Effects of Virtual Reality Physical and Cognitive Training Intervention On Cognitive Abilities of Elders with Mild Cognitive Impairment
Abstract
Background:
Virtual reality (VR) technology has become increasingly used for assessment and intervention in the neuroscience field.
Objective:
We aimed to investigate the effects of a VR Training System, named VRADA (VR Exercise App for Dementia and Alzheimer’s Patients), on the cognitive functioning of older people with mild cognitive impairment (MCI).
Methods:
In this intervention study, 122 older adults with MCI were randomly assigned to five groups (the VRADA group (n = 28), a bike group (n = 11), a physical exercise group (n = 24), a mixed group (physical and cognitive exercise) (n = 31), and a non-contact control group (n = 28). The VRADA group underwent 32 physical and cognitive training sessions, performed 2 or 3 times weekly for 12 weeks in the VR environment. All participants had detailed neuropsychological assessments before and after intervention.
Results:
A series of linear regression models revealed that the VRADA group showed improvement or no deterioration in cognitive decline in global cognitive function (MMSE), verbal memory (Rey Auditory Verbal Learning Test and WAIS forward test), and executive functions, mental flexibility (Trail Making Test B).
Conclusions:
This interventionstudy indicates that the VRADA system improves the cognitive function of elders with MCI.
INTRODUCTION
Individuals with mild cognitive impairment (MCI), which is a prodromal phase of dementia, are experiencing cognitive impairments (in the domains of memory, attention, orientation, and executive functions) but relatively fewer difficulties in daily functioning [1–4]. The risk of a person with MCI developing dementia is ten times higher than that of a person of the same age [5]. The risk of developing dementia increases with age and ranges from 8% for those with MCI between 55- to 59-year-olds, 9.5% between 60- to 69-year-olds, 14.6% between 70- to 79-year-olds, and 23.6% for those 80 and older [6, 7]. Living with cognitive impairment may adversely affect one’s quality of life and physical and mental health [8, 9]. More specifically, individuals with MCI exhibit higher levels of depression and anxiety that can accelerate the progression of dementia [8–10]. Nevertheless, not all individuals with MCI develop dementia. Instead, some remain at the same cognitive level or even improve [11, 12], which suggests that the incidence of dementia may be attenuated by delaying the progression of MCI. Thus, early and effective intervention is essential to delay or prevent further deterioration [13]. In other words, the progressive development of dementia and its adverse effects cannot be cured given the absence of effective pharmacological treatment [13, 14] non-pharmacological interventions are needed.
Physical activity is described as “any movement by skeletal muscles that leads to energy expenditure” to improve or maintain physical function [15]. Physical exercise (PE) is a common strategy for both prevention of risk for cognitive decline [16] and intervention [17]. It has recently been shown that PE and a high number of steps per day (over 10,000 steps), in particular, may be optimally associated with a lower risk of dementia [18]. Moreover, studies of PE intervention—that include aerobic exercise, resistance training, strength training, or postural balance—have shown improvements in cognitive abilities, like global cognition, logical memory, inhibitory control, attention, and executive functions, like Symbol Digit tests, Verbal Fluency, and Stroop [17, 19–23]—motor abilities, quality of life and reductions in psychological symptoms, like depression and anxiety in older adults with MCI [24]. In addition, a cognitive intervention is either cognitive stimulation (containing a group of activities with the view to increase cognitive and social functioning), cognitive rehabilitation (individual intervention designed to address cognitive and behavioral deficits), or cognitive training (which includes tasks that aim to provide a set of standardized tasks) [14, 25].
A growing body of evidence has found that comprehensive, combined, and multi-component non-pharmacological intervention, including cognitive, social, and physical activities, positively impacted elders with MCI [13, 26–28]. More specifically, this intervention has improved global cognitive function and sustained physical activity in older adults with MCI [13, 24, 26–28], considering both cognitive plasticity and learning abilities may be unaffected [29].
Recently, information and communications technologies, especially virtual reality (VR), have become increasingly used for assessment and intervention in the neuroscience field [30]. VR technology offers a pleasant, easy, and interactive environment and allows the patient to be trained physically and cognitively in conditions unsuitable by conventional programs [4, 31]. VR-based therapeutic intervention programs have been tested and found to be promising and potentially highly effective tools for managing patients (for a review, see Kim and colleagues [32] and Zhu and colleagues [33]).
Hassandra and colleagues asked 27 older individuals with MCI and 30 healthy university students to perform a dual-task training protocol that combined physical and cognitive exercises in two different training conditions (i.e., a cycling task in a lab environment while performing oral math calculations, single-digit additions and subtractions, and a cycling task in the VR environment while performing calculations that appeared within the VR app). Participants were interviewed after testing in both conditions and expressed a significant preference for the VR condition. They found the program a good, easy-to-use, and tolerable system [34]. Several studies have also incorporated VR to formulate multi-component intervention programs for individuals with MCI, with promising results in well-being, physical fitness, and cognition [35, 36]. Talassi et al. [37] conducted a computerized intervention in 54 individuals with MCI and mild dementia. They reported improvements in cognitive and affective status (including the Mini-Mental State Examination (MMSE) [38], forward and backward digit span, phonemic and semantic verbal fluency, episodic memory from the Rivermead Behavioral Memory Test [39], and the Geriatric Depression Scale [40]).
Similarly, Barnes and colleagues [41], when performing a pilot randomized, controlled trial of intensive, computer-based cognitive training in 47 patients with MCI using a comprehensive test battery that assessed various cognitive domains (e.g., attention, language, memory, spatial and executive functions) found that intensive, computer-based mental activity was more beneficial than more passive computer activities (reading, listening, visuospatial game) which the control group performed. In addition, Hwang and Lee [42] used a VR training four-week program in 24 individuals with MCI to examine possible changes in memory and balance using various measures (the Visual Span Test, the Word Color Test, and the Limit of Stability test). They found that the experimental group exhibited statistically significant differences in all test categories compared to the control group, which was subject to traditional occupational therapy. Similarly, Mrakic-Sposta et al. [4] assessed the impact of a VR-based program combining aerobic exercise (i.e., riding a bike in a park and avoiding cars while crossing a road) and cognitive training in 10 patients with MCI. They found improved cognitive abilities (i.e., visual-constructive, visuospatial attention, memory functions, and verbal fluency). Liao and colleagues [43] assessed the effects of VR-based physical and cognitive training on executive function (Stroop Color and Word Test and Trail Making Test (TMT)) and dual-task gait performance in older adults with MCI. In addition, they compared VR-based physical and cognitive training with traditional combined physical and cognitive training in 34 elders with MCI. They reported significant improvements in dual-task gait performance for individuals with MCI trained in the VR environment, which may be related to improvements in executive function [43]. In a pilot, randomized clinical trial, Doniger and colleagues [44] also tested the ability of a VR motor-cognitive training to improve cognitive abilities and cerebral blood flow in middle-aged (40- to 65-year-old) individuals at high Alzheimer’s disease (AD) risk. They included four groups (the experimental VR group with cognitive and physical exercise, a control group trained only cognitively in the VR- environment, an active control group with conventional cognitive and physical exercise, and a control group in which participants engaged in their usual everyday activities). They found that cognitive training improved the cognitive function of patients at risk of AD. Park and colleagues also reported significant improvement in the cognitive and motor function of 40 elders with MCI after a virtual reality-based cognitive–motor rehabilitation intervention program [45]. More specifically, they compared two intervention programs, a VR-based program and a conventional cognitive program, a six-week intervention. They assessed their participants using the Montreal Cognitive Assessment (MoCA) scale, the TMT-A and B, and the Digit Span Test forward and backward. After the intervention, the VR group showed significantly more improvement in the MoCA, TMT, and Digit Span Test forward and backward compared to the non-contact control group.
In summary, several studies have tested, to date, the possible beneficial effects of VR technology to improve the physical and cognitive functioning of patients with MCI or dementia [32, 33]. However, research that includes combined physical and cognitive training in individuals with MCI still needs to be completed, given the limited number of studies including combined intervention [33] and the limited number of studies that tested the effects of VR intervention [34]. In addition, the heterogeneity across intervention characteristics, such as the type, intensity, frequency, and duration of exercise, leads to difficulty in drawing conclusions and formulating guidelines [32, 33]. Therefore, our knowledge of the effectiveness of cognitive and physical VR-based training in those patients still needs to be improved.
MATERIALS AND METHODS
Study design and objectives
This intervention study assesses a Virtual Reality Training System, VRADA (VR Exercise App for Dementia and Alzheimer’s Patients), in older people with MCI. The study spanned over three months (a 12-week program) to evaluate the efficacy of VRADA intervention in reducing cognitive decline and/or even improving general cognitive function and specific cognitive abilities, such as memory and executive functions. We hypothesized that at the end of the intervention:
Hypothesis 1: Individuals with MCI, trained via VRADA, will perform better in neuropsychological tests that tap general cognitive function and specific cognitive abilities like memory and executive function compared to the other groups.
Hypothesis 2: Individuals with MCI will perform significantly better in functional abilities, depressive symptoms, and anxiety than the other groups.
To implement the intervention and evaluate the effect of physical exercise within the virtual environment, five different groups participated (a) patients with MCI who comprised the VRADA group, (b) patients with MCI who comprised the bike group, (c) patients with MCI who comprised the PE group, (d) patients with MCI who comprised the physical and cognitive exercise group (i.e., the mixed group), and (e) patients with MCI who were the non-contact control group (i.e., patients who did not participate in any intervention program and were passive). Participants were allocated to one of these five groups independently of each other.
All the study participants gave written informed consent following the Declaration of Helsinki. Additionally, the study was approved by the Scientific and Ethics Committee of Alzheimer Hellas. The effects of training were verified through pre and post-intervention evaluations. More specifically, the evaluation took place two weeks before the beginning of the intervention and at the end of the experimental 12 weeks. Neuropsychological evaluation was carried out at these points in the experimental and the rest of the groups.
Participants
One hundred twenty-two patients with MCI were included (23 males and 99 females). Their ages ranged from 43 to 88 years (Mage = 70.66, SD = 8.58), and most participants were retired (92.2%). The total study sample consisted of the visitors of the Greek Association of Alzheimer’s Disease and Related Disorders’ Day Care Centre “Saint Helen” (Alzheimer Hellas, DCCSH) in Thessaloniki, from 2020 to 2021.
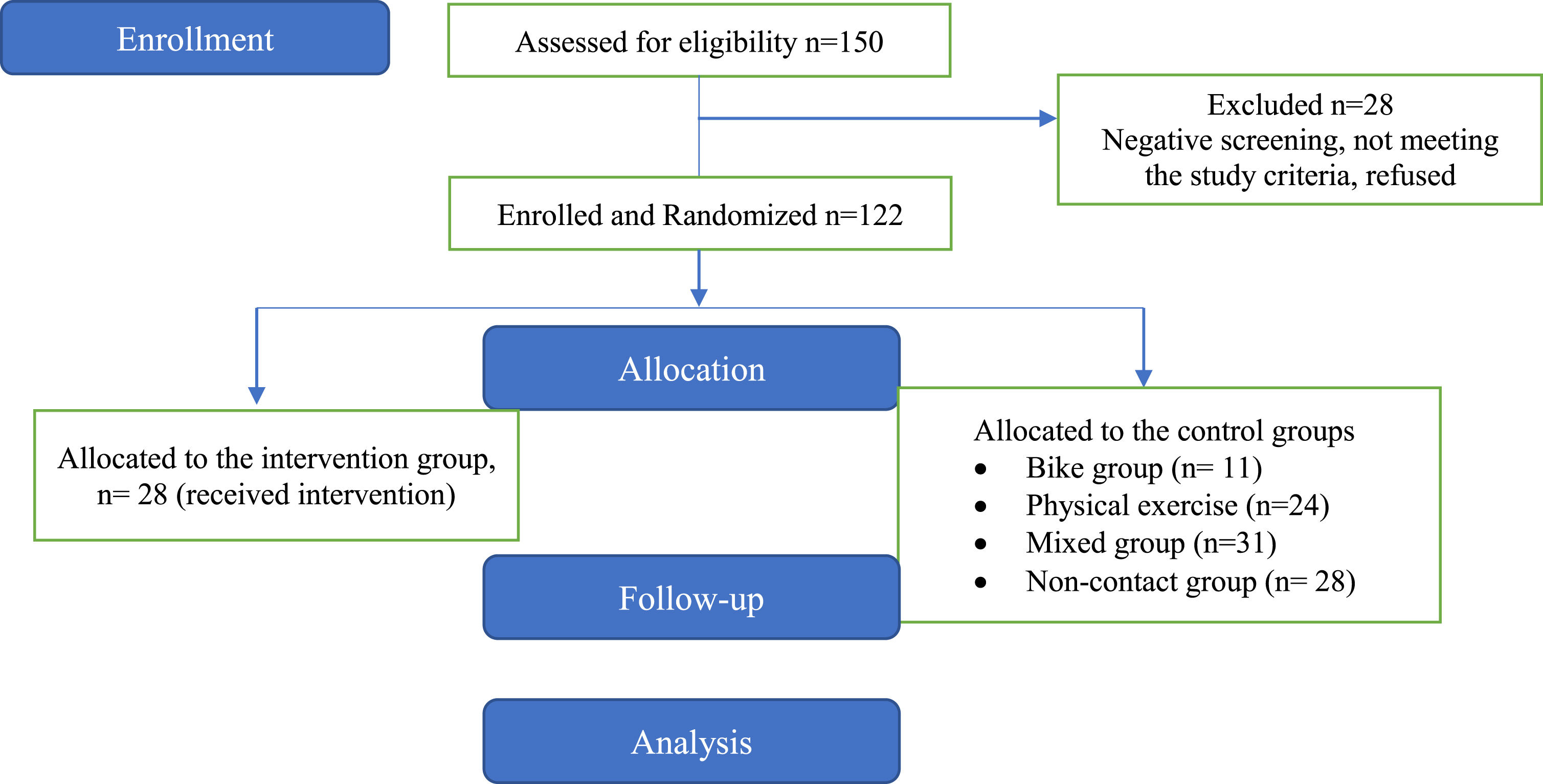
Their MCI diagnosis was the result of a multidisciplinary evaluation by specialized health professionals who are considered to be experts in the field of neurocognitive disorders and through a complete neurological, neuropsychological, and neuropsychiatric assessment, neuroimaging, and blood tests according to the criteria for the of Minor Neurocognitive Disorders of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [46] Participants were eligible if they met the criteria for MCI as defined by Petersen [47]. More specifically, inclusion criteria were: (a) a total score of 26 in the MMSE; (b) 0.5 on the global Clinical Dementia Rating scale [48] (stage 3 of the disease); (c) 1.5 standard deviations (SD) below the normal mean depending on education and age, in at least one cognitive area following the neuropsychological testing that was administered along with a neurological assessment [48]. The exclusion criteria included: (a) participants with other neurological disorders; (b) severe behavioral or neuropsychiatric symptoms (e.g., irritability or/and aggressiveness, apathy); (c) severe depression and/or anxiety; (d) severe mental illness; (e) epilepsy, stroke or hydrocephalus; (f) sensory deficits (such as uncontrolled hearing or visual issues); (g) severe hypertension or terminal illness and finally (h) participation in other intervention programs. Figure 1 describes the participants’ flow chart.
Fig. 1
Flow of the participants.
Procedure
Initially, 150 patients with MCI were screened for eligibility and underwent a neuropsychological evaluation. Of the initial 150 patients, 28 did not meet the eligibility criteria and were excluded from the study. Participant randomization was conducted following a simple random sampling scheme. Therefore, the remaining 122 patients (23 males and 99 females) were randomly selected by a statistician who was not involved in the execution and analysis of the study using SPSS software, version 25. Each eligible patient was assigned a unique identifier and was allocated through the SPSS algorithm to the respective training groups. Due to the simple random selection process, the group sizes were uneven (Fig. 2). Allocation was concealed from all investigators except for the statistician. The study staff was not blinded. The sample study, which consisted of these 122 patients with MCI, was based on the convenience and availability of eligible patients who were visitors of the Day Care Centre “Saint Helen” (Alzheimer Hellas, DCCSH) and consented to participate. We aimed to include the largest possible number of participants within the resources and time available for the study.
Fig. 2
Diagram for the Cognitive Assessment.
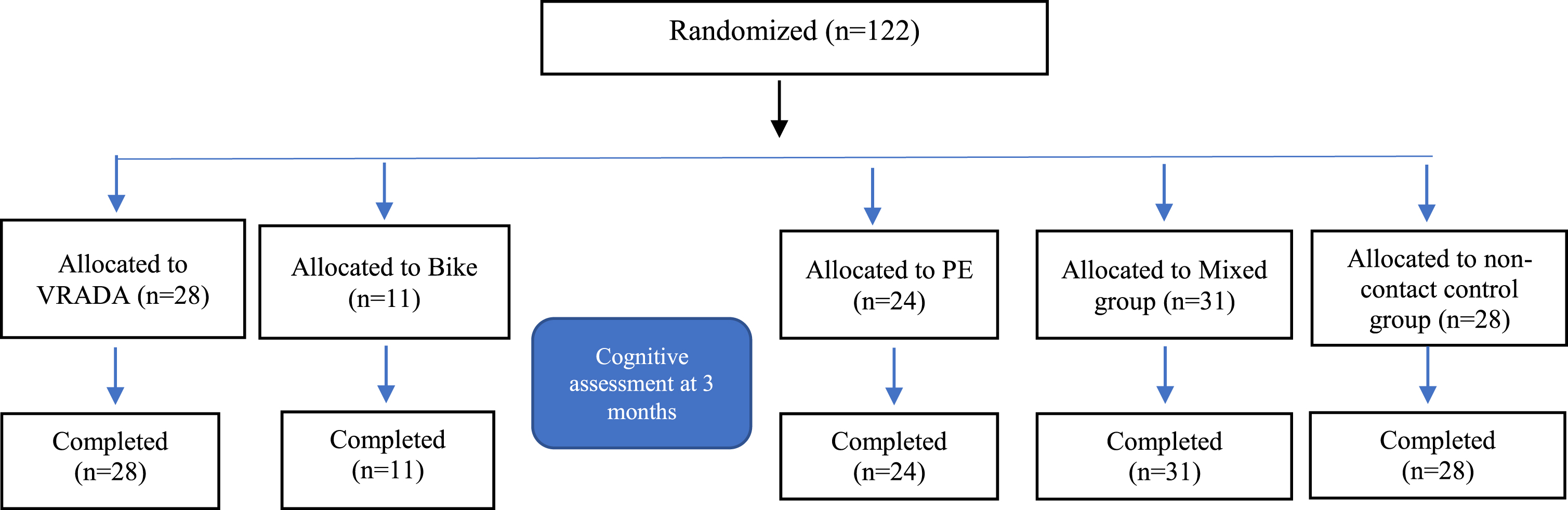
Furthermore, before the beginning of the intervention, the 122 participants underwent blood sugar, lactic acid, weight, and height measurements. In addition, blood was drawn from a specific number of participants from each group to determine the effect of exercise on specific biomarkers associated with cognitive decline. Of the total number of participants, 95 patients also agreed to donate blood before and after the end of the study (3-month follow-up). Furthermore, all participants were asked to complete a series of questionnaires concerning their health status, psychological state, fear of falling, physical activity, attitude and intention to exercise, enjoyment, and, for the VRADA group, the functionality and friendliness of the system. Finally, the neuropsychological assessment included a series of psychometric tests that were administered at the beginning of the intervention and three months afterward (pre- and post-intervention) by trained, experienced psychologists of the Day Care Centre “Saint Helen” (Alzheimer Hellas, DCCSH).
Design and development of the VRADA training system
A multidisciplinary team of experts, such as dementia experts, computer scientists, engineers, and healthcare professionals, provided expertise to encourage patients with dementia to improve their physical and cognitive skills [34]. Therefore, the VRADA system was developed based on a person-centered design, a systematic approach to developing usable products, systems, or services focusing explicitly on the target user [34]. It is known that individuals with MCI are characterized by apathy, low motivation, and low interest in daily activities, increasing the risk of disease progression [49]. To overcome this low motivation for exercise by patients with MCI, a series of motivational techniques originated from the self-determination theory was integrated into the VRADA system [34]. These techniques involved: (a) Goal setting, giving the participant a choice of the duration of the exercise at the beginning of each session; (b) Feedback on performance: after each session, the participant received informative feedback with regards to their exercise, such as duration, total cycling distance and the total number of correct answers on the cognitive exercises included in the session; (c) Task crafting: the ability to choose music to listen during the exercise; (d) self-monitoring of behavior: screen indicator of time, speed and distance [34]. Therefore, the VRADA system was developed to motivate and encourage MCI patients to exercise physically and cognitively via a user-friendly, effective, and safe system [34].
After the development of the VRADA prototype, Hassandra and colleagues conducted two feasibility studies to evaluate the accessibility, usability, and tolerability of the initial application in both University students (n = 37) (named VR1a) and patients with MCI (n = 20) (named VR2b) [34]. Both studies, VR2a and VR2b, showed that the scales included had high internal consistency (Cronbach’s alpha between 0.79 and 0.89), except for the System Usability Scale, which had slightly lower yet acceptable internal consistency (Cronbach’s alpha = 0.067 for VR2a and 0.068 VR2b, respectively) [34].
Equipment and training system
The hardware devices composing the training system were a cycle-ergometer (stationary seated bike type; Toorx, ChronoLine, BRX R 300), a VR head-mounted display (the Oculus Go headset) with a single 3DOF controller (see Fig. 1) [34]. In addition, the VRADA application was based on the ORamaVR MAGES platform [50, 51]. An enjoyable virtual environment (VR) was developed to emulate the exercise in a forest. At the beginning of each session, each user was required to choose the duration of their virtual exercise, i.e., cycling. For this purpose, we adapted a ray cast from the VR controller that allowed each user to choose a response simply by pointing and pressing the ray at the button on the controller (Fig. 2) [34]. The forest is dynamically generated as the participant cycles along the forest path. In parallel, animals appear in the forest during the exercise, and the user must remember the memory game at the end of the session [34]. Finally, users were trained to perform math calculations that appeared within the VR app (single-digit additions and subtractions) [34]. At the end of the session, each participant’s performance data were shown on the bike display, i.e., the number of correct or/and wrong answers, along with a visual and verbal reward (see Fig. 3) [34].
Fig. 3
The Oculus Go Head-Mounted display.

Therefore, the VRADA system, in an integrated manner, allows participants with MCI to complete cognitive tasks while cycling.
Training protocols
The VRADA and the bike group had to complete 32 physical and cognitive training sessions performed 2 or 3 times a week for 12 weeks. The VR-based intervention session carried out in a quiet room of the Day Care Centre “Saint Helen” was the same for each participant and lasted 20 min during the first five sessions and at a specific speed (20 km/h) with a progressive increase in time up to 30 min and speed up to 30 km/h (mild to moderate intensity). During each session, participants were instructed to complete (a) 20 simple numerical calculations (single-digit additions and subtractions) while cycling and (b) a memory game (how many animals appeared in the forest) (see Fig. 4) [34]. To answer, patients used a remote control [34].
Fig. 4
Selection by the user of the duration of the training session.
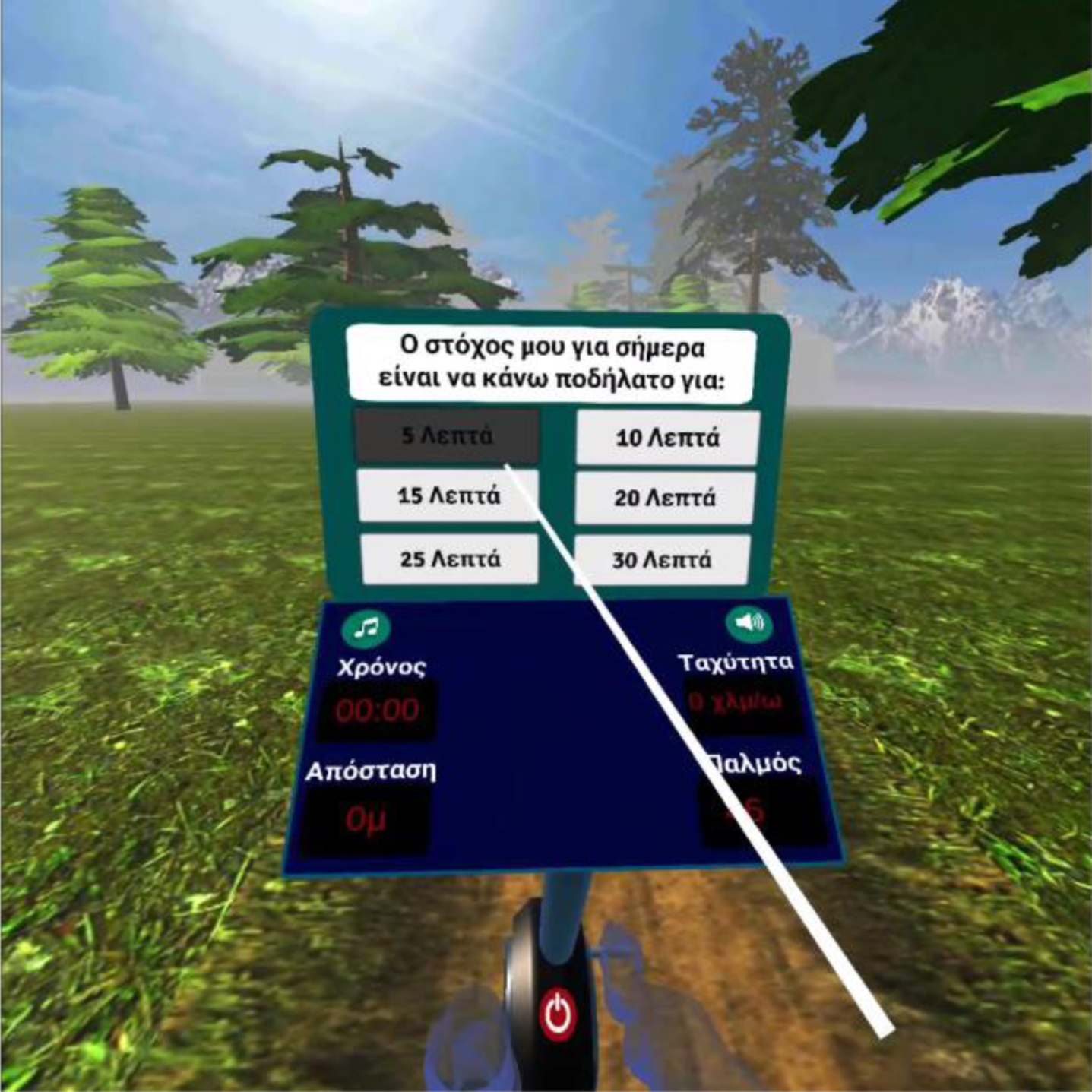
Fig. 5
User selects the correct answer.
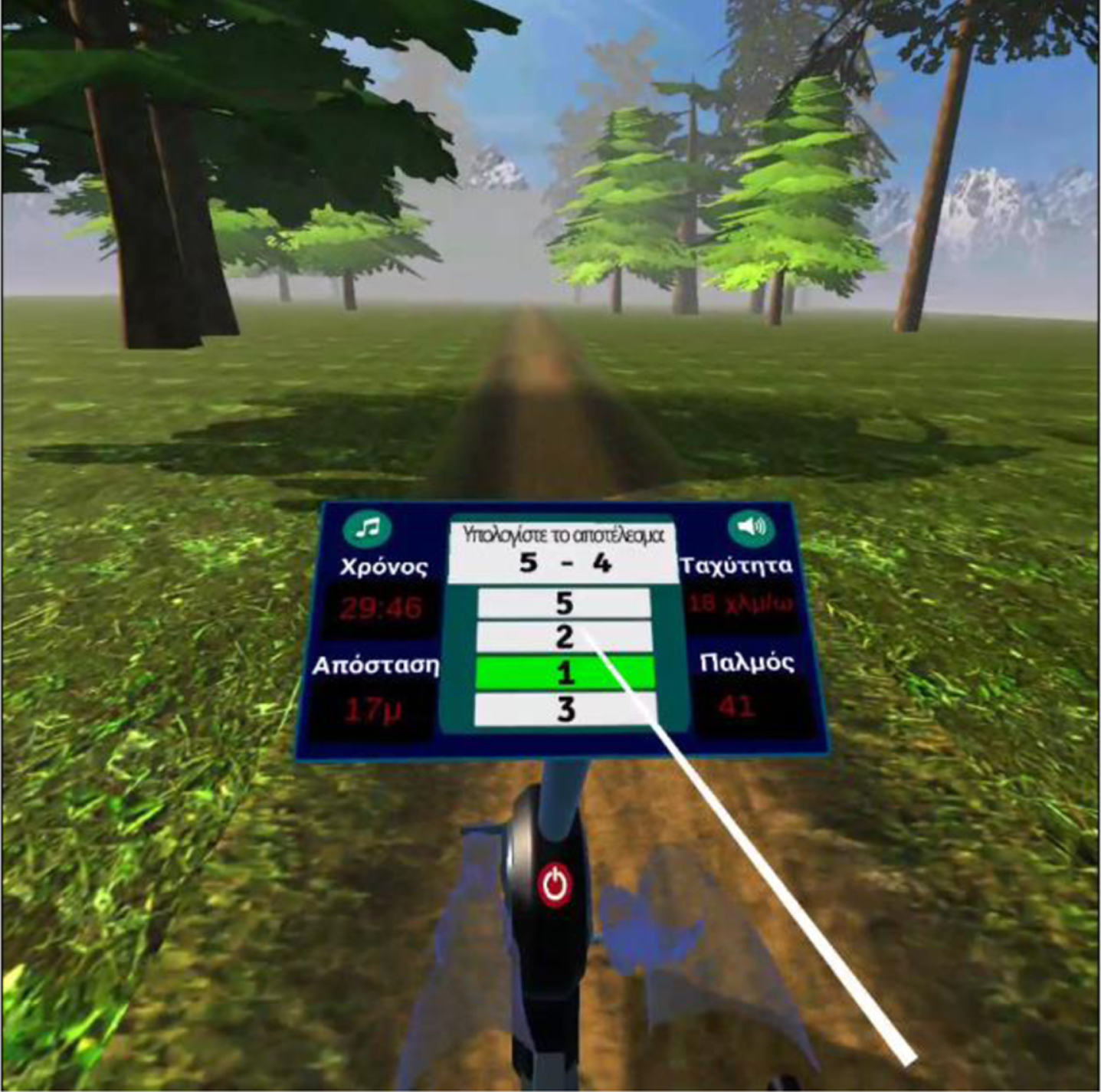
Fig. 6
Closing scene after each session.
Fig. 7
The forest where the animals appeared.

The bike group also had 32 sessions over 12 weeks, performed 2-3 times per week for 20–30 min each. While cycling at a mild to moderate intensity (starting at 20 km per hour for the first five sessions and up to 30 km per hour for the remaining ones), participants were asked by the experimenter to complete 20 simple numerical calculations (single-digit additions and subtractions).
The PE group had 32 sessions over 12 weeks, performed 2 to 3 times per week for 45 min each. Participants engaged in simple and complex exercises, following simple, rhythmic, verbal instructions. The routines included exercises for the head and neck, exercises for the shoulders, and a double workout for the hands and hips.
The Mixed group underwent 32 sessions of simple and complex physical exercise routines while seated over 12 weeks, held 2-3 times weekly for 45 min each. The routines included: (a) turning the head and neck while counting time and repetitions; (b) shoulder exercises (shrug both shoulders x 10 (counting each repetition either using digits or the alphabet letters); (c) hands and hips (dual-task) (raise the right foot slightly while making a fist, lower the foot and relax the hand, raise the left foot and stretch hands, lower the foot and relax hand x 10 (counting each repetition either using digits or the alphabet letters) a dual-task, hands, and hips exercise with counting).
Finally, the non-contact control group did not attend any intervention, and it was passive.
At the end of the session, the same assessments were administered to the participants to determine the effect of physical and cognitive exercise on the participants’ cognitive and physical abilities and specifically which groups differed from their initial condition and which type of intervention played the most crucial role (see Table 1 for details on the interventions for each group).
Table 1
Training protocol and intervention details per group
| Group | Duration | Frequency | Intervention details |
| VRADA | 12 weeks, 32 sessions, 20–30 min | 2-3 times per week | VR combined intervention: physical and cognitive exercises. Physical exercise (cycling): 20 min during the first 5 sessions (20 km/h) with a progressive increase in time up to 30 min and speed up to 30 km/h (mild to moderate intensity). Cognitive exercises while cycling: 20 simple numerical calculations (single-digit additions and subtractions); memory game (participants asked to remember the number of animals that appeared in the forest), max. Number of animals 5). |
| Bike | 12 weeks, 32 sessions, 20–30 min | 2-3 times per week | Physical exercise (cycling): 20 min. During the first 5 sessions (20 km/h), with a progressive increase in time up to 30 min and speed up to 30 km/h (mild to moderate intensity). Cognitive exercise: 20 simple numerical calculations (single-digit additions and subtractions), asked orally by the researcher. |
| Physical exercise | 12 weeks, 32 sessions, 45 min | 2-3 times per week | Physical simple and complex exercise routines at a seated position, participants followed simple, verbal, rhythmic instructions: (a) head and neck exercises, (b) shoulder exercises, and (c) a dual-task, hands, and hips exercise |
| Mixed | 12 weeks, 32 sessions, 45 min | 2-3 times per week | Physical simple and complex exercise routines at a seated position, participants followed simple, rhythmic, verbal instructions: (a) turning the head and the neck while counting time and repetitions; (b) shoulders (shrug both shoulders x 10 (counting each repetition either using digits or the alphabet letters); (c) Hands and Hips (dual-task): Raise right foot slightly while making a fist, lower the foot and relax the hand, raise the left foot and stretch hands, lower the foot and relax hand x 10 (counting each repetition either using digits or the alphabet letters) |
| Non-contact control | No intervention |
Assessments
To test the effectiveness of the VRADA intervention, the MMSE [38, 52] as a measure of general cognitive function, the TMT-B [53–55] as a measure of executive functions, the Rey auditory verbal learning test (RAVLT) [56–59], and the Digit Span Forward test [60, 61], as measures of memory, were performed. These tests are presented in detail below.
Mini-Mental State Examination. The MMSE comprises ten sub-items that measure varied brain domains: orientation to time, orientation to place, registration of three words, attention and calculation, recall of three words, language, and visual construction [38, 52]. The maximum score indicates good cognitive functioning, 30 points, while the lowest score is 0, indicating severe cognitive deficits. The threshold for the diagnosis of dementia in the Greek population is 23/24 [52]. The Greek version of the MMSE has been proved to be valid during test and retest with a Spearman’s coefficient p = 0.98 (p < 0.001) [53]. At the score level of 23/24, sensitivity was proved to be 90.80, specificity 90.62, and positive predictive value of 92.94 [52].
Trail Making Test-B. The TMT-B [53–55] examines mental flexibility, executive control, perception speed, concentration, working memory, mental tracking, and attention alternation. It involves five conditions: visual scanning, number sequence, letter sequence, number-letter alternation, and movement speed. The task requires participants to draw lines to connect, 25 numbered (1-13) and letters (A-M) (i.e., 1-A-2-B-3-C and so on) arranged in circles and randomly distributed on a paper surface from 1 to 25 (i.e., 1-2-3-4). The score represents the time required to complete the test (time in seconds) and the accuracy (number of errors); therefore, higher scores indicate worse performance. The average score is 75, and a deficient score is over 273 s. If the participant cannot complete the test in 5 min, the test is discontinued [55]. The test was administered and adapted in Greek-speaking elders with and without dementia and helps identify healthy individuals from patients with dementia [54].
Rey auditory verbal learning test. The RAVLT test [56–59] is designed to evaluate the verbal memory request of the subject in recalling 15 unrelated words over five trials, followed by a second list to serve as interference and subsequent short- and long-delay recall of the original list. Three scores were computed: the immediate recall score (maximum: 15), the total score for three recalls (maximum: 45), and the delayed recall score (maximum: 15). The Greek version of the RAVLT was recently adapted to an elderly Greek population with MCI, AD, and without dementia (from 60 to 89 years old) and discriminative and validity data were established [60]. In the group of participants with and without MCI and between 60 and 69 years old, sensitivity for the total score (cutoff score: 40.5/45) was 84.2%, and specificity was 61.1%. The respective scores for the group of the same participants from 70 to 79 years old (cutoff score: 35.5/45) were 81.9% and 39.3% [58]. Finally, for the age group of 80 to 89 (cutoff score: 27.5/45), sensitivity was 77.6%, and specificity was 45.6% [58].
Digit Span Forward test. The Digit Span Forward test, a subtest of the Wechsler Memory Scale [60, 61], was administered to assess short-term memory. Strings of numbers were read to participants, who had to repeat the digits in the same order they were presented. The number of correctly repeated rows was recorded. The maximum possible score is 16. As mentioned, the Wechsler Adult Intelligence Scale (WAIS) is standardized in the Greek population above 16, showing very high validity and reliability. The reliability of repeated measures (Pearson’s correlation coefficient r between the first and second administration scores) ranged between 0.87 and 0.94, and internal consistency (Cronbach’s alpha from 0.90 to 0.97) [61].
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 26 (IBM Corp. Armonk, NY). First, after the data were checked for homogeneity of variance and normality of distribution, demographic variables were compared across groups using one-way ANOVA for continuous (i.e., age, body weight, and height) and Chi-Square test for categorical variables (i.e., gender and educational level), respectively. Second, a series of Generalized Linear Models, specifically linear regressions, were conducted for differences in performance between groups while controlling for covariates (age, educational level, and baseline score). The significance level was set at p≤0.05.
RESULTS
Demographic characteristics
The demographic characteristics of the study sample are presented in Table 2. The five groups had a statistically significant difference in age, F(4,117) = 3.852, p = 0.006, gender χ2(4, 122) =13.425, p = 0.009, with female participants outnumbering male participants. Furthermore, there were differences in educational level between groups χ2(12,120) = 22.291, p = 0.034. However, there were no statistically significant differences in body weight between groups, F(4,106) = 0.649, p = 0.629, and height between participants F(4,107) = 1.356, p = 0.254) (see Table 2 for demographic characteristics of participants by group).
Table 2
Demographic and anthropometric characteristics by group in experimental and control groups
| VRADA group | Bike group | PE group | Mixed group | Non-contact control group | |||||||
| (N = 28) | (N = 11) | (N = 24) | (N = 31) | (N = 28) | |||||||
| M (SD) | Range | M (SD) | Range | M (SD) | Range | M (SD) | Range | M (SD) | Range | p | |
| Age (y) | 66.07(10.04) | 49–87 | 73.00 (8.54) | 58–87 | 70.96 (7.95) | 43–82 | 70.39 (7.36) | 62–85 | 74.36 (7.04) | 43–88 | 0.006 |
| Weight (kg) | 73.08(15.14) | 51–114 | 72.83 (13.62) | 53–97 | 80.77 (25.73) | 57–177 | 73.29 (13.46) | 49–118 | 74.31 (25.73) | 43–88 | 0.629 |
| Height (cm) | 162.5 (7.65) | 149–180 | 164.7 (10.27) | 146–180 | 161.4 (9.54) | 149–172 | 160.5 (6.55) | 130–178 | 158.29 (8.46) | 145–173 | 0.254 |
| Gender (F/M) | 21/7 | 5/6 | 20/4 | 29/2 | 24/4 | 0.009 | |||||
| Occupation (%) | 74.1% retired | 100% retired | 95.8% retired | 96.8% retired | 95.8% retired | 0.030 | |||||
| 22.2% employed | 3.2% employed | 4.2% employed | |||||||||
| 3.7% unemployed | 3.7% unemployed | ||||||||||
| Educational level (y) (%) | 17.9% (Primary school) | 30% (primary school) | 16.7% (primary school) | 19.4% (primary school) | 25.9% (primary school) | 0.034 | |||||
| 35.7% (high school) | 10% (Junior high school) | 20.8% (Junior high school) | 45.2% (high school) | 40.7% (high school) | |||||||
| 46.4% (University) | 10% (high school) | 33.3% (high school) | 35.5% (University) | 33.3% (University) | |||||||
| 50% (University) | 29.2% (University) | ||||||||||
M, mean; SD, standard deviation; p, statistical significance; PE, physical exercise.
Group differences
To test scores between the VRADA group and the comparison groups in the neuropsychological tests Pre- and Post-intervention, first, we computed the mean scores of the difference between pre- to post-intervention in each neuropsychological test (see Table 3 for the means and standard deviation in neuropsychological measures in pre- and post- assessment), and afterward, we conducted a series of linear regression models. Regression models revealed statistically significant differences between the VRADA group and the other groups in several domains, including (a) general cognitive function as measured by the MMSE test; (b) memory as assessed by the RAVLT and the WAIS (digits forward) test; and, (c) executive functions and mental flexibility specifically, as measured by the TMT-B test.
Table 3
Means and Standard Deviations on Neuropsychological tests (in the difference between pre- to post-intervention)
| VRADA group | Bike group | PE group | Mixed group | Non-contact control group | ||||||
| (N = 28) | (N = 11) | (N = 24) | (N = 31) | (N = 28) | ||||||
| M (SD) | Range | M (SD) | Range | M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| MMSE (diff) | –0.210 (0.41) | –1.00–0.00 | 0.363 (1.20) | 0.00–4.00 | 0.00 (0.43) | –1.0–1.00 | 0.040 (0.53) | –1.00–1.00 | –0.041 (0.35) | –1.00–1.00 |
| RAVLT-IV (diff) | 0.038 (6.59) | –11.0–26.0 | 9.54 (15.60) | –3.0–42.00 | 0.36 (9.01) | –17.0–33.0 | –1.28 (4.14) | –11.0–6.00 | 0.518 (4.45) | –9.0–16.0 |
| WAISforward (diff) | –0.210 (0.41) | –1.00–0.00 | 0.363 (1.20) | 0.0–4.00 | 0.00 (0.43) | –1.00–1.00 | 0.040 (0.53) | –1.00–1.00 | –0.041 (0.35) | –1.00–1.00 |
| TMT-B | 16.81 (47.31) | –30.0–194.0 | 26.54 (61.82) | –25–194.0 | 0.619 (73.14) | –122–177 | 21.79 (76.54) | –161–268 | –20.18 (72.54) | –241–88 |
M, mean; SD, standard deviation; MMSE, Mini-Mental State Examination; RAVLT-IV, Rey auditory verbal learning test; TMT-B, Trail Making Test B; WAISforward, WAIS Digit Span Forward test; PE, physical exercise.
In more detail, as shown in Table 4, there was a statistically significant difference in the performance in the MMSE test of the bike group compared to the VRADA group after controlling for age, educational level, and MMSE pre-intervention score. More specifically, the VRADA group significantly improved its global cognitive function compared to the bike group. Specifically, the bike group (M = 0.363, SD = 1.20) (p = 0.006, ηp2 = 0.072) did not improve their performance after the intervention, as their performance before the intervention exceeded their post-intervention. In contrast, the VRADA group improved their performance post-intervention (M = –0.210, SD = 0.41) (see Table 4).
Table 4
Results from the general linear model for the difference in the performance between the VRADA and the control groups in the MMSE test in relation to age, educational level, and MMSE pre-score
| Parameter | b | SE | Wald χ2 | df | p |
| Intercept | 0.642 | 0.949 | 0.457 | 1 | 0.499 |
| Non-contact control group | 0.178 | 0.190 | 0.876 | 1 | 0.349 |
| Mixed group | 0.282 | 0.178 | 2.502 | 1 | 0.114 |
| PE group | 0.199 | 0.187 | 1.118 | 1 | 0.290 |
| Bike group | 0.628 | 0.229 | 7.455 | 1 | 0.006 |
| VRADA group (reference group) | – | – | – | – | – |
| Age | 0.000 | 0.071 | 0.000 | 1 | 0.997 |
| Educational level | 0.016 | 0.015 | 1.122 | 1 | 0.289 |
| MMSE pre | –0.038 | 0.292 | 1.714 | 1 | 0.190 |
The table shows the regression coefficients (b), standard errors (SE), Wald χ2 test with appropriate degrees of freedom (df) and statistical significance (p). MMSE, Mini-Mental State Examination; PE, physical exercise.
Subsequently, in the memory area, as assessed by the RAVLT, the linear regression analysis showed a statistically significant difference in the performance of the bike group as compared to the VRADA group after controlling for age and educational level. More specifically, the bike group (M = 9.54, SD = 15.60) (p = 0.007, ηp2 = 0.142) did not improve its performance after the intervention (as the performance before the intervention exceeded its performance after the intervention, while the VRADA group seemed to maintain its performance at almost the same level after the intervention) (M = 0.038, SD = 6.59) (Table 5).
Table 5
Results from the general linear model for the difference in the performance between the VRADA group and the other groups in the RAVLT-IV test in relation to age, educational level, and RAVLT-IV pre
| Parameter | b | SE | Wald χ2 | df | p |
| Intercept | –20.21 | 4.343 | 21.671 | 1 | < 0.001 |
| Non-contact control group | –1.398 | 1.419 | 0.970 | 1 | 0.325 |
| Mixed group | –3.075 | 1.370 | 5.039 | 1 | 0.025 |
| PE group | –2.783 | 1.438 | 3.741 | 1 | 0.053 |
| Bike group | 5.023 | 1.860 | 7.290 | 1 | 0.007 |
| VRADA group (reference group) | – | – | – | – | – |
| Age | 0.167 | 0.574 | 8.502 | 1 | 0.004 |
| Educational level | 0.061 | 0.120 | 0.254 | 1 | 0.614 |
| RAVLT-IV pre | 0.929 | 0.073 | 157.8 | 1 | < 0.001 |
The table shows the regression coefficients (b), standard errors (SE), Wald χ2 test with appropriate degrees of freedom (df) and statistical significance (p). RAVLT, Rey auditory verbal learning test; PE, physical exercise.
Concerning the WAIS forward test, the regression coefficients showed a statistically significant difference in the performance of the bike group as compared to the VRADA group. More specifically, the bike group exhibited, on average, higher performance before the intervention (M = 3.09, SD = 10.25) (p = 0.003, ηp2 = 0.091) as compared to the VRADA group, whose performance improved after the intervention (M = –0.210, SD = 0.41) after controlling for age and educational level (see Table 6).
Table 6
Results from the general linear model for the difference in the performance between the VRADA group and the other groups in the WAISforward test in relation to age, educational level, and WAISforward pre-score
| Parameter | b | SE | Wald χ2 | df | p |
| Intercept | –5.648 | 3.401 | 2.756 | 1 | 0.097 |
| Non-contact control group | 0.174 | 1.097 | 0.300 | 1 | 0.584 |
| Mixed group | 0.257 | 1.025 | 0.114 | 1 | 0.736 |
| PE group | 0.224 | 1.067 | 0.013 | 1 | 0.909 |
| Bike group | 3.616 | 1.331 | 8.754 | 1 | 0.003 |
| VRADA group (reference group) | – | – | – | – | – |
| Age | 0.000 | 0.039 | 0.000 | 1 | 1.000 |
| Educational level | –0.045 | 0.083 | 0.288 | 0.002 | |
| WAISforward pre | 1.143 | 0.373 | 9.359 | 1 | 0.591 |
The table shows the regression coefficients (b), standard errors (SE), Wald χ2 test with appropriate degrees of freedom (df) and statistical significance (p). WAISforward, WAIS Digit Span Forward test; PE, physical exercise.
Regarding executive functions and mental flexibility, in particular, as assessed with TMT-B, the regression coefficients showed a statistically significant difference in the performance of the non-contact control group and the VRADA group after controlling for age and educational level. More specifically, the VRADA group (M = 16.81, SD = 47.31) seems to maintain almost the same performance in mental flexibility as compared to the non-contact control group, in which a statistically significant improvement is shown after the intervention (M =–20.18, SD = 72.54) (p = 0.016, ηp2 = 0.066) (Table 7).
Table 7
Results from the general linear model for the difference in performance of the VRADA group from the other groups in TMT-B, in relation to age, educational level, and TMT-B pre
| Parameter | b | SE | Wald χ2 | df | p |
| Intercept | –63.31 | 51.90 | 1.448 | 1 | 0.222 |
| Non-contact control group | –41.24 | 17.15 | 5.780 | 1 | 0.016 |
| Mixed group | –3.96 | 15.97 | 0.061 | 1 | 0.804 |
| PE group | –10.69 | 17.02 | 0.395 | 1 | 0.530 |
| Bike group | 12.26 | 21.33 | 0.331 | 1 | 0.565 |
| VRADA group (reference group) | – | – | – | – | – |
| Age | –0.107 | 0.716 | 0.022 | 1 | 0.881 |
| Educational level | 0.281 | 1.465 | 0.037 | 1 | 0.848 |
| TMT-B pre | 0.564 | 0.087 | 41.85 | 1 | < 0.001 |
The table shows the regression coefficients (b), standard errors (SE), Wald χ2 test with appropriate degrees of freedom (df), and statistical significance (p). PE, physical exercise; TMT-B, Trail Making Test.
Summary
Overall, the results supported our hypothesis that MCI patients of the VRADA group had statistically significant improvements in several cognitive measures after the intervention compared to those of the groups. More specifically, the VRADA group showed significant improvements in general cognitive function, as measured by the MMSE test, in contrast to the bike group, which did not demonstrate such improvement. In addition, the VRADA group performed significantly better in the RAVLT, which assessed memory. In contrast, the bike group did not show a similar performance. Finally, the VRADA group performed significantly better in executive functions and mental flexibility, as tested with the TMT-B. However, they did not perform as well as the non-contact control group. Our results confirmed our hypotheses that VRADA intervention may positively impact cognitive abilities such as general cognitive skills, memory, and executive functions of elders with MCI.
DISCUSSION
VR is increasingly becoming a technology that can support individuals with dementia by providing fun, interest, and real-time feedback [34, 35, 45, 62, 63]. People with MCI have been supported through VR-based interventions and showed either improvement or at least no deterioration. This study aimed to assess the effects of a VR-based cognitive and physical training program, VRADA, on cognitive status in elders with MCI and compare training via VR technology to a more traditional form of intervention (i.e., both physical and cognitive). Based on previous research, we expected improvements in the cognitive status of individuals with MCI trained in the VR environment. Our results confirmed our hypothesis, indicating that VR-based multi-component intervention improved patients’ cognitive function with MCI more than other intervention modalities. These findings are in line with previous studies like Mrakic-Sposta et al. [4], Barnes et al. [41], Hwang and Lee [42], Liao and colleagues [43, 64], Doniger et al. [44], and Park et al. [28, 45]. Furthermore, they report significant improvements in cognitive abilities before and after VR-based intervention for individuals with MCI. More specifically, our findings align with Park and colleagues [45]. After implementing a VR-based cognitive–motor rehabilitation intervention program for six weeks in 40 individuals with MCI, they found significant improvements in their executive functions, global cognitive function (assessed via MoCA scale), and memory (i.e., mental flexibility, executive control, perception speed, concentration, working memory, mental tracking, and attention alternation) (assessed through the TMT-A and -B) and in their short-term and working memory (assessed through Digit Span Test forward and backward)—similarly, our results line with Mrakic-Sposta and colleagues [4]. After providing multidimensional physical and cognitive VR intervention, they also found improvements in the executive functions of patients with MCI (verbal fluency, memory, and attention).
In addition, our results indicated that even though in some cognitive abilities, like short-term memory (as measured through the RAVLT), the VRADA group did not show improvement, their performance remained unchanged before and after the intervention compared to the bike group. One possible explanation for this finding is that VR training (combined with computerized cognitive training) may effectively delay cognitive decline [31, 65]. Some improvements were also noticed in the active-control groups (i.e., the PE and the mixed group) in the areas of language skills (as measured with the writing subscale of the BDAE test) or global functioning (MMSE), which is excepted as it is well known that physical exercise affects cognitive functions by raising the level of neurotrophic factor that comes from the brain and blood flow to the hippocampus, with beneficial metabolic effects as a result [43–45]. However, training in a VR environment is more beneficial as it also increases the motivation for participation, which in turn activates thinking via the activation of brain neurotransmitter pathways like cholinergic and dopaminergic systems, which have been shown to contribute to better concentration and memory in the older people [35, 45, 65].
Conclusions, limitations, and relevance
Limitations of this intervention study are linked mainly to the COVID-19 pandemic era in that it was conducted (i.e., with a reduced sample in some groups). In addition, the gender distribution of the study was skewed as there were more female patients than male ones. Therefore, a replication of this study with a larger sample, more evenly divided between the genders, and long-term follow-ups are necessary for estimating and evaluating rehabilitation efficacy. Despite the limited number of participants—in some groups—the strength of this study includes a multimodal intervention approach, the comparison of the VR-based combined intervention with different types of physical and cognitive intervention (e.g., physical aerobic exercise but without the VR component or physical exercise) to allow comparison of the impact of each component. Future research should include a more in-depth evaluation of the efficacy of the VRADA system via a randomized controlled trial with larger sample sizes, extended intervention period, and the effectiveness of VR-based interventions, like VRADA, in other measures like quality of life or social skills.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the ongoing support from the Greek Association of Alzheimer’s Disease and Related Disorders during the VRADA project.
FUNDING
This research is co-funded by the European Regional Development Fund of the E.U. and Greek national funds through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH–CREATE–INNOVATE (project code: T1EDK-01448). The funding agency played no role in the design or execution of the study, analysis or interpretation of the data, or decision to submit the results.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data sharing does not apply to this article as no datasets were generated or analyzed during this study.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230099.
REFERENCES
[1] | Petersen RC , Roberts RO , Knopman DS , Boeve BF , Geda YE , Ivnik RJ , Smith GE , Jack CR ((2009) ) Mild cognitive impairment: Ten years later. Arch Neurol 66: , 1447–1455. |
[2] | Sachdev PS , Lipnicki DM , Crawford J , Reppermund S , Kochan NA , Trollor JN , Wen W , Draper B , Slavin MJ , Kang K , Lux O , Mather KA , Brodaty H , Sydney Memory, Ageing Study Team ((2013) ) Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: A population-based study. PLoS One 8: , e59649. |
[3] | Michaud TL , Su D , Siahpush M , Murman DL ((2017) ) The risk of incident mild cognitive impairment and progression to dementia considering mild cognitive impairment subtypes. Dement Geriatr Cogn Dis Extra 7: , 15–29. |
[4] | Mrakic-Sposta S , Di Santo SG , Franchini F , Arlati S , Zangiacomi A , Greci L , Moretti S , Jesuthasan N , Marzorati M , Rizzo G , Sacco M , Vezzoli A ((2018) ) Effects of combined physical and cognitive virtual reality-based training on cognitive impairment and oxidative stress in MCI patients: A pilot study. Front Aging Neurosci 10: , 282. |
[5] | Huh Y , Yang EJ , Lee SA , Lim JY , Kim KW , Paik NJ ((2011) ) Association between executive function and physical performance in older Korean adults: Findings from the Korean Longitudinal Study on Health and Aging (KLoSHA). Arch Gerontol Geriatr 52: , e156–e161. |
[6] | Moretti F , De Ronchi D , Palmer K , Forlani C , Morini V , Ferrari B , Dalmonte E , Atti AR ((2013) ) Prevalence and characteristics of mild cognitive impairment in the general population. Data from an Italian population-based study: The Faenza Project. Aging Ment Health 17: , 267–275. |
[7] | Rossini PM , Miraglia F , Vecchio F ((2022) ) Early dementia diagnosis, MCI-to-dementia risk prediction, and the role of machine learning methods for feature extraction from integrated biomarkers, in particular for EEG signal analysis. Alzheimers Dement 18: , 2699–2706. |
[8] | Hussenoeder FS , Conrad I , Roehr S , Fuchs A , Pentzek M , Bickel H , Moesch E , Weyerer S , Werle J , Wiese B , Mamone S , Brettschneider C , Heser K , Kleineidam L , Kaduszkiewicz H , Eisele M , Maier W , Wagner M , Scherer M , König HH , Riedel-Heller SG ((2020) ) Mild cognitive impairment and quality of life in the oldest old: A closer look. Qual Life Res 29: , 1675–1683. |
[9] | Ma L ((2020) ) Depression, anxiety, and apathy in mild cognitive impairment: Current perspectives. Front Aging Neurosci 12: , 9. |
[10] | Kuring JK , Mathias JL , Ward L ((2018) ) Prevalence of depression, anxiety and PTSD in people with dementia: A systematic review and meta-analysis. Neuropsychol Rev 28: , 393–416. |
[11] | Roberts RO , Knopman DS , Mielke MM , Cha RH , Pankratz VS , Christianson TJ , Geda YE , Boeve BF , Ivnik R J , Tangalos EG , Rocca WA , Petersen RC ((2014) ) Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 82: , 317–325. |
[12] | Lyu Q , Cheung DSK , Lai H , Wang X , Qiu J , Huang Y , Zeng Y ((2020) ) A multi-component integrative intervention to slow down the progression of mild cognitive impairment: A protocol for a randomized controlled trial. Res Nurs Health 43: , 307–316. |
[13] | Yang C , Moore A , Mpofu E , Dorstyn D , Li Q , Yin C ((2020) ) Effectiveness of combined cognitive and physical interventions to enhance functioning in older adults with mild cognitive impairment: A systematic review of randomized controlled trials. Gerontologist 60: , 633–642. |
[14] | Gómez-Soria I , Marin-Puyalto J , Peralta-Marrupe P , Latorre E , Calatayud E ((2022) ) Effects of multi-component non-pharmacological interventions on cognition in participants with mild cognitive impairment: A systematic review and meta-analysis. Arch Gerontol Geriatr 103: , 104751. |
[15] | Lautenschlager NT , Cox KL , Ellis KA ((2019) ) Physical activity for cognitive health: What advice can we give to older adults with subjective cognitive decline and mild cognitive impairment? Dialogues Clin Neurosci 21: , 61–68. |
[16] | Baumgart M , Snyder HM , Carrillo MC , Fazio S , Kim H , Johns H ((2015) ) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11: , 718–726. |
[17] | Cammisuli D , Innocenti A , Franzoni F , Pruneti C ((2017) ) Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch Ital Biol 155: , 54–62. |
[18] | del Pozo Cruz B , Ahmadi M , Naismith SL , Stamatakis E ((2022) ) Association of daily step count and intensity with incident dementia in 78 430 adults living in the U.K. JAMA Neurol 79: , 1059–1063. |
[19] | Baker LD , Frank LL , Foster-Schubert K , Green PS , Wilkinson CW , McTiernan A , Plymate SR , Fishel MA , Watson GS , Cholerton BA , Duncan GE , Mehta PD , Craft S ((2010) ) Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol 67: , 71–79. |
[20] | Nagamatsu LS , Chan A , Davis JC , Beattie BL , Graf P , Voss MW , Sharma D , Liu-Ambrose T ((2013) ) Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: A 6-month randomized controlled trial. J Aging Res 2013: , 861893. |
[21] | Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Ito K, Shimokata H, Washimi Y, Endo H, Kato T ((2013) ) A randomized controlled trial of multi-component exercise in older adults with mild cognitive impairment. PLoS One 8: , e61483. |
[22] | Donnezan LC , Perrot A , Belleville S , Bloch F , Kemoun G ((2018) ) Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment Health Phys Act 15: , 78–87. |
[23] | Steichele K , Keefer A , Dietzel N , Graessel E , Prokosch H-U , Kolominsky-Rabas PL ((2022) ) The effects of exercise programs on cognition, activities of daily living, and neuropsychiatric symptoms in community-dwelling people with dementia—a systematic review. Alzheimers Res Ther 14: , 97. |
[24] | Lu Y , Liu C , Yu D , Fawkes S , Ma J , Zhang M , Li C ((2021) ) Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: A meta-analysis and systematic review. BMC Geriatr 21: , 10. |
[25] | Clare L , Woods B ((2003) ) Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev, CD003260. |
[26] | Bae S , Lee S , Lee S , Jung S , Makino K , Harada K , Shinkai Y , Chiba I , Shimada H ((2019) ) The effect of a multi-component intervention to promote community activity on cognitive function in older adults with mild cognitive impairment: A randomized controlled trial. Complement Ther Med 42: , 164–169. |
[27] | Özbe D , Graessel E , Donath C , Pendergrass A ((2019) ) Immediate intervention effects of standardized multi-component group interventions on people with cognitive impairment: A systematic review. J Alzheimers Dis 67: , 653–670. |
[28] | Park H , Park JH , Na H.R. , Hiroyuki S , Kim GM , Jung MK , Kim WK , Park KW ((2019) ) Combined intervention of physical activity, aerobic exercise, and cognitive exercise intervention to prevent cognitive decline for patients with mild cognitive impairment: A randomized controlled clinical study. J Clin Med 8: , 940. |
[29] | Li H , Li J , Li N , Li B , Wang P , Zhou T ((2011) ) Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Res Rev 10: , 285–96. |
[30] | Coyle H , Traynor V , Solowij N ((2015) ) Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: Systematic review of the literature. Am J Geriatr Psychiatry 23: , 335–359. |
[31] | Rizzo A , Kim GJ ((2005) ) A SWOT analysis of the field of virtual reality rehabilitation and therapy. Presence 14: , 119–146. |
[32] | Kim O , Pang Y , Kim JH ((2019) ) The effectiveness of virtual reality for people with mild cognitive impairment or dementia: A meta-analysis. BMC Psychiatry 19: , 219. |
[33] | Zhu S , Sui Y , Shen Y , Zhu Y , Ali N , Guo C , Wang T ((2021) ) Effects of virtual reality intervention on cognition and motor function in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Front Aging Neurosci 13: , 586999. |
[34] | Hassandra M , Galanis E , Hatzigeorgiadis A , Goudas M , Mouzakidis C , Karathanasi EM , Petridou N , Tsolaki M , Zikas P , Evangelou G , Papagiannakis G , Bellis G , Kokkotis C , Panagiotopoulos SR , Giakas G , Theodorakis YA ((2021) ) A virtual reality app for physical and cognitive training of older people with mild cognitive impairment: Mixed methods feasibility study. JMIR Serious Games 9: , e24170. |
[35] | D’Cunha NM , Nguyen D , Naumovski N , McKune AJ , Kellett J , Georgousopoulou EN , Frost J , Isbel S ((2019) ) A mini-review of virtual reality-based interventions to promote well-being for people living with dementia and mild cognitive impairment. Gerontology 65: , 430–440. |
[36] | Kim BR , Chun MH , Kim LS , Park JY ((2011) ) Effect of virtual reality on cognition in stroke patients. Ann Rehabil Med 35: , 450–459. |
[37] | Talassi EM , Guerreschi M , Feriani V , Fedi A , Bianchetti M , Trabucchi M ((2007) ) Effectiveness of a cognitive rehabilitation program in mild dementia (MD) and mild cognitive impairment (MCI): A case-control study. Arch Gerontol Geriatr 44: , 391–399. |
[38] | Folstein M.F. , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[39] | Wilson B , Cockburn J , Baddeley A , Hiorns R ((1989) ) The development and validation of a test battery for detecting and monitoring everyday memory problems. J Clin Exp Neuropsychol 11: , 855–870. |
[40] | Yesavage JA , Brink TL , Rose TL , Lum O , Huang V , Adey M , Leirer VO ((1983) ) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: , 37–49. |
[41] | Barnes DE , Yaffe K , Belfor N , Jagust WJ , DeCarli C , Reed BR , Kramer JH ((2009) ) Computer-based cognitive training for mild cognitive impairment: Results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord 23: , 205–210. |
[42] | Hwang J , Lee S ((2017) ) The effect of virtual reality program on the cognitive function and balance of the people with mild cognitive impairment. J Phys Ther Sci 29: , 1283–1286. |
[43] | Liao Y-Y , Chen I-H , Lin Y-J , Chen Y , Hsu W-C ((2019) ) Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front Aging Neurosci 11: , 162. |
[44] | Doniger GM , Beeri MS , Bahar-Fuchs A , Gottlieb A , Tkachov A , Kenan H , Livny A , Bahat Y , Sharon H , Ben-Gal O , Cohen M , Zeilig G , Plotnik M ((2018) ) Virtual reality-based cognitive-motor training for middle-aged adults at high Alzheimer’s disease risk: A randomized controlled trial. Alzheimers Dement (N Y) 4: , 118–129. |
[45] | Park JS , Jung YJ , Lee G ((2020) ) Virtual reality-based cognitive–motor rehabilitation in older adults with mild cognitive impairment: A randomized controlled study on motivation and cognitive function. Healthcare (Basel) 8: , 335. |
[46] | Edition F ((2013) ) Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc 21: , 591–643. |
[47] | Petersen RC , Morris JC ((2005) ) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62: , 1160–1163. |
[48] | Hughes CP , Berg L , Danziger W , Coben LA , Martin RL ((1982) ) A new clinical scale for the staging of dementia. Br J Psychiatry 140: , 566–572. |
[49] | Fan Z , Wang L , Zhang H , Lv X , Tu L , Zhang M , Yan C , Yu X , Wang H ((2021) ) Apathy as a risky neuropsychiatric syndrome of progression from normal aging to mild cognitive impairment and dementia: A systematic review and meta-analysis. Front Psychiatry 12: , 792168. |
[50] | Papagiannakis G , Lydatakis N , Kateros S , Georgiou S , Zikas P ((2018) ) Transforming medical education and training with vr using mages. SIGGRAPH Asia 2018 Posters, pp. 1–2. |
[51] | Papagiannakis G , Zikas P , Lydatakis N , Kateros S , Kentros M , Geronikolakis E , Kamarianakis M , Evangelou G , Kartsonaki I , Apostolou A , Birrenbach T , Exadaktylos KA , Sauter CT , Papapagiannakis G ((2020) ) IMAGES 3.0: Tying the knot of medical VR. ACM SIGGRAPH 2020 Immersive Pavilion, pp. 1–2. |
[52] | Fountoulakis KN , Tsolaki M , Chantzi H , Kazis A ((2000) ) Mini mental state examination (MMSE): A validation study in Greece. Am J Alzheimers Dis Other Demen 15: , 342–345. |
[53] | Arbuthnott K , Frank J ((2000) ) Trail Making Test, Part B as a measure of executive control: Validation using a set-switching paradigm. J Clin Exp Neuropsychol 22: , 518–528. |
[54] | Poptsi E , Kounti F , Karagiozi K , Eleuftheriou M , Agogiatou C , Bacoglidou E , Tsolaki M ((2007) ) The administration of trail making test to Greek healthy elderly, to patients with mild cognitive impairment, preclinical dementia and mild dementia. In 2nd International Conference of the Psychological society of Nothern Greece, Psychological Assessment, Poptsi E, Kounti F, Karagiozi K, Eleuftheriou M, Agogiatou C, Bacoglidou E, Nikolaidou E, Nakou S, Zafiropoulou M, Kioseoglou G, Tsolaki M, eds.pp. 157–158. |
[55] | Ciolek CH , Lee SY ((2019) ) Cognitive issues in the older adult. Guccione’s Geriatric Physical Therapy E-Book, 425. |
[56] | Efklides A , Yiultsi E , Kangellidou T , Kounti F , Dina F , Tsolaki M ((2002) ) Wechsler Memory Scale, Rivermead Behavioral Memory Test, and Everyday Memory Questionnaire in healthy adults and Alzheimer’s patients. Eur J Psychol Assess 18: , 63. |
[57] | Rey A ((1958) ) L’examenclinique en psychologie [The psychological examination]. Presses Universitaires de France, Paris. |
[58] | Kounti F , Tsolaki M , Nikolaides E , Zafeiropoulou M , Kazis A , Kiosseoglou G , Efklides A ((2004) ) The administration of Rey Auditory Verbal Learning test to Greek healthy, mildly cognitively impaired and demented elderly. 1st International Conference of Quality of Life and Psychology, Thessaloniki, 110. |
[59] | Messinis L , Tsakona I , Malefaki S , Papathanasopoulos P ((2007) ) Normative data and discriminant validity of Rey’s Verbal Learning Test for the Greek adult population. Arch Clin Neuropsychol 22: , 739–752. |
[60] | Wechsler D ((1987) ) Wechsler memory scale-revised. The Psychological Corporation, New York. |
[61] | Stogiannidou A ((2014) ) Wechsler Adult Intelligence Scale–Fourth Edition (WAIS-IV GR). Standardization in Greek. Athens. |
[62] | Riaz W , Khan Z Y , Jawaid A , Shahid S ((2021) ) Virtual reality (VR)-based environmental enrichment in older adults with mild cognitive impairment (MCI) and mild dementia. Brain Sci 11: , 1103. |
[63] | Hill NT , Mowszowski L , Naismith SL , Chadwick VL , Valenzuela M , Lampit A ((2017) ) A systematic review and meta-analysis of computerized cognitive training in older adults with mild cognitive impairment or dementia. Am J Psychiatry 174: , 329–340. |
[64] | Liao Y-Y , Tseng H-Y , Lin Y-J , Wang C-J , Hsu W-C ((2020) ) Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment: A randomized controlled trial. Eur J Phys Rehabil Med 56: , 47–57. |
[65] | Yang QH , Lyu X , Lin QR , Wang ZW , Tang L , Zhao Y , Lyu QY ((2022) ) Effects of a multi-component intervention to slow mild cognitive impairment progression: A randomized controlled trial. Int J Nurs Stud 125: , 104110. |




