A Hierarchical Multi-Dimensional Cognitive Training Program for Preventive Cognitive Decline in Acute Ischemic Stroke Patients: Study Protocol for a Randomized Controlled Trial
Abstract
Background:
One of the most popular ways to address cognitive decline is cognitive training. The fact that cognitive deterioration is permanent is one of the main issues. This issue might be resolved by preventive cognitive training when it is acute. As a result, this study aims to design and assess how well stroke patients respond to hierarchical, multi-dimensional preventative cognitive training.
Objective:
To describe the study design of this center implementation trial.
Methods:
Participants in the study will be recruited from a hospital in China and randomly assigned to the intervention group or the usual care group. Interventions will include four-week hierarchical multi-dimensional preventive cognitive training through a WeChat program. for Primary outcome measures will be the Montreal Cognitive Assessment, Mini-Mental State Examination, and Post-Stroke Cognitive Impairment (PSCI) Incidence. The secondary outcome measure will include the Hamilton Depression Scale, Hamilton Anxiety Scale, Modified Barthel Index, and National Institutes of Health Neurological Deficit Score. Outcomes will be measured at baseline, 12 weeks, and 24 weeks from the baseline.
Results:
We expect that the hierarchical multi-dimensional preventive cognitive training program will be easy to implement, and the cognitive function, cognitive psychology, ability of daily living will vary in each setting.
Conclusions:
The results will provide evidence highlighting differences in a new strategy of cognitive training through the WeChat program, which allows the home-based practice, puts forward an advanced idea of preventive cognitive training in the acute stage, and has the highest effectiveness of reducing cognitive impairment, and Alzheimer’s disease.
INTRODUCTION
Stroke remains a leading cause of disability around the world [1]. With the development of effective treatments in an acute stage, the outcomes have improved in global trends [2, 3], but post-stroke cognitive impairment (PSCI) and dementia remain highly prevalent. PSCI is a clinical syndrome characterized by cognitive impairment that occurs after a stroke and persists for 3 to 6 months. PSCI is divided into post-stroke cognitive impairment non-dementia and post-stroke dementia [4]. Cognitive deficits are present in over 70% of stroke survivors [5]. About one-half of patients develop PSCI within the first year after stroke [5, 6]. Recent extensive international cohort studies have reported that the incidence of PSCI is 24%–53.4% [7, 8], among which the incidence of cognitive impairment after stroke without dementia is 14%–29% and the incidence of dementia after stroke is 11%–42% [9]. A study in China shows that the incidence of PSCI is 53.1% [10]. Cognitive impairment progressively worsens following stroke, with 20–30% of patients developing dementia [11]. It can be seen that the incidence of PSCI is high, which is a significant health problem.
The mortality rate of stroke patients complicated with PSCI is significantly higher than that of patients without cognitive impairment [12]. The 5-year survival rate of patients with post-stroke dementia is only 39%, while the survival rate of stroke patients without dementia of the same age is 75% [13]. In addition, patients with PSCI will have long-term disability, a significant decline in the self-care ability of daily living, quality of life and mental health status, poor social participation ability, increased care pressure, and morbidity [4, 14]. If not intervened in time, it will pose a major burden to the family and society. Therefore, preventing the occurrence and delaying the progression of PSCI is an important task that must be solved urgently.
Rehabilitation is an important intervention to delay the progression of PSCI, aiming to promote the remodeling of the central nervous system, and the main method is early and multi-dimensional cognitive function training [13]. Some studies have confirmed that computerization, multi-cognitive domains, and adaptive cognitive training (7 consecutive weeks, 5 days a week, 30 min a day) can significantly improve the global cognitive function of patients with cognitive impairment after subcortical stroke with non-dementia [15]. A recent systematic review showed that cognitive training can improve patients’ cognitive function and daily living ability with mild PSCI [16]. It is also certain efficacious in global cognition, select cognitive domains, and psychosocial functioning in people with mild PSCI [17]. Conversely, evidence for efficacy in people with dementia is weak [17]. It can be seen that cognitive training is not effective for all stages of PSCI patients, and the effect of cognitive training is minimal for patients who have developed severe cognitive impairment or even dementia [17, 18]. That is to say, once PISC has developed, the effect of the intervention will become weaker. This suggests that cognitive intervention for acute ischemic stroke (AIS) patients may achieve the purpose of preventing the occurrence of PSCI or delaying the progression of PSCI. Therefore, this study innovatively proposed the concept of preventive cognitive training for AIS patients.
The way of cognitive training is developed from traditional to computer cognitive training, and this transfer increased the convenience and reduced the costs. But mature computer software for cognitive training need to be improved in China. Most of the cognitive training is carried out through the traditional way, or with the help of foreign online software, like the classic cognitive training software such as Brain-HQ, Cognifitim, Mindsparke, Gogmed, and so on, without considering the Chinese cultural background and living habits. Some software only provides a foreign language interface, which is not universal. WeChat is more convenient than computers in daily life, not limited by time and space, and WeChat has a higher penetration rate among the Chinese population.
In summary, based on literature research [13, 15, 19], the present protocol remodifies the cognitive training program, combined with the views of cognitive experts and rehabilitation experts, and builds a hierarchical multi-dimensional cognitive training program for preventive cognitive decline in AIS patients. The multi-dimensional cognitive training contains attention, memory, numeracy, thinking response, and perception, each dimension has two levels of difficulty. It is convenient for users to select appropriate items and the difficulty level based on the impairment dimensions and degrees. To evaluate the effect of the cognitive training program, cognitive function scale, psycho-psychological scale, self-care ability scale, and other tools are used. This study aims to clarify whether acute, preventive cognitive training can improve PSCI and provide a practical basis for the prevention and intervention of PSCI.
THE STUDY
Aim and research objectives
To evaluate the effectiveness of a hierarchical multi-dimensional cognitive training program to prevent stroke patients’ cognitive decline.
The objective of the study is to evaluate the effectiveness of a hierarchical multi-dimensional cognitive training program on:
(1) Cognitive function, such as Montreal Cognitive Assessment (MoCA), Mini-Mental State Examination (MMSE), and PSCI incidence.
(2) Psychiatric, such as Hamilton Depression Scale (HAMD), and Hamilton Anxiety Scale (HAMA).
(3) Function, such as Modified Barthel index (MBI), and National Institutes of Health Neurological Deficit Score (NIHSS).
Hypotheses
The study is designed to test the hypothesis at a.05 significance level. The hypotheses formulated are as follows:
(1) There will be a significant difference in cognitive function, such as MoCA, MMSE, and PSCI incidence between the intervention and the control arms.
(2) There will be a significant difference in the psychiatric, such as HAMD, and HAMA between the intervention and the control arms.
(3) There will be a significant difference in the function, such as MBI, and NIHSS between the intervention and the control arms.
Methodology
Design
This study is a prospective, two-arm, single-center, randomized control trial. The participants will be randomly allocated to either the intervention or the control arm. The intervention arm will receive a hierarchical multi-dimensional cognitive training program, whereas the control arm will receive routine treatment, rehabilitation, and care. The study participants will be followed up for 24 weeks and the outcomes are assessed before and after the intervention and at 12, 24 weeks. Table 1 summarizes all the items included in the trial registry.
Table 1
Brief structured summary of the trial
| Data category | Information |
| Trial identification number | ClinicalTrials.gov NCT05648149 |
| Date of registration | 23 November 2022 |
| Scientific title | Effect of preventive cognitive training on cognitive impairment after stroke in acute stage |
| Country of recruitment | China |
| Health condition studies | Stroke in acute stage |
| Interventions | Experimental: a hierarchical multi-dimensional cognitive training |
| Control: routine treatment, rehabilitation and care | |
| Inclusion/exclusion criteria | Eligible age: 18–80 years; eligible gender: males and females. |
| Inclusion criteria: Patients diagnosed as ischemic stroke by CT or MRI and meeting World Health Organization diagnostic criteria First onset, within 7 days of onset; There were no contraindications in MRI examination, and the examination was completed with good image quality and complete clinical data; Conscious (NIHSS consciousness level 0, 1); Informed consent. | |
| Exclusion Criteria: Patients with previous cognitive impairment; Aphasia or severe dysarthria; Previous cerebral atrophy or white matter lesions; History of severe cardiopulmonary dysfunction, craniocerebral trauma, etc. | |
| Study type | Interventional |
| Allocation: randomized; Intervention model: parallel assignment; masking: outcomes assessor, external evaluator, data analyst and principal investigator | |
| Primary purpose: Improving cognitive function | |
| Date of the first enrolment | 01/01/2023 |
| Target sample size | 136 (68 participants in each group) |
| Recruitment status | Actively recruiting |
| Primary outcomes | MoCA; MMSE; PSCI Incidence |
| Secondary outcomes | HAMD; HAMA; MBI; NIHSS |
CT, computed tomography; MRI, magnetic resonance imaging; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; HAMD, Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; MBI, Modified Barthel Index; NIHSS, National Institutes of Health Neurological Deficit Score; PSCI, post-stroke cognitive impairment.
Setting and participants
Participants’ identification and recruitment will be carried out in the neurology department at one hospital in China that formally agrees to participate in this research. Specifically, researchers will identify potentially eligible participants and inform them about the study. Interested patients will sign the written informed consent and eligibility criteria will be verified.
Inclusion criteria include: 1) Patients diagnosed as having ischemic stroke by CT or MRI and meeting WHO diagnostic criteria; 2) First onset, within 7 days of onset; 3) There were no contraindications in MRI examination, and the examination was completed with good image quality and complete clinical data; 4) Between 18 and 80 years old; 5) Conscious (NIHSS consciousness level 0, 1); 6) Informed consent.
Exclusion criteria include: 1) Patients with previous cognitive impairment; 2) Aphasia or severe dysarthria; 3) Previous cerebral atrophy or white matter lesions; 4) History of severe cardiopulmonary dysfunction, craniocerebral trauma, etc.
Sample size
The sample size is estimated for repeated measures considering the primary outcome variable (MoCA). According to the preliminary test results, σ= 3.83, δ= 2.28. Accepting an alpha risk of 0.05, a beta risk <0.10 (two-sided), and accounting for a 15% drop-out rate, 68 subjects will be needed in each group to detect a minimum difference between the two groups.
Randomization
The participants will be randomly allocated (1:1) to either an intervention or a control arm with randomization. The random allocation sequence is generated from https://www.sealedenvelope.com. The allocation concealment is done with sequentially numbered, opaque sealed envelopes prepared by the person who is not a part of the study. Assessors are blinded in this study.
Description of a hierarchical multi-dimensional cognitive training
According to foreign classic cognitive training software such as Brain-HQ, Cognifitim, Mindsparke, Gogmed, and so on, the dimensions and items of cognitive training are set independently. The hierarchical multi-dimensional cognitive training will be determined by the expert meeting method. The expert meeting will be held twice offline, one to confirm the content and the other for the final draft. 10 experts will include: 2 stroke experts, 3 Cognitive neuroscience experts, 3 cognitive psychology experts, 2 Cognitive neuroscience nursing experts. All the experts have more than 10 years of professional work and can attend offline meetings. 2 researchers will be responsible for taking notes, contacting experts, and scheduling and initiating expertmeetings.
The program includes: (1) Five main categories of cognitive function, which are attention, memory, numeracy, thinking response, and perception; (2) There are 10 training items, which are drawing, quick matching, flash memory, number memory, face memory, color tracking, number span, word naming, symbol number form, quick arithmetic; (3) Each training item is divided into two levels of difficulties, which are basic and advanced. Only the correct rate of basic training reaches more than 80%, can enter the advanced level; (4) The item flash memory and color tracking will have two colors; (5) To facilitate patient training, all items will be embedded in the WeChat program, and corresponding cognitive training will be done through the patient’s WeChat program; (6) All the items have a time progress bar, which can achieve deep brain training; (7) Small program background can monitor training results and send reminder messages; (8) Cognitive training will carry out by the patients with the help of three trained and certified nurses in the cognitive training program. During hospitalization, the patients and their families will be taught how to use cognitive training programs. After discharge, nurses will be assigned to supervise and urge the patients to conduct cognitive training through the background; (9) Twice a day, 30 min each time, for 4 weeks (Table 2).
Table 2
Components of the intervention
| Categories | Item | Duration | Quality control |
| Attention Memory Numeracy Thinking response Perception | drawing, quick matching, flash memory, number memory, face memory, color tracking, number span, word naming, symbol number form, quick arithmetic | Twice a day, 30 min each time, for 4 weeks | 1) Cognitive training was carried out in the We-Cat program 2) Each training item is divided into basic and advanced training; 3) Only the correct rate of the basic training reaches more than 80%, can the advanced training be entered; 4) Special personnel were assigned to supervise and urge the training. 5) Program can monitor training results and send reminder messages. |
Description of the control intervention
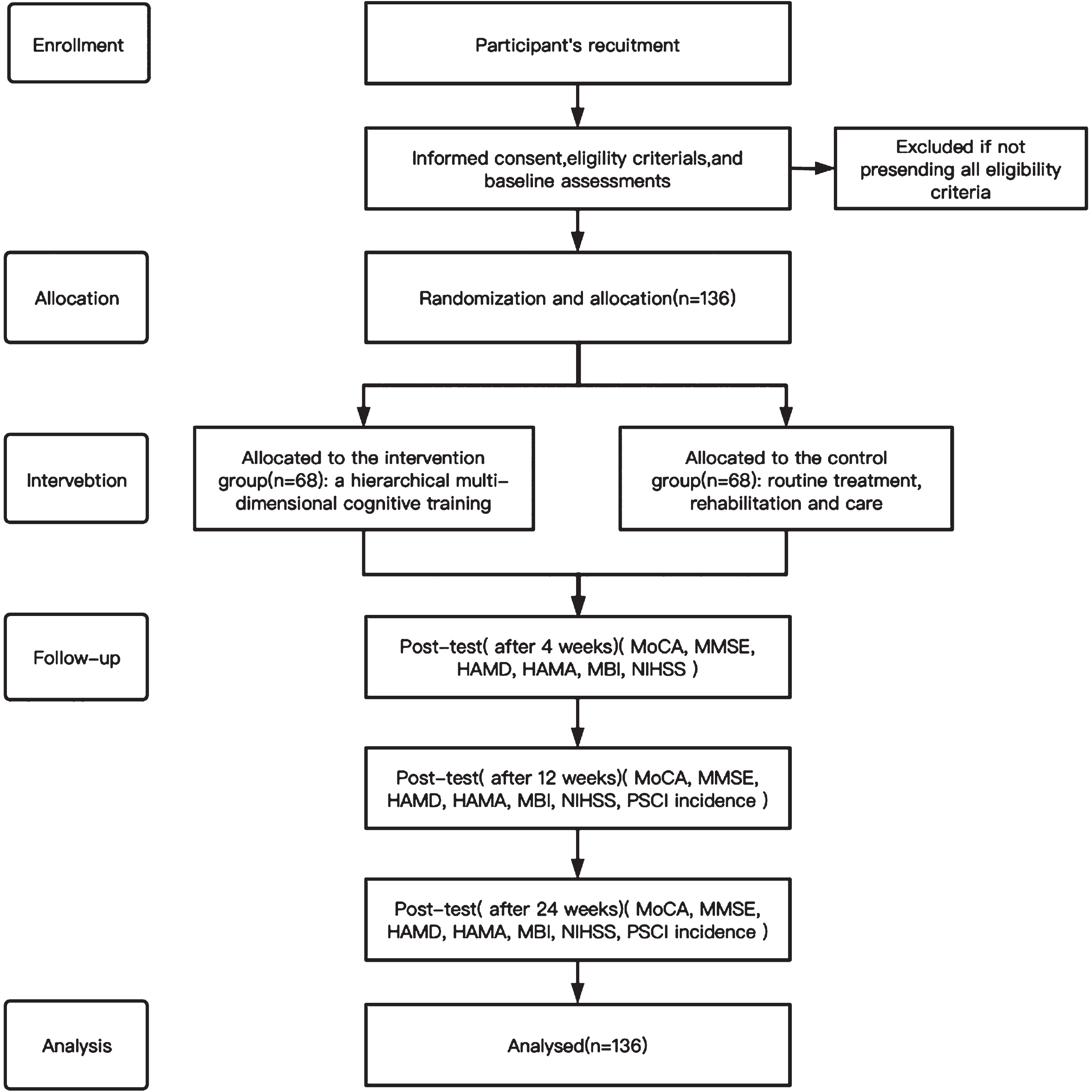
Routine treatment, rehabilitation, and care. The details on how the trial will be conducted are presented in the Consolidated Standards of Reporting Clinical Trial (CONSORT) flow chart [20] (Fig. 1).
Fig. 1
Study flow diagram. Consolidated Standards of Reporting Clinical Trial (CONSORT) flow diagram in this study. MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; HAMD, Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; MBI, Modified Barthel Index; NIHSS, National Institutes of Health Neurological Deficit Score; PSCI, post-stroke cognitive impairment.
Outcome measures
The study’s outcome measures include clinical outcomes such as MoCA, MMSE, PSCI Incidence, HAMD, HAMA, MBI, and NIHSS. The MoCA, MMSE, HAMD, HAMA, MBI, and NIHSS will be assessed before and after the intervention and at 12, 24 weeks. PSCI incidence will be assessed at 12, 24 weeks. Table 3 provides an overview of the schedule of enrolment, interventions, main study visits, and participants assessment measures.
Table 3
Participants main visits and assessment schedules
| Visit | Main visits and assessment schedules | |||
| V0 | V1 | V2 | V3 | |
| Time point1 | 0w | 4w | 12w | 24w |
| Informed consent | X | |||
| Inclusion/exclusion criteria | X | |||
| Randomization | X | |||
| Baseline data2 | X | |||
| Initiation of the intervention3 | X | X | ||
| Cognitive function assessment4 | X | X | X | |
| PSCI Incidence | X | X | ||
| Mental assessment5 | X | X | X | |
| Functional assessment6 | X | X | X | |
1. Time point: w = week. 2. Baseline data: socio-demographic and clinical characteristics, MoCA, MMSE, HAMD, HAMA, MBI, and NIHSS. 3. Initiation of the intervention: a hierarchical multi-dimensional cognitive training for the experimental group, routine treatment, rehabilitation and care for the control group. 4. Cognitive function assessment: Montreal Cognitive Assessment and Mini-mental State Examination. 5. Mental assessment: Hamilton Depression Scale and Hamilton Anxiety Scale. 6. Functional assessment: Modified Barthel Index and National Institutes of Health Neurological Deficit Score.
Measurements
(1) MoCA. An assessment tool for rapid screening of cognitive impairment [21]. Several validation studies have been conducted on the MoCA, in various clinical populations. The total score is 30 points, and the test result shows that the normal value is ≥23 points [22].
(2) MMSE. An assessment tool to reflect the subjects’ mental state and the degree of cognitive impairment [23]. The total score ranges from 0–30 points. Scores are closely related to the level of education, and the normal cut-off is defined as >17 points for illiteracy, >20 points for primary school, and >24 points for junior high school and above [24].
(3) PSCI Incidence. PSCI Incidence = number of PSCI cases/total number of enrolled cases.
(4) HAMD. An assessment tool for assessing depression status. Total score <7: normal; The total score of 7 to 17: depression may be present; The total score of 17 to 24: definitely depression; Total score >24: severe depression [25, 26].
(5) HAMA. It is mainly used to assess the severity of neurosis and anxiety symptoms in people [27]. A total score of more than 29 may indicate severe anxiety; if more than 21 points, there must be significant anxiety; if more than 14 points, there is anxiety; More than 7 points, may have anxiety; If the score is less than 7, there are no symptoms of anxiety [28].
(6) MBI. The MBI consists of 10 items and 5 different weights of rating scales: unable, attempts but unsafe, moderate help, minimal help, and fully independent [29]. There is a score range of 0 to 5 for bathing, and grooming; a score range of 0 to 10 for feeding, dressing, bowels, bladder, toilet, and stairs; and a score range of 0 to 15 for chair/bed transfers, walking. A higher score represents a higher degree of ADL independence [30].
(7) NIHSS. The NIHSS will assess the degree of the neurological deficit [31]. For all 11 parameters, the top score is 42 points, a score of 0 and 1 is normal, and higher scores mean a worse neurological deficit [32].
Data collection procedure
People in the age group of 18–80 years old, diagnosed with ischemic stroke, and admitted under the neurology department will be screened for eligibility based on the inclusion and exclusion criteria. The study will be explained to the people who meet the eligibility criteria using the participant information sheet that informs the benefits and the risks associated with participation in the study. Those who are willing to participate will be enrolled with written informed consent. An external member (neurology department nurse) allocates the participants to the intervention and the control arm. The participant who with a sealed envelope with ‘1’ will be assigned to the intervention arm, and with ‘2’ to the control arm.
The baseline data on socio-demographic and clinical characteristics, MoCA, MMSE, HAMD, HAMA, MBI, and NIHSS will be assessed using the questionnaires within two days after admission. The follow-up data will be assessed when the participants visit the hospital.
Statistical analysis
The data will be analyzed using the Statistical Package for Social Sciences (SPSS, version 24). Descriptive statistics such as frequency, percentage, mean, and standard deviation will describe socio-demographic and clinical characteristics. The chi-square (χ2) and the independent sample t-test will be used to compare the baseline variables of the intervention and the control arms. An intention-to-treat analysis will be adopted to manage the missing data and repeated measures ANOVA will be used to analyze the outcome variables across the time.
Ethical considerations
The Institutional Ethics Committee (IR2022373) approved the trial in 2022. Administrative permission has been obtained from the hospital where the study will be conducted. Participants will have the right to consent voluntarily or decline to participate and withdraw their participation at any time in between without any effect on their treatment. The privacy, anonymity, and confidentiality of participants will be secured throughout the study. All clinical experiments have been conducted according to the principles expressed in the Declaration of Helsinki.
Pilot study
The feasibility of recruitment, randomization, retention, and assessment procedures will be evaluated by performing a pilot study during the initial phase of the trial including the first 40 randomized patients. Pilot results will give information on the viability of the trial and possible modifications needed to be implemented.
Validity and reliability
This randomized controlled trial (RCT) will provide strong evidence on cognitive interventions for cognitive decline prevention in AIS patients. The study will include a representative sample of people with stroke allowing for generalizability of results. Internal validity will be warranted by a randomization process based on allocation sequence generation, blinded to the researchers involved in the intervention. Moreover, an external evaluator will assess the main outcome, and will be blinded to patient allocation to reduce biases in evaluating the intervention. Lastly, type 1 and 2 errors associated with sample size will also be minimized as the adequacy of statistical power was carefully assessed. The study uses validated and reliable tools for data collection, the interventions are based on official guidelines, the latest scientific evidence, and the views of the experts. A pilot study will be carried out during the initial phase of the trial to evaluate recruitment and logistical procedures.
RESULTS
In principle, the hierarchical multi-dimensional preventive cognitive training program will improve cognitive function. Additionally, we assume the intervention will be more effective in the acute stage.
DISCUSSION
The RCT will provide new insight into preventive cognitive intervention, and the effectiveness of a hierarchical multi-dimensional cognitive training program for preventative cognitive decline in AIS patients.
An essential strength of this research is preventive cognitive interventions. Early cognitive impairment after stroke predicts outcome with activity and participation at 6–12 months post-injury [33]. Prevention of cognitive deterioration is urgent. Previous studies have shown that interventions to address cognitive impairment are a restorative and remedial approach (often called a cognitive remediation approach), a compensatory and adaptive approach, or a combination of both [34]. A cognitive remediation approach is generally lagged, the effect is limited due to the irreversibility of cognitive impairment. As noted above, we propose that cognitive interventions in the acute stage can prevent or delay cognitive impairment.
Moreover, cognitive training can improve the cognitive function of mild PSCI patients [9, 10]. However, cognitive training is not effective for all stages of PSCI patients, especially serious cognitive impairment or dementia [9, 10]. This also suggests to us that cognitive training should be implemented at an early stage. So preventive cognitive interventions are rationality and innovation.
The proposed intervention would offer a convenient way with the WeChat program, which can be trained at any time and anywhere, and also can be trained for a long time. The items are designed with full consideration of the degree of cognitive impairment. The difficulty of training is rated in three levels, suitable for stroke patients with different needs. The match of patients and items can improve the effect of training and also work on encouraging them to keep on training.
Finally, it would allow for cognitive training for many people, including the old and socially vulnerable, making it acceptable to both stroke patients and healthcare workers. The study design is an RCT and has the potential to provide high-quality evidence on the benefit of the intervention proposed.
Limitations
The study has certain limitations. First, the study participants are selected from a single center. Secondly, the intervention was not long enough.
CONCLUSION
This study will contribute to the evidence on the effectiveness of cognitive training programs on AIS patients. If this cognitive training program is effective, the nurses working at all levels of healthcare could be trained to carry out the cognitive function training program for AIS patients. This program could be implemented in clinical practice to delay cognitive function and improve clinical outcomes. Furthermore, the program could be used to plan adaptive interventions based on the extent of cognitive impairment and the effect of training.
ACKNOWLEDGMENTS
The authors would like to thank the members of the research team. Also, Thanks to my husband and daughter for their support.
FUNDING
This project is funded by the medical and health science and technology planning project of the Zhejiang province (Project ID: 2023KY757).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
REFERENCES
[1] | GBD 2017 DALYs and HALE Collaborators ((2018) ) Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: , 1859–1922. |
[2] | Mendelson SJ , Prabhakaran S ((2021) ) Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. JAMA 325: , 1088–1098. |
[3] | Herpich F , Rincon F ((2020) ) Management of acute ischemic stroke. Crit Care Med 48: , 1654–1663. |
[4] | Rost NS , Brodtmann A , Pase MP , van Veluw SJ , Biffi A , Duering M , Hinman JD , Dichgans M ((2022) ) Post-stroke cognitive impairment and dementia. Circ Res 130: , 1252–1271. |
[5] | Pendlebury ST , Rothwell PM , Oxford Vascular Study ((2019) ) Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular Study. Lancet Neurol 18: , 248–258. |
[6] | Barbay M , Taillia H , Nédélec-Ciceri C , Bompaire F , Bonnin C , Varvat J , Grangette F , Diouf M , Wiener E , Mas JL , Roussel M , Godefroy O , GRECOG-VASC Study Group ((2018) ) Prevalence of poststroke neurocognitive disorders using national institute of neurological disorders and stroke-Canadian stroke network, VASCOG criteria (vascular behavioral and cognitive disorders), and optimized criteria of cognitive deficit. Stroke 49: , 1141–1147. |
[7] | Lo JW , Crawford JD , Desmond DW , Godefroy O , Jokinen H , Mahinrad S , Bae HJ , Lim JS , Köhler S , Douven E , Staals J , Chen C , Xu X , Chong EJ , Akinyemi RO , Kalaria RN , Ogunniyi A , Barbay M , Roussel M , Lee BC , Srikanth VK , Moran C , Kandiah N , Chander RJ , Sabayan B , Jukema JW , Melkas S , Erkinjuntti T , Brodaty H , Bordet R , Bombois S , Hénon H , Lipnicki DM , Kochan NA , Sachdev PS ; Stroke and Cognition (STROKOG) Collaboration ((2019) ) Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 93: , e2257–e2271. |
[8] | Douiri A , Rudd AG , Wolfe CD ((2013) ) Prevalence of poststroke cognitive impairment: South London stroke register 1995-2010. Stroke 44: , 138–145. |
[9] | Munthe-Kaas R , Aam S , Ihle-Hansen H , Lydersen S , Knapskog AB , Wyller TB , Fure B , Thingstad P , Askim T , Beyer MK , Næss H , Seljeseth YM , Ellekjær H , Pendlebury ST , Saltvedt I ((2020) ) Impact of different methods defining post-stroke neurocognitive disorder: The Nor-COAST study. Alzheimers Dement (N Y) 6: , e12000. |
[10] | Ding MY , Xu Y , Wang YZ , Li PX , Mao YT , Yu JT , Cui M , Dong Q ((2019) ) Predictors of cognitive impairment after stroke: A prospective stroke cohort study. J Alzheimers Dis 71: , 1139–1151. |
[11] | Pendlebury ST , Rothwell PM ((2009) ) Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol 8: , 1006–1018. |
[12] | Huang YY , Chen SD , Leng XY , Kuo K , Wang ZT , Cui M , Tan L , Wang K , Dong Q , Yu JT ((2022) ) Post-stroke cognitive impairment: Epidemiology, risk factors, and management. J Alzheimers Dis 86: , 983–999. |
[13] | Wang K , Dong Q ((2021) ) Expert consensus on management of cognitive impairment after stroke 2021. Chinese J Stroke 16: , 376–389. |
[14] | Rohde D , Gaynor E , Large M , Mellon L , Hall P , Brewer L , Bennett K , Williams D , Dolan E , Callaly E , Hickey A ((2019) ) The impact of cognitive impairment on poststroke outcomes: A 5-year follow-up. J Geriatr Psychiatry Neurol 32: , 275–281. |
[15] | Tang Y , Xing Y , Zhu Z , He Y , Li F , Yang J , Liu Q , Li F , Teipel SJ , Zhao G , Jia J ((2019) ) The effects of 7-week cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE study): A randomized controlled trial. Alzheimers Dement 15: , 605–614. |
[16] | Nie P , Liu F , Lin S , Guo J , Chen X , Chen S , Yu L , Lin R ((2022) ) The effects of computer-assisted cognitive rehabilitation on cognitive impairment after stroke: A systematic review and meta-analysis. J Clin Nurs 31: , 1136–1148. |
[17] | Hill NT , Mowszowski L , Naismith SL , Chadwick VL , Valenzuela M , Lampit A ((2017) ) Computerized cognitive training in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Am J Psychiatry 174: , 329–340. |
[18] | Loetscher T , Potter KJ , Wong D , das Nair R ((2019) ) Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev 2019: , CD002842. |
[19] | Khaleghi A , Aghaei Z , Mahdavi MA ((2021) ) A gamification framework for cognitive assessment and cognitive training: Qualitative study. JMIR Serious Games 9: , e21900. |
[20] | Schulz KF , Altman DG , Moher D ; CONSORT Group ((2010) ) CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med 152: , 726–732. |
[21] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[22] | Carson N , Leach L , Murphy KJ ((2018) ) A re-examination of montreal cognitive assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry 33: , 379–388. |
[23] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[24] | Trivedi D ((2017) ) Cochrane review summary: Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Prim Health Care Res Dev 18: , 527–528. |
[25] | Hamilton M ((1960) ) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: , 56–62. |
[26] | Rosenberg LI ((2022) ) The Ham-D is not Hamilton’s depression scale. Psychopharmacol Bull 52: , 117–153. |
[27] | Rabinowitz J , Williams JBW , Hefting N , Anderson A , Brown B , Fu DJ , Kadriu B , Kott A , Mahableshwarkar A , Sedway J , Williamson D , Yavorsky C , Schooler NR ((2023) ) Consistency checks to improve measurement with the Hamilton Rating Scale for Anxiety (HAM-A). J Affect Disord 325: , 429–436. |
[28] | Rodriguez-Seijas C , Thompson JS , Diehl JM , Zimmerman M ((2020) ) A comparison of the dimensionality of the Hamilton Rating Scale for anxiety and the DSM-5 anxious-distress specifier interview. Psychiatry Res 284: , 112788. |
[29] | Shah S , Vanclay F , Cooper B ((1989) ) Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 42: , 703–709. |
[30] | Leung SO , Chan CC , Shah S ((2007) ) Development of a Chinese version of the Modified Barthel Index-validity and reliability. Clin Rehabil 21: , 912–922. |
[31] | Dewey HM , Donnan GA , Freeman EJ , Sharples CM , Macdonell RA , McNeil JJ , Thrift AG ((1999) ) Interrater reliability of the National Institutes of Health Stroke Scale: Rating by neurologists and nurses in a community-based stroke incidence study. Cerebrovasc Dis 9: , 323–327. |
[32] | Chalos V , van der Ende NAM , Lingsma HF , Mulder MJHL , Venema E , Dijkland SA , Berkhemer OA , Yoo AJ , Broderick JP , Palesch YY , Yeatts SD , Roos YBWEM , van Oostenbrugge RJ , van Zwam WH , Majoie CBLM , van der Lugt A , Roozenbeek B , Dippel DWJ ; MR CLEAN Investigators ((2020) ) National Institutes of Health Stroke Scale: An alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke 51: , 282–290. |
[33] | Mole JA , Demeyere N ((2020) ) The relationship between early post-stroke cognition and longer term activities and participation: A systematic review. Neuropsychol Rehabil 30: , 346–370. |
[34] | Gibson E , Koh CL , Eames S , Bennett S , Scott AM , Hoffmann TC ((2022) ) Occupational therapy for cognitive impairment in stroke patients. Cochrane Database Syst Rev 3: , CD006430. |




