Davis HE , McCorkell L , Vogel JM , Topol EJ ((2023) ) Long COVID: Major findings, mechanisms and recommendations. Nat Rev Microbiol 21: , 133–146. |
[6] | Kubota T , Kuroda N , Sone D ((2023) ) Neuropsychiatric aspects of long COVID: A comprehensive review. Psychiatry Clin Neurosci 77: , 84–93. |
[7] | Jha NK , Ojha S , Jha SK , Dureja H , Singh SK , Shukla SD , Chellappan DK , Gupta G , Bhardwaj S , Kumar N , Jeyaraman M , Jain R , Muthu S , Kar R , Kumar D , Goswami VK , Ruokolainen J , Kesari KK , Singh SK , Dua K ((2021) ) Evidence of coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: A review on neurological impairments and manifestations. J Mol Neurosci 71: , 2192–2209. |
[8] | Mahboubi Mehrabani M , Karvandi MS , Maafi P , Doroudian M ((2022) ) Neurological complications associated with Covid-19; molecular mechanisms and therapeutic approaches. Rev Med Virol 32: , e2334. |
[9] | Liu Y-H , Wang Y-R , Wang Q-H , Chen Y , Chen X , Li Y , Cen Y , Xu C , Hu T , Liu X-D , Yang L-L , Li S-J , Liu X-F , Liu C-M , Zhu J , Li W , Zhang L-L , Liu J , Wang Y-J ((2021) ) Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener 16: , 48. |
[10] | Balcom EF , Nath A , Power C ((2021) ) Acute and chronic neurological disorders in COVID-19: Potential mechanisms of disease. Brain 144: , 3576–3588. |
[11] | Grover S , Sahoo S , Mishra E , Gill KS , Mehra A , Nehra R , Suman A , Bhalla A , Puri GD ((2021) ) Fatigue, perceived stigma, self-reported cognitive deficits and psychological morbidity in patients recovered from COVID-19 infection. Asian J Psychiatry 64: , 102815. |
[12] | Chen C , Haupert SR , Zimmermann L , Shi X , Fritsche LG , Mukherjee B ((2022) ) Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J Infect Dis 226: , 1593–1607. |
[13] | Möller M , Borg K , Janson C , Lerm M , Normark J , Niward K ((2023) ) Cognitive dysfunction in post-COVID-19 condition: Mechanisms, management, and rehabilitation. J Intern Med 294: , 563–581. |
[14] | Cecchetti G , Agosta F , Canu E , Basaia S , Barbieri A , Cardamone R , Bernasconi MP , Castelnovo V , Cividini C , Cursi M , Vabanesi M , Impellizzeri M , Lazzarin SM , Fanelli GF , Minicucci F , Giacalone G , Falini A , Falautano M , Rovere-Querini P , Roveri L , Filippi M ((2022) ) Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J Neurol 269: , 3400–3412. |
[15] | Ceban F , Ling S , Lui LMW , Lee Y , Gill H , Teopiz KM , Rodrigues NB , Subramaniapillai M , Di Vincenzo JD , Cao B , Lin K , Mansur RB , Ho RC , Rosenblat JD , Miskowiak KW , Vinberg M , Maletic V , McIntyre RS ((2022) ) Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 101: , 93–135. |
[16] | Shimohata T ((2022) ) Neuro-COVID-19. Clin Exp Neuroimmunol 13: , 17–23. |
[17] | Hampshire A , Chatfield DA , MPhil AM , Jolly A , Trender W , Hellyer PJ , Giovane MD , Newcombe VFJ , Outtrim JG , Warne B , Bhatti J , Pointon L , Elmer A , Sithole N , Bradley J , Kingston N , Sawcer SJ , Bullmore ET , Rowe JB , Menon DK ((2022) ) Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. eClinicalMedicine 47: , 101417. |
[18] | Jaywant A , Vanderlind WM , Alexopoulos GS , Fridman CB , Perlis RH , Gunning FM ((2021) ) Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology 46: , 2235–2240. |
[19] | Crivelli L , Palmer K , Calandri I , Guekht A , Beghi E , Carroll W , Frontera J , García-Azorín D , Westenberg E , Winkler AS , Mangialasche F , Allegri RF , Kivipelto M ((2022) ) Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement 18: , 1047–1066. |
[20] | Almeria M , Cejudo JC , Sotoca J , Deus J , Krupinski J ((2020) ) Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health 9: , 100163. |
[21] | Vannorsdall TD , Brigham E , Fawzy A , Raju S , Gorgone A , Pletnikova A , Lyketsos CG , Parker AM , Oh ES ((2022) ) Cognitive dysfunction, psychiatric distress, and functional decline after COVID-19. J Acad Consult Liaison Psychiatry 63: , 133–143. |
[22] | Zhou H , Lu S , Chen J , Wei N , Wang D , Lyu H , Shi C , Hu S ((2020) ) The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res 129: , 98–102. |
[23] | Frolli A , Ricci MC , Di Carmine F , Lombardi A , Bosco A , Saviano E , Franzese L ((2021) ) The impact of COVID-19 on cognitive development and executive functioning in adolescents: A first exploratory investigation. Brain Sci 11: , 1222. |
[24] | Li G , Huang S , Xu W , Jiao W , Jiang Y , Gao Z , Zhang J ((2020) ) The impact of mental fatigue on brain activity: A comparative study both in resting state and task state using EEG. BMC Neurosci 21: , 20. |
[25] | Kopańska M , Ochojska D , Muchacka R , Dejnowicz-Velitchkov A , Banaś-Ząbczyk A , Szczygielski J ((2022) ) Comparison of QEEG findings before and after onset of post-COVID-19 brain fog symptoms. Sensors 22: , 6606. |
[26] | Douaud G , Lee S , Alfaro-Almagro F , Arthofer C , Wang C , McCarthy P , Lange F , Andersson JLR , Griffanti L , Duff E , Jbabdi S , Taschler B , Keating P , Winkler AM , Collins R , Matthews PM , Allen N , Miller KL , Nichols TE , Smith SM ((2022) ) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604: , 697–707. |
[27] | Rahman MA , Islam K , Rahman S , Alamin M ((2021) ) Neurobiochemical cross-talk between COVID-19 and Alzheimer’s disease. Mol Neurobiol 58: , 1017–1023. |
[28] | Etter MM , Martins TA , Kulsvehagen L , Pössnecker E , Duchemin W , Hogan S , Sanabria-Diaz G , Müller J , Chiappini A , Rychen J , Eberhard N , Guzman R , Mariani L , Melie-Garcia L , Keller E , Jelcic I , Pargger H , Siegemund M , Kuhle J , Oechtering J , Eich C , Tzankov A , Matter MS , Uzun S , Yaldizli Ö , Lieb JM , Psychogios M-N , Leuzinger K , Hirsch HH , Granziera C , Pröbstel A-K , Hutter G ((2022) ) Severe neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: A prospective cross-sectional study. Nat Commun 13: , 6777. |
[29] | Ollila H , Pihlajamaa J , Martola J , Kuusela L , Blennow K , Zetterberg H , Salmela V , Hokkanen L , Tiainen M , Hästbacka J ((2024) ) Brain magnetic resonance imaging findings six months after critical COVID-19: A prospective cohort study. J Crit Care 80: , 154502. |
[30] | Meyer PT , Hellwig S , Blazhenets G , Hosp JA ((2022) ) Molecular imaging findings on acute and long-term effects of COVID-19 on the brain: A systematic review. J Nucl Med 63: , 971–980. |
[31] | Yang AC , Kern F , Losada PM , Agam MR , Maat CA , Schmartz GP , Fehlmann T , Stein JA , Schaum N , Lee DP , Calcuttawala K , Vest RT , Berdnik D , Lu N , Hahn O , Gate D , McNerney MW , Channappa D , Cobos I , Ludwig N , Schulz-Schaeffer WJ , Keller A , Wyss-Coray T ((2021) ) Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595: , 565–571. |
[32] | Zhou Y , Xu J , Hou Y , Leverenz JB , Kallianpur A , Mehra R , Liu Y , Yu H , Pieper AA , Jehi L , Cheng F ((2021) ) Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimer Res Therapy 13: , 110. |
[33] | Hanson BA , Visvabharathy L , Ali ST , Kang AK , Patel TR , Clark JR , Lim PH , Orban ZS , Hwang SS , Mattoon D , Batra A , Liotta EM , Koralnik IJ ((2022) ) Plasma biomarkers of neuropathogenesis in hospitalized patients with COVID-19 and those with postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm 9: , e1151. |
[34] | Frontera JA , Boutajangout A , Masurkar AV , Betensky RA , Ge Y , Vedvyas A , Debure L , Moreira A , Lewis A , Huang J , Thawani S , Balcer L , Galetta S , Wisniewski T ((2022) ) Comparison of serum neurodegenerative biomarkers among hospitalized COVID-19 patients versus non-COVID subjects with normal cognition, mild cognitive impairment, or Alzheimer’s dementia. Alzheimers Dement 18: , 899–910. |
[35] | Reiken S , Sittenfeld L , Dridi H , Liu Y , Liu X , Marks AR ((2022) ) Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement 18: , 955–965. |
[36] | He Y , Bai X , Zhu T , Huang J , Zhang H ((2021) ) What can the neurological manifestations of COVID-19 tell us: A meta-analysis. J Transl Med 19: , 363. |
[37] | Rogers JP , Chesney E , Oliver D , Pollak TA , McGuire P , Fusar-Poli P , Zandi MS , Lewis G , David AS ((2020) ) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7: , 611–627. |
[38] | Girard TD , Jackson JC , Pandharipande PP , Pun BT , Thompson JL , Shintani AK , Gordon SM , Canonico AE , Dittus RS , Bernard GR , Ely EW ((2010) ) Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 38: , 1513–1520. |
[39] | Ollila H , Pihlaja R , Koskinen S , Tuulio-Henriksson A , Salmela V , Tiainen M , Hokkanen L , Hästbacka J ((2022) ) Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: A comprehensive controlled neuropsychological study. Crit Care 26: , 223. |
[40] | Evans RA , McAuley H , Harrison EM , Shikotra A , Singapuri A , Sereno M , Elneima O , Docherty AB , Lone NI , Leavy OC , et al. ((2021) ) Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respiratory Med 9: , 1275–1287. |
[41] | Hensley MK , Markantone D , Prescott HC ((2022) ) Neurologic manifestations and complications of COVID-19. Annu Rev Med 73: , 113–127. |
[42] | Taquet M , Geddes JR , Husain M , Luciano S , Harrison PJ ((2021) ) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 8: , 416–427. |
[43] | Liu Y-H , Chen Y , Wang Q-H , Wang L-R , Jiang L , Yang Y , Chen X , Li Y , Cen Y , Xu C , Zhu J , Li W , Wang Y-R , Zhang L-L , Liu J , Xu Z-Q , Wang Y-J ((2022) ) One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: A longitudinal cohort study. JAMA Neurol 79: , 509. |
[44] | Hastie CE , Lowe DJ , McAuley A , Winter AJ , Mills NL , Black C , Scott JT , O’Donnell CA , Blane DN , Browne S , Ibbotson TR , Pell JP ((2022) ) Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun 13: , 5663. |
[45] | Huang L , Li X , Gu X , Zhang H , Ren L , Guo L , Liu M , Wang Y , Cui D , Wang Y , Zhang X , Shang L , Zhong J , Wang X , Wang J , Cao B ((2022) ) Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir Med 10: , 863–876. |
[46] | Yang X , Yu Y , Xu J , Shu H , Xia J , Liu H , Wu Y , Zhang L , Yu Z , Fang M , Yu T , Wang Y , Pan S , Zou X , Yuan S , Shang Y ((2020) ) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 8: , 475–481. |
[47] | Li J , Long X , Huang H , Tang J , Zhu C , Hu S , Wu J , Li J , Lin Z , Xiong N ((2020) ) Resilience of Alzheimer’s disease to COVID-19. J Alzheimers Dis 77: , 67–73. |
[48] | Al-Lami RA , Urban RJ , Volpi E , Algburi AMA , Baillargeon J ((2020) ) Sex hormones and novel corona virus infectious disease (COVID-19). Mayo Clin Proc 95: , 1710–1714. |
[49] | Naughton SX , Raval U , Pasinetti GM ((2020) ) Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J Alzheimers Dis 76: , 21–25. |
[50] | Inal J ((2020) ) Biological factors linking ApoE ɛ4 variant and severe COVID-19. Curr Atheroscler Rep 22: , 70. |
[51] | Herridge MS , Moss M , Hough CL , Hopkins RO , Rice TW , Bienvenu OJ , Azoulay E ((2016) ) Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 42: , 725–738. |
[52] | Rezaei N , ed. ((2021) ) Coronavirus Disease - COVID-19, Springer International Publishing, Cham. |
[53] | Verma K , Amitabh , Prasad DN , Kumar B , Kohli E ((2020) ) Brain and COVID-19 crosstalk: Pathophysiological and psychological manifestations. ACS Chem Neurosci 11: , 3194–3203. |
[54] | Beyrouti R , Adams ME , Benjamin L , Cohen H , Farmer SF , Goh YY , Humphries F , Jäger HR , Losseff NA , Perry RJ , Shah S , Simister RJ , Turner D , Chandratheva A , Werring DJ ((2020) ) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 91: , 889–891. |
[55] | Global Burden of Disease Long COVID Collaborators, Wulf Hanson S , Abbafati C , Aerts JG , Al-Aly Z , Ashbaugh C , Ballouz T , Blyuss O , Bobkova P , Bonsel G , et al. ((2022) ) Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328: , 1604–1615. |
[56] | Palao M , Fernández-Díaz E , Gracia-Gil J , Romero-Sánchez CM , Díaz-Maroto I , Segura T ((2020) ) Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord 45: , 102377. |
[57] | Meinhardt J , Radke J , Dittmayer C , Franz J , Thomas C , Mothes R , Laue M , Schneider J , Brünink S , Greuel S , Lehmann M , Hassan O , Aschman T , Schumann E , Chua RL , Conrad C , Eils R , Stenzel W , Windgassen M , Rößler L , Goebel H-H , Gelderblom HR , Martin H , Nitsche A , Schulz-Schaeffer WJ , Hakroush S , Winkler MS , Tampe B , Scheibe F , Körtvélyessy P , Reinhold D , Siegmund B , Kühl AA , Elezkurtaj S , Horst D , Oesterhelweg L , Tsokos M , Ingold-Heppner B , Stadelmann C , Drosten C , Corman VM , Radbruch H , Heppner FL ((2021) ) Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24: , 168–175. |
[58] | Sánchez KE , Rosenberg GA ((2022) ) Shared inflammatory pathology of stroke and COVID-19. IJMS 23: , 5150. |
[59] | Venkataramani V , Winkler F ((2022) ) Cognitive deficits in long Covid-19. N Engl J Med 387: , 1813–1815. |
[60] | Allen WE , Blosser TR , Sullivan ZA , Dulac C , Zhuang X ((2023) ) Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell 186: , 194–208.e18. |
[61] | Baig AM , Khaleeq A , Ali U , Syeda H ((2020) ) Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11: , 995–998. |
[62] | Paniz-Mondolfi A , Bryce C , Grimes Z , Gordon RE , Reidy J , Lednicky J , Sordillo EM , Fowkes M ((2020) ) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 92: , 699–702. |
[63] | Baruch K , Deczkowska A , David E , Castellano JM , Miller O , Kertser A , Berkutzki T , Barnett-Itzhaki Z , Bezalel D , Wyss-Coray T , Amit I , Schwartz M ((2014) ) Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346: , 89–93. |
[64] | Lee M-H , Perl DP , Nair G , Li W , Maric D , Murray H , Dodd SJ , Koretsky AP , Watts JA , Cheung V , Masliah E , Horkayne-Szakaly I , Jones R , Stram MN , Moncur J , Hefti M , Folkerth RD , Nath A ((2021) ) Microvascular injury in the brains of patients with Covid-19. N Engl J Med 384: , 481–483. |
[65] | Hernández-Fernández F , Sandoval Valencia H , Barbella-Aponte RA , Collado-Jiménez R , Ayo-Martín Ó , Barrena C , Molina-Nuevo JD , García-García J , Lozano-Setién E , Alcahut-Rodriguez C , Martínez-Martín Á , Sánchez-López A , Segura T ((2020) ) Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain 143: , 3089–3103. |
[66] | DeTure MA , Dickson DW ((2019) ) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14: , 32. |
[67] | Ramachandran AK , Das S , Joseph A ((2021) ) Crosstalk between Covid-19 and associated neurological disorders: A review. Curr Neuropharmacol 19: , 1688–1700. |
[68] | Burtscher J , Mallet RT , Burtscher M , Millet GP ((2021) ) Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res Rev 68: , 101343. |
[69] | Serebrovska ZO , Chong EY , Serebrovska TV , Tumanovska LV , Xi L ((2020) ) Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin 41: , 1539–1546. |
[70] | Suri V , Pandey S , Singh J , Jena A ((2021) ) Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep 14: , e245816. |
[71] | Franke C , Boesl F , Goereci Y , Gerhard A , Schweitzer F , Schroeder M , Foverskov-Rasmussen H , Heine J , Quitschau A , Kandil FI , Schild A-K , Finke C , Audebert HJ , Endres M , Warnke C , Prüss H ((2023) ) Association of cerebrospinal fluid brain-binding autoantibodies with cognitive impairment in post-COVID-19 syndrome. Brain Behav Immun 109: , 139–143. |
[72] | Sánchez-Morales AE , Urrutia-Osorio M , Camacho-Mendoza E , Rosales-Pedraza G , Dávila-Maldonado L , González-Duarte A , Herrera-Mora P , Ruiz-García M ((2021) ) Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Childs Nerv Syst 37: , 2305–2312. |
[73] | Troyer EA , Kohn JN , Hong S ((2020) ) Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun 87: , 34–39. |




