Norms for the Triana Test: A Story Recall Test Based on Emotional Material
Abstract
Background:
The “Triana Test” is a novel story recall test based on emotional material with demonstrated accuracy in diagnosing mild cognitive impairment patients.
Objective:
This study aims to obtain normative data for the “Triana Test”.
Methods:
A normative study was conducted at a university hospital in Spain. Partners of patients were systematically recruited if eligible (age ≥50, no memory complaints, and a total TMA-93 score at or above the 10th percentile). The “Triana Test” was administered and scored. For developing the normative data, a regression-based method was followed.
Results:
The final sample included 362 participants (median age = 66, range = 50–88; 64.9% females). A model including age and educational level better predicted the total scores. Combinations of these variables resulted in different 10th percentile scores.
Conclusions:
Norms for using the “Triana Test” are now available. The provided cutoffs for the 10th percentile will aid in the diagnosis of prodromal Alzheimer’s disease.
INTRODUCTION
Over the last decade, there has been a conceptual shift in the early diagnosis of Alzheimer’s disease (AD), focusing on high-sensitivity memory tests before confirming the diagnosis using biomarkers [1, 2].
Common memory tests usually evaluate signs that suggest hippocampal dysfunction, such as recognition failure in list learning tests [3], or semantic clue facilitation loss in the Free and Cued Selective Reminding Test [4]. In this hippocampal line, and for detecting early AD, a new generation of tests has emerged, designed to pinpoint deficits with even greater sensitivity [5]. Among these innovative tests are the Loewenstein-Acevedo Scale for Semantic Interference and Learning [6, 7], that evaluates the semantic interference paradigm; the Memory Binding Test [8], that assess binding by recalling pairs of words that share a common semantic category, drawn from two different word lists; or the Face Name Associative Memory Test [9], that delves into cross-modal associative memory, specifically evaluating the ability to link names with faces.
An alternative approach, less explored, challenges the amygdala using emotional memory tests [10–13]. Studies on patients with selective bilateral amygdala lesions have shown that the amygdala plays a crucial role in memorizing emotional material [6, 7]. Since amygdala and hippocampal neurodegeneration coexist in AD [14–16], emotional memory tests represent an intriguing research direction.
Emotional memory has been studied in patients with affective disorders mainly using tests based on lists of words with positive, negative, or neutral valence, such as the Emotional Verbal Learning Test [17, 18], the Affective Auditory Verbal Learning Test [19], or the Verbal Affective Memory Test [20].
The “Triana Test” is a recently developed story recall test with a different approach [21]. It aims to diagnose prodromal AD by presenting a captivating love and heartbreak story between a flamenco dancer and a Japanese student. Designed to capture examinees’ attention, this emotional story is expected to facilitate learning [22–25]. Since emotional arousal enhances declarative memory in healthy older individuals but inconsistently in cognitively impaired patients [26–29], the “Triana Test” hypothesizes that the story could effectively discriminate between patients with amnestic mild cognitive impairment (aMCI) and healthy controls (HCs). In this way, in a preliminary validation study that included 55 patients with aMCI and 69 HCs, the “Triana Test”, particularly its delayed free recall component, showed good diagnostic utility for discriminating between the diagnostic groups [21]. Through receiver operating characteristic curve analysis, the area under the curve (AUC) for the delayed free recall reached 0.86 (95% CI = 0.79–0.92). This level of accuracy compares favorably with that of other story recall tests [21].
Encouraged by the validation study’s results, we conducted a normative study to obtain “Triana Test” norms from educationally diverse cognitively unimpaired older individuals and analyze the impact of socio-demographic variables on the test.
MATERIAL AND METHODS
Design
We conducted a cross-sectional, observational, and prospective normative study of the “Triana Test” in older Spanish adults.
Study population
Participants were recruited from partners of patients attending the general neurology outpatient clinic at the University Hospital Virgen del Rocio in Seville, Andalusia, Spain.
Inclusion criteria were: 1) age between 50 and 90 years; 2) no memory complaints; 3) no memory impairment: score at or above the 10th percentile on the Memory Associative Test of the district of Seine-Saint-Denis (TMA-93), a binding memory test with normative data for Spain [30–32]; 4) independent functioning.
The following were the exclusion criteria: 1) history of neurological disease potentially causing cognitive impairment; 2) significant systemic disease that might affect cognitive evaluation; 3) poor vision or hearing; 4) severe psychiatric disease (active major depression, schizophrenia, or bipolar disorder); 5) psychotropic medications, and/or a history of use of psychoactive substances.
Age, gender, and educational attainment (categorized as “incomplete primary studies”, “primary studies completed”, or “studies higher than primary”) were considered socio-demographic variables. Recruitment was systematic, and eligible individuals were invited consecutively each day. Recruitment was closed once a minimum of 75 individuals were enrolled in each group of age (categorized as below or above 65 years), educational attainment, and gender. This ensured a baseline sample size of 225 participants which could be expanded until the criterion was met.
Procedures: TMA-93 and TT
TMA-93
The TMA-93 was administered to determine cognitive status and eligibility. Administration and scoring followed the instructions given by its authors [30]. When the participants scored at or above the 10th percentile on the TMA-93, according to the Spanish normative data [31, 32], they were considered cognitively unimpaired and eligible for this study.
Triana test
In the “Triana Test”, the examinee is instructed to attentively listen to a narrative [21]. The examiner reads the story aloud, and immediately afterward, the examinee is asked to recall it word-for-word. This phase is referred to as immediate free recall and it is scored based on the successful recall of 12 specific items from the story, with a maximum score of 12 points. Following this, the examinee is presented with 12 yes/no questions about the same story items. This immediate recognition recall phase also has a maximum score of 12 points. After a 20-min delay, during which non-memory tests may be conducted, both types of recalls, now called “delayed free recall” and “delayed recognition recall”, are assessed again and scored in a similar manner [21].
Ethics
This study was approved by the ethics committee of the Virgen del Rocio Hospital (Seville, Spain), and participants provided informed consent.
Statistical study
Descriptive results are presented as frequency (percent) for dichotomous and categorical variables, mean (±SD, range) for normally-distributed continuous variables, and median (interquartile range [IQR], range) for non-normally distributed continuous variables. Normality was checked by a Shapiro-Wilk test. Between-group comparison of continuous variables were performed using Student’s t-test or one-way analysis of variance (ANOVA; or their non-parametric alternatives: the Mann-Whitney U-test and Kruskal-Wallis ANOVA, respectively). Categorical variables were compared using the chi-square test.
Spearman correlation was used to estimate associations between continuous variables.
To establish the normative data, we followed a regression-based approach [33, 34]. We standardized the age of the participants by deducting the mean age of the group from the chronological age of each subject. We created four multiple regression models (one for each “Triana Test” recall), with the cognitive score as the dependent variable. The independent variables included age standardization, gender (male = 0, female = 1), and educational level (categorized into three levels: incomplete primary studies = 0, primary studies completed = 1, studies higher than primary = 2). We utilized a stepwise method, with a significance level of p < 0.05 for the beta coefficient to retain a predictor in the model. The constant and coefficients were used to compute predicted scores based on the regression equation. By subtracting each possible value of the cognitive score and each possible expected score (using the relevant predictors for each variable) residuals were calculated. Subsequently, these residuals were converted into z-scores by dividing them by the standard deviation of the regression model. For the reference data, we present tables containing percentiles that are commonly used. Each table displays the theoretical raw scores corresponding with each percentile value.
The analysis was performed using R software version 4.2.2. Statistical significance was set at p < 0.05, and 95% confidence intervals were estimated.
RESULTS
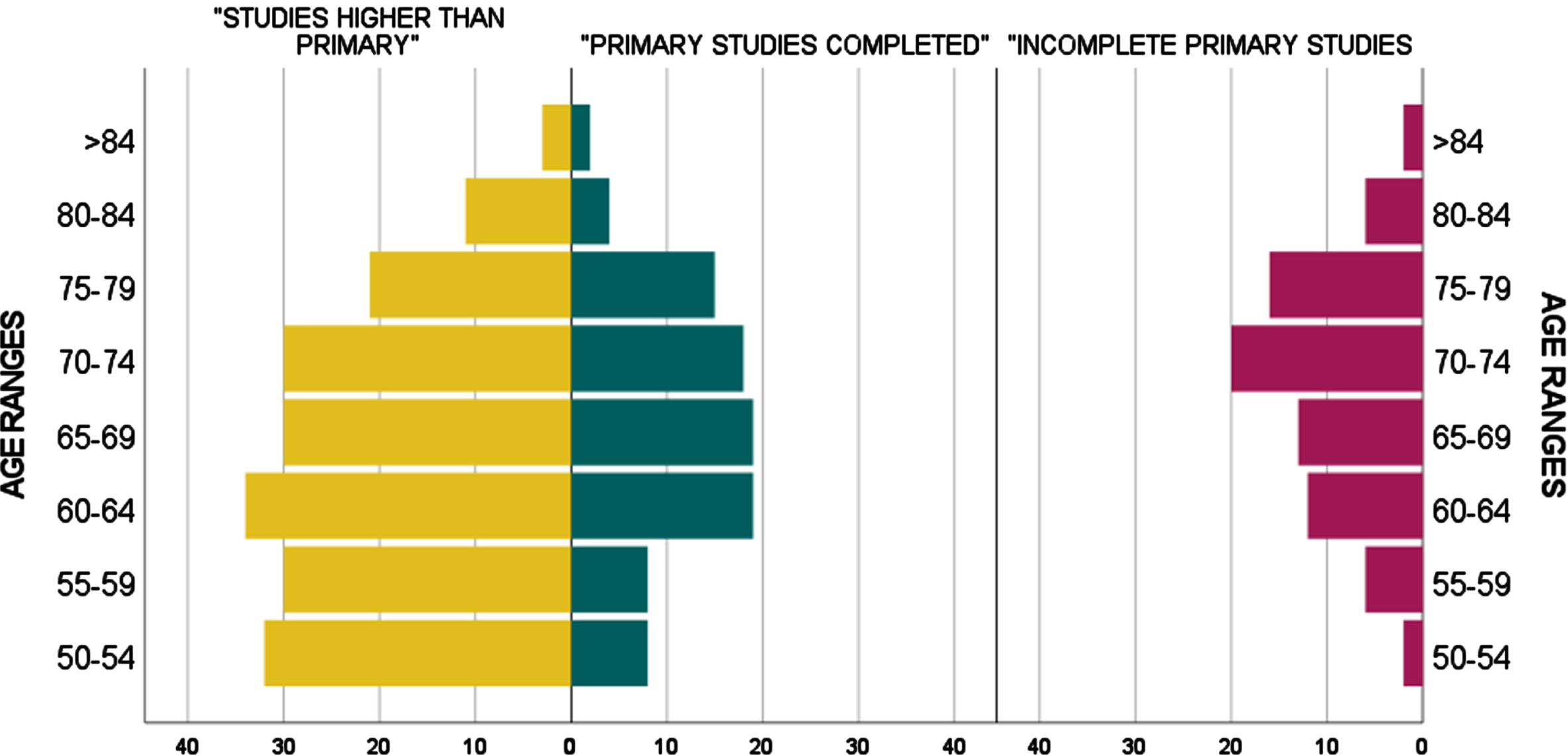
Between October 2019 and July 2022, we invited 371 eligible individuals to participate in the study. Nine participants were excluded due to scoring below the 10th percentile on the TMA-93. The socio-demographic characteristics and the neuropsychological background of the final sample (n = 362) are presented in Table 1. Participants had an average of 66.7 years (SD = 9.0, median = 66, range = 50–88), with 235 of them (64.9%) being female. Concerning educational attainment, 77 individuals (21.3%) had incomplete primary studies, 93 individuals (25.7%) had primary studies completed, and 192 (53%) had studies higher than primary. Figure 1 graphically exhibits the total sample distribution by age ranges and educational attainment.
Table 1
Socio-demographic characteristics and neuropsychological background of the final sample
| Age (y) | 66/60–74/50–88 |
| Gender | Male, 127 (35.1%) |
| Female, 235 (64.9%) | |
| Educational level | “Incomplete primary studies”: 77 (21.3%) |
| “Primary studies completed”: 93 (25.7%) | |
| “Studies higher than primary”: 192 (53%) | |
| TMA–93 | 30/29–20/21–30 |
| Triana Test Immediate Free Recall | 6/5–8/0–12 |
| Triana Test Immediate Recognition Recall | 11/10–11/2–12 |
| Triana Test Delayed Free Recall | 7/5–8/0–12 |
| Triana Test Delayed Recognition Recall | 11/10–12/5–12 |
Categorical variables are presented as frequencies. Continuous variables are presented as median/ interquartile range = P25-P75 / range.
Fig. 1
Total sample (n = 362): Distribution by age ranges and educational attainment.
The TMA-93 total score (median = 30, IQR = 29–30, range = 21–30) was consistent with a sample composed of cognitively unimpaired people (Table 1). Table 1 also displays the total scores for the “Triana Test” recalls.
Spearman correlation showed a significant, weak, and positive association between age and each “Triana Test” recall: immediate free recall (r = –0.21, p < 0.001); immediate recognition recall (r = –0.22, p < 0.001); delayed free recall (r = –0.26, p < 0.001); delayed recognition recall (r = –0.23, p < 0.001). Total scores on “Triana Test” recalls showed significant differences by educational attainment (p < 0.001), but not by gender.
Consistent with correlation and the between-group comparisons results, the multiple linear regression analysis showed that total score for each “Triana Test” recall were better predicted by a model that retained age and educational level while excluding gender (Table 2). Thus, the final stratification was based on age and educational level.
Table 2
Multiple regression linear analysis: effect of socio-demographic variables on total scores for Triana Test recalls
| β±Std. Error | t | p | ||
| Immediate Free Recall | Constant | 5.38±0.21 | 25.56 | <0.001 |
| R2: 0.11 | Age (centered) | –0.03±0.01 | –2.93 | <0.001 |
| R2 adjusted: 0.10 | ||||
| p < 0.001 | Educational attainment | 0.70±0.14 | 5.11 | <0.0005 |
| Immediate Recognition Recall | Constant | 9.92±0.16 | 59.85 | <0.001 |
| R2: 0.07 | Age (centered) | –0.03±0.01 | –3.61 | <0.001 |
| R2 adjusted: 0.06 | ||||
| p < 0.001 | Educational attainment | 0.29±0.11 | 2.69 | <0.01 |
| Delayed Free Recall | Constant | 5.72±0.23 | 25.17 | <0.001 |
| R2: 0.15 | Age (centered) | –0.06±0.01 | –4.69 | <0.001 |
| R2 adjusted: 0.15 | ||||
| p < 0.001 | Educational attainment | 0.79±0.15 | 5.32 | <0.001 |
| Delayed Recognition Recall | Constant | 9.88±0.17 | 57.84 | <0.001 |
| R2: 0.07 | Age (centered) | –0.04±0.01 | –3.78 | <0.001 |
| R2 adjusted: 0.06 | ||||
| p < 0.001 | Educational attainment | 0.30±0.11 | 2.65 | <0.001 |
Std.Error, Standard Error; t, Student’s t-test. For applying the regression equations (Predicted Score = Constant + b1* Age centered + b2* Educational attainment), Age should be centered to 66.7 (average), and Educational attainment entered as: “Incomplete primary studies” = 0; “Primary studies completed” = 1; “Studies higher than primary” = 2. Signification (p) and explained variance (R2, R2 adjusted) for each regression model are displayed in the first column of the table.
In the Supplementary Material, four tables ( Supplementary Tables 1–4), each one providing norms for each “Triana Test” recall, are presented. Tables show the theorical raw scores associated with each percentile value (percentiles 1, 2, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 95, 98), according to age and educational level ((categorized into the three levels: incomplete primary studies = 0, primary studies completed = 1, studies higher than primary = 2). The data analysis revealed variations in the scores for the 10th percentile for all “Triana Test” recalls by age and education. For instance, within the delayed free recall category, the total score ranged from 2/12 for 84-year-old individual with incomplete primary studies to 5/12 for another individual aged 50 with studies higher than primary. On the other hand, total scores for the recognition recalls (immediate and delayed), in contrast to the free recalls, showed less dispersion and some ceiling effect, ranging from 10 to 11 within the 50th to 60th percentiles, regardless of age and educational level.
DISCUSSION
In this normative study for the “Triana Test” which included cognitively unimpaired people, comprehensive norms for the test were established, covering a wide age range and the entire spectrum of educational backgrounds. These norms will prove valuable when evaluating patients with memory complaints using the test.
The results indicate that the performance on the “Triana Test” is influenced by both age and education. The percentile-based reference data obtained closely align with combinations of age and educational background. Across all the “Triana Test” recalls, variations in total scores at the 10th percentile, which serves as a crucial cut-off point for diagnosing memory impairment, range from one to three points. When evaluating patients with memory complaints, it is essential to consider these variations before determining whether a total recall score falls within the pathological range.
Although women typically outperform men in verbal memory tests, including those assessing verbal emotional memory [17, 20], we did not observe significant gender-based differences in any of the “Triana Test” scores. For those verbal emotional memory tests, it is worth noting that gender differences have been identified in validation studies [17, 20], that are focused on discriminative validity. On the contrary, normative studies are intentionally designed to investigate the influence of socio-demographic variables on scores.
As expected from a normative study focusing on cognitively unimpaired individuals, the total scores for the recognition recalls (both immediate and delayed) exhibited less variability and showed a slight ceiling effect, with scores ranging from 10 to 11 within the 50th to 60th percentiles. These distributions suggest that the total scores on free recalls may be more effective in discriminating between normal and impaired memory compared to the scores on recognition recalls. This finding is consistent with the results of the previous validation study, where free recalls (both immediate and delayed) demonstrated better diagnostic accuracy, with an AUC greater than 0.80, whereas recognition recalls had an AUC greater than 0.70 [21]. This pattern is consistent with other memory tests used to diagnose memory impairment, where scores on free recalls tend to decline earlier than those on cued or recognition recalls [35–37].
This normative study offers a reliable interpretative framework for using the “Triana Test” without excluding patients with lower levels of education, effectively addressing the increasing need for neuropsychological tests that can accommodate cultural and educational diversity [38, 39]. Notably, 21.43% of participants in this study had “incomplete primary studies”, and all of them completed the test, which demonstrated its practical feasibility, as was also observed in the previous validation study [21].
One of the strengths of this study was the use of the TMA-93 to assess the cognitive status of the participants and determine their eligibility for the research. The TMA-93 has been validated by AD biomarkers, making it a reliable tool for diagnosing early AD and accurately distinguishing between patients with aMCI and healthy controls [40, 41]. Moreover, its administration takes less than three minutes in healthy individuals [42] which offers a time-saving advantage during normative studies conducted in typical clinical practice settings. Since the TMA-93 examines binding by images, its administration should not interfere with that of an audio-verbal test based on learning a story. Lastly, the TMA-93 provides valuable normative data for individuals with lower levels of education [31, 32].
Among the limitations of this study is that the sample was not exactly representative of the Spanish population since the recruitment was based on partners of patients who were attending a general neurology outpatient clinic in a specific city in southern Spain and were aged 50 and over. However, this recruitment strategy ensured the inclusion of a diverse range of individuals in terms of educational backgrounds, all within the age group at risk for AD. While participants with a history of severe psychiatric conditions were excluded, another limitation of this normative study was that a depression scale with a defined cutoff to detect and exclude individuals with suspected moderate or severe depression was not included among the selection procedures.
An important question to address is where the “Triana Test” fits within the broad spectrum of memory tests. The “Triana Test” is a story recall test, similar to the Weschler Memory Scale Logical Memory Test or the Story Recall Test [22, 23]. According to a meta-analysis, story recall tests have a lower diagnostic utility in discriminating between patients with aMCI and HCs compared to tests that evaluate cueing efficiency, such as the Free and Cued Selective Reminding Test, or “binding”, as the Memory Binding Test [37, 43, 44]. However, story recall tests offer certain advantages. They closely mimic real-life memory demands encountered in everyday conversations or media interactions, making them valuable additions to neuropsychological batteries for optimizing memory domain evaluation [37]. Additionally, in situations where visual impairment may limit the use of visual memory tests, audio-verbal tests become more feasible. In such cases, and according to previous results [21, 45], the recall of a story with emotional material could be more useful in diagnosing memory impairment than the recall of a story with relatively neutral material. However, a direct comparison is yet to be conducted to fully elucidate their respective merits and limitations.
In conclusion, this study provides valuable norms for implementing the “Triana Test” in clinical settings to diagnose memory impairment. The total scores on the test are influenced by both age and educational background. Importantly, the test is applicable and feasible for individuals with lower levels of education. Moving forward, the next steps in the development of the “Triana Test” will involve validating it using AD biomarkers and conducting direct comparisons with other story recall tests to further evaluate its diagnostic utility and potential benefits.
ACKNOWLEDGMENTS
We are thankful to all participants involved in this research.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Silvia Rodrigo-Herrero and Emilio Franco-Macías are the authors of the “Triana Test”.
DATA AVAILABILITY
Data derived from this research are available and may be shared.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230096.
REFERENCES
[1] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , Dekosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol 13: , 614–629. |
[2] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[3] | Libon DJ , Bondi MW , Price CC , Lamar M , Eppig J , Wambach DM , Nieves C , Delano-Wood L , Giovannetti T , Lippa C , Kabasakalian A , Cosentino S , Swenson R , Penney DL ((2011) ) Verbal serial list learning in mild cognitive impairment: A profile analysis of interference, forgetting, and errors. J Int Neuropsychol Soc 17: , 905–914. |
[4] | Grober E , Buschke H , Crystal H , Bang S , Dresner R ((1988) ) Screening for dementia by memory testing. Neurology 38: , 900–903. |
[5] | Pavisic IM , Suarez-Gonzalez A , Pertzov Y ((2020) ) Translating visual short-term memory binding tasks to clinical practice: From the theory to practice. Front Neurol 11: , 458. |
[6] | Loewenstein DA , Curiel RE , Wright C , Sun X , Alperin N , Crocco E , Czaja SJ , Raffo A , Penate A , Melo J , Capp K , Gamez M , Duara R ((2017) ) Recovery from proactive semantic interference in mild cognitive impairment and normal aging: Relationship to atrophy in brain regions vulnerable to Alzheimer’s disease. J Alzheimers Dis 56: , 1119–1126. |
[7] | Matías-Guiu JA , Curiel RE , Rognoni T , Valles-Salgado M , Fernández-Matarrubia M , Hariramani R , Fernández-Castro A , Moreno-Ramos T , Loewenstein DA , Matías-Guiu J ((2017) ) Validation of the Spanish Version of the LASSI-L for diagnosing mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 56: , 733–742. |
[8] | Buschke H (2013) The rationale of the Memory Binding Test. In Dementia and Memory, Nilsson LG, Ohta N, eds. Psychology Press, New York, pp. 55-71. |
[9] | Rentz DM , Amariglio RE , Becker JA , Frey M , Olson LE , Frishe K , Carmasin J , Maye JE , Johnson KA , Sperling RA ((2011) ) Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia 49: , 2776–2783. |
[10] | LeDoux JE ((1993) ) Emotional memory systems in the brain. Behav Brain Res 58: , 69–79. |
[11] | Adolphs R , Cahill L , Schul R , Babinsky R ((1997) ) Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem 4: , 291–300. |
[12] | Cahill L , Babinsky R , Markowitsch HJ , McGaugh JL ((1995) ) Amygdala and emotional memory. Nature 377: , 295–296. |
[13] | LaBar KS , Cabeza R ((2006) ) Cognitive neuroscience of emotional memory. Nat Rev Neurosci 7: , 54–64. |
[14] | Braak H , Braak E ((1991) ) Neuropathological stageing of Alzheimer related changes. Acta Neuropathol 82: , 239–259. |
[15] | Hyman BT , Van Hoesen GW , Damasio AR ((1990) ) Memory-related neural systems in Alzheimer’s disease: An anatomic study. Neurology 40: , 1721–1730. |
[16] | Murray AN , Chandler HL , Lancaster TM ((2021) ) Multimodal hippocampal and amygdala subfield volumetry in polygenic risk for Alzheimer’s disease. Neurobiol Aging 98: , 33–41. |
[17] | Strauss GP , Allen DN ((2013) ) Emotional verbal learning test: Development and psychometric properties. Arch Clin Neuropsychol 28: , 435–451. |
[18] | Mah L , Anderson ND , Verhoeff NP , Pollock BG ((2017) ) Negative emotional verbal memory biases in mild cognitive impairment and late-onset depression. Am J Geriatr Psychiatry 25: , 1160–1170. |
[19] | Snyder KA , Harrison DW ((1997) ) The affective auditory verbal learning test. Arch Clin Neuropsychol 12: , 477–482. |
[20] | Jensen CG , Hjordt LV , Stenbaek D , Andersen E , Back SK , Lansner J , Hageman I , Dam H , Nielsen AP , Knudsen GM , Frokjaer VG , Hasselbalch SG ((2016) ) Development and psychometric validation of the verbal affective memory test. Memory 24: , 1208–1223. |
[21] | Luque-Tirado A , Rodrigo-Herrero S , Bernal Sánchez-Arjona M , Franco-Macías E ((2021) ) Preliminary validation of the Triana Test: A new story recall test based on emotional material. Am J Alzheimers Dis Other Demen 36: , 15333175211025911. |
[22] | Wechsler D (1945) Wechsler Memory Scale. Psychological Corporation. |
[23] | Baek MJ , Kin HJ , Sangyun K ((2012) ) Comparison between the story recall test and the word-list learning test in Korean patients with mild cognitive impairment and early stage of Alzheimer’s disease. J Clin Exp Neuropscyhol 34: , 396–404. |
[24] | Baek MJ , Kim HJ , Ryu HJ , Lee SH , Han SH , Na HR , Chang Y , Chey JY , Kim S ((2011) ) The usefulness of the story recall test in patients with mild cognitive impairment and Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 18: , 214–229. |
[25] | Burke A , Heuer F , Reisberg D ((1992) ) Remembering emotional events. Mem Cognit 20: , 277–290. |
[26] | Sharot T , Phelps EA ((2004) ) How arousal modulates memory: Disentangling the effects of attention and retention. Cogn Affect Behav Neurosci 4: , 294–306. |
[27] | Bradley MM , Greenwald MK , Petry MC , Lang PJ ((1992) ) Remembering pictures: Pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn 18: , 379–390. |
[28] | Kazui H , Mori E , Hashimoto M , Hirono N , Imamura T , Tanimukai S , Hanihara T , Cahill L ((2000) ) Impact of emotion on memory. Controlled study of the influence of emotionally charged material on declarative memory in Alzheimer’s disease. Br J Psychiatry 177: , 343–347. |
[29] | Mori E , Ikeda M , Hirono N , Kitagaki H , Imamura T , Shimomura T ((1999) ) Amygdalar volume and emotional memory in Alzheimer’s disease. Am J Psychiatry 156: , 216–222. |
[30] | Maillet D , Narme P , Amieva H , Matharan F , Bailon O , Le Cléiau H , Belin C ((2017) ) The TMA-93: A new memory test for Alzheimer’s disease in illiterate and less educated people. Am J Alzheimers Dis Other Dement 32: , 461–467. |
[31] | Rodrigo-Herrero S , Sánchez-Benavides G , Ainz-Gómez L , Luque-Tirado A , Graciani-Cantisán E , Sánchez-Arjona MB , Jiménez-Hernández MD , Franco-Macías E ((2020) ) Norms for testing visual binding using the Memory Associative Test (TMA-93) in older educationally-diverse adults. J Alzheimers Dis 75: , 871–878. |
[32] | García-Roldán E , Arriola-Infante JE , Méndez-Barrio C , Montiel-Herrera F , Mendoza-Vázquez G , Marín-Cabañas AM , Rodrigo-Herrero S , Luque-Tirado A , Sánchez-Arjona MB , Maillet D , Franco-Macías E ((2022) ) Testing visual binding by the TMA-93 in people aged 75 and over. J Alzheimers Dis 88: , 503–512. |
[33] | Lenhard W , Lenhard A ((2021) ) Improvement of norm score quality via regression-based continuos norming. Educ Psychol Measure 81: , 229–261. |
[34] | Brugulat-Serrat A , Cañas-Martínez A , Canals-Gispert L , Marne P , Gramunt N , Milà-Alomà M , Suárez-Calvet M , Arenaza-Urquijo EM , Grau-Rivera O , González-de-Echávarri JM , Minguillon C , Fauria K , Kollmorgen G , Suridjan I , Zetterberg H , Blennow K , Gispert JD , Molinuevo JL , Sánchez-Benavides G ((2021) ) Enhancing the sensitivity of memory tests: Reference data for the Free and Cued Selective Reminding Test and the Logical Memory Task from cognitively healthy subjects with normal Alzheimer’s disease cerebrospinal fluid biomarkers levels. J Alzheimer Dis 84: , 119–128. |
[35] | Grober E , Lipton RB , Hall C , Crystal H ((2000) ) Memory impairment on free and cued selective reminding predicts dementia. Neurology 54: , 827–832. |
[36] | Ivanoiu A , Adam S , Van Der Linden M , Salmon E , Juillerat AC , Mulligan R , Seron X ((2005) ) Memory evaluation with a new cued recall test in patients with mild cognitive impairment and Alzheimer’s disease. J Neurol 252: , 47–55. |
[37] | De Simone MS , Perri R , Fadda L , Caltagirone C , Carlesino GA ((2019) ) Predicting progression to Alzheimer's disease in subjects with amnestic mild cognitive impairment using performance on recall an recognition test. J Neurol 266: , 102–111. |
[38] | Brucki SM ((2010) ) Illiteracy and dementia. Dement Neuropsychol 4: , 153–157. |
[39] | Ardila A , Bertolucci PH , Braga LW , Castro-Caldas A , Judd T , Kosmidis MH , Matute E , Nitrini R , Ostrosky-Solis F , Rosselli M ((2010) ) Illiteracy: The neuropsychology of cognition without reading. Arch Clin Neuropsychol 25: , 689–712. |
[40] | Rodrigo-Herrero S , Luque-Tirado A , Méndez-Barrio C , García-Solís D , Bernal Sánchez-Arjona M , Oropesa-Ruíz JM , Maillet D , Franco-Macías E ((2021) ) TMA-93 validation by Alzheimer’s disease biomarkers: A comparison with the Free and Cued Selective Reminding Test. J Alzheimers Dis 82: , 401–410. |
[41] | Rodrigo-Herrero S , Carnero-Pardo C , Méndez-Barrio C , De Miguel-Tristancho M , Graciani-Cantisán E , Sánchez-Arjona MB , Maillet D , Jiménez-Hernández MD , Franco-Macías E ((2019) ) TMA-93 for diagnosing amnestic mild cognitive impairment: A comparison with the Free and Cued Selective Reminding Test. Am J Alzheimers Dis Other Demen 34: , 322–328. |
[42] | Franco-Macías E , Rodrigo-Herrero S , Luque-Tirado A , Méndez-Barrio C , Medina-Rodríguez M , Graciani-Cantisán E , Sánchez-Arjona MB , Maillet D ((2020) ) Reliability and feasibility of the memory associative test TMA-93. J Alzheimers Dis Rep 4: , 431–440. |
[43] | Buschke H (2014) Rationale of the Memory Binding Test. In Dementia and Memory, Nilsson LG, Ohta N, eds. Psychology Press, pp. 55-71. |
[44] | Weissberger GH , Strong JV , Stefanidis KB , Summers MJ , Bondi MW , Stricker NH ((2017) ) Diagnostic accuracy of memory measures in Alzheimer’s dementia and mild cognitive impairment: A systematic review and meta-analysis. Neuropsychol Rev 27: , 354–388. |
[45] | Chapman KR , Bing-Canar H , Alosco ML , Steinberg EG , Martin B , Chaisson C , Kowall N , Tripodis Y , Stern RA ((2016) ) Mini Mental State Examination and Logical Memory scores for entry into Alzheimer’s disease trials. Alzheimers Res Ther 8: , 9. |




