Weight Change and Neuropsychiatric Symptoms in Alzheimer’s Disease and Frontotemporal Dementia: Associations with Cognitive Decline
Abstract
Weight changes, neuropsychiatric symptoms (NPS), and cognitive decline often coincide in Alzheimer’s disease (AD) and frontotemporal dementia (FTD); however, the direction of their relationship remains unclear. This study aims to clarify the connection between weight changes, NPS, and cognition in AD and FTD. We found that cognitive decline was associated with decreased body mass index (BMI) in AD, while BMI gain was associated with increased conversion to FTD. Elevated NPS were associated with decreased BMI in AD and increased BMI in FTD. Identifying early changes in NPS and BMI may facilitate the detection of cognitive decline, providing an opportunity for early intervention.
INTRODUCTION
Detecting signals of impending cognitive decline is a challenge in dementia care, complicating efforts to forecast disease progression. Changes in body mass index (BMI), however, have emerged as a clinical indicator of accelerating decline in both Alzheimer’s disease (AD) and frontotemporal dementia (FTD) [1–3]. Despite recognizing this indicator, the relationship between BMI changes and cognition across dementia types remains incompletely understood. Clarifying the nature of weight changes associated with cognitive decline in AD and FTD and their association with neuropsychiatric symptoms (NPS) could provide opportunities for individualized prognosis and pre-emptive intervention.
AD is commonly associated with decreases in appetite and subsequent weight loss [4]. In contrast, FTD is typically associated with hyperorality and overeating, often leading to weight gain [5]. While recent work has shown that both increases and decreases in BMI may be related to cognitive decline in dementia, studies have tended not to differentiate dementia subtypes [1]. Neuropsychiatric symptoms, including depression, anxiety, apathy, disinhibition, and psychosis, are also common in AD and FTD, leading to significant patient suffering and caregiver distress [6]. In both diseases, a link between NPS and changes in eating behavior has been established [6, 7]. However, research has not yet confirmed the temporal connection between increased NPS, changes in weight, and cognitive decline. If NPS coincide with changes in eating behavior, then there may be a role for targeting NPS to forestall weight changes, thereby tempering the rate of cognitive decline.
With this background in mind, the primary aim of this study is to examine the relationship between NPS, BMI, and cognitive decline in AD and FTD. We hypothesize the following: 1) increases in NPS will be associated with weight changes in both AD and FTD, 2) increased NPS will lead to decreases in BMI in AD and increases in FTD, and 3) decreased BMI will coincide with cognitive decline in AD, while increases in BMI will coincide with cognitive decline in FTD. We hope to use insights from this study to help clarify the connection between NPS, BMI, and cognition in AD and FTD, with the goal of developing precision therapies to target difficult-to-treat symptoms in a vulnerable patient population.
METHODS
Study sample
We performed a retrospective analysis of data drawn from the National Alzheimer’s Coordinating Center (NACC), which maintains the Uniform Data Set (UDS) and includes participants from Alzheimer’s Disease Centers (ADCs) across the United States [8–10]. The UDS is the product of the National Institute on Aging’s (NIA) ADC program, which oversees an ongoing prospective, standardized, multi-site, longitudinal study of participants with dementia, mild cognitive impairment, and healthy controls. We included all participants enrolled in NACC-UDS between June 2005 and February 2021 with a final diagnosis of Alzheimer’s disease (AD, n = 3,831) or behavioral variant frontotemporal dementia (FTD, n = 417), including those entering the study with normal cognition or mild cognitive impairment (MCI) who subsequently converted to a diagnosis of AD or FTD. Written informed consent was provided by all participants and their informants and approved by local institutional review boards (IRBs).
Demographics and diagnosis
Demographic characteristics included age, sex, race, years of education, and years of follow-up. The diagnosis for each participant was based on the etiologic diagnosis at the final visit. Participants who entered the study with either normal cognition or MCI and later transitioned to a diagnosis of AD or FTD were considered ‘converters.’
Cognition
All participants underwent standardized cognitive testing at each study visit. Global cognition was captured using the Clinical Dementia Rating scale (CDR), Mini-Mental State Exam (MMSE), and Montreal Cognitive Assessment (MoCA) [11–13]. We captured memory using immediate and delayed logical memory tests and attention using the digit span backward test [14]. We used the difference in scores between the trail-making A and trail-making B tests to capture executive functioning [14, 15].
Medical comorbidity
We captured individual levels of medical comorbidity by computing each participant’s Revised Framingham Stroke Risk Profile (rFSRP) score. The rFSRP is a valuable metric to control for cognitive changes associated with cerebrovascular disease [16, 17]. The rFSRP includes age, sex, systolic blood pressure, smoking status, cardiovascular disease, diabetes, and antihypertensive medication use. We used this composite variable to control for the influence of cerebrovascular disease on cognitive decline.
Neuropsychiatric symptoms
We captured NPS using the Neuropsychiatric Inventory Questionnaire (NPI-Q), which includes items on depression, anxiety, psychosis, apathy, disinhibition, irritability, elation, and sleep disturbance/nighttime behaviors. The NPI-Q is a widely used and well-validated informant-report questionnaire used to assess psychiatric symptoms in subjects with various types of dementia, including AD and FTD [6, 18–20]. The Geriatric Depression Scale, a validated and reliable self-report metric of depressive symptoms in older adults, was also used to capture depressive states [21].
Table 1
Demographic and clinical characteristics in AD and FTD
| AD (n = 3831) | FTD (n = 417) | t or χ2 | p | |
| Age, mean (SD) | 74.4 (9.8) | 62.9 (9.5) | 22.8 | <0.001 |
| Sex, % female | 60.3 | 42.5 | 49.5 | <0.001 |
| Race, % non-white | 22.9 | 9.8 | 47.5 | <0.001 |
| Education, mean (SD) | 14.8 (8.2) | 16.9 (11.1) | –4.9 | <0.001 |
| MMSE, mean (SD) | 19.6 (7.3) | 19.2 (8.4) | 0.63 | 0.53 |
| CDR Sum, mean (SD) | 5.7 (4.6) | 7.9 (4.6) | –9.5 | <0.001 |
| FSRP10, mean (SD) | 0.34 (0.34) | 0.09 (0.16) | 14.2 | <0.001 |
| Systolic BP, mean (SD) | 136 (20) | 130 (18) | 6.0 | <0.001 |
| HTN Medication, % yes | 53.9 | 35.5 | 50.9 | <0.001 |
| Smoking, % yes | 39.2 | 29.7 | 14.2 | <0.001 |
| Cardiac Disease, % yes | 15.5 | 7.2 | 20.7 | <0.001 |
| Hypercholesterolemia, % yes | 39.7 | 32.4 | 18.4 | <0.001 |
AD, Alzheimer’s disease; FTD, frontotemporal dementia; MMSE, Mini-Mental State Examination; CDR Sum, Clinical Dementia Rating – sum of boxes; FSRP, Framingham Stroke Risk Profile; BP, blood pressure; HTN, hypertension.
Body mass index
BMI was calculated based on measured weight and height (weight in kilograms divided by squared height in centimeters) at baseline and at each follow-up visit. Body mass index change was measured between each visit and from the baseline to the final visit.
Statistical analyses
Our primary outcome measure was change in BMI across study visits. The main independent variables in our analyses were cognition, psychiatric symptoms, and rFSRP. We used mixed effects time-averaged models with a random intercept testing associations of cognitive status and psychiatric symptom burden at the previous visit as predictors of subsequent BMI adjusting for rFSRP and years of education. We also used Cox proportional hazard regression models using change in BMI as a predictor of converting from normal cognition or MCI to a dementia state with adjustment for rFSRP score and level of education. Alpha was set at 0.05. We used STATA SE 17 (StataCorp LP, College Station, TX) for all analyses.
RESULTS
Demographics
Table 1 displays demographic data from our study population. Our study had 3,831 participants with a final diagnosis of AD and 417 participants with a final diagnosis of FTD. Participants with AD tended to be older and were more likely to be female than participants with FTD. Both AD and FTD participants were predominately white; however, a greater proportion of AD participants were non-white. Participants with FTD had more years of education and higher CDR sum of boxes scores, while participants with AD had higher MMSE scores. Participants with AD also had higher baseline systolic blood pressure, antihypertensive use, smoking rates, cardiac disease, and hypercholesterolemia, which elevated Framingham risk scores.
Cognition and BMI
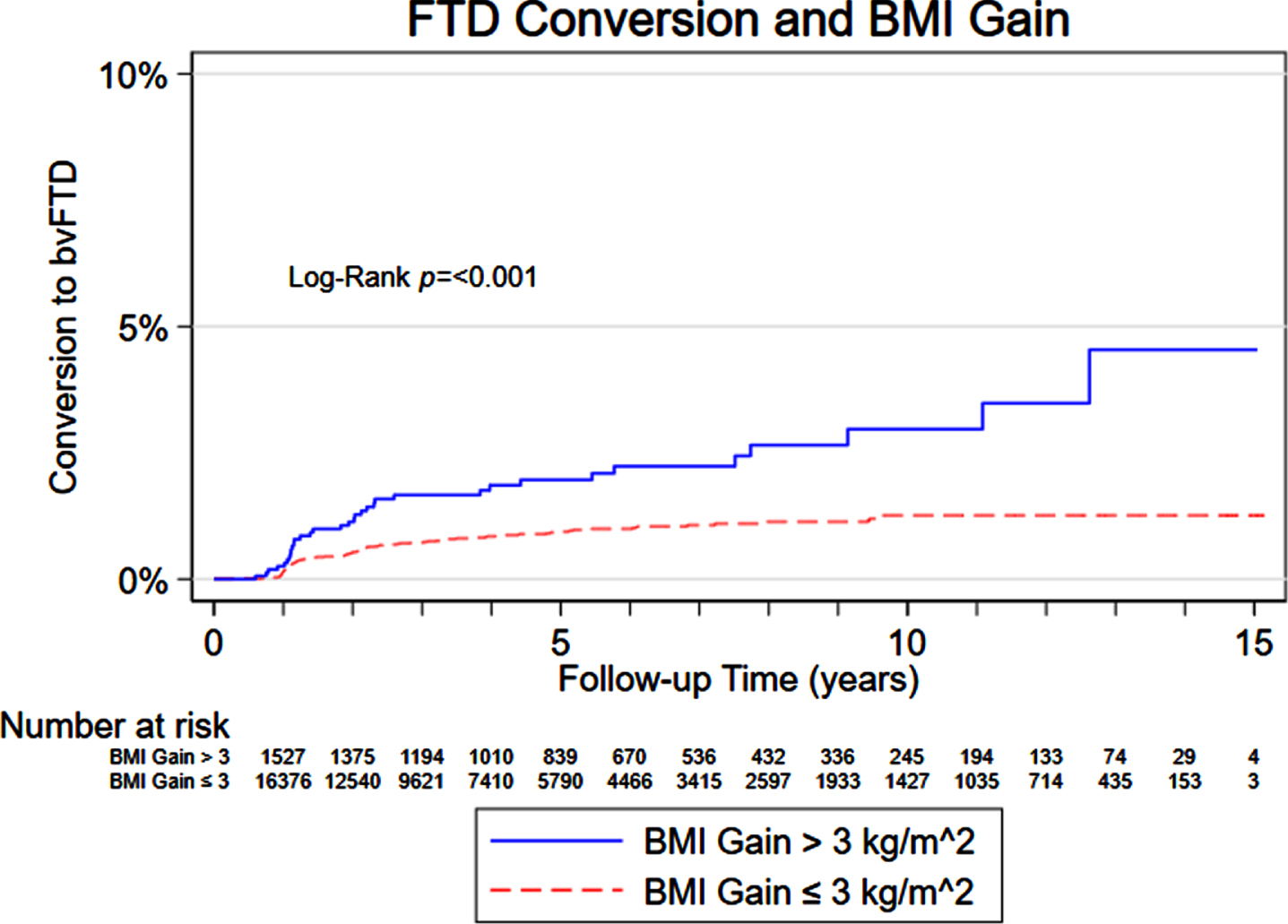
Results from our mixed-effects regression model assessing the association of cognition on subsequent BMI in AD and FTD are displayed in Table 2. A decrease in MMSE score was associated with a lower subsequent BMI for participants with AD. Higher trail making test difference scores (worse performance) were also associated with lower subsequent BMI in AD. There was no statistically significant association between cognitive test scores and subsequent BMI in participants with FTD. However, participants with an initial diagnosis of MCI had a 1.16 increased hazard of converting to FTD when there were BMI gains between the initial visit and the first visit with a dementia diagnosis. A Kaplan-Meier curve showing the increased risk of conversion to FTD overtime in those with an increase in BMI greater than 3 kg/m2 compared to those without a BMI increase greater than 3 kg/m2 is displayed in Fig. 1.
Table 2
Lagged Cognitive and Psychiatric Symptoms and BMI in AD and FTD
| AD BMI Coefficient (SE) | FTD BMI Coefficient (SE) | |
| Cognitive Measures MMSE | 0.01 (0.004) p = 0.001 | –0.01 (0.018) p = 0.52 |
| MoCA | 0.01 (0.01) p = 0.28 | –0.03 (0.04) p = 0.51 |
| Memory Factor | 0.05 (0.03) p = 0.17 | –0.05 (0.16) p = 0.73 |
| Digit Span Backwards | –0.007 (0.009) p = 0.44 | –0.02 (0.05) p = 0.75 |
| Trail Making Test Difference Score | –0.001 (0.0003) p = 0.02 | 0.02 (0.004) p = 0.66 |
| Neuropsychiatric Measures NPI Total | 0.001 (0.005) p = 0.84 | 0.05 (0.019) p = 0.01 |
| NPI - Appetite | –0.10 (0.03) p = 0.003 | 0.33 (0.16) p = 0.04 |
| NPI - Apathy | 0.05 (0.03) p = 0.11 | 0.44 (0.17) p = 0.01 |
| Ritualistic/Compulsive Behavior | –1.3 (0.78) p = 0.10 | 0.09 (0.31) p = 0.76 |
| Hyperorality | –1.6 (0.99) p = 0.11 | 0.54 (0.28) p = 0.05 |
| Disinhibition | 0.40 (0.96) p = 0.68 | 0.90 (0.40) p = 0.03 |
| GDS | –0.016 (0.007) p = 0.02 | 0.034 (.036) p = 0.36 |
Cognitive model adjusted for Framingham Risk Factors and years of education. Neuropsychiatric model adjusted for Framingham Risk Factors, cognition (CDR global), and education. AD, Alzheimer’s disease; FTD, frontotemporal dementia; BMI, body mass index; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NPI, Neuropsychiatric Inventory; GDS, Geriatric Depression Scale; CDR, Clinical Dementia Rating.
Fig. 1
Risk of converting to FTD based on BMI gain.
Neuropsychiatric symptoms and BMI
Results from our mixed-effects regression model assessing the association of NPS on subsequent BMI in AD and FTD are also displayed in Table 2. In participants with AD, increased symptoms of depression were associated with lower subsequent BMI. There was no association between depressive symptoms and BMI in participants with FTD; however, a higher total NPI score was associated with subsequent increases in BMI in those with FTD. Changes in appetite, as rated on the NPI-Q, were associated with subsequent decreases in BMI in the AD group and subsequent increases in BMI in the FTD group. Apathy was associated with subsequent BMI increases in the FTD group. Disinhibition was also associated with subsequent BMI increases in the FTD group. There was no association between total NPI score, apathy, disinhibition, or hyperorality and subsequent BMI in participants with AD.
DISCUSSION
Overview
In this study, we present evidence of a temporal association between cognitive decline, changes in BMI, and neuropsychiatric symptoms in AD and FTD. We found that a reduction in cognition was associated with decreased BMI in AD, while increased BMI was associated with an increased risk of conversion to FTD. Increased depressive symptoms were associated with declines in BMI in AD, while apathy, appetite changes, and disinhibition were associated with increases in BMI in FTD.
Origins of weight loss in dementia
Our finding that increases in NPS are associated with changes in BMI adds to the evidence that weight changes in dementia are multifactorial, extending beyond just changes in appetite. As outlined in the European Society for Clinical Nutrition and Metabolism (ESPEN) dementia guidelines, the origins of weight loss in dementia are complex, with proposed mechanisms including atrophy of regions responsible for appetite regulation, inflammatory processes, genetic factors, and changes in olfaction among other causes [22–27]. The underlying neurodegenerative process giving rise to dementia syndromes likely leads to early psychiatric symptoms and weight changes, prior to significant cognitive changes, providing a possible window for early intervention. The existing ESPEN guidelines provide a framework for careful screening to guide nutritional interventions in patients with dementia to help maintain a healthy weight [27]. Our study supports these guidelines while reinforcing the importance of NPS screening in dementia alongside nutritional monitoring to facilitate early intervention.
Implications for disease mechanism
In addition to aiding in early detection and treatment, attention to NPS and weight changes may also provide insights into underlying disease mechanisms in AD and FTD. While our study shows that increased NPS and changes in BMI often precede cognitive declines in patients with AD and FTD, some evidence suggests that weight changes may precede cognitive impairment by several years, representing a prodromal disease state [28]. In patients eventually diagnosed with AD, weight loss preceding cognitive impairment has been linked to nutrient deficiencies and subsequent oxidative damage, increased cortisol levels, and elevated free radicals, all of which are implicated in AD pathogenesis [29–31]. Alterations in leptin levels have also been associated with AD, with low leptin levels and weight loss conferring an increased risk of AD [32, 33]. In FTD, eating abnormalities have diverse and multifactorial origins. Network degeneration and structural atrophy in the right insula, striatum, and orbitofrontal cortex have been implicated in eating behavior changes in FTD [34, 35]. Hypothalamic degeneration with subsequent disruption of orbitofrontal and cortical reward pathways has also been implicated in early abnormal eating behavior and weight gain in FTD prior to cognitive impairment [36, 37]. Similarly, neuro-endocrine changes like dysregulated levels of leptin, ghrelin, cholecystokinin, peptide tyrosine tyrosine, and agouti-related peptide have been associated with early eating abnormalities in FTD, representing initial manifestations of the underlying neurodegenerative process [5, 36, 38]. Given these findings, changes in BMI in both AD and FTD may serve not only as signs of impending cognitive decline but also enhance understanding of disease mechanisms, providing potential targets for future therapies.
Forecasting cognitive decline
Overall, our findings provide valuable insights for understanding how BMI, cognition, and neuropsychiatric symptoms move together in AD and FTD. Predicting cognitive decline remains a core challenge in dementia care, as the goal is to help patients and families prepare to avoid complications from financial errors, falls, automobile accidents, and other consequences of cognitive change. Unfortunately, forecasting individual-level rates of disease progression is challenging even with tools like the MMSE, MoCA, neuroimaging, and biomarkers [39–42]. By necessity, care is often reactionary, responding to the consequences of cognitive decline instead of being preventative.
Implications for preventative screening
Our results suggest that careful attention to BMI and neuropsychiatric symptom status may help detect subtle changes in cognition, facilitating a more preventative approach to care. Under this approach, early treatment of psychiatric symptoms alongside nutritional interventions and safeguards against financial mistakes, falls, and other injuries could positively impact patients living with early AD and FTD and their caregivers. Increasing the frequency of neuropsychiatric symptom screens and weight checks could lead to early detection of cognitive change, facilitating a more preventative approach to care. Tools allowing for home measurement of neuropsychiatric state and BMI between visits may warrant development, to better promote monitoring of cognition and preemptive intervention at the first signals of cognitive change.
Limitations
While our study has many strengths, including the use of a large, multi-site (>40), longitudinal cohort of subjects with AD and FTD, some limitations merit consideration. Cases in this study rely on clinical diagnosis of AD and FTD rather than pathological diagnosis. However, diagnostic reliability in NACC has been validated in previous studies, suggesting that the clinical diagnoses are largely accurate [43]. We were also unable to assess the association of risk of conversion to dementia with weight changes in subjects diagnosed with AD in the same way we were able to so with FTD, as the AD data violated assumptions of the statistical model. This challenge complicates comparisons between the two disease states; however, using the lagged BMI and cognition model for AD and FTD facilitates an alternative comparison. Finally, the population consisted primarily of white participants, and findings may not generalize to other groups.
Conclusion
Dementia remains a leading cause of disability and death worldwide [44]. While advances in the field portend disease-modifying therapy, patients and families living with dementia face many obstacles, including the uncertain timing and significant consequences of cognitive decline. Our study provides preliminary insights into how increased attention to changes in neuropsychiatric symptoms and BMI may facilitate early detection of cognitive change and provide an opportunity for proactive intervention. These findings will need to be replicated and expanded upon in future studies to better clarify the connection between NPS, BMI, and cognition and to help elucidate the neurobiology underpinning their relationship. While all forms of dementia are relentlessly progressive, our study identifies opportunities to customize and improve care for patients and families facing these illnesses.
ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD).
FUNDING
CM is funded by KL2TR003099. Dr. Kamath was supported by KL2TR001077 during the conduct of this work and is currently funded by R01AG064093 and R01NS108452.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
DATA AVAILABILITY
The data supporting the findings of this study are openly available from NACC at https://naccdata.org/requesting-data/data-request-process.
REFERENCES
[1] | Beeri MS , Tirosh A , Lin HM , Golan S , Boccara E , Sano M , Zhu CW ((2022) ) Stability in BMI over time is associated with a better cognitive trajectory in older adults. Alzheimers Dement 18: , 2131–2139. |
[2] | Faxén-Irving G , Fereshtehnejad SM , Falahati F , Cedergren L , Göranzon H , Wallman K , García-Ptacek S , Eriksdotter M , Religa D ((2014) ) Body mass index in different dementia disorders: Results from the Swedish Dementia Quality Registry (SveDem). Dement Geriatr Cogn Dis Extra 4: , 65–75. |
[3] | Alhurani RE , Vassilaki M , Aakre JA , Mielke MM , Kremers WK , Machulda MM , Geda YE , Knopman DS , Petersen RC , Roberts RO ((2016) ) Decline in weight and incident mild cognitive impairment: Mayo Clinic Study of Aging. JAMA Neurol 73: , 439–446. |
[4] | Soysal P , Tan SG , Rogowska M , Jawad S , Smith L , Veronese N , Tsiptsios D , Tsamakis K , Stewart R , Mueller C ((2021) ) Weight loss in Alzheimer’s disease, vascular dementia and dementia with Lewy bodies: Impact on mortality and hospitalization by dementia subtype. Int J Geriatr Psychiatry 37: , doi: 10.1002/gps.5659. |
[5] | Ikeda M , Brown J , Holland AJ , Fukuhara R , Hodges JR ((2002) ) Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 73: , 371–376. |
[6] | Da Silva TBL , Ordonez TN , Bregola AG , Bahia VS , Cecchini MA , Guimarães HC , Gambogi LB , Caramelli P , Balthazar MLF , Damasceno BP , Brucki SMD , de Souza LC , Nitrini R , Yassuda MS ((2021) ) Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and Alzheimer’s disease: A 12-month follow-up study. Front Neurol 12: , 728108. |
[7] | Morrow CB , Chaney G-AS , Capuzzi D , Bakker A , Onyike CU , Kamath V ((2022) ) Hyperorality in frontotemporal dementia: Cognitive and psychiatric symptom profiles in early-stage disease. J Alzheimers Dis 89: , 1203–1209. |
[8] | Morris JC , Weintraub S , Chui HC , Cummings J , Decarli C , Ferris S , Foster NL , Galasko D , Graff-Radford N , Peskind ER , Beekly D , Ramos EM , Kukull WA ((2006) ) The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20: , 210–216. |
[9] | Beekly DL , Ramos EM , Lee WW , Deitrich WD , Jacka ME , Wu J , Hubbard JL , Koepsell TD , Morris JC , Kukull WA , NIA Alzheimer’s Disease Centers ((2007) ) The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 21: , 249–258. |
[10] | Weintraub S , Salmon D , Mercaldo N , Ferris S , Graff-Radford NR , Chui H , Cummings J , DeCarli C , Foster NL , Galasko D , Peskind E , Dietrich W , Beekly DL , Kukull WA , Morris JC ((2009) ) The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis Assoc Disord 23: , 91–101. |
[11] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[12] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[13] | Nasreddine ZS , Phillips NA , Bedirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[14] | Cauthen NR ((1977) ) Extension of the Wechsler Memory Scale norms to older age groups. J Clin Psychol 33: , 208–211. |
[15] | Llinàs-Reglà J , Vilalta-Franch J , López-Pousa S , Calvó-Perxas L , Torrents Rodas D , Garre-Olmo J ((2017) ) The trail making test. Assessment 24: , 183–196. |
[16] | Pelcher I , Puzo C , Tripodis Y , Aparicio HJ , Steinberg EG , Phelps A , Martin B , Palmisano JN , Vassey E , Lindbergh C , McKee AC , Stein TD , Killiany RJ , Au R , Kowall NW , Stern RA , Mez J , Alosco ML ((2020) ) Revised Framingham Stroke Risk Profile: Association with cognitive status and MRI-derived volumetric measures. J Alzheimers Dis 78: , 1393–1408. |
[17] | Dufouil C , Beiser A , McLure LA , Wolf PA , Tzourio C , Howard VJ , Westwood AJ , Himali JJ , Sullivan L , Aparicio HJ , Kelly-Hayes M , Ritchie K , Kase CS , Pikula A , Romero JR , D’Agostino RB , Samieri C , Vasan RS , Chene G , Howard G , Seshadri S ((2017) ) Revised Framingham Stroke Risk Profile to reflect temporal trends. Circulation 135: , 1145–1159. |
[18] | Saari T , Koivisto A , Hintsa T , Hänninen T , Hallikainen I ((2022) ) Psychometric properties of the neuropsychiatric inventory: A review. J Alzheimers Dis 86: , 1485–1499. |
[19] | Fieldhouse JLP , Gossink FT , Feenstra TC , de Boer SCM , Lemstra AW , Prins ND , Bouwman F , Koene T , Rhodius-Meester HFM , Gillissen F , Teunissen CE , van der Flier WM , Scheltens P , Dols A , Vijverberg EGB , Pijnenburg YAL ((2021) ) Clinical phenotypes of behavioral variant frontotemporal dementia by age at onset. J Alzheimers Dis 82: , 381–390. |
[20] | Kaufer DI , Cummings JL , Ketchel P , Smith V , MacMillan A , Shelley T , Lopez OL , DeKosky ST ((2000) ) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12: , 233–239. |
[21] | Albinski R , Kleszczewska-Albinska A , Bedynska S ((2011) ) Geriatric Depression Scale (GDS). Validity and reliability of different versions of the scale–review. Psychiatr Pol 45: , 555–562. |
[22] | Gillette Guyonnet S , Abellan Van Kan G , Alix E , Andrieu S , Belmin J , Berrut G , Bonnefoy M , Brocker P , Constans T , Ferry M , Ghisolfi-Marque A , Girard L , Gonthier R , Guerin O , Hervy MP , Jouanny P , Laurain MC , Lechowski L , Nourhashemi F , Raynaud-Simon A , Ritz P , Roche J , Rolland Y , Salva T , Vellas B , International Academy on Nutrition and Aging Expert Group ((2007) ) IANA (International Academy on Nutrition and Aging) Expert Group: Weight loss and Alzheimer’s disease. J Nutr Health Aging 11: , 38–48. |
[23] | Albanese E , Taylor C , Siervo M , Stewart R , Prince MJ , Acosta D ((2013) ) Dementia severity and weight loss: A comparison across eight cohorts. The 10/66 study. Alzheimers Dement 9: , 649–656. |
[24] | Grundman M , Corey-Bloom J , Jernigan T , Archibald S , Thal LJ ((1996) ) Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology 46: , 1585–1591. |
[25] | Engelhart MJ , Geerlings MI , Meijer J , Kiliaan A , Ruitenberg A , van Swieten JC , Stijnen T , Hofman A , Witteman JC , Breteler MM ((2004) ) Inflammatory proteins in plasma and the risk of dementia: The rotterdam study. Arch Neurol 61: , 668–672. |
[26] | Stanciu I , Larsson M , Nordin S , Adolfsson R , Nilsson LG , Olofsson JK ((2014) ) Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: A longitudinal, population-based study. J Int Neuropsychol Soc 20: , 209–217. |
[27] | Volkert D , Chourdakis M , Faxen-Irving G , Fruhwald T , Landi F , Suominen MH , Vandewoude M , Wirth R , Schneider SM ((2015) ) ESPEN guidelines on nutrition in dementia. Clin Nutr 34: , 1052–1073. |
[28] | Sergi G , De Rui M , Coin A , Inelmen EM , Manzato E ((2013) ) Weight loss and Alzheimer’s disease: Temporal and aetiologic connections. Proc Nutr Soc 72: , 160–165. |
[29] | Luchsinger JA , Mayeux R ((2004) ) Dietary factors and Alzheimer’s disease. Lancet Neurol 3: , 579–587. |
[30] | Salem N , Jr. , Niebylski CD ((1995) ) The nervous system has an absolute molecular species requirement for proper function. Mol Membr Biol 12: , 131–134. |
[31] | Yen PK ((2005) ) Relationship of dementia and body weight. Geriatr Nurs 26: , 79–80. |
[32] | Morrison CD ((2009) ) Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta 1792: , 401–408 . |
[33] | Lieb W , Beiser AS , Vasan RS , Tan ZS , Au R , Harris TB , Roubenoff R , Auerbach S , DeCarli C , Wolf PA , Seshadri S ((2009) ) Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 302: , 2565–2572. |
[34] | Ahmed RM , Irish M , Piguet O , Halliday GM , Ittner LM , Farooqi S , Hodges JR , Kiernan MC ((2016) ) Amyotrophic lateral sclerosis and frontotemporal dementia: Distinct and overlapping changes in eating behaviour and metabolism. Lancet Neurol 15: , 332–342. |
[35] | Woolley JD , Gorno-Tempini ML , Seeley WW , Rankin K , Lee SS , Matthews BR , Miller BL ((2007) ) Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 69: , 1424–1433. |
[36] | Ahmed RM , Latheef S , Bartley L , Irish M , Halliday GM , Kiernan MC , Hodges JR , Piguet O ((2015) ) Eating behavior in frontotemporal dementia: Peripheral hormones vs hypothalamic pathology. Neurology 85: , 1310–1317. |
[37] | Ahmed RM , Halliday G , Hodges JR ((2021) ) Hypothalamic symptoms of frontotemporal dementia disorders. Handb Clin Neurol 182: , 269–280. |
[38] | Woolley JD , Khan BK , Natesan A , Karydas A , Dallman M , Havel P , Miller BL , Rankin KP ((2014) ) Satiety-related hormonal dysregulation in behavioral variant frontotemporal dementia. Neurology 82: , 512–520. |
[39] | Yoong SQ , Lu J , Xing H , Gyanwali B , Tan YQ , Wu XV ((2021) ) The prognostic utility of CSF neurogranin in predicting future cognitive decline in the Alzheimer’s disease continuum: A systematic review and meta-analysis with narrative synthesis. Ageing Res Rev 72: , 101491. |
[40] | Dansson HV , Stempfle L , Egilsdottir H , Schliep A , Portelius E , Blennow K , Zetterberg H , Johansson FD , Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Predicting progression and cognitive decline in amyloid-positive patients with Alzheimer’s disease. Alzheimers Res Ther 13: , 151. |
[41] | Jang H , Park J , Woo S , Kim S , Kim HJ , Na DL , Lockhart SN , Kim Y , Kim KW , Cho SH , Kim SJ , Seong JK , Seo SW , Alzheimer’s Disease Neuroimaging Initiative ((2019) ) Prediction of fast decline in amyloid positive mild cognitive impairment patients using multimodal biomarkers. Neuroimage Clin 24: , 101941. |
[42] | Staffaroni AM , Quintana M , Wendelberger B , Heuer HW , Russell LL , Cobigo Y , Wolf A , Goh SM , Petrucelli L , Gendron TF , Heller C , Clark AL , Taylor JC , Wise A , Ong E , Forsberg L , Brushaber D , Rojas JC , VandeVrede L , Ljubenkov P , Kramer J , Casaletto KB , Appleby B , Bordelon Y , Botha H , Dickerson BC , Domoto-Reilly K , Fields JA , Foroud T , Gavrilova R , Geschwind D , Ghoshal N , Goldman J , Graff-Radford J , Graff-Radford N , Grossman M , Hall MGH , Hsiung GY , Huey ED , Irwin D , Jones DT , Kantarci K , Kaufer D , Knopman D , Kremers W , Lago AL , Lapid MI , Litvan I , Lucente D , Mackenzie IR , Mendez MF , Mester C , Miller BL , Onyike CU , Rademakers R , Ramanan VK , Ramos EM , Rao M , Rascovsky K , Rankin KP , Roberson ED , Savica R , Tartaglia MC , Weintraub S , Wong B , Cash DM , Bouzigues A , Swift IJ , Peakman G , Bocchetta M , Todd EG , Convery RS , Rowe JB , Borroni B , Galimberti D , Tiraboschi P , Masellis M , Finger E , van Swieten JC , Seelaar H , Jiskoot LC , Sorbi S , Butler CR , Graff C , Gerhard A , Langheinrich T , Laforce R , Sanchez-Valle R , de Mendonca A , Moreno F , Synofzik M , Vandenberghe R , Ducharme S , Le Ber I , Levin J , Danek A , Otto M , Pasquier F , Santana I , Kornak J , Boeve BF , Rosen HJ , Rohrer JD , Boxer AL , Frontotemporal Dementia Prevention Initiative (FPI) Investigators ((2022) ) Temporal order of clinical and biomarker changes in familial frontotemporal dementia. Nat Med 28: , 2194–2206. |
[43] | Beach TG , Monsell SE , Phillips LE , Kukull W ((2012) ) Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol 71: , 266–273. |
[44] | Heron M ((2021) ) Deaths: Leading causes for 2019. Natl Vital Stat Rep 70: , 1–114. |




