The Effect of Adding Mirtazapine to Quetiapine on Reducing Agitation in Patients with Alzheimer’s Disease
Abstract
Background:
Alzheimer’s disease (AD) is one of the most debilitating diseases in old age, associated with cognitive decline and behavioral symptoms.
Objective:
This study aimed to investigate the effect of adding mirtazapine to quetiapine in reducing agitation among patients with AD.
Methods:
Thirty-seven elderly patients (18 cases and 19 controls) with AD, diagnosed according to National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria, were enrolled at Nezam-Mafi Clinic. Inclusion criteria comprised a minimum of two years post-diagnosis, a Cohen-Mansfield Agitation and Aggression Questionnaire (CMAI) score above 45, and treatment with 100–150 mg of quetiapine. Patients were randomly assigned to receive mirtazapine (15 mg at night, increased to 30 mg at night after two weeks) or a placebo. Cognitive changes were assessed at weeks 0 and 6 using the Mini-Mental State Examination instrument. Furthermore, symptoms of agitation and aggression were evaluated using the CMAI questionnaire at weeks 4 and 6.
Results:
In this study, the mean duration of AD in the control group was 4.68 years, and in the case group, it was 5.05 years. Although the total agitation score showed no significant change at the end of the study compared to the control group, the rate of physical non-aggressive behavior showed a significant decrease (p < 0.05).
Conclusions:
According to this study, adding mirtazapine to the antipsychotic drug regimen may not be an effective treatment for agitation in AD patients.
INTRODUCTION
Dementia is the most common cognitive disorder, serving as an umbrella term that encompasses a range of symptoms resulting from conditions affecting memory, thinking, behavior and emotion. The most common form of dementia is Alzheimer’s disease (AD), impacting 50–60% of individuals diagnosed with dementia. Dementia has a significant higher prevalence among the elderly population, with 5–7% of people over 60 years exhibiting symptoms [1, 2]. Behavioral disorders in AD patients pose significant challenges, causing distress to the patients, their caregivers, and the broader support system [3, 4]. Among these behavioral symptoms, agitation is one of the most challenging behaviors exhibited by these patients [5].
Agitation is a common behavioral disorder characterized by exaggerated feeling of irritability or verbal and/or physical restlessness, disrupting activities of daily living. The prevalence of agitation in AD patients ranges from 30–50%, with a higher occurrence in the moderate to severe stages of the disease [6]. Agitation adversely affects cognitive function and the quality of life for patients, making their care more challenging [7, 8]. Additionally, agitation is associated with an increased hospital or care center admissions, higher use of medications, and higher mortality rates [9]. Therefore, effective management of aggressive and agitated behaviors is essential in patients with dementia.
While non-pharmacologic interventions aimed at addressing underlying causes of agitations are considered the first line of treatment, situations may arise where the underlying cause is unclear or these interventions prove ineffective, necessitating second line treatments. Antipsychotic drugs are the mainstay of drug therapy for agitated AD patients. However, alternative medications, such as acetyl cholinesterase inhibitors, anticonvulsants, antidepressants, and benzodiazepines have been prescribed with varying, and often limited efficacy [10].
Mirtazapine, a noradrenergic and specific serotonergic antidepressant, ranks among the most commonly prescribed antidepressants for older people and those with dementia. Mirtazapine’s effect in enhancing serotonergic neurotransmission may be associated with the anti-agitation property of this drug [11]. While there have been a few studies exploring the impact of mirtazapine on reducing agitation or aggression in AD [10–12], there remains a need for further investigations to comprehensively understand the potential role of mirtazapine in managing behavioral symptoms and to determine its effective dosage. Therefore, this study aimed to determine the effect of adding mirtazapine to quetiapine on reducing agitation in patients with AD.
METHODS
Study design and setting
This randomized, double-blind clinical trial was conducted at the Nezam-Mafi Medical Rehabilitation Clinic, Tehran, Iran.
Sample size and sampling technique
All outpatients referred to the Nezam-Mafi Medical Rehabilitation Clinic affiliated to the University of Social Welfare and Rehabilitation Sciences (Tehran, Iran) between August 2020 and June 2021 who met the inclusion criteria were enrolled. Demographic information, including age and sex, were collected. The number of participants at the beginning of the research was 40. Using a table of random numbers, participants were then allocated into either the intervention or control groups with 20 individuals in each group. Group A was designated as the intervention group, while group B served as the control group. Conscious consent was obtained from all participants.
Throughout the study, two patients in intervention group and one patient in control group were excluded due to unwanted side effects, and in the control group, one participant was excluded due to dizziness. Consequently, after excluding these participants, the final sample size was reduced to 37. The selection criteria of study participants are shown in Fig. 1.
Fig. 1
Flow Chart CONSORT.
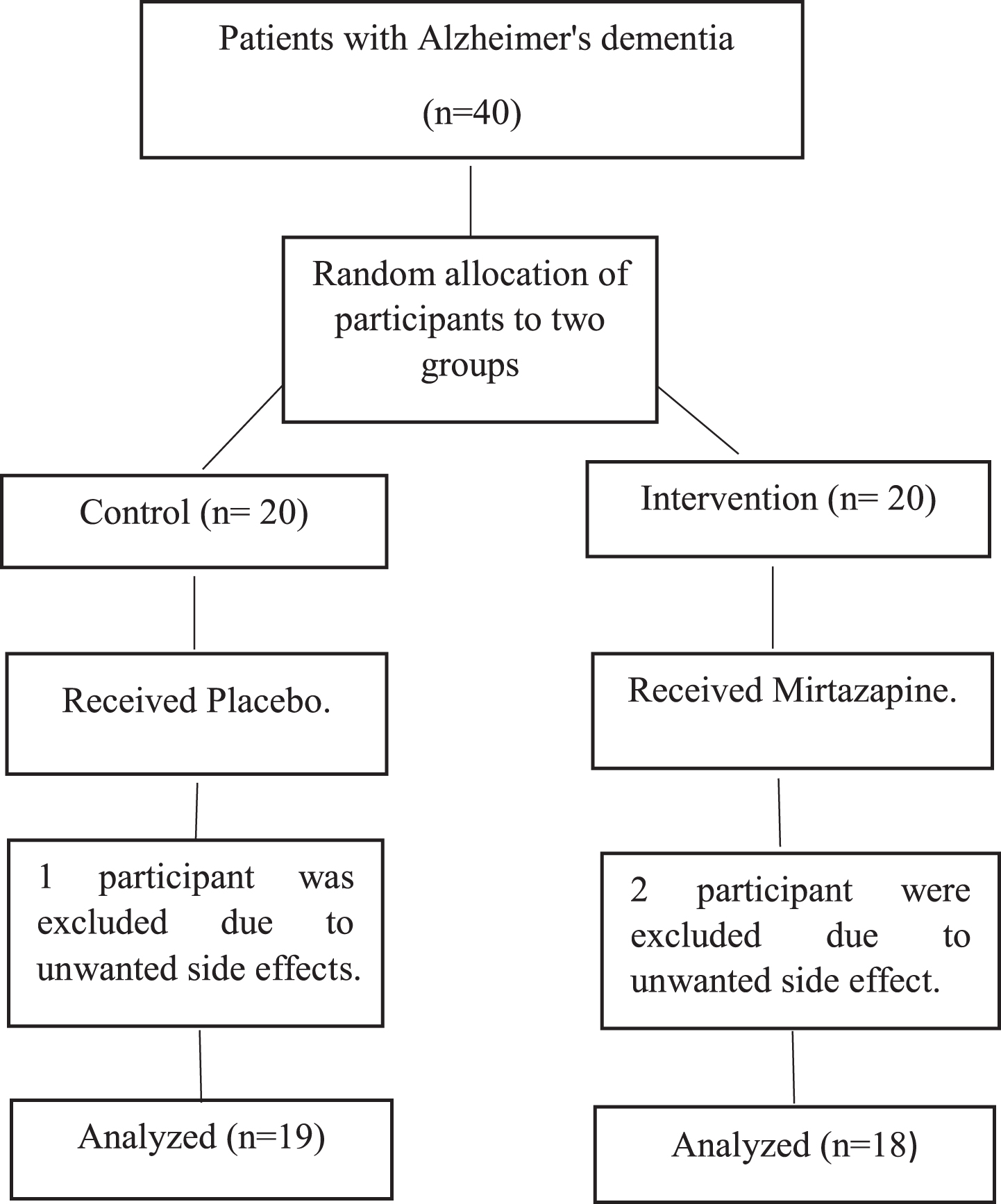
Eligibility criteria
The study population comprised adult patients (aged 18 years and above) with AD who met the 2011 National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic criteria, as confirmed by a neurologist. Also, participants had a disease onset of at least two years, and their Cohen-Mansfield Agitation Inventory (CMAI) test score was above 45. Other inclusion criteria encompassed the absence of medical, psychiatric, or neurological conditions significantly impacting cognition, the absence of mental retardation, no substance abuse within the previous six months, and not currently receiving other antidepressants or antipsychotics. For patients on medication, an appropriate washout period was considered. Patients were excluded from the study if they met any of the following criteria: 1) There was a need to discontinue or change the patient’s antipsychotic drug for any reason during the study; 2) Presence of second-degree or higher heart block; 3) Prolonged QT syndrome; 4) The patient exhibited critical behavioral status (such as suicide or harm to others) that could not be randomized; 5) Any contraindications to the administration of mirtazapine, such as drug hypersensitivity; 6) Occurrence of any unwanted side effects leading to clinical or psychiatric disorders for the patient (common side effects include: sedation, fatigue, dizziness, weight gain, orthostatic hypotension, headache, constipation, increased triglycerides, and alanine transaminase); 7) The patient or caregiver’s unwillingness to continue cooperating and refusal to answer questions during the interview.
Measurement tools
Cohen-Mansfield Agitation Inventory (CMAI). This questionnaire identifies and prioritizes destructive behaviors and evaluates the effectiveness of various pharmacological treatments, especially non-pharmacological interventions. The 29-item scale rates questions from one (never) to seven (several times in an hour). Responses are based on the observations from the last two weeks, categorizing behaviors into four groups: physical/aggressive, physical/non-aggressive, verbal/aggressive, and verbal/non-aggressive behaviors. The Persian version of this questionnaire was standardized by Maryam Zare et al. [13].
Mini-Mental State Examination (MMSE). The MMSE is a test to assess the quality of consciousness by diagnosing and screening of dementia with a maximum score of 30. This test consists of 3 items with 5 points, three items with 3 points, one with 2 points, and four items with 1 point. Its 5-point items include orientation to time, orientation to place and serial seven (for evaluation of attention and calculation); 3-point items include execution of three-step command, registration and recall of three words; the 2-point item include naming of two objects and its 1-point items also include read, write, repeat and shape. The minimum normal score is 29 for people with higher education, 27 for people with secondary education, 25 for people with primary education, and 19 for illiterate people. The Persian form of instrument was structured by Seyedian et al. In 2008, and the Cronbach’s alpha coefficient for the whole test was 0.81. Rock curve analysis identified a score of 22 as the cut-off point, and the test had a sensitivity of 90% and a specificity of 93.5% [14].
Study procedure
Group A (intervention group) received 15 mg of mirtazapine tablets at night, which increased to 30 mg at night after two weeks and continued until the sixth week. Group B (control group) in addition to atypical antipsychotic drug therapy, was given placebo tablets, taking one tablet overnight for the first two weeks and two pills overnight after two weeks. The packaging and shape of the two tablets were similar to maintain the blindness of the study. Patients were followed up weekly for six weeks using telephone visits and were monitored for side effects, especially central nervous system such as sedation or dizziness. Furthermore, they were assessed to be excluded from the study in case of severe side effects. Psychiatric symptoms were recorded according to the CMAI scale at the beginning of the study, the end of the fourth week, and the sixth week. Moreover, cognitive symptoms were evaluated by the MMSE test at the beginning and end of the study. The primary outcome of the study was a decrease in CMAI score at weeks 4 and 6 of drug initiation in the intervention group compared to the control and the secondary outcome was an increase in MMSE score at week 6.
Statistical analysis
The data were analyzed using descriptive (mean and standard deviation) and inferential (repeated measurement tests and analysis of covariance) statistics in SPSS software version 22.
Ethical considerations
This study was registered by Iranian Registry of Clinical Trials (Code: IRCT20210613051565N1). The written informed consent was obtained from all participants prior to the study.
RESULTS
The findings of this study revealed a significant decrease in the rate of physical non-aggressive behavior at the end of the study compared to the control group (p < 0.05). However, other variables such as verbal aggressive/non-aggressive behaviors, physical aggression, total agitation score and cognitive symptoms did not show a significant difference.
Demographic variables
A total of 37 people (18 in the intervention group and 19 in the control group) completed the study. As shown in Table 1, the study population had a mean age of 69.47±5.91 years and 72.16±5.37 years in control and intervention groups, respectively. The average disease duration was 4.68±2.21 years in control group and 5.05±1.89 years in intervention group.
Table 1
The characteristics of the intervention and control groups
| Characteristics | Control | Case | |
| Age | 69.47±5.91 (60–82) | 72.16±5.37 (63–83) | |
| Mean of disease duration | 4.68±2.21 (2–10) | 5.05±1.89 (2–9) | |
| Sex | Male | 8 (42%) | 10 (55.5%) |
| Female | 11 (58%) | 8 (44.5%) | |
Evaluation of the rate of cognitive disorder between weeks 0 and 6 indicated no significant difference in both groups (p = 0.360). Furthermore, physical/aggressive, verbal/aggressive, and verbal/non-aggressive behaviors revealed no significant difference between weeks 0, 4, and 6 in both groups. While the physical non-aggressive behavior score was similar between consecutive weeks in the control group, a significant difference was observed in the intervention group (p = 0.005) showing a decline in physical non-aggression (restlessness). A consequent analysis in cases revealed a higher mean of physical non-aggression in week 0 (p = 0.006) and 4 (p = 0.037) compared with week 6. Results are presented in Table 2.
Table 2
Assessment of aggressive and non-aggressive behaviors and cognitive symptoms between case and control patients with Alzheimer’s disease
| Feature | Groups | Mean | SD | t | df | p |
| Cognitive disorder | Controls | 0.21 | 0.97 | 0.940 | 18 | 0.360 |
| Cases | −0.05 | 0.99 | −0.236 | 17 | 0.816 | |
| Sum of Squares | Mean squares | F | df | p | ||
| Verbal/aggressive behavior | Controls | 6.21 | 2.526 | 0.577 | 2 | 0.565 |
| Cases | 2.815 | 1.407 | 0.280 | 2 | 0.757 | |
| Physical/aggressive behavior | Controls | 57.879 | 28.895 | 0.857 | 2 | 0.430 |
| Cases | 41.815 | 20.907 | 0.644 | 2 | 0.530 | |
| Aggressive behavior | Controls | 21.158 | 10.579 | 1.106 | 2 | 0.338 |
| Cases | 10.954 | 5.477 | 0.572 | 2 | 0.568 | |
| Verbal/non-aggressive behavior | Controls | 3.895 | 1.947 | 0.109 | 2 | 0.897 |
| Cases | 8.111 | 4.056 | 0.251 | 2 | 0.779 | |
| Physical/non-aggressive behavior | Controls | 0.456 | 0.228 | 0.004 | 2 | 0.996 |
| Cases | 186.926 | 93.463 | 5.818 | 2 | 0.005 | |
| Non-aggressive behavior (Restlessness) | Controls | 0.956 | 0.478 | 0.020 | 2 | 0.980 |
| Cases | 30.954 | 15.477 | 1.277 | 2 | 0.288 |
Comparing all parameters between case and intervention groups pointed out that physical non-aggressive behaviors were significantly different in week 6 (p = 0.014). All other factors were similar between groups between weeks (p > 0.05). Results are shown in Table 3.
Table 3
Assessment of rate of cognitive disorder, aggressive and non-aggressive behaviors between week 0, 4, and 6 in case and control patients with Alzheimer’s disease
| Feature | Week | Mean | F | t | df | p | |
| Control | Case | ||||||
| Verbal/aggressive behavior | 0 | 6.21 | 6.11 | 0.004 | 0.142 | 34.731 | 0.888 |
| 4 | 6.05 | 5.88 | 1.743 | 0.231 | 33.318 | 0.819 | |
| 6 | 5.57 | 6.44 | 0.096 | −1.217 | 34.506 | 0.232 | |
| Physical/aggressive behavior | 0 | 32.31 | 33.33 | 0.002 | −0.0573 | 34.941 | 0.570 |
| 4 | 30.26 | 31.38 | 0.036 | −0.0587 | 34.950 | 0.561 | |
| 6 | 30.10 | 31.55 | 0.267 | −0.734 | 34.963 | 0.468 | |
| Aggressive behavior | 0 | 19.26 | 19.72 | <0.001 | −0.442 | 34.966 | 0.661 |
| 4 | 18.15 | 18.63 | 0.001 | −0.0489 | 34.854 | 0.628 | |
| 6 | 17.84 | 19.00 | <0.001 | −1.125 | 34.810 | 0.268 | |
| Verbal/non-aggressive behavior | 0 | 10.26 | 9.05 | 0.933 | 0.953 | 34.566 | 0.347 |
| 4 | 9.73 | 9.61 | 0.193 | 0.092 | 34.999 | 0.927 | |
| 6 | 9.68 | 10.00 | 0.036 | −0.220 | 34.573 | 0.827 | |
| Physical/non-aggressive behavior | 0 | 20.68 | 19.94 | 2.812 | 0.381 | 28.597 | 0.706 |
| 4 | 20.84 | 19.00 | 3.126 | 0.951 | 28.508 | 0.350 | |
| 6 | 20.63 | 15.61 | 2.596 | 2.614 | 27.283 | 0.014 | |
| Non-aggressive behavior | 0 | 15.47 | 14.50 | 3.31 | 0.701 | 31.908 | 0.489 |
| 4 | 15.28 | 14.30 | 2.80 | 0.699 | 32.684 | 0.490 | |
| 6 | 15.15 | 12.80 | 2.32 | 1.699 | 32.803 | 0.099 | |
| Cognitive symptoms | 0 | 17.26 | 17.00 | 0.030 | 0.247 | 34.780 | 0.806 |
| 6 | 17.05 | 17.05 | 0.045 | −0.003 | 34.754 | 0.998 | |
| Total score | 0 | 69.47 | 68.44 | 1.344 | 0.228 | 34.374 | 0.821 |
| 4 | 66.89 | 65.94 | 0.343 | 0.233 | 34.900 | 0.817 | |
| 6 | 66.00 | 63.61 | 0.133 | 0.560 | 34.973 | 0.579 | |
Assessing aggressive behaviors revealed a significant difference in its rate between weeks (p = 0.005); however, adding mirtazapine to quetiapine in the treatment of patients and examining the interaction between time and mirtazapine add-on did not show any significant effect (Table 4). On the other hand, while mirtazapine had no general impact on non-aggressive behaviors, time (p < 0.001) and also time-mirtazapine interaction (p < 0.001) indicated a significant effect (Table 4).
Table 4
The effect of adding mirtazapine to quetiapine on cognitive symptoms and aggressive/non-aggressive behaviors in patients with Alzheimer’s disease
| Features | Variables | Sum of Squares | df | Mean squares | F | p | Effect size |
| Aggressive behaviors | Time | 28.918 | 1.845 | 15.673 | 5.946 | 0.005 | 0.145 |
| Mirtazapine | 13.561 | 1 | 13.561 | 0.568 | 0.456 | 0.016 | |
| Mirtazapine-Time | 2.918 | 1.845 | 1.581 | 0.600 | 0.539 | 0.017 | |
| Non-aggressive behaviors | Time | 21.095 | 1.832 | 11.517 | 17.752 | >0.001 | 0.337 |
| Mirtazapine | 57.233 | 1 | 57.233 | 1.066 | 0.309 | 0.030 | |
| Mirtazapine-Time | 11,626 | 1.832 | 6.348 | 9.784 | >0.001 | 0.218 | |
| Cognitive symptoms | Mirtazapine | 0.008 | 1 | 0.008 | 0.009 | 0.925 | – |
Furthermore, no significant effect was found on cognitive symptoms when adding mirtazapine to routine therapy. Besides, these findings were not influenced by sex and duration of the disease Results are presented in Table 5.
Table 5
The effect of adding mirtazapine to quetiapine on cognitive symptoms and aggressive/non-aggressive behaviors in patients with Alzheimer’s disease based on sex and duration of the disease
| Factor | Feature | Sum of Squares | df | Mean squares | F | p |
| Sex | Aggression | 2.872 | 1 | 2.872 | 0.692 | 0.419 |
| Agitation | 4.533 | 1 | 4.533 | 0.689 | 0.401 | |
| Cognitive | 3.243 | 1 | 3.243 | 0.712 | 0.302 | |
| Duration of the disease | Aggression | 3.742 | 1 | 3.742 | 0.721 | 0.256 |
| Agitation | 6.234 | 1 | 6.234 | 0.527 | 0.329 | |
| Cognitive | 5.613 | 1 | 5.613 | 0.844 | 0.425 |
Two patients in the intervention group withdrew from the study due to side effects (both patients experiences severe sedation). One patient in the control group was excluded from the study due to dizziness. The most common side effects in the case group were sedation (4 cases, 22%), dizziness (3 cases, 16%), and headache (1 case, 5%), which were mild and disappeared with continued treatment. Complaints of patients in the control group included transient sedation (n = 1), dizziness (n = 2), and fatigue (n = 1). These side effects did not show significant differences between the two groups.
DISCUSSION
Our study showed that, in general, adding mirtazapine to quetiapine did not significantly impact the agitation of patients with AD. However, the assessment of sub-behaviors indicated a modest reduction in physical non-aggressive behaviors after six weeks of treatment. Nevertheless, this difference was not significantly associated with gender and the duration of the disease. The adverse effects of mirtazapine were mild in this study, and patients generally tolerated it well. Appetite changes and hypotension, reported in previous studies, were not observed in our patients [11].
Pharmacologic interventions for controlling dementia-related agitation have not yielded effective results, posing a significant clinical challenge for healthcare providers and caregivers [6]. While most clinicians prescribe atypical antipsychotics to reduce patients’ aggressive behaviors, the side effects of these drugs, such as cerebrovascular accidents and extrapyramidal symptoms, limit their use. The U.S. Food and Drug Administration (U.S. FDA) issued black box warnings against the use of antipsychotics in dementia in 2005 and 2008 due to the increased risk of morbidity and mortality. This led to a reduction in their prescription rates, highlighting the need for safer and more effective pharmacological alternatives. However, alternative medications have also shown limited efficacy [10]. Some studies have suggested that psychotropic drugs with serotonergic enhancement, like mirtazapine, may effectively control dementia-related agitation [15]. Mirtazapine is increasingly used in the elderly and could be a viable option for managing behavioral symptoms of dementia. Some studies attribute the reduction in agitation to mirtazapine’s sedative effects, improving sleep disturbances in dementia patients throughout the day, even in those without evident depression. [16].
In our study, adding mirtazapine to antipsychotic treatment did not have a significant impact on overall aggressive/non-aggressive patient behavior. While mirtazapine did reduce physical non-aggressive behavior after six weeks compared to the control group, this effect appeared to be clinically weak. Contrary to the findings of our study, several studies have reported the positive effects of mirtazapine on the symptoms of dementia patients, including agitation/aggression. In the first such study, Cakir et al. showed that mirtazapine reduced agitation scores in patients with dementia and recommended it an option for treatment of agitation in patients with AD. The side-effect profile of mirtazapine was mild in this study [11]. Romeo et al. found that mirtazapine, compared to sertraline, improved behavioral disorders, reduced care costs, and shortened care time for dementia patients due to its anti-anxiety and sedative effects [17]. In another survey, Varia et al. reported that mirtazapine improved depression, insomnia, anxiety, agitation, physical symptoms, and some aspects of quality of life in depressed elderly individuals with medical conditions [18].
A recent a randomized, double-blind, placebo-controlled study of mirtazapine for agitated behaviors in dementia (SYMBAD) showed that mirtazapine is no more effective than a placebo and might even increase mortality, which is in line with our findings [10]. In this multicenter study, 204 AD patients were included and randomly placed in two mirtazapine and placebo groups. The mean CMAI score was not significantly different in the two groups after 12 weeks. Adverse event rates were similar in the two groups, although more deaths were reported in the mirtazapine group after 16 weeks. This is the largest study that has so far evaluated the effects of mirtazapine on the agitation of AD patients and its results were negative. However, this study has differences from our study: Mirtazapine was used as monotherapy and at a higher dose (45 mg), and patients were treated for twice the duration as in our study (12 weeks). Although the results of this large study were against the use of mirtazapine in dementia patients, the modest beneficial effect of the drug on some behavioral symptoms of dementia in our study and some previous study [11], suggests that short-term use of mirtazapine at an average dose, aimed at minimizing side effects, may alleviate symptoms in patients for whom sleep disturbances are the primary cause of agitation and those with predominant non-aggressive symptoms.
Mirtazapine, as an add-on therapy, may be effective in selected AD patients with non-aggressive agitated behavior, although further investigation through well-designed, larger studies is warranted. There was no significant change in the severity of the cognitive symptoms at the end of our study Similar to our findings, some studies evaluating cognitive changes in dementia patients have reported limited improvements, with mirtazapine primarily impacting sleep, appetite, anxiety, and weight but not significantly affecting cognitive symptoms [19]. Furthermore, in the Cakir et al. study, mirtazapine did not significantly improve the cognitive symptoms of dementia patients [11]. Therefore, this medication does not seem to have a significant effect on improving cognitive symptoms. However, to confirm this finding, larger studies with extended follow-up periods are needed.
Limitations
Our study has several potential limitations, including a small sample size. Future studies with larger sample sizes are needed to validate our results. Additionally, the relatively short follow-up period may not fully capture the chronic nature of restlessness in dementia patients, warranting longer-term investigations. Lastly, our findings may not be directly generalizable to patients admitted to hospitals or care centers, as our study was conducted in an outpatient setting.
Conclusion
In conclusion, our study suggests that adding mirtazapine to quetiapine may not be an effective treatment for agitation in AD patients. However, it does show potential for a positive effect on specific physical non-aggressive behaviors, which should be further investigated through well-designed, larger studies.
ACKNOWLEDGMENTS
We thank the patients who participated in this study. We would also like to thank the Clinical Research Development Center of Rofeideh Rehabilitation Hospital, Tehran, Iran for their support, cooperation, and assistance throughout the period of study.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
[1] | Prince M , Bryce R , Albanese E , Wimo A , Ribeiro W , Ferri CP ((2013) ) The global prevalence of dementia: A systematic review and metaanalysis, Alzheimers Dement 9: , 63–75.e2. |
[2] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[3] | Levenson RW , Sturm VE , Haase CM ((2014) ) Emotional and behavioralsymptoms in neurodegenerative disease: A model for studying theneural bases of psychopathology., Annu Rev Clin Psychol 10: , 581–606. |
[4] | Latifian M , Abdi K , Raheb G , Islam SM , Alikhani R ((2023) ) The experiences of bipolar patients’ families regarding stigma coping strategies in Tehran: A qualitative study, Curr Psychol 17: . https://doi.org/10.1007/s12144-023-04620-2 |
[5] | Koenig AM , Arnold SE , Streim JE ((2016) ) Agitation and irritability inAlzheimer’s disease: Evidenced-based treatments and the black-boxwarning. Curr Psychiatry Rep 18: , 3. |
[6] | Carrarini C , Russo M , Dono F , Barbone F , Rispoli MG , Ferri L , DiPietro M , Digiovanni A , Ajdinaj P , Speranza R , Granzotto A ((2021) ) Agitation and dementia: Prevention and treatment strategies in acuteand chronic conditions. Front Neurol 12: , 644317. |
[7] | Cheng S-T ((2017) ) Dementia caregiver burden: A research update and critical analysis. Curr Psychiatry Rep 19: , 64. |
[8] | Sörensen S , Conwell Y ((2011) ) Issues in dementia caregiving:Effects on mental and physical health, intervention strategies, and research needs. Am J Geriatr Psychiatry 19: , 491–496. |
[9] | Kongpakwattana K , Sawangjit R , Tawankanjanachot I , Bell JS , Hilmer SN , Chaiyakunapruk N ((2018) ) Pharmacological treatments for alleviating agitation in dementia: A systematic review and network meta-analysis. Br J Clin Pharmacol 84: , 1445–1456. |
[10] | Banerjee S , High J , Stirling S , Shepstone L , Swart AM , Telling T , Henderson C , Ballard C , Bentham P , Burns A , Farina N ((2021) ) Study of mirtazapine for agitated behaviours in dementia (SYMBAD): A randomised, double-blind, placebo-controlled trial. Lancet 398: , 1487–1497. |
[11] | Cakir S , Kulaksizoglu IB ((2008) ) The efficacy of mirtazapine in agitated patients with Alzheimer’s disease: A 12-week open-label pilot study. Neuropsychiatr Dis Treat 4: , 963–966. |
[12] | Marcinkowska M , Śniecikowska J , Fajkis N , Paśko P , Franczyk W , Kołaczkowski M ((2020) ) Management of dementia-related psychosis, agitation and aggression: A review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs 34: , 243–268. |
[13] | Zare M , Shayeghian Z , Birashk B , Afkham Ebrahimi A ((2012) ) Reliability, validity and factor analysis of Cohen-Mansfield Agitation Inventory (CMAI). Iran J Psychiatry Clin Psychol 18: , 67–73. |
[14] | Seyedian M , Falah M , Nourouzian M , Nejat S , Delavar A , Ghasemzadeh HA ((2008) ) Validity of the farsi version of mini-mental state examination. J Med Council Iran 25: , 408–414. |
[15] | Aga VM ((2019) ) When and how to treat agitation in Alzheimer’s disease dementia with citalopram and escitalopram. Am J Geriatr Psychiatry 27: , 1099–1107. |
[16] | Herrmann N , Wang HJ , Song BX , Bawa KK , Lanctôt KL ((2022) ) Risks and benefits of current and novel drugs to treat agitation in Alzheimer’s disease. Expert Opin Drug Saf 21: , 1289–1301. |
[17] | Romeo R , Knapp M , Hellier J , Dewey M , Ballard C , Baldwin R , Bentham P , Burns A , Fox C , Holmes C , Katona C ((2013) ) Cost-effectiveness analyses for mirtazapine and sertraline in dementia: Randomised controlled trial. Br J Psychiatry 202: , 121–128. |
[18] | Varia I , Venkataraman S , Hellegers C , Gersing K , Doraiswamy PM ((2007) ) Effect of mirtazapine orally disintegrating tablets on health-related quality of life in elderly depressed patients with comorbid medical disorders: A pilot study. Psychopharmacol Bull 40: , 47–56. |
[19] | Raji MA , Brady SR ((2001) ) Mirtazapine for treatment of depression and comorbidities in Alzheimer disease. Ann Pharmacother 35: , 1024–1027. |




