Prevalence of Mild Cognitive Impairment in Southern Regions of Colombia
Abstract
Background:
Recent reports suggest that by 2050 there will be an increase of around 310% of cases affected by dementia in Latin American countries. A previous study in a Southern region reported one of the highest prevalences of dementia in Latin America.
Objective:
To investigate the prevalence of mild cognitive impairment associated with low education, rurality, and demographic characteristics.
Methods:
A cross-sectional study recruited a community-dwelling sample of 823 adults from rural and urban areas of two Southern provinces of Colombia from 2020–2022. Participants were assessed with a neuropsychological protocol validated in Colombia. To obtain general and region-specific prevalence rates, age, sex, schooling, and socioeconomic level were considered and controlled for.
Results:
Most of the participants reported low education and socioeconomic level, the participation of women was higher. It was determined that the prevalence of mild cognitive impairment (MCI) was 53.6%, with 56.6% in the province of Caquetá followed by 51.9% in the province of Huila. The amnestic MCI represented 42.6%, the amnestic multi-domain was 39%, the non-amnestic 16.55%, and the non-amnestic multi-domain 1.81%. Our participants reported comorbidities such as diabetes and hypertension. We also observed a relationship between exposure to pesticides and MCI.
Conclusions:
We observed one of the highest prevalences of MCI in Latin America reported to date. Variables such as age, gender, and education proved risk factors for MCI in the explored regions. Our findings are very much in line with recent studies that highlight the influence of non-canonical risk factors of dementia in underrepresented countries from Latin America.
INTRODUCTION
Recent reports on the prevalence of dementia forecast that by 2050 there will be an increase of around 310% of cases affected by this epidemic disorder in Latin American countries. Colombia seems to be well on its way to meet such a forecast as a previous study reported one of the highest prevalences of dementia in a Latin America. It is important to explore current prevalence rates of dementia risk conditions such as mild cognitive impairment (MCI). This was the aim of this study. Although MCI has been largely associated with dementia [1], other studies have found it co-existing with other health conditions known to reduce cognitive functioning including head trauma [2], depression [3], environmental pollution by pesticides [4], chronic diseases such as hypertension [5], and diabetes [6]. We also address the presence and influences of such risk factors.
It has been suggested that the classification of MCI in different subtypes (amnestic MCI single domain (aMCIs), amnestic MCI multiple domains (aMCIm), non-amnestic MCI single domain (naMCIs), and non-amnestic MCI multiple domains (naMCIm)) [7] can aid the identification of risk profiles and the prediction of the underlying disease, i.e., type of dementia the affected person will develop. Accrued knowledge suggests that approximately 14.9% of patients with aMCI will go on to develop dementia [8]. Considering the imminent growth of the older population, which is anticipated to be more dramatic in low- and middle-income countries (LMIC) than in high-income countries (HIC) [9, 10], it becomes imperative to undertake an epidemiological characterization of this health problem in Latin America. Recently, it has been acknowledged [11, 12] that the lack of such studies has precluded the contribution of data from Latin America to major global initiatives such as the 10/66 study [11–13]. This is unfortunate as changes in demographic structures in Latin America Countries (LAC) due to increase in life expectancy, better health care, and others, are leading to an unprecedented raise in the number of older adults who develop neurodegenerative diseases such as dementia [11, 14].
The available evidence indicates that the prevalence of all type of MCI in LAC ranges from 6.8% to 25.5%, approximately [15]. Two prevalence studies have been conducted in Colombia. The first one, was a cross-sectional study among older adults over the age of 50 which reported a prevalence of MCI of 9.7% [16]. The other, completed between 2012 and 2014, identified a prevalence of MCI of 34% and of dementia of 23% [17]. However, such studies were conducted in two main capital cities of Colombia. Therefore, these earlier results are not representative of the entire Colombian population let alone of the population living in more deprived conditions (i.e., towns with low income, rural areas). In 2015, the SABE Survey (Health, Well-being, and Aging) was carried out in Colombia using a probabilistic sample that included a large amount of data [18]. The study included 27.1% of participants from the central region where the departments of Huila and Caquetá are located [19]. The study determined a prevalence of MCI of 17.93% among older adults over 60 years old [20]. It is worth noting that the presence of MCI was supported via the use of the Mini-Mental State Examination (MMSE). This methodological choice might have potentially limited the strength of the evidence as the sensitivity and specificity of the MMSE for the diagnosis MCI is limited [21]. The evidence shows that the MMSE is not a sensitive tool to screen for cognitive decline in individuals with a low educational level [22].
As previously mentioned, it is known that demographic factors such as years of schooling and living in rural areas modify the risk of developing MCI and dementia and, therefore, more studies are needed that involve populations exposed to such factors. A previous relevant study in a region targeted by this study (Neiva, Huila) reported a dementia prevalence of 23.6% [23]. This figure was strikingly high, in fact higher than that reported by other countries. Given this earlier local evidence and that reviewed with respect to the global picture, an updated characterization of MCI in a broader region of southern Colombia is overdue. This was the objective of the present study. Based on the findings from previous research in the region, we have formulated a hypothesis where we anticipate an equally high prevalence of MCI, particularly of the amnesic subtype that we consider may have a certain relationship with low education, rurality, and demographic characteristics typical of the southern Colombian region.
METHODS
A community-based cross-sectional study was carried out between 2020 and 2022 in two municipalities in southern Colombia, Huila and Caquetá. We have assessed the present study and its report against the ten methodological evaluation criteria for prevalence studies presented by Zachary Munn [24] (see Supplementary Material 1). The recruitment process involved two main strategies. First, a group of psychologists with training in neuropsychology approached older adults who regularly attended community centers for the elderly in the target municipalities. These centers run government programs where older adults participate to improve their quality of life. We provided information about the study and invited those who expressed interest to participate. The older adults who attend these centers are functionally independent. Those who agreed to be involved were transported to a neuropsychological assessment facility to undertake the study assessments.
Second, we used open invitations on social networks and institutional pages to reach people over 50 who might be interested in participating in the study. The goal was to ensure that the sample we collected was representative of the general population. As our age limit was rather low (50), there was the possibility that individuals at risk of early onset dementia were also recruited. The participants were evaluated by an interdisciplinary team comprising neuropsychologists, psychiatrists, and neurologists who carried out the assessments at the clinics following the study protocols.
The inclusion criteria were as follows: Participants who were aged 50 years or older, had normal or corrected-to-normal vision and hearing, and could complete the neuropsychological assessment were included. Those who had uncontrolled chronic diseases even if medicated and a history of neurological diseases or psychiatric illnesses were excluded. The study was conducted in compliance with ethical standards, specifically Bioethics and Research Committee number 002-006 of the University Hospital of the University Surcolombiana as well as the Declaration of Helsinki. The informed consent was signed by the participant following the information session during which the procedures involved in the research project were explained and discussed. A family member or caregiver (witness) of the participant was also invited to consent. The informed consent included the signature of the participant as well as that of a witness. In the consenting stage, the principles of confidentiality, respect for autonomy, beneficence and non-maleficence were mentioned to the participants.
Sample size
Although our sample was not selected through a probabilistic method, we have taken rigorous measures to ensure it was as representative as possible. To ascertain this, we conducted a power calculation to determine the appropriate sample size for our study by utilizing a previously reported MCI prevalence of 34% [18]. OpenEpi®, an open-source software for statistics in epidemiology, indicated that we needed a sample size of 596 adults over 50 years of age in both municipalities to achieve the desired level of statistical power, with a 99% confidence level, an absolute precision of 5% (d = 0.05), and a design effect of 1.0. This approach ensures that, despite our non-probabilistic sampling method, our results would be robust and supported by sufficient power, thereby enhancing the overall representativeness of our results.
Procedures
Older adults who agreed to participate in the study were evaluated in two sessions, a psychologist with knowledge and training in neuropsychology administered an assessment protocol to every participant. This helped to detect the presence of cognitive impairment and classify participants accordingly. The assessment comprised a clinical interview, and an interview with a caregiver and/or accompanying person which could be completed via a telephone call, neuropsychological and functional assessments. The criteria used to detect MCI were those described by Petersen [7]. We explored such criteria in participants whose MMSE was 23 or above at the screening point and who were also independent in their activities of daily living. The decision of using 23 as a MMSE cut-off score for dementia stemmed from Colombian normative studies carried out to validate the same tools we used in our protocol (all the normative groups above 50 years of age and with 0 years of education or more had a minimum average MMSE of 28 points [25]). Furthermore, a MMSE cut-off of 23 for dementia is line with that reported by other authors in similar Latin American populations. The diagnostic criteria involved the: 1) Presence of subjective memory complaint, 2) accompanied by objective cognitive decline as informed by neuropsychological assessment (scores≤1.5SD below the norms) [25], 3) preserved abilities to perform basic Activities of Daily Living (ADL) and relatively preserved abilities to perform instrumental ADL, and 4) absent of criteria for dementia. Cases that were difficult to classify were discussed through case studies among expert panels, which involved medics, neuropsychologists, and psychologists who are collaborators of the longitudinal study on the early detection of dementias in the south of Colombia. Figure 1 shows the algorithm followed to identify and classify participants.
Fig. 1
Flow chart illustrating the recruitment process.
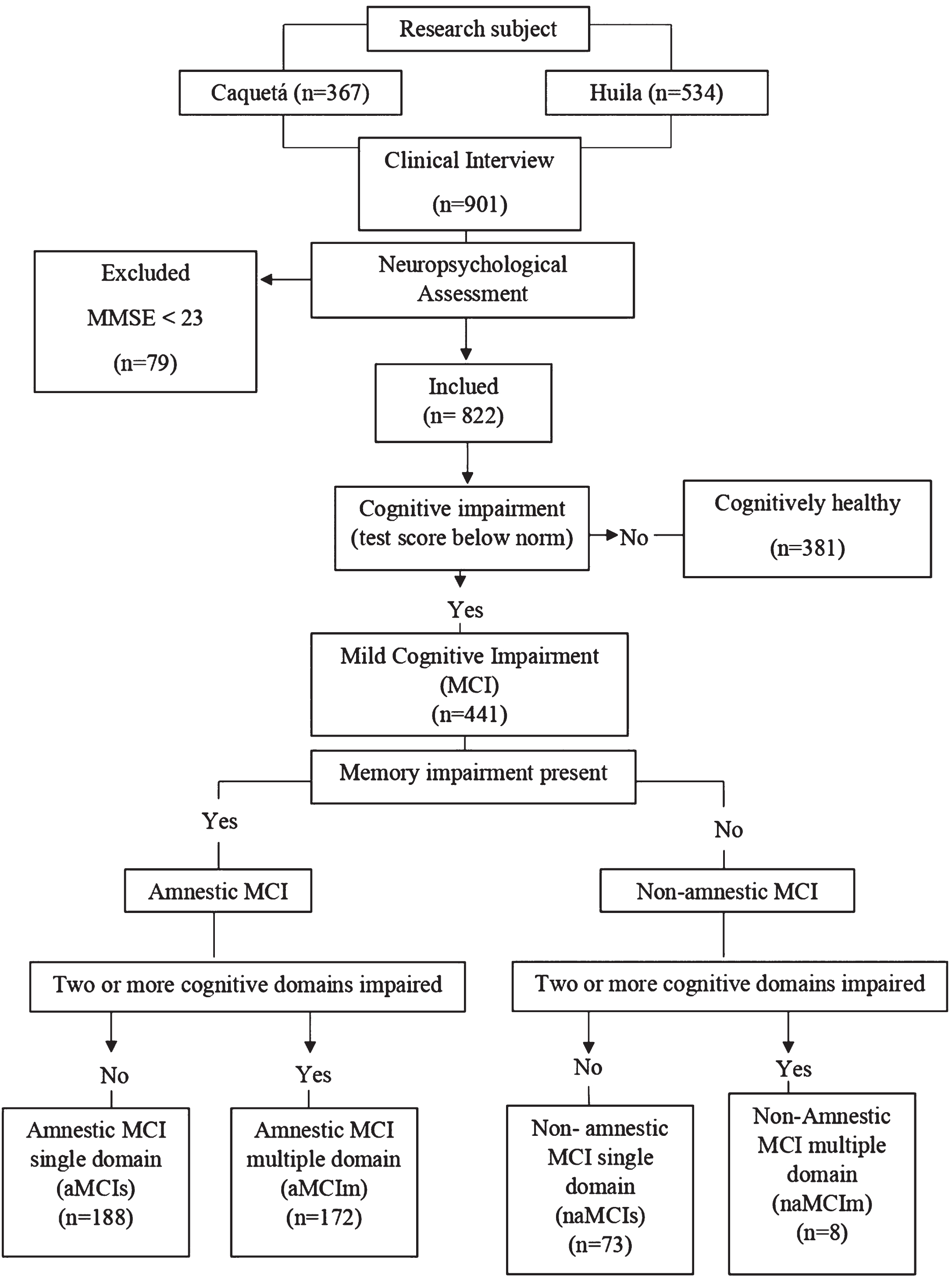
To ensure a comfortable and effective evaluation, we divided questionnaires and tests into two sessions, making them shorter when needed. The format was engaging, tailored to participants’ characteristics. Feedback and results were shared to boost satisfaction. Sessions lasted about an hour, with breaks for older adults, respecting individual needs and promoting participation.
Cognitive assessment
Addenbrooke’s Cognitive Examination - Revised ACER-R Colombia: It is a brief, sensitive and specific test battery to detect early cognitive dysfunction. It evaluates five cognitive domains: attention/orientation, memory, fluency, language, and visuospatial skills [26]. The version used was adapted and validated for Colombia [27] and the cut-off score according to the ROC curve was 87 points with sensitivity of 92.72% and specificity of 90.54%.
The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): is a neuropsychological screening battery originally developed in English [28], for the neurocognitive assessment we used the Colombian validation of CERAD-Col conducted by the Neuroscience Group of Antioquia (GNA) [24] and the updated normative data [20]. The CERAD tests used include: the MMSE to assess the cognitive areas of orientation, fixation, concentration, calculation, memory, and language; the Boston Naming test-15 to assess language (naming); the Word List Memory task that assesses learning; the Word List Recall to assess free recall; the Word List Recognition task; the Praxis Copy and Evocation task; Semantic Fluency and Phonological Fluency; Rey-Osterrieth Figure Copy and Evocation; and Trail Making Test A and B. For the analysis of the internal consistency of the CERAD-Col, the authors reported that the scores of each of the tests of the battery were used. The Cronbach’s α was 0.83 (95% CI = 0.78-0.88) for the general population sample, and 0.87 (95% CI = 0.83-0.9) for the sample of patients with Alzheimer’s disease, indicating a high level of homogeneity and equivalence of responses to all tests.
The Yesavage Scale for Geriatric Depression-GDS-15 [29]: the scale quantifies depressive symptoms in older adults. In the study of consistency and factorial structure in the Colombian population, it is reported that the GDS-15 showed an acceptable internal consistency of 0.78. A systematic review showed that the 15-item version has better diagnostic accuracy and clinical utility than the 30-item version.
Data analysis
Our protocol comprised an independent variable (Group) with two levels (healthy older adults (HOA) and MCI). The allocation of participants to such groups was described above (see Fig. 1). All the other variables were dependent. To describe the sociodemographic and clinical characteristics of the participants, absolute and relative frequencies were used in the case of qualitative variables, and measures of central tendency with dispersion were used in the case of quantitative variables. The overall and specific prevalence was estimated by age group, sex, education, and socioeconomic level. Chi square analyzes were performed to explore categorical variables. Multivariate logistic regression analysis was performed using as predictors Age, education, place of residence, diabetes, hypertension, cerebrovascular disease, malnutrition, exposure to pesticides and smoking and Group as the dependent variable.
RESULTS
Before we report on the core outcomes from our study, it is worth mentioning that we subjected our design and protocol to a self-assessment focused on the ten methodological criteria recommended for prevalence studies [24]. Our study successfully met such criteria (see Supplementary Material 2). We recruited 823 older adults into the study thus exceeding our estimated sample size. Of these, 73.3% were women. After administering the above protocol, we determined that 53.6% of those evaluated had cognitive impairment. Our sample was drawn from two cities, 503 participants were recruited in the department of Huila and 320 in Caquetá. Of the sample in Huila, 261 participants (51.9%) met the MCI criteria while in Caquetá 181 participants (56.6%) met such criteria.
Table 1 shows the distribution of the sample across Group (HOA and MCI) and socio-demographic variables. Of note, 103 (38.8%) participants with MCI were between 50-60 years of age, 250 out of 326 adults were in the lowest education band (1 and 5 years), and 86% were in low socioeconomic status. Regarding comorbidities, 60.7% (n = 68) MCI patients had diabetes and 55.5% (n = 176) had high blood pression. Regarding environmental risk factors, 128 (15.5%) participants (out of 823) reported exposure to agricultural pesticides of whom 73% (n = 57) met MCI criteria (see Table 1).
Table 1
Demographics variables
| Variable | Total sample | MCI group | HOA group | p |
| 822 | 441 (53.6) | 381 (46.3) | ||
| Sex | ||||
| Male | 218 (26.4) | 122 (56) | 96 (44) | 0.424 |
| Female | 604 (73.3) | 319 (52.8) | 285 (47.2) | |
| Age | ||||
| 50–59 | 265 (32.2) | 103 (38.8) | 162 (61.2) | 0.000 |
| 60–69 | 385 (46.7) | 215(55.8) | 170 (44.2) | |
| >70 | 172 (20.9) | 123(71.5) | 49 (28.5) | |
| Years of Education | ||||
| 1–5 | 326 (39.6) | 250 (76.7) | 75 (23.1) | 0.000 |
| 6–11 | 267 (32.4) | 121 (45.3) | 145 (54.3) | |
| >12 | 227 (27.5) | 67 (29.5) | 160 (70.5) | |
| Socioeconomic status* | ||||
| One | 286 (34.7) | 185 (64.6) | 101 (35.4) | 0.000 |
| Two | 424 (51.5) | 215 (50.7) | 209 (49.3) | |
| Three | 82 (9.9) | 32 (39) | 50 (61) | |
| Four | 30 (3.6) | 9 (30) | 21 (70) | |
| Place of residence | ||||
| Rural | 120 (14.5) | 75 (62.5) | 45 (37.5) | 0.932 |
| Urban | 703 (85.4) | 367 (52.2) | 336 (47.8) | |
| Department | ||||
| Huila | 503 (61.1) | 261 (51.9) | 242 (48.1) | 0.204 |
| Caquetá | 320 (38.8) | 181 (56.6) | 139 (43.4) | |
| Chronic disease | ||||
| Diabetes | 112 (13.6) | 68 (60.7) | 44 (39.3) | 0.107 |
| Arterial hypertension | 317 (38.5) | 176 (55.5) | 141 (44.5) | 0.394 |
| Cerebrovascular disease | 17 (2) | 11 (64.7) | 6 (35.3) | 0.356 |
| Malnutrition | 15 (1.8) | 9 (60) | 6 (40) | 0.619 |
| Heart disease | 38 (4.6) | 25 (65.8) | 13 (34.2) | 0.124 |
| Tobacco | 258 (31.4) | 163 (63.2) | 95 (36.8) | 0.000 |
| Depression | ||||
| Yes | 64 (7) | 42 (65.6) | 22 (34.4) | 0.031 |
| No | 759 (92) | 399 (52.6) | 360 (47.4) | |
| Pesticide exposure | ||||
| Yes | 128 (15.5) | 73 (57) | 55 (43) | 0.405 |
| No | 692 (84) | 367 (53) | 325 (47) |
*The classification of the SES is based on the classification of the Administrative Planning Departments, which classify the municipality’s housing and/or land (Law 142 of 1994) in the following categories: one (low-low), two (low), three (medium-low), four (medium), five (medium-high) and six (high). This classification is based on the characteristics of the community dwellers and their urban-rural environments.
In the analysis of the association between age and the presence of MCI, significant patterns were observed. People aged 50-59 years represented 38.8% of the MCI group and 61.2% of the control group. In the 60-69 age group, 55.8% showed MCI, while 44.2% were HOA. Finally, in the age group over 70 years, 71.5% had MCI, compared to 28.5% of the HOA. The chi-square analysis revealed a highly significant association between age (χ2 = 46.099, p < 0.001) and the presence of MCI (χ2 = 4.649, p < 0.005). These findings indicate a statistically significant relationship between age and MCI, suggesting that MCI tends to increase with older age. Table 2 shows the neuropsychological characteristics of the groups. Of the evaluated sample, 188 (42.6%) met criteria for amnestic MCI, 172 (39%) for amnestic multi-domain MCI, 73 (16.55%) for non-amnestic MCI, and 8 (1.81%) for non-amnestic multi-domain.
Table 2
Frequency distribution of subtypes of MCI
| MCI | Frequency (n = 441) | Female | Male | Percentage (100) |
| Amnestic MCI | 188 | 130 | 58 | 42.6 |
| Multi-domain amnestic MCI | 172 | 123 | 49 | 39 |
| Non-amnestic MCI | 73 | 58 | 15 | 16.55 |
| Multi-domain non-amnestic MCI | 8 | 8 | 0 | 1.81 |
In the logistic regression analysis, multiple variables were evaluated to determine their impact on the likelihood of individuals belonging to the MCI group. Statistically significant results were found for age (p = 0.009). With one year increase in age, there was approximately 3.0% increase in the probability of belonging to the MCI group (OR = 1.030 CI: 0.834 - 0.887). Years of education also proved significant (p < 0.001), indicating that each additional year of education was associated with a 14.0% decrease in the probability of being in the MCI group (OR = 0.860; CI = 0.834-0.887). Individuals who smoked had approximately 42.6% higher odds of developing MCI compared to non-smokers (OR = 1.426; CI = 1.015-2.003), although these results were only marginally significant. Other variables such as place of residence, chronic diseases, diabetes, hypertension, vascular disease, malnutrition, and pesticide exposure did not show statistically significant associations with the probability of MCI (see Table 3).
Table 3
Results from the logistic regression
| Variables | B | SE | Wald | Sig. | OR | 95% CI | |
| Age | 0.029 | 0.011 | 6.865 | 0.009 | 1.03 | 1.007 | 1.052 |
| Years of education | –0.15 | 0.016 | 90.222 | 0.000 | 0.86 | 0.834 | 0.887 |
| Place of residence | 0.137 | 0.223 | 0.377 | 0.539 | 1.147 | 0.741 | 1.775 |
| Diabetes | 0.211 | 0.233 | 0.82 | 0.365 | 1.235 | 0.782 | 1.948 |
| Hypertension | –0.142 | 0.167 | 0.725 | 0.394 | 0.868 | 0.625 | 1.203 |
| Vascular disease | 0.414 | 0.565 | 0.537 | 0.464 | 1.513 | 0.5 | 4.579 |
| Malnutrition | –0.401 | 0.555 | 0.522 | 0.47 | 0.67 | 0.226 | 1.987 |
| Pesticide exposure | 0.217 | 0.224 | 0.938 | 0.333 | 1.242 | 0.801 | 1.927 |
| Smoking | 0.355 | 0.174 | 4.178 | 0.041 | 1.426 | 1.015 | 2.003 |
DISCUSSION
The present study investigated the hypothesis that high prevalence of MCI would be found in Colombian regions where the prevalence of dementia had been reported to be among the highest in Latin America. We anticipated that such prevalence rates would be associated to low education, rurality, and demographic characteristics typical of the southern Colombian region. The prevalence of MCI was 51.9% for the province of Huila and 56.6% for the province of Caquetá (average regional prevalence of 53.6%). This greatly surpasses the prevalence reported by other studies. Differences in prevalence rates of MCI may be attributed to the diagnostic criteria, study populations, methodologies and instruments used [15]. The screening tools traditionally used to identify subjects with suspected MCI have proved both limited and challenging (e.g., MMSE). Such tools can explain the low prevalence reported by other studies. The fact that for the present study we relied on an assessment protocol widely used and validated in Colombia [25] grant us confidence in the reliability of our results.
Our current results are consistent with the prevalence of dementia established for this population in 2003, corresponding to 23.6% in population over 60 years [23]. The prevalence of MCI here reported would match conversion rates for dementia in members of these Colombian populations. When estimating the conversion rate from MCI to dementia based on age and other healthcare factors, an annual rate between 10-15% % is expected [30]; about 1 in 4 people with MCI will be at risk of dementia [31]. Although the prevalence of MCI here reported might look high, other studies had also identified extremely high prevalence. For example, Mohan et al. [32] reported that the prevalence of MCI varies widely between 3% and 42% across different regions of the world. Artero et al. [33] reported a prevalence of MCI of 42% which they found to be consistently high across three recruitment sites (43%, 47%, and 28%). MCI prevalence of 32.9% was reported among community-dwelling elderly Koreans aged 74.3±16.7 [34]. The prevalence of MCI is a highly inconsistent epidemiological figure of a highly heterogeneous nature [32]. In the case of our study, we feel confident to endorse our identified prevalence rate. Our previous characterization of the dementia prevalence in Huila [23] together with the strict methodological approach followed in the current study indicate that the magnitude of such a problem in this region of Colombia is alarming [35].
The MCI sample found in our study showed that the amnestic type was the most common form for both genders and that the Multi-domain non-Amnestic MCI was not found in men. Regarding risk factors, we found that both age and education were significant predictors of group membership. These results are not entirely new. Recent studies have explored the social determinants of health and aging in Latin America [36] and have suggested a set of non-canonical risk factors driving aging trajectories in the region. Studies from developed countries has reported that limited access to formal education grant higher risk of anormal aging [20, 37, 38]. Recently Santamaria-Garcia et al. [36] reported that this is also true for Latin American countries and our study confirm it does apply to Colombia; the population targeted by this study is located in regions of Colombia where the factors described by Santamaria-Garcia et al. [36] are rather abundant (see Supplementary Material 2 for a detailed description of the targeted Colombian regions). Regarding comorbidities and environmental factors, it was found that more than 50% of our patients had other chronic diseases being diabetes (n = 68, 60.7%) and cardiovascular diseases (hypertension, n = 176, 55.5%) the most common comorbidities. This evidence is relevant as Livingston et al. [10] reported that diabetes is a key risk factor for dementia later in life accounting for a potential percentage reduction in dementia prevalence of 1%. Hypertension also figures among the midlife risk factors for dementia accounting for a potential percentage reduction in dementia prevalence of 2% [39]. Therefore, by tackling these risk factors, we could potentially reduce 3% of future dementia cases. However, in the multivariate regression analyses we did not find significant associations between chronic diseases, such as diabetes, hypertension, and vascular accidents, and the risk of MCI. This contrasts with previous studies, which have identified stroke, depression, and reduced physical activity (less than half an hour) as independent risk factors for cognitive decline in older individuals with diabetes [40].
While a direct link between depression and MCI demonstrated a statistically significant association, multivariate logistic regression analysis, controlling for variables including age, education, chronic diseases, and pesticide exposure, failed to provide evidence supporting a connection between ‘depression’ and cognitive deterioration. These findings suggest that cognitive decline may be more comprehensively explained by the interplay of these other variables, which have received extensive attention in the existing literature. Other studies have reported that depression is a significant risk factor for dementia in individuals with type 2 diabetes [41]. Pink and colleagues found a relationship between amyloid deposition in patients who tested positive for positron emission tomography (PET) and depression but not with clinical anxiety [42].
Finally, we observed a remarkably high number of MCI cases exposed to pesticides, although without statistically significant associations at this stage. We had 75 cases of MCI (62.5%) who reported living in the rural area in both departments and 128 participants (15.5%) had been exposed to agricultural pesticides. This is not surprising as we are aware that this population is imminently agricultural. What is surprising is that Livingston et al [10] did not identify environmental factors as potential sources of dementia risk. It is known that the prevalence of MCI tends to be higher in rural areas [43]. Evidence is accruing suggesting the presence of learning and memory deficits following repeated occupational exposures to organophosphate [44]. Therefore, it is important to inquire about these aspects, specifically in areas highly exposed to agricultural pesticides.
The study faces some limitations. For example, the type of non-probabilistic sampling in this study may limit the generalization of our results. Older adults who are experiencing cognitive decline may be more prompted to enroll due to a desirability factor. Another limitation is the lack of dementia biomarkers to achieve a higher diagnostic accuracy.
To conclude, the prevalence of MCI found in this study was higher than that reported in Latin America, for example in Perú aMCI 17.9% [45] and Costa Rica 9% [46]. Low educational level and chronic diseases are relevant factors which have been identified by others. We are the first group to report pesticide exposure as a potential factor worth investigating in Latin American populations in the future. As highlighted above, the forecast on new dementia cases for LMIC, including Latin America, is not encouraging [35]. The dementia epidemic will have a more serious impact on our countries than in developed countries [11, 14]. It is essential to conduct further studies to explore other factors related to dementia risk among these populations. For example, given that these individuals are still in a productive stage of their lives, it is important to consider the potential impact of environmental factors such as exposure to toxic agents.
ACKNOWLEDGMENTS
We thank the older adults for their participation and willingness to carry out the research, the community clubs of Huila and Caquetá.
FUNDING
This work was supported by The Science, Technology, and Innovation Fund- Huila of the General System of Royalties through the Ministry of Science, Technology, and Innovation of Colombia [BPIN 2020000100011] Universidad Surcolombiana and Universidad de la Amazonia, and [CONADI - Universidad Cooperativa de Colombia in agreement with Universidad Surcolombiana] under Grant [INV2384].
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings are available on reasonable requests via the corresponding authors.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230041.
REFERENCES
[1] | Bonilla-Santos J , Zea-Romero EY , González-Hernández A , Cala-Martínez DY ((2021) ) Cognitive, biological, anatomical and behavioral markers of mild cognitive impairment and Alzheimer’s disease. A systematic review. Rev Ecuatoriana Neurol 30: , 57–67. |
[2] | LoBue C , Woon FL , Rossetti HC , Hynan LS , Hart J , Cullum CM ((2018) ) Traumatic brain injury history and progression from mild cognitive impairment to Alzheimer disease. Neuropsychology 32: , 401–409. |
[3] | Ferrer-Cairols I , Montoliu T , Crespo-Sanmiguel I , Pulopulos MM , Hidalgo V , Gómez E , López-Cuevas R , Cuevas A , Martín N , Baquero M , Salvador A ((2022) ) Depression and suicide risk in mild cognitive impairment: The role of Alzheimer’s disease biomarkers. Psicothema 34: , 553–561. |
[4] | Fuhrimann S , Farnham A , Staudacher P , Atuhaire A , Manfioletti T , Niwagaba CB , Namirembe S , Mugweri J , Winkler MS , Portengen L , Kromhout H , Mora AM ((2021) ) Exposure to multiple pesticides and neurobehavioral outcomes among smallholder farmers in Uganda. Environ Int 152: , 106477. |
[5] | Heizhati M , Li N , Wang L , Hong J , Li M , Yang W , Yao L , Lin M , Pan F , Yang Z , Wang Z , Abudereyimu R ((2021) ) Association of hypertension with mild cognitive impairment in population from less-developed areas of multiethnic northwest China. Neuroepidemiology 55: , 407–415. |
[6] | Ciudin A , Espinosa A , Simó-servat O , Ruiz A ((2017) ) Type 2 diabetes is an independent risk factor for dementia conversion in patients with mild cognitive impairment. J Diabetes Complications 31: , 1272–1274. |
[7] | Petersen RC ((2004) ) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: , 183–194. |
[8] | Petersen RC , Lopez O , Armstrong MJ , Getchius TSD , Ganguli M , Gloss D , Gronseth GS , Marson D , Pringsheim T , Day GS , Sager M , Stevens J , Rae-Grant A ((2018) ) Practice guideline update summary: Mild cognitive impairment report of theguideline development, dissemination, and implementation. Neurology 90: , 126–135. |
[9] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[10] | Livingston G , Sommerlad A , Orgeta V , Costafreda SG , Huntley J , Ames D , Ballard C , Banerjee S , Burns A , Cohen-Mansfield J , Cooper C , Fox N , Gitlin LN , Howard R , Kales HC , Larson EB , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2017) ) Dementia prevention, intervention, and care. Lancet 390: , 2673–2734. |
[11] | Parra MA , Baez S , Allegri R , Nitrini R , Lopera F , Slachevsky A , Custodio N , Lira D , Piguet O , Kumfor F , Huepe D , Cogram P , Bak T , Manes F , Ibanez A ((2018) ) Dementia in Latin America Assessing the present and envisioning the future. Neurology 90: , 222–231. |
[12] | Parra MA , Baez S , Sedeño L , Gonzalez Campo C , Santamaría-García H , Aprahamian I , Bertolucci PHF , Bustin J , Camargos Bicalho MA , Cano-Gutierrez C , et al. ((2020) ) Dementia in Latin America: Paving the way toward a regional action plan. Alzheimers Dement 17: , 295–313. |
[13] | Sosa AL , Albanese E , Stephan BCM , Dewey M , Acosta D , Ferri CP , Guerra M , Huang Y , Jacob KS , Jiménez-Velázquez IZ , Rodriguez JJ , Salas A , Williams J , Acosta I , González-Viruet M , Guerra Hernandez MA , Shuran L , Prince MJ , Stewart R ((2012) ) Prevalence, distribution, and impact of mild cognitive impairment in Latin America, China, and India: A 10/66 population-based study. PLoS Med 9: , e1001170. |
[14] | Mukadam N , Sommerlad A , Huntley J , Livingston G ((2019) ) Population attributable fractions for risk factors for dementia in low-income and middle-income countries: An analysis using cross-sectional survey data. Lancet Glob Health 7: , e596–e603. |
[15] | Ribeiro FS , Teixeira-santos AC , Leist AK ((2022) ) The prevalence of mild cognitive impairment in Latin America and the Caribbean: A systematic review and meta-analysis. Aging Ment Health 26: , 1710–1720. |
[16] | Henao-Arboleda E , Aguirre-Acevedo DC , Muñoz C , Pineda DA , Lopera F ((2008) ) Prevalence of mild cognitive impairment, amnestic-type, in a Colombian population. Rev Neurol 46: , 709–713. |
[17] | Pedraza OL , Montes AMS , Sierra FA , Montalvo MC , Muñoz Y , Díaz JM , Lozano A , Piñeros C ((2017) ) Mild cognitive impairment (MCI) and dementia in a sample of adults in the city of Bogotá. Dement Neuropsychol 11: , 262–269. |
[18] | Gomez F , Corchuelo J , Curcio C , Calzada M , Mendez F ((2016) ) SABE Colombia: Survey on Health, Well-Being, and Aging in Colombia. Curr Gerontol Geriatr Res 2016: , 7910205. |
[19] | Ortega-Lenis D , Mendez F ((2019) ) Survey on health, well-being and aging. SABE Colombia 2015: Technical report. Colomb Med 50: , 128–138. |
[20] | O’Donovan G , Hamer M , Sarmiento OL , Hessel P ((2020) ) Education in early life markedly reduces the probability of cognitive impairment in later life in Colombia. Sci Rep 10: , 17685. |
[21] | Zhuang L , Yang Y , Gao J ((2021) ) Cognitive assessment tools for mild cognitive impairment screening. J Neurol 268: , 1615–1622. |
[22] | Arévalo SP , Kress J , Rodriguez FS ((2020) ) Validity of cognitive assessment tools for older adult Hispanics: A systematic review. J Am Geriatr Soc 68: , 882–888. |
[23] | Gooding M , Amaya E , Parra M , Rios A ((2006) ) Prevalencia de las demencias en el municipio de Neiva 2003-2005. Acta Neurol Colomb 22: , 243–248. |
[24] | Munn Z , MClinSc SM , Lisy K , Riitano D , Tufanaru C ((2015) ) Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 13: , 147–153. |
[25] | Torres VL , Vila-castelar C , Bocanegra Y , Baena A , Aguirre-Acevedo DC , Tirado V , Munoz C , Moreno S , Giraldo M , Acosta N , Romenets SR , Langbaum JB , Cho W , Reiman EM , Tariot PN , Rosselli M , Quiroz YT , Lopera F , Raton B , Hospital MG , Fransisco SS , Hospital MG ((2021) ) Normative data stratified by age and education for a Spanish neuropsychological test battery: Results from the Colombian Alzheimer’s Prevention Initiative Registry. Appl Neuropsychol Adult 28: , 230–244. |
[26] | Mioshi Eneida , Dawson Kate , Joanna Mitchell RA and JRH ((2006) ) The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21: , 1078–1085. |
[27] | Ospina NA ((2015) ) Adaptación y validación en Colombia del Addenbrooke’s Cognitive Examination-Revisado (ACE-R) en pacientes con deterioro cognoscitivo leve y demencia. |
[28] | Morris JC , Mohs RC , Rogers H , Fillenbaum G , Heyman A ((1989) ) Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Parte I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39: , 1159–1165. |
[29] | Campo-Arias A , Mendoza YU , Morales TS , Pino AJV , Cogollo Z ((2008) ) Consistencia interna, estructura factorial y confiabilidad del constructo de la Escala de Yesavage para depression geriátrica (GDS-15) en Cartagena (Colombia). Salud Uninorte 24: , 2–9. |
[30] | Chen Y , Qian X , Zhang Y , Su W , Huang Y , Wang X , Chen X , Zhao E , Han L , Ma Y ((2022) ) Prediction models for conversion from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis. Front Aging Neurosci 14: , 840386. |
[31] | McGrattan AM , Pakpahan E , Siervo M , Mohan D , Reidpath DD , Prina M , Allotey P , Zhu Y , Shulin C , Yates J , Paddick SM , Robinson L , Stephan BCM ((2022) ) Risk of conversion from mild cognitive impairment to dementia in low- and middle-income countries: A systematic review and meta-analysis. Alzheimers Dement (N Y) 8: , e12267. |
[32] | Mohan D , Iype T , Varghese S , Usha A , Mohan M ((2019) ) A cross-sectional study to assess prevalence and factors associated with mild cognitive impairment among older adults in an urban area of Kerala, South India. BMJ Open 9: , e025473. |
[33] | Artero S , Ancelin ML , Portet F , Dupuy A , Berr C , Dartigues JF , Tzourio C , Rouaud O , Poncet M , Pasquier F , Auriacombe S , Touchon J , Ritchie K ((2008) ) Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry 79: , 979–984. |
[34] | Choi SJ , Jung SS , You YS , Shin BS , Kim JE , Yoon SW , Jeon DW , Baek JH , Park SW , Lee JG , Kim YH ((2008) ) Prevalence of Alzheimer’s dementia and its risk factors in community-dwelling elderly Koreans. Psychiatry Investig 5: , 78–85. |
[35] | Prince M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London. |
[36] | Santamaria-Garcia H , Sainz-Ballesteros A , Hernandez H , Moguilner S , Maito M , Ochoa-Rosales C , Corley M , Valcour V , Miranda JJ , Lawlor B , Ibanez A ((2023) ) Factors associated with healthy aging in Latin American populations. Nat Med 29: , 2248–2258. |
[37] | Stern Y ((2021) ) How can cognitive reserve promote cognitive and neurobehavioral health? Arch Clin Neuropsychol 36: , 1291–1295. |
[38] | Stern Y , Barulli D ((2019) ) Cognitive reserve. Handb Clin Neurol 167: , 181–190. |
[39] | Kumar H , Arokiasamy P , Selvamani Y ((2020) ) socioeconomic disadvantage, chronic diseases and their association with cognitive functioning of adults in India: A multilevel analysis. . J Popul Ageing 13: , 285–303. |
[40] | Xiu S , Liao Q , Sun L , Chan P ((2019) ) Risk factors for cognitive impairment in older people with diabetes: A community-based study. Ther Adv Endocrinol Metab 10: , 2042018819836640. |
[41] | Carr AL , Sluiman AJ , Grecian SM , Forster R , McLachlan S , Strachan MWJ , Price JF ((2021) ) Depression as a risk factor for dementia in older people with type 2 diabetes and the mediating effect of inflammation. Diabetologia 64: , 448–457. |
[42] | Pink A , Krell-Roesch J , Syrjanen JA , Vassilaki M , Lowe VJ , Vemuri P , Stokin GB , Christianson TJ , Kremers WK , Jack CR , Knopman DS , Petersen RC , Geda YE ((2022) ) A longitudinal investigation of Aβ, anxiety, depression, and mild cognitive impairment. Alzheimers Dement 18: , 1824–1831. |
[43] | Chuang YF , Liu YC , Tseng HY , Lin PX , Li CY , Shih MH , Lin KC , Yang TYO , Yan SH , Chiu YL ((2021) ) Urban-rural differences in the prevalence and correlates of mild cognitive impairment in community-dwelling older adults in Taiwan: The EMCIT study. J Formos Med Assoc 120: , 1749–1757. |
[44] | Sarailoo M , Afshari S , Asghariazar V , Safarzadeh E , Dadkhah M ((2022) ) Cognitive impairment and neurodegenerative diseases development associated with organophosphate pesticides exposure: A review study. Neurotox Res 40: , 1624–1643. |
[45] | Sánchez SS , Abanto J , Sanchez-Boluarte A , Boluarte-Carbajal A , Sanchez-Coronel D , Custodio-Capuñay N , Samalvides-Cuba F ((2019) ) Frequency and associated factors of amnestic mild cognitive impairment at four senior citizen clubs in Lima, Peru. Dement Neuropsychol 13: , 321–328. |
[46] | Wesseling C , Román N , Quirós I , Páez L , García V , Mora AM , Juncos JL , Steenland KN ((2013) ) Parkinson’s and Alzheimer’s diseases in Costa Rica: A feasibility study toward a national screening program. Glob Health Action 6: , 23061. |




