Genetic Risk for Alzheimer’s Disease Alters Perceived Executive Dysfunction in Cognitively Healthy Middle-Aged and Older Adults
Abstract
Background:
Subjective cognitive complaints (SCC) may be an early indicator of future cognitive decline. However, findings comparing SCC and objective cognitive performance have varied, particularly in the memory domain. Even less well established is the relationship between subjective and objective complaints in non-amnestic domains, such as in executive functioning, despite evidence indicating very early changes in these domains. Moreover, particularly early changes in both amnestic and non-amnestic domains are apparent in those carrying the Apolipoprotein-E ɛ4 allele, a primary genetic risk for Alzheimer’s disease (AD).
Objective:
This study investigated the role of the ɛ4 allele in the consistency between subjective and objective executive functioning in 54 healthy, cognitively intact, middle-aged and older adults.
Methods:
Participants (Mage = 64.07, SD = 9.27, range = 48–84; ɛ4+ = 18) completed the Frontal Systems Behavior Scale (FrSBe) Executive Dysfunction Scale (EXECDYS) to measure subjective executive functioning (SEF) and multiple executive functioning tasks, which were condensed into a single factor.
Results:
After accounting for age, depression, and anxiety, objective executive functioning performance significantly predicted SEF. Importantly, ɛ4 moderated this effect. Specifically, those carrying the ɛ4 allele had significantly less accurate self-awareness of their executive functioning compared to ɛ4 non-carriers.
Conclusions:
Utilizing an approach that integrates self-evaluation of executive functioning with objective neurocognitive assessment may help identify the earliest signs of impending cognitive decline, particularly in those with genetic risk for AD. Such an approach could sensitively determine those most prone to future cognitive decline prior to symptom onset, when interventions could be most effective.
INTRODUCTION
Aging is accompanied by a myriad of changes related to physical, mental, and cognitive health. From a cognitive perspective, a modest degree of objective cognitive decline across older adulthood is well- documented and considered typical during the aging process [1, 2]. However, some of these individuals will exhibit severe cognitive decline that progresses to dementia. Prior to this progression, a subset of these elders will perceive changes in their cognition that are not clinically meaningful on neuropsychological tests. These perceived changes are referred to as subjective cognitive complaints (SCC) [3, 4].
Although SCC are prevalent and are not unique to any one etiology underlying cognitive decline, mounting evidence suggests they may specifically portend a diagnosis of Alzheimer’s disease (AD) [5–7]. Specifically, individuals who endorse these complaints are 4.5 times more likely than others to later convert to either mild cognitive impairment (MCI) [8], a transitional stage between normal cognitive aging and dementia, or to dementia, within a seven-year timeframe [9, 10]. Over four years, a recent meta-analysis of longitudinal SCC studies demonstrated the 4-year rate of conversion to MCI was 27% while rate of conversion to dementia was 14% [11]. SCC endorsement is also correlated with biomarkers indicative of impending AD, including hippocampal atrophy [12], elevated amyloid-β deposition (i.e., amyloid burden) [13–21], and altered brain activity [17, 22].
Although the SCC construct is still somewhat controversial due to heterogeneity in the literature, comprehensive reviews and meta-analyses support a significant relationship between SCC and objective cognitive functioning; other factors such as age, sex, and depression add further to the effect sizes [23, 24]. Recent large sample studies of elders with objectively intact cognition have furthermore shown even stronger effects linking SCC to poorer cognition, both globally and in specific domains such as episodic memory, semantic memory/language, and processing speed [19, 25, 26], as well as greater cognitive decline over time in these domains and in visuospatial processing and working memory [25]. These effects held when controlling for depression and demographic factors. However, it is also important to acknowledge that metacognition is required for SCC to accurately reflect cognitive functioning [27]. Notably, anosognosia, the impairment in awareness of deficits in cognitive functioning, is a common feature of AD, particularly in the early stage [28–31], including in approximately 50% of those diagnosed with MCI, a prodromal phase of AD [32, 33]. Other studies report that elders with MCI [34] and also cognitively intact elders have hypernosognosia [35], or hyperawareness of their true deficits, but these findings have not yet successfully replicated [36]. Regardless, anosognosia, measured as the discrepancy between objectively and subjectively assessed cognition, may present an important opportunity for early detection of AD-related cognitive decline. Even the newest, promising pharmaceutical treatments for AD, which act by clearing amyloid deposition, are far more effective in the early stage [37], making any adjuvant predictors incredibly valuable.
The Apolipoprotein E (APOE) ɛ4 allele is a key non-modifiable risk factor for late-onset AD, the most common form of AD. In fact, ɛ4 is the most significant risk factor other than age [38], with carriers of at least one ɛ4 allele having three to four times increased likelihood of developing AD and carriers of both ɛ4 alleles having approximately 12 times increased likelihood [39, 40]. In contrast to other AD biomarkers, APOE ɛ4 is quickly, non-invasively, and inexpensively measured [38]. Furthermore, it is associated with lower cognitive scores and greater amyloid burden after accounting for other AD risks such as sex, education, and lifestyle factors [41, 42]. Importantly, SCC has been shown to be indicative of elevated amyloid burden in younger elders, in particular, in ɛ4-carriers [43]. Moreover, APOE ɛ4 is a primary predictor of future objective cognitive decline in SCC endorsers [44, 45]. Cognitively healthy ɛ4 carriers exhibit compensatory brain activity [46, 47] and accelerated brain atrophy rates [48, 49], as well as a significantly faster rate of cognitive decline overall [10, 41, 42, 50, 51]. While the neuropathology of AD (e.g., amyloid and tau burden) is a primary focus in the current literature, these markers only approach clinical significance, on average, six years before diagnosis of AD [10]. Given that APOE ɛ4 is static and indicative of amyloid burden in those who endorse SCC, the combination of SCC and ɛ4 may provide a valuable proxy for early assessment of AD risk.
Notably absent in the literature are studies of SCC outside of the domain of memory. Although SCC is not limited to memory functions, memory has been the focus of SCC research due to the ubiquity of episodic memory impairment in AD [52, 53]. However, there is accumulating evidence that very early changes also occur in non-amnestic domains, such as in executive functioning [19, 25, 26, 54], including in cognitively intact elders who have family history of AD [55] or who carry ɛ4 [46, 47]. Executive functioning is an umbrella term for multiple interacting cognitive processes that underlie goal-directed behaviors [56], including working memory (updating), task switching, and countermanding dominant or prepotent responses (i.e., inhibitory control) [57, 58]. These processes, predominantly mediated by the prefrontal cortex [59, 60], play an important role in the successful completion of activities of daily living, a fundamental component of the AD diagnosis. Importantly, Grober et al. [61] found tests of executive functioning to have comparable predictive validity to tests of memory function, particularly several years before an MCI or AD diagnosis, which may coincide with the onset of neural dysfunction (i.e., compensation) [62, 63] and when individuals begin to endorse SCC. Some evidence suggests that, amongst those with SCC, baseline executive functioning and language are more closely associated with progression to non-amnestic MCI, while verbal memory is more associated with amnestic MCI [64].
Despite an absence of objective memory impairment, individuals with SCC in memory have evidenced poorer performance, in some cases comparable to those diagnosed with MCI, on non-amnestic cognitive tasks that tap frontal executive networks including, sustained and divided attention [65], verbal fluency [66–68], visual working memory [69], inhibition and interference [67, 68]. The link between memory complaints and executive functioning also corresponds to greater AD neuropathology, such as amyloid burden [70, 71] and future dementia [72]. Cognitively normal individuals with elevated amyloid burden have also been shown to endorse more SCC specific to language and executive functioning than those with low amyloid burden [68]. As such, subjective executive functioning (SEF) is a gap in the literature that needs study as a predictor of risk for AD.
The current study examined the relationship between subjective and objective executive functioning (i.e., awareness of executive functioning), in cognitively intact middle aged and older adults who were stratified by risk for AD using the APOE ɛ4 allele. We hypothesized that the relationship between subjective and objective executive functioning would be moderated by APOE ɛ4, such that individuals at a greater risk for AD (APOE ɛ4 carriers; ɛ4+) would have reduced awareness of their executive functioning relative to non-carriers (ɛ4-).
MATERIALS AND METHODS
Participants
Participants were recruited via various forms of advertising in the metro Milwaukee area for studies measuring multi-dimensional aspects of cognitive aging. Participants were carefully screened to ensure that they did not engage in concurrent studies that could appreciably influence the outcomes of the present study. Cognitive status was assessed with an initial phone screen followed by a cognitive battery that included the Mini-Mental State Examination (MMSE) [73] and Mattis Dementia Rating Scale-2 (DRS-2) [74]. Cognitively intact adults (determined by a DRS-2 score ≥130) were included in the current study. Thus, the current sample was 54 cognitively intact adults (Mage = 64.07, SD = 9.27, range = 48-84), 18 of whom were carriers of APOE ɛ4 allele (ɛ4/ɛ4 : 1; ɛ3/ɛ4 : 15; ɛ2/ɛ4 : 2). All participants were compensated for their participation. Procedures, approved by the Marquette University Institutional Review Board, were in accord with the 1975 Declaration of Helsinki.
Inclusion and exclusion criteria
Inclusion criteria included a minimum age of 45, right-handed, English-speaking, good general health by self-report, and intact cognition (DRS-2 score ≥130). Exclusion criteria included history or evidence of neurologically relevant illness or disorder (e.g., head trauma with > 30 min loss of consciousness, cerebrovascular disease or disorder, cardiovascular disease, cerebral palsy, epilepsy, brain tumor, neurodegenerative disease or disorder, dementia, etc.); other severe illness or conditions that may affect brain function (e.g., untreated hypertension, insulin-dependent diabetes, substance abuse history, etc.); major psychiatric disturbance meeting DSM-IV Axis I criteria; current use of psychoactive medications; MMSE score < 26; and Geriatric Depression Scale (GDS) score ≥12. Nicotine and alcohol use were restricted within 24 hours of testing.
Measures and instruments
Frontal systems behavior scale (FrSBe)
The FrSBe [75] is a 46-item behavior rating scale that measures behavior associated with damage to frontal systems of the brain. The questions assess current and retrospective (i.e., previous 10 years) behavior in three frontal systems behavioral domains: apathy (n = 14), disinhibition (n = 15), and executive dysfunction (n = 17). For the present study, only the self-reported current assessment on the Executive Dysfunction (EXECDYS) subscale was used, which evaluates planning, organization, error correction, perseveration, flexibility, judgement, and awareness of behavior and cognition, using a 5-point Likert-type scale (1, Almost Never; 2, Seldom; 3, Sometimes; 4, Frequently; and 5, Almost Always). As such, higher EXECDYS ratings correspond to greater perceived dysfunction (i.e., SEF). Current EXECDYS was specifically chosen for this study to assess current perceived frequency of concerns related to executive dysfunction that most closely mirrors the objective tests that were administered. Perceived retrospective frequency of problems was considered potentially confounded by limitations in awareness (a focus of this study).
Geriatric depression scale (GDS)
The GDS is a frequently used self-report scale assessing symptoms of depression in middle age and older adults [76]. It consists of 30 items (i.e., Are you basically satisfied with your life?) with yes/no response options. A score ≥12 indicates more than minimal depression, which was an exclusionary criterion for recruitment.
Beck anxiety inventory (BAI)
The BAI is a 21-item scale and was used to assess anxiety severity [77, 78]. It has high internal consistency (α=0.92) and good test-retest reliability over one week interval. A score ≥16 indicates more than minimal/mild anxiety. While not considered during recruitment, no participant met criteria for more than minimal/mild anxiety.
Executive functioning tests
A short battery of standardized executive functioning measures was used for the current study. The Trail-Making Tests (TMT) [79] measures attention, psychomotor speed, visuospatial search, and target-directed motor tracking (Part A) and set-switching (Part B) [80]. In Part A, the subject uses a pencil to connect quasi-randomly ordered circles in numerical order, as quickly and accurately as possible, without lifting the pencil. In Part B, numbers and letters must be alternated (i.e., 1, A, 2, B, etc.). The primary variable is time to completion (seconds, maximum of 300). The Symbol Digit Modalities Test (SDMT) [81] measures processing speed and efficiency. The subject is given a key that pairs each of nine symbols with a digit, followed by rows in which only the symbols are shown; the subject is to fill in the missing associated digits as quickly as possible. The score is the number of items completed within 90 s. The Rey Auditory Verbal Learning Test (RAVLT) [82] was given as a measure of verbal learning and retention. Only List B was used in this paper; it is also known as the interference trial, which requires executive functioning to encode and retrieve a new list of words following five trials with a prior list [83, 84]. Finally, three language tests whose performances required executive functioning were also included [85, 86]. Phonemic fluency was measured with the Controlled Oral Word Association Test (COWAT) [87], in which the subject is asked to generate as many unique words that begin with a specific letter as possible in 1 min; three stimulus letters are used (e.g., F, A, S, 1 min each). Category (semantic) fluency was assessed by asking the participant to generate as many unique items as possible from a stimulus category (e.g., animals) in 1 min [88, 89]. A 15-item alternating items subset of the Boston Naming Test (BNT) [90] was given, in which participants name the object depicted in line drawings. Scores were converted to reflect the standard 30-item test.
APOE genotyping
APOE genotyping was performed using genetic material from a mouth swab (buccal cells) [40, 91] using Sample to SNP kits (Applied Biosystems, Foster City, CA). DNA for APOE genotyping was performed using TaqMan assays (ABI) in the largest batches possible, with known genotyped controls run with each batch. Specifically, for APOE allele determination, two separate SNP genotyping used the polymorphisms rs7412 and rs429358 in order to distinguish between alleles (i.e., ɛ2 /ɛ4, ɛ3/ɛ3, ɛ3 /ɛ4 and ɛ4/ɛ4). Those with one or more ɛ4 allele were deemed ɛ4-carriers (ɛ4+); all other allele combinations were deemed ɛ4-non-carriers (ɛ4-). APOE ɛ4 carrier status was not revealed to participants.
Procedures and analyses
Recruited participants attended a single session in the laboratory, which lasted approximately 120 min, where they completed the cognitive screening measures (i.e., MMSE and DRS-2), neuropsychological assessment, surveys, and buccal cell collection. APOE ɛ4+ and ɛ4- participants were compared across demographic and other measured variables using independent samples t-tests and χ2 to characterize the overall study sample (SPSS v28; SPSS Inc, Chicago, IL). All the included executive functioning tests were significantly intercorrelated in this sample (|r| = 0.272 to 0.631, p < 0.05). Principal Components Analysis reduced this set to a single factor solution, which accounted for 49.4% of the variance (eigenvalue = 3.5), which was used as a predictor on the subsequent analyses (EF_Factor). A moderation model was performed to predict SEF (EXECDYS subscale as the dependent variable) with EF_Factor (i.e., objective performance), genetic risk for AD (i.e., APOE ɛ4), and their interaction as the predictors (PROCESS 4.3) [92]. Covariates were included when descriptive statistics suggested they were relevant; these are described in the results.
RESULTS
Participant characteristics
Table 1 presents the demographic and descriptive statistics by APOE ɛ4 group. The groups did not significantly differ on any measure except age (t (53) = –2.67, p = 0.01); ɛ4+ were older than ɛ4-. Although not statistically significant, ɛ4+ also had a larger proportion of females than ɛ4-. Objective executive functioning performance was within normal age and education limits. Although performance was comparable between the ɛ4 groups on individual tests, the factor analysis showed ɛ4+ had marginally poorer executive performance than in ɛ4- group overall.
Table 1
Descriptive statistics (mean (±SD))
| Full sample | APOE ɛ4+ | APOE ɛ4- | p | |
| (n = 54) | (n = 18) | (n = 36) | ||
| Age (y) | 64.07 (9.27) | 68.67 (9.72) | 61.78 (8.24) | 0.01* |
| Education (y) | 15.70 (2.26) | 15.33 (2.03) | 15.89 (2.38) | 0.40 |
| Sex (n (%) female) | 33 (61%) | 14 (78%) | 19 (53%) | 0.08 |
| MMSE | 29.44 (.95) | 29.67 (.77) | 29.33 (1.01) | 0.23 |
| GDS | 1.39 (2.04) | 1.39 (2.55) | 1.39 (1.78) | 1.00 |
| BAI | 4.04 (4.09) | 4.28 (5.42) | 3.92 (3.32) | 0.76 |
| EXECDYS | 31.61 (7.22) | 30.39 (5.78) | 32.22 (7.85) | 0.38 |
| Trail-making Tests-A | 28.92 (9.2) | 32.56 (9.2) | 27.10 (8.8) | 0.26 |
| Trail-making Tests-B | 65.31 (27.5) | 76.00 (35.9) | 59.96 (20.7) | 0.20 |
| SDMT | 47.76 (9.3) | 44.89 (9.0) | 49.19 (9.2) | 0.64 |
| COWAT | 42.59 (10.8) | 42.11 (10.3) | 42.83 (11.2) | 0.94 |
| Category Fluency | 20.81 (4.0) | 21.00 (4.3) | 20.72 (3.9) | 0.55 |
| Boston Naming Test | 28.09 (2.6) | 27.61 (1.9) | 28.33 (2.9) | 0.47 |
| AVLT List B | 5.54 (2.0) | 4.67 (2.0) | 5.97 (1.9) | 0.11 |
| EF_Factor | 0.00 (1.00) | -0.36 (1.10) | 0.16 (0.92) | 0.07 |
p based on between groups t-test (χ2 for sex); MMSE, Mini-Mental State Exam total score; GDS, Geriatric Depression Scale total; BAI, Beck Anxiety Inventory total; EXECDYS, Frontal Assessment Battery executive dysfunction subscore (subjective executive functioning); SDMT, Symbol-digit Modalities Test; COWAT, Controlled Oral Word Association Test; AVLT, Rey Auditory Verbal Learning Test (List B = interference list); EF_Factor, factor score of objective executive functioning battery; test score rotated loadings on EF-Factor = 0.57 to 0.81. *95% CI [-11.5, -1.6], d = 0.74.
Intercorrelations
Exploratory correlations were performed between demographic variables and SEF (Table 2). Despite very low overall depression and anxiety scores, the GDS and BAI were significantly correlated with EXECDYS. Specifically, participants with greater EXECDYS scores (i.e., higher endorsement of SEF) had higher (albeit low) depression and anxiety scores. Notably, however, the correlation with depression was due primarily to the ɛ4+ group (r ɛ4 + = 0.72; r ɛ4 - = 0.31), while the correlation with anxiety was significant in both groups (r ɛ4 + = 0.51; r ɛ4 - = 0.40). As such, the GDS and BAI were included as covariates in subsequent analyses. Although age did not significantly correlate with the measure of SEF, it was also included as a covariate as a precaution because of the age difference between the APOE ɛ4 groups, and its correlation with objective executive performance. Although the ɛ4+ group had a somewhat higher percentage of females than the ɛ4- group, the difference was not statistically significant and sex did not significantly correlate with any other variable. Thus, sex was not a covariate in subsequent analyses.
Table 2
Exploratory correlations
| All (n = 54) | EXECDYS | Age | Sex | APOE ɛ4 | MMSE | EF_Factor | GDS |
| EXECDYS | |||||||
| Age | –0.05 | ||||||
| Sex | –0.23 | –0.09 | |||||
| APOE ɛ4 | –0.03 | 0.35 | 0.24 | ||||
| MMSE | –0.25 | –0.09 | 0.04 | 0.18 | |||
| EF_Factor | –0.01 | –0.48 | –0.05 | 0.06 | 0.06 | ||
| GDS | 0.38 | –0.32 | –0.06 | –0.1 | –0.1 | –0.07 | |
| BAI | 0.36 | –0.25 | –0.03 | –0.04 | –0.04 | 0.35 | 0.26 |
Significant correlations (p < 0.05) are in bold; all correlations are Pearson r except with sex (Spearman rho); EXECDYS, Frontal Assessment Battery executive dysfunction subscore (subjective executive functioning); APOE, Apolipoprotein-E; MMSE, Mini-Mental State Exam total score; EF_Factor, single factor score of objective executive functioning tests; GDS, Geriatric Depression Scale total; BAI, Beck Anxiety Inventory total.
Moderation model
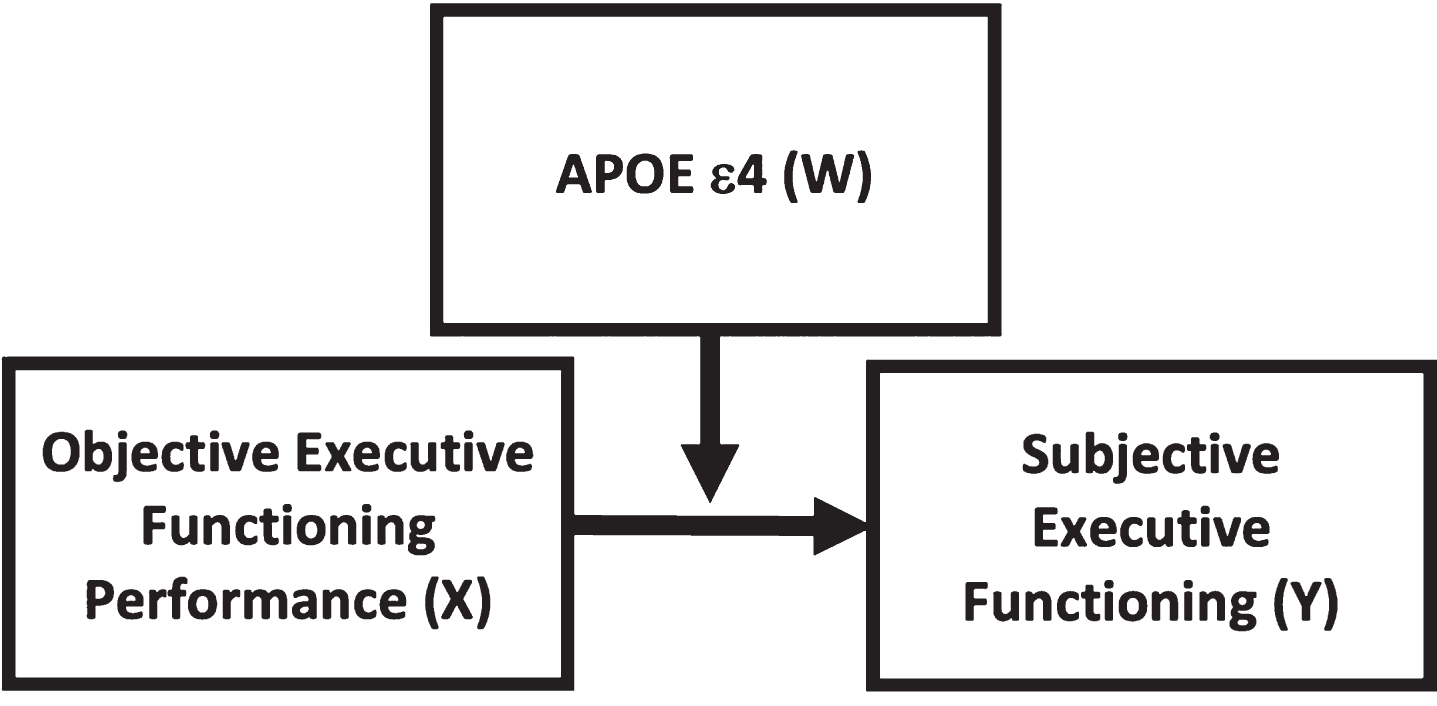
We hypothesized that the relationship between objective executive functioning and SEF would be moderated by APOE ɛ4, such that ɛ4+ older adults would be less aware of their actual executive functioning than ɛ4- participants. This theoretical model is depicted in Fig. 1.
Fig. 1
Theoretical model depicting APOE ɛ4 (W) moderating the relationship between objective executive functioning (X, predictor) and subjective executive functioning (Y, outcome).
The results of the moderation analysis are presented in Table 3. After accounting for the contributions of age, depression (GDS) and anxiety (BAI), objective executive function performance (EF_Factor) was significantly associated with subjective executive function (EXECDYS), and APOE ɛ4 moderated this relationship. Specifically, those with poorer objectively assessed executive function had greater perceived executive dysfunction, but this was clarified by an interaction with ɛ4. In ɛ4-, those with poorer objective executive functioning also had greater perceived executive dysfunction, while in ɛ4+, those with poorer objective executive functioning had less perceived executive dysfunction. Thus, ɛ4+ were less accurate in assessing their executive functioning than ɛ4-. These differing patterns are shown in Fig. 2.
Table 3
Moderation model
| Model summary | ||||||
| R | R2 | MSE | F(6,47) | p | ||
| EXECDYS | 0.58 | 0.34 | 39.12 | 3.94 | 0.003 | |
| Model parameters | ||||||
| Coeff. | se | t | p | LLCI | ULCI | |
| Constant | 21.557 | 7.692 | 2.803 | 0.007 | 6.083 | 37.031 |
| EF_Factor | –2.878 | 1.233 | –2.334 | 0.024 | –5.359 | –0.397 |
| ɛ4 | –1.793 | 1.973 | –0.986 | 0.368 | –5.762 | 2.176 |
| Interaction | 3.873 | 1.832 | 2.114 | 0.04 | 0.187 | 7.559 |
| Covariates | ||||||
| Age | 0.114 | 0.116 | 0.99 | 0.327 | –0.118 | 0.347 |
| GDS | 1.291 | 0.628 | 2.058 | 0.045 | 0.029 | 2.554 |
| BAI | 0.589 | 0.323 | 1.822 | 0.075 | –0.061 | 1.24 |
| Highest order unconditional interaction | ΔR2 | F(1,47) | p | |||
| EF_Factor X ɛ4 | 0.063 | 4.468 | 0.04 | |||
| Conditional effects | Effect | se | t | p | LLCI | ULCI |
| ɛ4- | –2.878 | 1.233 | –2.334 | 0.024 | –5.359 | –0.397 |
| ɛ4+ | 0.995 | 1.53 | 0.65 | 0.519 | –2.083 | 4.072 |
EXECDYS, Frontal Assessment Battery executive dysfunction subscore (subjective executive functioning); EF_Factor, factor score of objective executive functioning battery; ɛ4, APOE ɛ4; Coeff, unstandardized coefficient; MSE, mean square error; LLCI, lower-level confidence interval; ULCI, upper level confidence interval; GDS, Geriatric Depression Scale total; BAI, Beck Anxiety Inventory total.
Fig. 2
Moderation analysis [simple-slopes analysis via PROCESS; 92] showing the interaction of objectively measured executive functioning (EF_Factor) and APOE ɛ4 group, predicting subjective executive functioning (EXECDYS). Age, depression, and anxiety were covaried. APOE ɛ4 carriers (ɛ4+) were less accurate in identifying executive concerns than ɛ4 non-carriers (ɛ4-). Specifically, in ɛ4-, perceived executive dysfunction was greater in those with poorer objective executive functioning, while in ɛ4+, the opposite pattern was evident; perceived executive dysfunction was lower in those with poorer objective executive functioning.
![Moderation analysis [simple-slopes analysis via PROCESS; 92] showing the interaction of objectively measured executive functioning (EF_Factor) and APOE ɛ4 group, predicting subjective executive functioning (EXECDYS). Age, depression, and anxiety were covaried. APOE ɛ4 carriers (ɛ4+) were less accurate in identifying executive concerns than ɛ4 non-carriers (ɛ4-). Specifically, in ɛ4-, perceived executive dysfunction was greater in those with poorer objective executive functioning, while in ɛ4+, the opposite pattern was evident; perceived executive dysfunction was lower in those with poorer objective executive functioning.](https://content.iospress.com:443/media/adr/2024/8-1/adr-8-1-adr230166/adr-8-adr230166-g002.jpg)
Post-hoc analysis of subjective memory functioning
This study was not designed to evaluate perceived memory functioning. However, our participants did complete memory testing (RAVLT) and depression screening (GDS); the GDS includes the following question, “Do you feel you have more problems with memory than most? (yes or no)”. As many studies of SCC have used a single dichotomous response question to assess SCC [23, 93], we performed a post-hoc analysis using these measures to compare subjective memory functioning to our primary results with SEF. These results are shown in the Supplementary Material. There were 13 participants who endorsed memory dysfunction (2 APOE ɛ4 carriers, 11 non-carriers). Perceived memory dysfunction did not significantly correlate with other study variables, including SEF or objective memory functioning, and no differences or relationships were attributable to ɛ4. Furthermore, substituting objective memory functioning for objective executive functioning in the primary model produced a non-significant model with no significant predictors. Thus, the executive functioning awareness results were specific to executive functioning, rather than general cognitive or memory functioning.
DISCUSSION
Mounting evidence suggests that SCC might foreshadow an AD diagnosis [5–7, 9, 10] and its related accumulating neuropathology [13–21, 94]. Moreover, there has been little study of perceived dysfunction in non-amnestic cognitive domains, despite the demonstrated importance of domains such as executive functioning in the earliest signs of impending dementia [70, 95]. Even less is known about the role of APOE ɛ4, a primary risk factor for AD [38], in non-amnestic SCC. Thus, this study examined the relationship between subjective and objective executive functioning (i.e., awareness) in healthy, cognitively intact, middle-aged and older adults, as a function of APOE ɛ4. The results demonstrated that SEF was significantly correlated with objectively measured executive function performance in cognitively intact older adults, and, as predicted, ɛ4 carriers exhibited significantly less accurate awareness of their executive functioning performance than ɛ4 non-carriers. A post-hoc speculative analysis also reinforced that these results are specific to executive functioning, rather than cognition in general or memory and suggest that executive functioning may be more sensitive to very early dysfunction in awareness. These results therefore support the use of subjective executive dysfunction along with APOE ɛ4 as possible sensitive, early markers of impending cognitive decline. Specifically, poor awareness of cognitive functioning, otherwise referred to as anosognosia, in cognitively intact ɛ4 carriers may specifically portend the development of AD. Given the promising new amyloid clearing pharmaceutical treatments for AD are primarily effective in the early stage [37], anosognosia in executive function, particularly in ɛ4 carriers may be a valuable early adjuvant to predicting AD risk.
Our SEF findings add to the growing evidence in the memory domain showing a significant relationship between subjective and objective cognitive performance. The largest memory SCC studies indeed also show poorer cognitive functioning and greater decline over time in multiple domains outside of memory in those who endorse SCC [19, 25, 26], particularly in complex cognitive functions that rely on frontal executive networks [65–69]. Thus, our findings extend the literature beyond the memory domain into executive functioning, and further reinforcing the possible importance of executive function in the earliest signs of impending dementia [70, 95].
Our results do not directly address AD neuropathology. However, our findings are consistent with studies showing greater amyloid burden and elevated risk of cognitive decline in cognitively intact ɛ4 carriers who endorse SCC [43–45]. Indeed, since cognitively normal individuals with significant amyloid burden have been shown to endorse SCC specifically in the executive domains [68], our results reinforce the potential value of assessing subjective executive dysfunction as an early risk for future dementia. Moreover, while altered memory awareness has been shown in AD risk via amyloid burden, our results suggest that these alterations are comparably detectable simply via APOE ɛ4, a cost-effective, non-invasive method of examining AD risk. Additional research is needed to determine whether ɛ4 alongside subjective executive dysfunction might be able to detect risk for future cognitive decline at an earlier stage than amyloid burden. Indeed, our findings further suggest that ɛ4 status might be crucial to a clear assessment of the relationship between SCC and AD risk because only ɛ4 carriers had poor awareness of their functioning. It is also noteworthy that despite their elevated risk, only about half of all ɛ4 carriers go on to develop AD [96]. Yet, since those who do develop AD exhibit an accelerated rate of decline [10, 41, 42], an early index of the degree of risk and need for early intervention could help to focus early assessment efforts on those most at risk, rather than attempting to indiscriminately evaluate all ɛ4 carriers. Thus, early impairment of awareness in ɛ4 carriers could possibly serve as a precursor sign for early assessment of amyloid and tau burden.
Our results are consistent with anosognosia [97, 98], rather than hypernosognosia [34–36], as a potential early indicator of future cognitive decline in high risk older adults who are currently cognitively intact. Yet, Cacciamani et al. [97] reported that although cognitively intact older adults with elevated amyloid burden had anosognosia, there was no difference in cognitive awareness between APOE ɛ4 carriers and noncarriers. Notably, while we used a self-appraisal of awareness, Cacciamani and colleagues operationalized awareness using the discrepancy between self- and informant appraisals, as a number of studies have suggested that informant appraisals may be more sensitive than self-appraisals [99–102]. However, a number of cross-sectional studies have also shown that self-report is as effective as informant reports when there is little actual impairment [103, 104], as is the case with our study. Indeed a review and meta-analysis concluded that self-reports may be better at indexing function prior to the transition to dementia, while informant reports may be better at the more advanced and transitional stage [102]. Thus, we can conclude that the ɛ4 carriers in our sample likely had more impaired awareness than in their sample. Perhaps more importantly, we note that the combination of subjective and objective assessment, as used in our study, might be more sensitive to anosognosia, and therefore to AD risk, than the discrepancy between two purely subjective assessments.
It is important to acknowledge that given the cognitively intact status of the participants and the cross-sectional design of the current study, it is not known which of these participants will ultimately exhibit cognitive decline or convert to MCI or AD, or whether they have elevated amyloid burden. However, the elevated risk for AD in the ɛ4 carriers is well established [39, 40, 105, 106]. Thus, longitudinal data that includes assessment prior to and after onset of symptoms would be helpful toward confirming the value of the assessment of subjective executive dysfunction to early AD prediction given the non-specific nature of SCC [107, 108]. Furthermore, consideration of life course factors, particularly those associated with cognitive reserve, will provide a more comprehensive understanding of variability in the trajectories of those with SCC. Menopause and estrogen are also potentially influential factors that were not considered in the current study. Additionally, we relied on a global subjective executive functioning score rather than perceived dysfunction related to individual processes of executive functioning. Synchronized assessment of both subjective and objective executive subprocesses might be even more illuminating, possibly with differential findings across executive subprocesses. Lastly, this study had a relatively small sample and did not assess the underlying neural mechanisms that might be responsible for subjective concerns or that might correlate earlier and more strongly with subjective dysfunction than objective testing. Understanding the relevant neural mechanisms may also better explain differences in awareness observed in cognitively intact older adults, including underestimation and overestimation of cognitive abilities.
In conclusion, the findings of the current study add to the small but growing body of literature on awareness of cognitive functioning, and specifically in executive functioning, in cognitively normal older adults who have elevated risk for AD. Our findings suggest that combined self-appraisal of executive functioning used alongside objective neurocognitive assessment may be helpful toward early identification of impending cognitive decline. This may be particularly valuable and important to discerning who amongst APOE ɛ4 carriers have substantively elevated risk for AD and thus may be more likely to exhibit future cognitive decline. Moreover, the current findings suggest that the assessment of SEF complements objective neurocognitive assessment of executive functioning and, taken with neuroimaging and other biomarkers, has potential to improve prediction of future cognitive decline while effective intervention is still possible.
CRediT AUTHOR STATEMENT
Sarah A. Evans (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing –original draft; Writing –review & editing); Elizabeth R. Paitel (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing –original draft; Writing –review & editing); Riya Bhasin (Data curation; Investigation; Writing –original draft; Writing –review & editing); Kristy A. Nielson (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing –original draft; Writing –review & editing).
ACKNOWLEDGMENTS
The authors wish to acknowledge the contributions of Katherine Reiter and Stephanie Potts toward portions of the data collection for this study.
FUNDING
This research was supported in part by a Way Klingler Sabbatical Research Fellowship from the Office of the Provost at Marquette University (KAN); a private contribution from Thomas J. Salentine to the Aging, Imaging and Memory Lab at Marquette University (KAN, Director); the National Science Foundation (under grant number NSF-1854158 KAN); any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation), and the National Institutes of Health (via the Clinical Translational Science Institute of SE Wisconsin, 8UL1TR000055, sub-5UL1RR031973-05 (KAN); the study contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230166.
REFERENCES
[1] | Hedden T , Gabrieli JDE ((2004) ) Insights into the ageing mind: A view from cognitive neuroscience., Nat Rev Neurosci 5: , 87–96. |
[2] | National Research Council (US) Committee on Future Directions for Cognitive Research on Aging (2000) The Aging Mind: Opportunities in Cognitive Research, Stern PC, Carstensen LL, eds. National Academies Press (US), Washington, DC. |
[3] | Petersen RC ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[4] | Reisberg B , Prichep L , Mosconi L , John ER , Glodzik-Sobanska L , Boksay I , Monteiro I , Torossian C , Vedvyas A , Ashraf N , Jamil IA , de Leon MJ ((2008) ) The pre–mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease., Alzheimers Dement 4: , S98–S108. |
[5] | Brigola AG , Manzini CSS , Oliveira GBS , Ottaviani AC , Sako MP , Vale FAC ((2015) ) Subjective memory complaints associated with depression and cognitive impairment in the elderly: A systematic review. Dement Neuropsychol 9: , 51–57. |
[6] | Buckley RF , Maruff P , Ames D , Bourgeat P , Martins RN , Masters CL , Rainey-smith S , Lautenschlager N , Rowe CC , Savage G , Villemagne VL , Ellis KA ((2016) ) Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimers Dement 12: , 796–804. |
[7] | Mitchell AJ , Beaumont H , Ferguson D , Yadegarfar M , Stubbs B ((2014) ) Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand 130: , 439–451. |
[8] | Cabeza R , Anderson ND , Locantore JK , McIntosh AR ((2002) ) Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage 17: , 1394–1402. |
[9] | Reisberg B , Shulman MB , Torossian C , Leng L , Zhu W ((2013) ) Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 6: , 1–27. |
[10] | Insel PS , Weiner M , Mackin RS , Mormino E , Lim YY , Stomrud E , Palmqvist S , Masters CL , Maruff PT , Hansson O ((2019) ) Determining clinically meaningful decline in preclinical Alzheimer disease., Neurology 93: , e322–e333. |
[11] | Rostamzadeh A , Bohr L , Wagner M , Baethge C , Jessen F ((2022) ) Progression of subjective cognitive decline to MCI or dementia in relation to biomarkers for Alzheimer disease: A meta-analysis., Neurology 99: , e1866–e1874. |
[12] | van der Flier WM , van Buchem MA , Weverling-Rijnsburger AWE , Mutsaers ER , Bollen ELEM , Admiraal-Behloul F , Westendorp RGJ , Middelkoop HAM ((2004) ) Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol 251: , 671–675. |
[13] | Amariglio RE , Becker JA , Carmasin J , Wadsworth LP , Lorius N , Sullivan C , Maye JE , Gidicsin C , Pepin LC , Sperling RA ((2012) ) Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50: , 2880–2886. |
[14] | Perrotin A , Mormino EC , Madison CM , Hayenga AO , Jagust WJ ((2012) ) Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol 69: , 223–229. |
[15] | Rowe CC , Ellis KA , Rimajova M , Bourgeat P , Pike KE , Jones G , Fripp J , Tochon-Danguy H , Morandeau L , O’Keefe G ((2010) ) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31: , 1275–1283. |
[16] | Chipi E , Bellomo G , Salvadori N , Montanucci C , Gaetani L , Paoletti FP , Parnetti L ((2023) ) Association between neuropsychological performance and CSF profile in subjective cognitive decline: Towards the diagnosis of preclinical AD. J Prev Alzheimers Dis 10: , 523–529. |
[17] | Chen X , Farrell ME , Rundle MM , Chan MY , Moore W , Wig GS , Park DC ((2021) ) The relationship of functional hippocampal activity, amyloid deposition, and longitudinal memory decline to memory complaints in cognitively healthy older adults. Neurobiol Aging 105: , 318–326. |
[18] | Wen C , Bi Y-L , Hu H , Huang S-Y , Ma Y-H , Hu H-Y , Tan L , Yu J-T ((2022) ) Association of subjective cognitive decline with cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: The CABLE study. J Alzheimers Dis 85: , 1143–1151. |
[19] | Wolfsgruber S , Kleineidam L , Guski J , Polcher A , Frommann I , Roeske S , Spruth EJ , Franke C , Priller J , Kilimann I ((2020) ) Minor neuropsychological deficits in patients with subjective cognitive decline., Neurology 95: , e1134–e1143. |
[20] | Sánchez-Benavides G , Suárez-Calvet M , Milà-Alomà M , Arenaza-Urquijo EM , Grau-Rivera O , Operto G , Gispert JD , Vilor-Tejedor N , Sala-Vila A , Crous-Bou M ((2021) ) Amyloid-βpositive individuals with subjective cognitive decline presentincreased CSF neurofilament light levels that relate to lowerhippocampal volume. Neurobiol Aging 104: , 24–31. |
[21] | Hedden T , Oh H , Younger AP , Patel TA ((2013) ) Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 80: , 1341–1348. |
[22] | Lista S , Molinuevo JL , Cavedo E , Rami L , Amouyel P , Teipel SJ , Garaci F , Toschi N , Habert M-O , Blennow K ((2015) ) Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline., J Alzheimers Dis 48: , S171–S191. |
[23] | Burmester B , Leathem J , Merrick P ((2016) ) Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol Rev 26: , 376–393. |
[24] | Crumley JJ , Stetler CA , Horhota M ((2014) ) Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychol Aging 29: , 250. |
[25] | Morrison C , Oliver MD ((2023) ) Subjective cognitive decline is associated with lower baseline cognition and increased rate of cognitive decline. J Gerontol B Psychol Sci Soc Sci 78: , 573–584. |
[26] | Marrero-Polegre D , Finke K , Roaschio N , Haupt M , Reyes-Moreno C , Ruiz-Rizzo AL ((2023) ) Lower visual processing speed relates to greater subjective cognitive complaints in community-dwelling healthy older adults. Front Psychiatry 14: , 1063151. |
[27] | Blackburn DJ , Wakefield S , Shanks MF , Harkness K , Reuber M , Venneri A ((2014) ) Memory difficulties are not always a sign of incipient dementia: A review of the possible causes of loss of memory efficiency. Br Med Bull 112: , 71–81. |
[28] | Gerretsen P , Chung JK , Shah P , Plitman E , Iwata Y , Caravaggio F , Nakajima S , Pollock BG , Graff-Guerrero A ((2017) ) Anosognosia is an independent predictor of conversion from mild cognitive impairment to Alzheimer’s disease and is associated with reduced brain metabolism. J Clin Psychiatry 78: , 805–805. |
[29] | Mak E , Chin R , Ng LT , Yeo D , Hameed S ((2015) ) Clinical associations of anosognosia in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry 30: , 1207–1214. |
[30] | Orfei MD , Blundo C , Celia E , Casini AR , Caltagirone C , Spalletta G , Varsi AE ((2010) ) Anosognosia in mild cognitive impairment and mild Alzheimer’s disease: Frequency and neuropsychological correlates. Am J Geriatr Psychiatry 18: , 1133–1140. |
[31] | Ries ML , Jabbar BM , Schmitz TW , Trivedi MA , Gleason CE , Carlsson CM , Rowley HA , Asthana S , Johnson SC ((2007) ) Anosognosia in mild cognitive impairment: Relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc 13: , 450–461. |
[32] | Senturk G , Bilgic B , Arslan AB , Bayram A , Hanagasi H , Gurvit H , Emre M ((2017) ) Cognitive and anatomical correlates of anosognosia in amnestic mild cognitive impairment and early-stage Alzheimer’s disease. Int Psychogeriatr 29: , 293–302. |
[33] | Tremont G , Alosco ML ((2011) ) Relationship between cognition and awareness of deficit in mild cognitive impairment. Int J Geriatr Psychiatry 26: , 299–306. |
[34] | Roberts JL , Clare L , Woods RT ((2009) ) Subjective memory complaints and awareness of memory functioning in mild cognitive impairment: A systematic review. Dement Geriatr Cogn Disord 28: , 95–109. |
[35] | Vannini P , Amariglio R , Hanseeuw B , Johnson KA , McLaren DG , Chhatwal J , Pascual-Leone A , Rentz D , Sperling RA ((2017) ) Memory self-awareness in the preclinical and prodromal stages of Alzheimer’s disease. Neuropsychologia 99: , 343–349. |
[36] | Vannini P , Hanseeuw B , Munro CE , Amariglio RE , Marshall GA , Rentz DM , Pascual-Leone A , Johnson KA , Sperling RA ((2017) ) Anosognosia for memory deficits in mild cognitive impairment: Insight into the neural mechanism using functional and molecular imaging. Neuroimage Clin 15: , 408–414. |
[37] | Sims JR , Zimmer JA , Evans CD , Lu M , Ardayfio P , Sparks J , Wessels AM , Shcherbinin S , Wang H , Nery ESM ((2023) ) Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330: , 512–527. |
[38] | Alzheimer’s Association ((2023) ) 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 19: , 1598–1695. |
[39] | Farrer LA , Cupples LA , Haines JL , Hyman B , Kukull WA , Mayeux R , Myers RH , Pericak-Vance MA , Risch N , Van Duijn CM ((1997) ) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA 278: , 1349–1356. |
[40] | Saunders AM , Strittmatter WJ , Schmechel D , George-Hyslop PHS , Pericak-Vance MA , Joo SH , Rosi BL , Gusella JF , Crapper-MacLachlanDR , Alberts MJ ((1993) ) Association of apolipoprotein E alleleɛ4 with late-onset familial and sporadic Alzheimer’sdisease. Neurology 43: , 1467–1467. |
[41] | Sperling RA , Donohue MC , Raman R , Sun C-K , Yaari R , Holdridge K , Siemers E , Johnson KA , Aisen PS , Team AS ((2020) ) Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol 77: , 735–745. |
[42] | Rodrigue K , Kennedy K , Devous M , Rieck J , Hebrank A , Diaz-Arrastia R , Mathews D , Park D β-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology ((2012) ) 78: , 387–395. |
[43] | Zwan MD , Villemagne VL , Doré V , Buckley R , Bourgeat P , Veljanoski R , Salvado O , Williams R , Margison L , Rembach A ((2016) ) Subjective memory complaints in APOE ɛ4 carriers areassociated with high amyloid-β burden. J AlzheimersDis 49: , 1115–1122. |
[44] | Ali JI , Smart CM , Gawryluk JR ((2018) ) Subjective cognitive declineand APOE ɛ4: A systematic review. J AlzheimersDis 65: , 303–320. |
[45] | Müller-Gerards D , Weimar C , Abramowski J , Tebrügge S , Jokisch M , Dragano N , Erbel R , Jöckel K-H , Moebus S , Winkler A ((2019) ) Subjective cognitive decline, APOE ɛ4, and incident mild cognitive impairment in men and women. Alzheimers Dement 11: , 221–230. |
[46] | Elverman KH , Paitel ER , Figueroa CM , McKindles RJ , Nielson KA ((2021) ) Event-related potentials, inhibition, and risk for Alzheimer’s disease among cognitively intact elders. J Alzheimers Dis 80: , 1413–1428. |
[47] | Paitel ER , Nielson KA ((2023) ) Cerebellar EEG source localizationreveals age-related compensatory activity moderated by genetic riskfor Alzheimer’s disease., Psychophysiology 60: , e14395. |
[48] | Kelly DA , Seidenberg M , Reiter K , Nielson KA , Woodard JL , Smith JC , Durgerian S , Rao SM ((2018) ) Differential 5-year brain atrophy rates in cognitively declining and stable APOE-ɛ4 elders. Neuropsychology 32: , 647–653. |
[49] | Reiter K , Nielson KA , Durgerian S , Woodard JL , Smith JC , Seidenberg M , Kelly DA , Rao SM ((2017) ) Five-year longitudinal brain volume change in healthy elders at genetic risk for Alzheimer’s disease. J Alzheimers Dis 55: , 1363–1377. |
[50] | Rao SM , Bonner-Jackson A , Nielson KA , Seidenberg M , Smith JC , Woodard JL , Durgerian S ((2015) ) Genetic risk for Alzheimer’s disease alters the five-year trajectory of semantic memory activation in cognitively intact elders. Neuroimage 111: , 136–146. |
[51] | Sugarman MA , Woodard JL , Nielson KA , Seidenberg M , Smith JC , Durgerian S , Rao SM ((2012) ) Functional magnetic resonance imaging of semantic memory as a presymptomatic biomarker of Alzheimer’s disease risk. Biochim Biophys Acta Mol Basis Dis 1822: , 442–456. |
[52] | Correa DD , Graves RE , Costa L ((1996) ) Awareness of memory deficit in Alzheimer’s disease patients and memory-impaired older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 3: , 215–228. |
[53] | Lacerda IB , Sousa MFB , Santos RL , Nogueira MML , Dourado MCN ((2016) ) Concepts and objects of awareness in Alzheimer’s disease: An updated systematic review. J Bras Psiquiatr 65: , 99–109. |
[54] | Chen P , Ratcliff G , Belle SH , Cauley JA , DeKosky ST , Ganguli M ((2001) ) Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. JAMA Psychiatry 58: , 853–858. |
[55] | Hazlett KE , Figueroa CM , Nielson KA ((2015) ) Executive functioning and risk for Alzheimer’s disease in the cognitively intact: Family history predicts Wisconsin Card Sorting Test performance. Neuropsychology 29: , 582–582. |
[56] | Elliott R ((2003) ) Executive functions and their disorders: Imaging in clinical neuroscience. Br Med Bull 65: , 49–59. |
[57] | Miyake A , Friedman NP , Emerson MJ , Witzki AH , Howerter A , Wager TD ((2000) ) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol 41: , 49–100. |
[58] | Smith EE , Jonides J ((1999) ) Storage and executive processes in the frontal lobes. Science 283: , 1657–1661. |
[59] | Chayer C , Freedman M ((2001) ) Frontal lobe functions. Curr Neurol Neurosci Rep 1: , 547–552. |
[60] | Fuster JM ((2000) ) Executive frontal functions. Exp Brain Res 133: , 66–70. |
[61] | Grober E , Hall CB , Lipton RB , Zonderman AB , Resnick SM , Kawas C ((2008) ) Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc 14: , 266–278. |
[62] | Paitel ER , Samii MR , Nielson KA ((2021) ) A systematic review of cognitive event-related potentials in mild cognitive impairment and Alzheimer’s disease. Behav Brain Res 396: , 112904. |
[63] | Paitel ER , Nielson KA (2021) Temporal dynamics of event-related potentials during inhibitory control characterize age-related neural compensation. Symmetry 13, 2323. |
[64] | Jester DJ , Vyhnálek M , Andel R , Marková H , Nikolai T , Laczó J , Matusková V , Cechová K , Sheardova K , Hort J ((2022) ) Progression from subjective cognitive decline to mildcognitive impairment or dementia: The role of baseline cognitiveperformance. J Alzheimers Dis 86: , 1763–1774. |
[65] | Saunders NLJ , Summers MJ ((2010) ) Attention and working memory deficits in mild cognitive impairment. J Clin Exp Neuropsychol 32: , 350–357. |
[66] | Kim WH , Kim BS , Chang SM , Lee DW , Bae JN ((2020) ) Relationship between subjective memory complaint and executive function in a community sample of South Korean elderly. Psychogeriatrics 20: , 850–857. |
[67] | Seo EH , Kim H , Lee KH , Choo IL ((2016) ) Altered executive function in pre-mild cognitive impairment. J Alzheimers Dis 54: , 933–940. |
[68] | Valech N , Tort-Merino A , Coll-Padrós N , Olives J , Leon M , Rami L , Molinuevo JL ((2018) ) Executive and language subjective cognitive decline complaints discriminate preclinical Alzheimer’s disease from normal aging. J Alzheimers Dis 61: , 689–703. |
[69] | Viviano RP , Hayes JM , Pruitt PJ , Fernandez ZJ , van Rooden S , van der Grond J , Rombouts SARB , Damoiseaux JS ((2019) ) Aberrant memory system connectivity and working memory performance in subjective cognitive decline. Neuroimage 185: , 556–564. |
[70] | Pérez-Cordón A , Monté-Rubio G , Sanabria A , Rodriguez-Gomez O , Valero S , Abdelnour C , Marquié M , Espinosa A , Ortega G , Hernandez I ((2020) ) Subtle executive deficits are associated with higher brain amyloid burden and lower cortical volume in subjective cognitive decline: The FACEHBI cohort. Sci Rep 10: , 17721. |
[71] | van Harten AC , Smits LL , Teunissen CE , Visser PJ , Koene T , Blankenstein MA , Scheltens P , van der Flier WM ((2013) ) Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology 81: , 1409–1416. |
[72] | Webster-Cordero F , Giménez-Llort L ((2022) ) The challenge ofsubjective cognitive complaints and executive functions inmiddle-aged adults as a preclinical stage of dementia: A systematicreview. Geriatrics 7: , 30. |
[73] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[74] | Jurica PJ , Leitten CL , Mattis S (1988) DRS-2 dementia rating scale-2: Professional manual, Psychological Assessment Resources. |
[75] | Grace J , Malloy PH (2001) Frontal systems behavior scale (FrSBe): Professional manual, Psychological Assessment Resources (PAR). |
[76] | Yesavage JA ((1988) ) Geriatric depression scale. Psychopharmacol Bull 24: , 709–711. |
[77] | Beck AT , Steer RA (1993) Beck Anxiety Inventory. Psychological Corporation, San Antonio, TX. |
[78] | Beck AT , Epstein N , Brown G , Steer RA ((1988) ) An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56: , 893–893. |
[79] | Reitan RM , Wolfson D (1993) The Halstead-Reitan neuropsychology battery: Theory and clinical interpretation, 2nd edition. Neuropsychology Press, Tucson, AZ. |
[80] | Varjacic A , Mantini D , Demeyere N , Gillebert CR ((2018) ) Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 115: , 78–87. |
[81] | Smith A (1982) Symbol Digit Modalities Test. Western Psychological Services, Los Angeles, CA. |
[82] | Schmidt M (1996) Rey auditory verbal learning test: A handbook.Western Psychological Services, Los Angeles, CA. |
[83] | Lezak MD , Howieson DB , Loring DW , Fischer JS (2004) Neuropsychological assessment. Oxford University Press, USA. |
[84] | Strauss E , Sherman EMS , Spreen O (2006) A compendium of neuropsychological tests: Administration, norms, and commentary. American Chemical Society. |
[85] | Whiteside DM , Kealey T , Semla M , Luu H , Rice L , Basso MR , Roper B ((2016) ) Verbal fluency: Language or executive function measure? , Appl Neuropsychol Adult 23: , 29–34. |
[86] | Lafleche G , Albert MS ((1995) ) Executive function deficits in mild Alzheimer’s disease. Neuropsychology 9: , 313. |
[87] | Benton AL , Hamsher Kd , Sivan AB (1976) Multilingual Aphasia Examination. University of Iowa, Iowa City. |
[88] | Gladsjo JA , Schuman CC , Evans JD , Peavy GM , Miller SW , Heaton RK ((1999) ) Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment 6: , 147–178. |
[89] | Heaton RK (2004) Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual. Psychological Assessment Resources. |
[90] | Goodglass H , Kaplan E , Weintraub S (1983) Boston naming test. Lea & Febiger. |
[91] | Hixson JE , Vernier DT ((1990) ) Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 31: , 545–548. |
[92] | Hayes AF (2022) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press. |
[93] | Reid LM , Maclullich MJ ((2006) ) Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord 22: , 471–485. |
[94] | Amariglio RE , Buckley RF , Mormino EC , Marshall GA , Johnson KA , Rentz DM , Sperling RA ((2018) ) Amyloid-associated increases in longitudinal report of subjective cognitive complaints. Alzheimers Dement 4: , 444–449. |
[95] | Clark LR , Schiehser DM , Weissberger GH , Salmon DP , Delis DC , Bondi MW ((2012) ) Specific measures of executive function predict cognitive decline in older adults. J Int Neuropsychol Soc 18: , 118–127. |
[96] | Landau S , Harvey D , Madison C , Reiman E , Foster N , Aisen P , Petersen RC , Shaw L , Trojanowski J , Jack C ((2010) ) Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75: , 230–238. |
[97] | Cacciamani F , Sambati L , Houot M , Habert M-O , Dubois B , Epelbaum S ((2020) ) Awareness of cognitive decline trajectories in asymptomatic individuals at risk for AD. Alzheimers Res Ther 12: , 129. |
[98] | Cacciamani F , Tandetnik C , Gagliardi G , Bertin H , Habert M-O , Hampel H , Boukadida L , Révillon M , Epelbaum S , Dubois B ((2017) ) Lowcognitive awareness, but not complaint, is a good marker ofpreclinical Alzheimer’s disease. J Alzheimers Dis 59: , 753–762. |
[99] | Bellaali Y , Woodard JL , Hanseeuw B , Ivanoiu A ((2021) ) Spouse-appraised memory functioning predicts memory decline better than subjective memory complaints in community dwelling older adults at genetic risk for Alzheimer’s disease. Front Psychiatry 12: , 167–167. |
[100] | Kuhn E , Perrotin A , La Joie R , Touron E , Dautricourt S , Vanhoutte M , Vivien D , de La Sayette V , Chételat G , Alzheimer’s DiseaseNeuroimaging Initiative ((2023) ) Association of the informant-reportedmemory decline with cognitive and brain deterioration through theAlzheimer clinical continuum., Neurology 100: , e2454–e2465. |
[101] | Munro CE , Buckley R , Vannini P , DeMuro C , Sperling R , Rentz DM , Johnson K , Gatchel JR , Amariglio R ((2022) ) Longitudinal trajectories of participant-and study partner-rated cognitive decline, in relation to Alzheimer’s disease biomarkers and mood symptoms. Front Aging Neurosci 13: , 806432. |
[102] | Perez-Blanco L , Felpete A , Patten SB , Mallo SC , Pereiro AX , Campos-Magdaleno M , Juncos-Rabadan O ((2022) ) Do informant-reported subjective cognitive complaints predict progression to mild cognitive impairment and dementia better than self-reported complaints in old adults? A meta-analytical study. Ageing Res Rev 82: , 101772. |
[103] | Buckley R , Saling M , Ellis K , Rowe C , Maruff P , Macaulay LS , Martins R , Masters C , Savage G , Rainey-Smith S ((2015) ) Self and informant memory concerns align in healthy memory complainers and in early stages of mild cognitive impairment but separate with increasing cognitive impairment. Age Ageing 44: , 1012–1019. |
[104] | Rueda AD , Lau KM , Saito N , Harvey D , Risacher SL , Aisen PS , Petersen RC , Saykin AJ , Farias ST , Initiative AsDN ((2015) ) Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimers Dement 11: , 1080–1089. |
[105] | Schmechel DE , Saunders AM , Strittmatter WJ , Crain BJ , Hulette CM , Joo SH , Pericak-Vance MA , Goldgaber D , Roses AD ((1993) ) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 90: , 9649–9653. |
[106] | Strittmatter WJ , Saunders AM , Schmechel D , Pericak-Vance M , Enghild J , Salvesen GS , Roses AD ((1993) ) Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90: , 1977–1981. |
[107] | Jester DJ , Andel R , Cechová K , Laczó J , Lerch O , Marková H , Nikolai T , Vyhnálek M , Hort J ((2021) ) Cognitive phenotypes ofolder adults with subjective cognitive decline and amnestic mildcognitive impairment: The Czech Brain Aging Study. J IntNeuropsychol Soc 27: , 329–342. |
[108] | Machulda MM , Lundt ES , Albertson SM , Kremers WK , Mielke MM , Knopman DS , Bondi MW , Petersen RC ((2019) ) Neuropsychological subtypes of incident mild cognitive impairment in the Mayo Clinic Study of Aging. Alzheimers Dement 15: , 878–887. |




