Diffusion Tensor Imaging Along Perivascular Spaces (DTI-ALPS) to Assess Effects of Age, Sex, and Head Size on Interstitial Fluid Dynamics in Healthy Subjects
Abstract
Diffusion tensor imaging along perivascular spaces (DTI-ALPS) is a novel MRI method for assessing brain interstitial fluid dynamics, potentially indexing glymphatic function. Failed glymphatic clearance is implicated in Alzheimer’s disease (AD) pathophysiology. We assessed the contribution of age and female sex (strong AD risk factors) to DTI-ALPS index in healthy subjects. We also for the first time assessed the effect of head size. In accord with prior studies, we show reduced DTI-ALPS index with aging, and in men compared to women. However, head size may be a major contributing factor to this counterintuitive sex difference.
INTRODUCTION
During normal brain functioning, neurons generate metabolic waste that must be cleared. The brain has an important fluid drainage system that involves the movement of cerebrospinal fluid (CSF) and interstitial fluid (ISF) through astrocytic water channels along paravascular spaces termed the glymphatic system [1, 2]. The paravascular glymphatic network of channels is where ISF containing neuronal waste products can mix with fresh CSF produced by the choroid plexus and be washed out of the brain. The glymphatic system is especially important for removing the waste protein amyloid-β (Aβ) from the brain, and glymphatic impairment has been hypothesized to be a key pathophysiological mechanism causing the buildup of Aβ plaques in Alzheimer’s disease (AD) [1, 3]. Glymphatic functioning decreases with aging, and this is considered to predispose to age-related diseases like AD [1]. Mechanisms for decreased glymphatic functioning with aging are poorly understood but based on studies in animal models, posited to relate to anatomical and functional defects such as decreased cerebrovascular pulsatility [4] (a driving force for paravascular clearance), reduced CSF production [5] and depolarization of astrocytic aquaporin-4 channels [1], which are a key anatomic component of the glymphatic system.
A recently developed technique called diffusion tensor image analysis along the perivascular space (DTI-ALPS) has been proposed to assess glymphatic system activity in vivo in humans [6]. Several prior studies have demonstrated reduced ALPS index in AD and other neurodegenerative disorders [7–12].
In this study, we apply DTI-ALPS to assess the effects of age and sex on interstitial fluid dynamics in a sample of healthy subjects (n = 49). Based on prior studies in humans [13–15] and animal models [1], we hypothesized that the ALPS index, a putative measure of glymphatic function, would correlate inversely with age in healthy subjects. Sex differences in glymphatic function have received little study. One rodent study found no sex difference [16] but two recent human studies found higher ALPS index in women than men [14, 17]. Understanding possible sex differences in glymphatic function could be relevant to why AD is significantly more common in women than men [18]. In addition to age and sex, we assess a possible association of head size with ALPS index.
MATERIALS AND METHODS
Subjects
All subjects provided written informed consent prior to participation, and all study activities were approved by Weill Cornell Medicine’s Institutional Review Board. This was a convenience sample of healthy subjects (n = 49) recruited through advertising to serve as controls in several different ongoing studies at Weill Cornell Medicine. All subjects were free from significant medical, psychiatric, neurologic or substance use disorder. Normal cognition was confirmed as follows: the majority of subjects (35/49) were enrolled in studies focused on aging and AD and were assessed using the National Alzheimer’s Coordinating Center (NACC) Uniform Dataset [19]. These subjects were assigned a diagnosis of “normal cognition” by a board-certified neurologist based on interview, exam and review of clinical data and neuropsychological testing results. The remaining subjects (14/49) were controls in a study of Traumatic Brain Injury. These subjects underwent a detailed screening interview and a multidomain neuropsychological testing battery that included the California Verbal Learning Test II (CVLT) [20] of memory as well as tests of attention and executive function. Raw neuropsychological test scores were converted to standardized scores adjusting for age, and education when available, using established normative data. A clinical neuropsychologist reviewed all participant scores and confirmed the absence of clinically significant cognitive impairment (i.e., 2 or more “extremely low” scores in a single domain based on the classification system of Guilmette et al. [21]). In particular, it was confirmed that all subjects had normal memory function (i.e., normal performance on the CVLT-II delayed recall retention, recall discriminability, and recognition discriminability).
Image acquisition
Multi-shell DTI was acquired on a 3T Siemens Prisma scanner with a single-shot spin-echo echo-planar pulse sequence, with 98 directions, TR/TE = 3230/89.20 ms, flip angle = 78°, FOV = 21×21 cm, matrix size = 140×140, voxel size = 1.5×1.5×1.5 mm, 92 axial slices, 3 b-values = 0, 1500, and 300 0 s/mm2, multiband factor = 6. Each DTI scan was acquired with an opposite phase encoding direction for geometric distortion correction. T1w used for co-registration and segmentation was acquired using MPRAGE sequence with TR/TE = 2400/2.96 ms, flip angle = 9°, FOV = 25.6×25.6 cm, matrix size = 256×256, 208 sagittal slices, voxel size = 0.5×0.5×0.5 mm.
Image processing
DTI-ALPS takes advantage of the known, orthogonal orientation of white matter tracts and blood vessels in deep white matter to isolate and quantify fluid diffusivity in the PVS surrounding deep medullary veins as an ALPS index. DTI-ALPS is based on the diffusion tensor (D) which is a mathematical model that describes the diffusion of water molecules in biological tissues, fractional anisotropy (FA) which quantifies the degree of directionality in this diffusion, and eigenvectors (V1, V2, V3) which describe the orientation of the underlying brain structures such as nerve fibers and vessels. Figure 1 is an illustration of the method. To calculate the ALPS index, diffusion images were first preprocessed to correct motion artifacts, eddy currents, distortions, and head movement using standard methods [22]. Using FSL dtifit command on the preprocessed DTI data, D, FA, and eigenvectors were obtained. FA maps were color-coded into RGB format. Using this color-coded FA map overlayed on anatomic images, bilateral 5 mm square regions of interest (ROIs) were manually placed on pure blue (projection) and pure green (association) fibers adjacent to ventricle on axial slices, avoiding any lesions apparent on anatomic images (Fig. 2). Diffusion directions were defined as: x = right-left; y = anterior-posterior, and z = inferior-superior. Mean x-, y-, and z-axis diffusivity within each ROI were measured and used to generate a left and right ALPS index for each subject. ALPS index is the ratio of the mean of the x-axis diffusivity in the projection area and the x-axis diffusivity in the association area to the mean of the y-axis diffusivity in the projection area and the z-axis diffusivity in the association area. Left and right ALPS indices were averaged, generating one value per subject. Lower ALPS index reflects reduced glymphatic function.
Fig. 1
A simplified drawing of DTI-ALPS. Projection fibers (blue), association fibers (green), and subcortical fibers (red) are presented along z-, y-, and x-axis, respectively. PVS is orthogonal to both projection and association fibers. (Adapted from [6]).
![A simplified drawing of DTI-ALPS. Projection fibers (blue), association fibers (green), and subcortical fibers (red) are presented along z-, y-, and x-axis, respectively. PVS is orthogonal to both projection and association fibers. (Adapted from [6]).](https://content.iospress.com:443/media/adr/2024/8-1/adr-8-1-adr230143/adr-8-adr230143-g001.jpg)
Fig. 2
Schematic diagram of image acquisition, processing, and measurement of DTI-ALPS index.
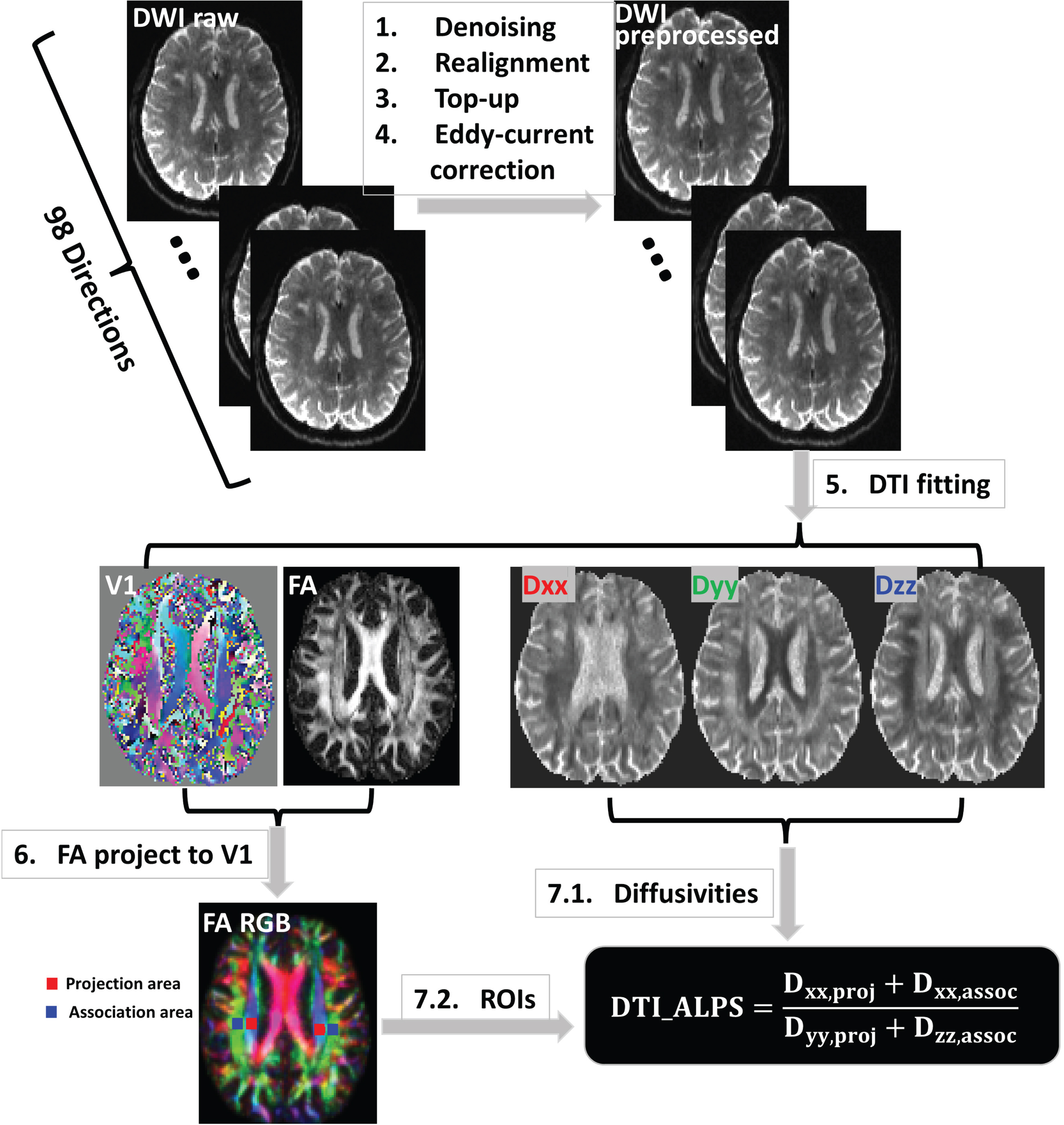
Each subject’s intracranial volume (ICV) was estimated on T1w using SPM’s three region (gray matter, white matter, and CSF) segmentation.
Statistical analyses
Analyses were performed in RStudio version 2002.07.2. Differences in age and ICV between men and women were assessed using 2-tailed t test. Linear regression was used to evaluate the contribution of age and sex to the ALPS index. The analysis was also performed with ICV as an additional variable. All regression assumptions (normality, linearity, homoscedasticity, absence of multicollinearity) were checked. Results were considered significant at p < 0.05.
RESULTS
Subject characteristics are presented in Table 1. There was no significant difference in age between women and men (t = 1.05; p = 0.31).
Table 1
Participant demographics
| Total | Women | Men | |
| Number (%) | 49 (100) | 34 (69.4) | 15 (30.6) |
| Mean age±SD (range) | 66.8±12.8 (23–86) | 68.5±8.0 (47–86) | 62.9±19.7 (23–86) |
A valid, significant regression model (R2 = 0.28, F = 8.95, p = 0.001) showed that ALPS decreased significantly with age (standardized beta [β] = −0.513, p < 0.001) (Fig. 3) and was greater in women than men when accounting for age (β = −0.211, p = 0.04; mean ALPS for women:1.40 [SD = 0.14]; men:1.35 [SD = 0.16]). There was no age by sex interaction (p = 0.63).
Fig. 3
Scatterplot of association between DTI-ALPS index and age.
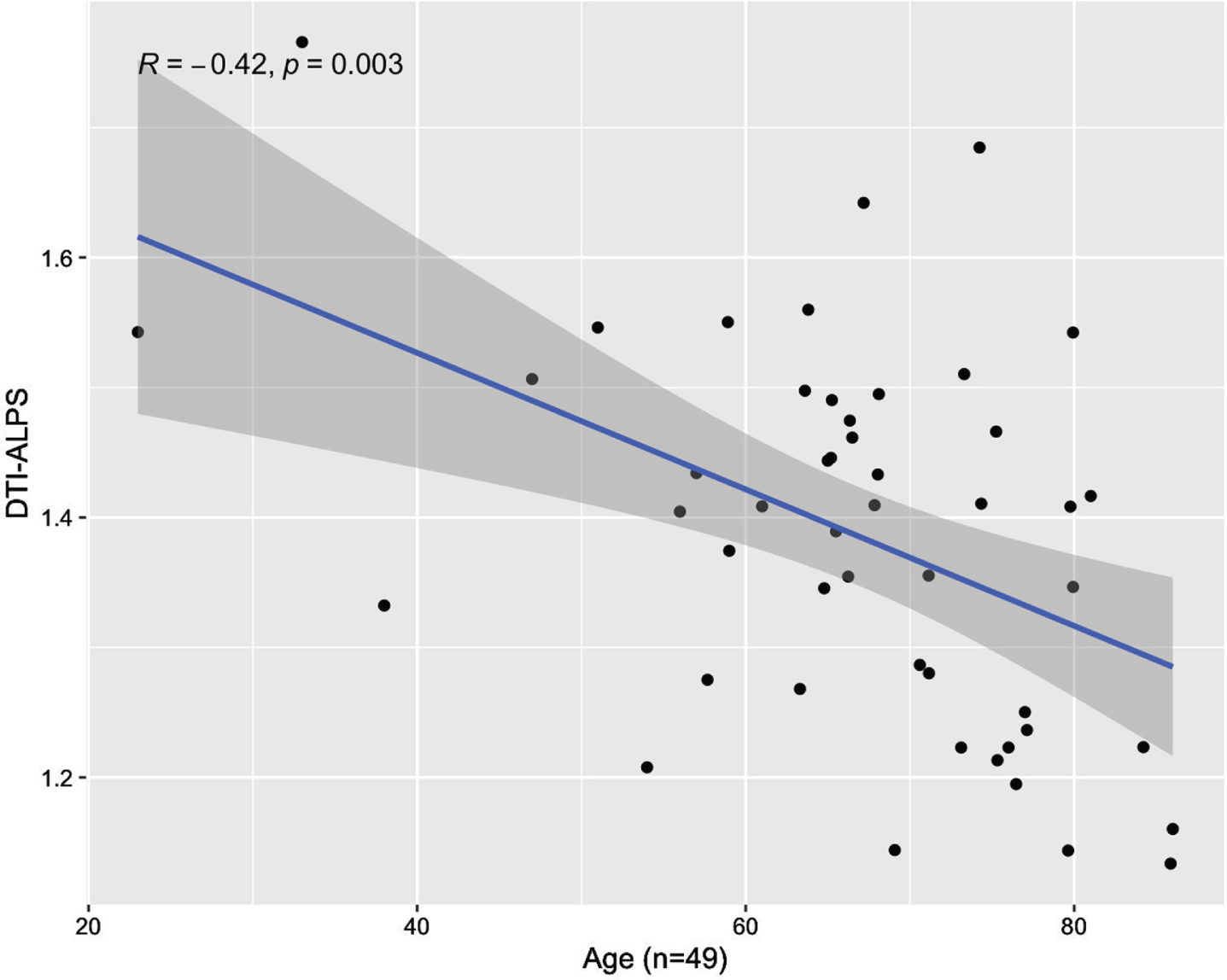
Addressing potential confounds related to the extremely wide age range of subjects, results were similar when limiting analysis to subjects over age 50 (n = 45; age: β = −0.342, p = 0.017; sex: β = −2.109, p = 0.041).
ICV was significantly greater in men than women, as expected (mean ICV in women: 1.430 L [SD 0.101]; men: 1.619 L [SD 0.125]; t = 5.59, p < 0.001). When ICV was added to the regression model predicting ALPS, the effect of age was unchanged (β = −0.512, p < 0.001) but sex was no longer significant (β = −0.266, p = 0.124). The effect of ICV itself was not significant (β = −0.006, p = 0.97).
DISCUSSION
We demonstrate in healthy subjects that the ALPS index inversely correlates with age and is lower in men as compared to women. This is in accord with several prior studies [14, 17]. However, this sex difference was no longer significant when ICV was included in the model. This is a novel finding. Because AD is much more common in women than men [18], and involves failed brain clearance as a key pathophysiological mechanisms, understanding how to accurately measure and interpret sex differences in brain clearance is essential.
Age effect
The glymphatic system plays a crucial role in the clearance of waste products from the brain, and glymphatic function has been shown to decline with aging in animal models [2]. Mechanisms for age-associated glymphatic dysfunction may relate to depolarization of astrocytic aquaporin-4 channels [1], reduced CSF production, decreased cerebrovascular pulsatility [6] and reduced CSF production [7] which is a key driver of fluid flow [23]. Measuring glymphatic function in humans is challenging, but essential because age-related glymphatic dysfunction is considered to predispose to the toxic protein deposition underlying AD and other neurologic disorders. Our demonstration of decreasing ALPS index with increasing age is in accord with other human studies [14, 17] and supports the use of ALPS as a reliable, noninvasive biomarker of glymphatic function that could one-day guide targeted treatments targeted at maintaining glymphatic function in older adults.
Effect of sex and ICV
In our study, women had significantly higher average ALPS index than men when controlling for age. Similar sex differences were demonstrated in two prior studies [14, 17]. However, women are known to have smaller heads than men [24]. This is an important potential confound that has not previously been addressed. When we controlled for ICV, sex differences in the ALPS index were no longer significant. Our results show that considering brain size is necessary when assessing possible sex differences in glymphatic function.
In our study, ICV was not a significant predictor of the ALPS index. A prior, larger study did demonstrate a significant negative correlation between ALPS Index and ICV, though did not explain or suggest potential clinical relevance of this finding [17]. It is possible that head size may be directly relevant to clearance, independent of sex. The distance between the nearest CSF spaces and different parts of brain parenchyma, which depends critically upon brain size, is known to affect fluid clearance efficiency and mechanisms [24]. Because human heads are much larger than rodent heads, greater attention to head and brain size may help resolve controversy concerning cross-species glymphatic differences [24] and facilitate translation of glymphatic knowledge (and, someday, therapies) from rodents to humans.
Limitations
There are several limitations of this study. First, although the ALPS index has been shown to correlate with a more invasive measure of glymphatic function in humans [6], it is based on two small ROIs at the level of the lateral ventricle and assesses diffusivity only along medullary veins, not the whole brain’s glymphatic system, and may be affected by blood flow and/or tissue property effects on DTI measures [25, 26]. More generally, there are many controversies concerning glymphatic function and how to measure it in humans and animals [23].
Uneven sex distribution among subjects is another limitation that could affect the generalizability of findings. The absence of sex differences in the ALPS index when accounting for ICV must be considered a preliminary finding given our small sample size, especially for the males (n = 15).
Conclusion
ALPS index, a diffusion MRI measure of interstitial fluid dynamics considered to reflect glymphatic function, was decreased in association with increasing age in 49 healthy subjects. ALPS index was higher in women than men when controlling for age, but this sex difference was no longer significant when controlling for head size, which was greater in men than women. These results indicate that it is necessary to account for head size when assessing sex differences in glymphatic function. In addition, because preclinical research suggests that head size may be directly relevant to brain fluid clearance efficiency and mechanisms, future studies should assess whether there is an independent contribution of head size to ALPS-measured glymphatic function. Understanding how factors such as age, sex and head size affect glymphatic function in healthy subjects is essential to understanding how glymphatic dysfunction may underly diseases such as AD.
AUTHOR CONTRIBUTIONS
Ilker Ozsahin (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing); Liangdong Zhou (Data curation; Formal analysis; Methodology; Writing – review & editing); Xiuyuan Wang (Formal analysis; Writing – review & editing); Jacob Garetti (Formal analysis; Writing – review & editing); Keith Jamison (Writing – review & editing); Ke Xi (Formal analysis; Writing – review & editing); Emily Tanzi (Data curation; Writing – review & editing); Abhishek Jaywant (Data curation; Writing – review & editing); Abigail Patchell (Data curation; Writing – review & editing); Thomas Maloney (Data curation; Writing – review & editing); Mony J. de Leon (Writing – review & editing); Amy Kuceyeski (Validation; Writing – review & editing); Sudhin A. Shah (Validation; Writing – review & editing); Yi Li (Validation; Writing – review & editing); Tracy A. Butler (Conceptualization; Formal analysis; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing).
ACKNOWLEDGMENTS
We acknowledge Dr. Steven Poulin for help with statistical analyses.
FUNDING
This work was funded by NIH R01NS102646, R01AG057848, RF1AG057570, R56AG058913, and R56NS111052.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
DATA AVAILABILITY
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request, without undue reservation.
REFERENCES
[1] | Kress BT , Iliff JJ , Xia M , Wang M , Wei Bs HS , Zeppenfeld D , Xie L , Hongyi Kang BS , Xu Q , Liew JA , Plog BA , Ding F , PhD RD , Nedergaard M ((2014) ) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: , 845–861. |
[2] | Iliff JJ , Wang M , Liao Y , Plogg BA , Peng W , Gundersen GA , Benveniste H , Vates GE , Deane R , Goldman SA , Nagelhus EA , Nedergaard M ((2012) ) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β.. Sci Transl Med 4: , 147ra111. |
[3] | Tarasoff-Conway JM , Carare RO , Osorio RS , Glodzik L , Butler T , Fieremans E , Axel L , Rusinek H , Nicholson C , Zlokovic BV , Frangione B , Blennow K , Ménard J , Zetterberg H , Wisniewski T , De Leon MJ ((2015) ) Clearance systems in the brain— implications for Alzheimer disease. Nat Rev Neurol 11: , 457–470. |
[4] | Zieman SJ , Melenovsky V , Kass DA ((2005) ) Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: , 932–943. |
[5] | Fleischman D , Berdahl JP , Zaydlarova J , Stinnett S , Fautsch MP , Allingham RR ((2012) ) Cerebrospinal fluid pressure decreases with older age. PLoS One 7: , e52664. |
[6] | Taoka T , Masutani Y , Kawai H , Nakane T , Matsuoka K , Yasuno F , Kishimoto T , Naganawa S ((2017) ) Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 35: , 172–178. |
[7] | Kamagata K , Andica C , Takabayashi K , Saito Y , Taoka T , Nozaki H , Kikuta J , Fujita S , Hagiwara A , Kamiya K , Wada A , Akashi T , Sano K , Nishizawa M , Hori M , Naganawa S , Aoki S ((2022) ) Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology 99: , e2648–e2660. |
[8] | Bae YJ , Choi BS , Kim JM , Choi JH , Cho SJ , Kim JH ((2021) ) Altered glymphatic system in idiopathic normal pressure hydrocephalus. Parkinsonism Relat Disord 82: , 56–60. |
[9] | Shen T , Yue Y , Ba F , He T , Tang X , Hu X , Pu J , Huang C , Lv W , Zhang B , Lai HY ((2022) ) Diffusion along perivascular spaces as marker for impairment of glymphatic system in Parkinson’s disease. NPJ Parkinsons Dis 8: , 174. |
[10] | Qin Y , He R , Chen J , Zhou X , Zhou X , Liu Z , Xu Q , Guo JF , Yan XX , Jiang N , Liao W , Taoka T , Wang D , Tang B ((2023) ) Neuroimaging uncovers distinct relationships of glymphatic dysfunction and motor symptoms in Parkinson’s disease. J Neurol 270: , 2649–2658. |
[11] | Zhou L , Butler TA , Wang XH , Xi K , Tanzi EB , Glodzik L , Chiang GC , De Leon MJ , Li Y (2023) Multimodal assessment of brain fluid clearance is associated with amyloid-beta deposition in humans. J Neuroradiol. doi: 10.1016/j.neurad.2023.10.009. |
[12] | Butler T , Zhou L , Ozsahin I , Wang XH , Garetti J , Zetterberg H , Blennow K , Jamison K , de Leon MJ , Li Y , Kuceyeski A , Shah SA ((2023) ) Glymphatic clearance estimated using diffusion tensor imaging along perivascular spaces is reduced after traumatic brain injury and correlates with plasma neurofilament light, a biomarker of injury severity. Brain Commun 5: , fcad134. |
[13] | Dai Z , Yang Z , Chen X , Zheng W , Zhuang Z , Liao Y , Li M , Chen S , Lin D , Wu X , Shen J ((2023) ) The aging of glymphatic system in human brain and its correlation with brain charts and neuropsychological functioning. Cereb Cortex 33: , 7896–7903. |
[14] | Hsiao WC , Chang HI , Hsu SW , Lee CC , Huang SH , Cheng CH , Huang CW , Chang CC ((2023) ) Association of cognition and brain reserve in aging and glymphatic function using diffusion tensor image-along the perivascular space (DTI-ALPS). Neuroscience 524: , 11–20. |
[15] | Park CJ , Kim SY , Kim JH , Son NH , Park JY , Jeong YH , Kim HJ , Park J , Kim WJ ((2023) ) Evaluation of glymphatic system activity using diffusion tensor image analysis along the perivascular space and amyloid PET in older adults with objectively normal cognition: A preliminary study. Front Aging Neurosci 15: , 1221667. |
[16] | Giannetto M , Xia M , Stæger FF , Metcalfe T , Vinitsky HS , Dang JAML , Xavier ALR , Kress BT , Nedergaard M , Hablitz LM ((2020) ) Biological sex does not predict glymphatic influx in healthy young, middle aged or old mice. Sci Rep 10: , 16073. |
[17] | Zhang Y , Zhang R , Ye Y , Wang S , Jiaerken Y , Hong H , Li K , Zeng Q , Luo X , Xu X , Yu X , Wu X , Yu W , Zhang M , Huang P ((2021) ) The influence of demographics and vascular risk factors on glymphatic function measured by diffusion along perivascular space. Front Aging Neurosci 13: , 393. |
[18] | Mielke MM , Vemuri P , Rocca WA ((2014) ) Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol 6: , 37–48. |
[19] | Besser L , Kukull W , Knopman DS , Chui H , Galasko D , Weintraub S , Jicha G , Carlsson C , Burns J , Quinn J , Sweet RA , Rascovsky K , Teylan M , Beekly D , Thomas G , Bollenbeck M , Monsell S , Mock C , Zhou XH , Thomas N , Robichaud E , Dean M , Hubbard J , Jacka M , Schwabe-Fry K , Wu J , Phelps C , Morris JC , NeuropsychologyWork Group, Directors, and Clinical Core leaders of the National Institute on Aging-funded US Alzheimer’s Disease Centers ((2018) ) Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 32: , 351–358. |
[20] | Delis DC , Kramer JH , Kaplan E , Ober BA (2000) California Verbal Learning Test-Second Edition (CVLT-II). Psychological Corporation, San Antonio, TX. |
[21] | Guilmette TJ , Sweet JJ , Hebben N , Koltai D , Mahone EM , Spiegler BJ , Stucky K , Westerveld M Conference Participants ((2020) ) American Academy of Clinical Neuropsychology consensus conference statement on uniform labeling of performance test scores. Clin Neuropsychol 34: , 437–453. |
[22] | Andersson JLR , Sotiropoulos SN ((2016) ) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125: , 1063–1078. |
[23] | Mestre H , Mori Y , Nedergaard M ((2020) ) The brain’s glymphatic system: Current controversies. Trends Neurosci 43: , 458–466. |
[24] | Ruigrok ANV , Salimi-Khorshidi G , Lai MC , Baron-Cohen S , Lombardo MV , Tait RJ , Suckling J ((2014) ) A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39: , 34–50. |
[25] | Naganawa S , Taoka T ((2022) ) The glymphatic system: A review of the challenges in visualizing its structure and function with MR imaging. Magn Reson Med Sci 21: , 182–194. |
[26] | Barisano G , Lynch KM , Sibilia F , Lan H , Shih NC , Sepehrband F , Choupan J ((2022) ) Imaging perivascular space structure and function using brain MRI. Neuroimage 257: , 119329. |




