Health Care Resource Use and Social Costs in Mild Cognitive Impairment and Mild Alzheimer’s Disease
Abstract
Background:
As the number of patients with dementia increases, so do the social costs. In recent years, attempts have been made to reduce risk to be dementia and treat it from the early stages of the disease, making it important to estimate the costs of the early stages.
Objective:
To estimate the medical and social costs of the early stages of Alzheimer’s disease (AD), which include mild cognitive impairment (MCI) due to AD and mild AD.
Methods:
Questionnaires were used to obtain basic information (e.g., age, cognitive function) and medical costs, social care costs, family caregiver medical costs, and family caregiver informal care costs from patients with MCI due to AD or mild AD who were attending a memory clinic. A comparison was then conducted between these two groups.
Results:
Patients with mild AD had higher total costs, patient medical costs, patient social care costs, and family caregiver informal care costs than did patients with MCI; however, only patient medical costs were significantly different (p = 0.022). A detailed analysis of patient medical costs revealed that anti-dementia drug treatment costs were significantly higher in patients with mild AD (p < 0.001).
Conclusion:
Compared with patients with mild AD, those with MCI may have lower patient and family caregiver costs. As it is important to reduce social costs through risk reduction and therapeutic interventions from the early stages of AD, the present findings could help estimate the social costs and verify the cost-effectiveness of early interventions for AD.
INTRODUCTION
Because of the rapid aging of society, the number of people with dementia is also rapidly increasing, and it is estimated that this number worldwide will reach 74.7 million by 2030 and 131.5 million by 2050 worldwide [1]. In Japan, the most rapidly aging society in the world, the numbers of people with dementia and MCI were estimated to be 4.6 million and 4.0 million, respectively, in 2012 [2]. Among the diseases that cause dementia, Alzheimer’s disease (AD), a progressive neurodegenerative disease, is the most common, reportedly accounting for up to 70% of all dementia cases [3].
The impact of AD on patients and family caregivers includes decreased quality of life (QoL), a substantial burden of illness, and high medical and long-term care costs [4]. Along with increased medical costs due to dementia and age-related comorbidities, family caregivers of such patients have reported a significantly increased risk of comorbidities such as depression, insomnia, anxiety, and pain, decreased QoL, significant reductions in productivity, and the increased use of social resources [4]. Therefore, the burden on patients with AD and their family caregivers could be predicted to lead to the increased use of social resources overall. The burden of unpaid care, or informal care, by family members and others close to the patient with AD also predicts the magnitude of the associated costs. Therefore, research on the economic burden due to dementia has been vigorously conducted [5–11]. A study in Japan found that the total social cost of dementia in 2014 was an estimated 14.5 trillion yen, with informal care costs accounting for 40% of that amount, or an estimated 6.2 trillion yen [2]. In 2015, the medical and social costs of dementia care worldwide, including the cost of directly caring for people with dementia and economic losses such as those resulting from family caregivers taking time off work to provide care, were estimated to be 1 trillion USD [12].
To address the socioeconomic challenges associated with the increasing prevalence of AD, data on the use and costs of AD-related social resources are needed. As the disease progresses, patients and their family caregivers are likely to require additional medical care services. Non-pharmacological interventions that seek to reduce the risks associated with MCI and earlier disease stages have recently been developed [13, 14], as have AD drugs that help slow disease progression from the early stages, including MCI [15, 16], and the medical and social care costs associated with these early stages are expected to be identified. However, to date, studies on the medical care costs associated with MCI and mild AD have been limited [17].
Therefore, the present study aimed to evaluate and compare the economic burden of MCI and mild AD in terms of patient medical costs, social care costs, family caregiver medical costs, and informal costs related to patient care.
MATERIALS AND METHODS
Participants
The study participants were patients with MCI due to AD (hereinafter abbreviated as MCI) and mild AD who were attending a memory clinic at Fujita Health University Hospital and were age 55– 85 years at the time of providing informed consent. A clinical diagnosis of MCI or mild AD was made using the National Institute on Aging-Alzheimer’s Association diagnostic criteria [18, 19]. In principle, MCI was defined as a Mini-Mental State Examination (MMSE) score of 24– 30 points and a Clinical Dementia Rating (CDR) of 0.5, and mild AD as an MMSE score of 20– 26 points and a CDR of 1.0. In addition to the MMSE and CDR, WMS-R logical memory test (immediate and delayed), executive function tests, visuospatial cognitive tests, and questions about instrumental activities of daily living (IADLs) were also administered. Family caregivers were defined as those responsible for the informal care of the patient, i.e., the patient’s family or close associates providing unpaid care, rather than remunerated care provided by a professional. The family caregiver is also the person who makes routine decisions for the patient as needed and bears most of the responsibility for providing home care. This study was approved by the ethics committee of Fujita Health University (HM18-372), and written informed consent was obtained from all study participants.
A survey was conducted between February 2019 and February 2020. The number of study participants was assumed to follow an exponential distribution of costs, and as a result, 43 patients each with MCI and mild AD were estimated to be needed for statistical analysis. However, 33 patients with MCI and 34 with mild AD were finally enrolled during the study period.
Evaluation method
To assess the economic burden on patients with MCI and mild AD quantitatively, it was necessary to obtain information from patients and their family caregivers regarding the utilized medical services and types of social care. Therefore, the Resource Utilization in Dementia (RUD) questionnaire, which is an internationally standardized instrument for calculating costs [20], was used in the present study.
The following items were assessed in interviews conducted with patients and their family caregivers after obtaining consent. Items related to the patients included basic attributes such as age and gender, cognitive function as evaluated using the MMSE, CDR, the use of anti-dementia medications, information on comorbidities and medications, support from the long-term care insurance system, and out-of-pocket costs. Items related to the family caregivers included age, gender, relationship with the patient, whether they lived with the patient, whether they were engaged in paid work, their own medical visits and comorbidities, the time needed to support the patients’ activities of daily living and IADLs, and the time needed to look after the patients.
From the above items, the average costs for patients and family caregivers over the last 30 days were estimated in a manner similar to previous studies in Japan [21]. The costs for patients and family caregivers were estimated for each patient by applying the average unit cost of Japan-specific services to the obtained information on the use of medical resources and additional data collected on treatments such as medications, the long-term care insurance system, and out-of-pocket costs [21]. Costs were divided into four cost components; the patient’s medical costs (e.g., outpatient visits, medications) related to the patient’s AD and comorbidities; the patient’s social care costs, including home care and day care services; the caregiver informal care costs which is estimated from contribution of family caregivers, including time spent caring for the patient. Caregiver informal care costs were also estimated the value of lost production time; the caregiver health care cost which is needed for the treatment of family caregivers’ comorbidities. In 2019, the average exchange rate of US $1 was 109.0 yen.
Statistical analysis
Descriptive statistics (e.g., mean, standard deviation [SD], prevalence rates) were used for the participants’ basic characteristics. Mean costs were calculated and compared between patients with MCI and mild AD. Differences in parameters among the groups were compared using the Mann– Whitney U test. The chi-square test was used for categorical variables. To identify outliers, we used a box-and-whisker plot and confirmed the presence of some outliers. However, in these cases, it appears that the main causes were not abnormal values or incorrect inputs, but rather individual differences and variations in the use of care services and daily life. Specifically, one caregiver for an MCI patient was recorded as providing 12 hours of supervision. In contrast, the other participants in this study spent less than 2 hours on supervision for both MCI and mild AD. Nevertheless, after considering the rule of filling up the RUD, which considers the time spent on sleeping and other factors, we deemed the family member’s supervision time acceptable. To ensure the robustness of our conclusions, we performed statistical calculations for the main part of the study, excluding this individual, and found no significant changes in the results. SPSS Statistics for Windows (version 27; IBM, Armonk, NY, USA) was used for all statistical analyses, with the level of significance set at 0.05.
RESULTS
Demographic characteristics of participants
The mean age±standard deviation of the MCI group (n = 33) was 79.1±4.9 years, with a mean MMSE score of 24.9±2.0, and the mean age of the mild AD group (n = 34) was 81.9±5.4 years, with a mean MMSE score of 21.3±2.4. No significant difference in the gender ratio or educational background was found between the two groups. Regarding the type of residence, most of the patients with MCI lived alone or cohabited with a spouse, while the patients with mild AD often cohabited with a spouse and children; however, no statistically significant differences were found between the two groups. In addition, no significant differences in visits to hospitals or clinics or in the presence or number of comorbidities were observed between the two groups. Anti-dementia drug treatment was significantly more common in the patients with mild AD. The number of people treated with anti-dementia medications was 18 with donepezil, 10 with galantamine, 3 with rivastigmine, and 2 with memantine in mild AD, one of whom was using both donepezil and memantine; in MCI it was 1 with donepezil and 6 with galantamine. No difference in psychotropic drug treatment was found between the two groups. Moreover, no significant differences in the certification of long-term care needs or the use of long-term care services were observed(Table 1).
Table 1
Basic characteristics of the patients with MCI and mild AD in the present study
| MCI (n = 33) | Mild AD (n = 34) | p | |
| Age, mean (SD) | 79.1 (4.9) | 81.9 (5.4) | 0.035 |
| Female, n (%) | 19 (57.6) | 20 (58.8) | 0.918 |
| Education, n (%) | |||
| ≤ 12 y | 24 (72.7) | 25 (73.5) | 0.941 |
| >12 y | 9 (27.3) | 9 (26.5) | |
| Marital status | |||
| Married/cohabiting, n (%) | 18 (54.5) | 24 (70.6) | 0.175 |
| Living situation, n (%) | |||
| Living alone | 7 (21.2) | 4 (11.8) | 0.078 |
| With spouse | 16 (48.5) | 14 (41.2) | |
| With spouse and child | 1 (3.0) | 9 (26.5) | |
| With child | 8 (24.2) | 7 (20.6) | |
| Other | 1 (3.0) | 0 (0.0) | |
| Hospital/clinic visit, yes (%) | 30 (90.9) | 27 (79.4) | 0.187 |
| GP clinic visit, mean (SD) | 2.6 (1.8) | 2.1 (2.4) | 0.413 |
| Comorbidity, yes (%) | 25 (78.8) | 26 (76.5) | 0.82 |
| Numbers of comorbidities, mean (SD) | 1.6 (1.1) | 1.2 (1.0) | 0.128 |
| MMSE, mean (SD) | 24.9 (2.0) | 21.3 (2.4) | <0.001 |
| AD treatment, yes (%) | 7 (21.2) | 32 (94.1) | <0.001 |
| Treatment with psychotropic drugs, yes (%) | 12 (36.4) | 6 (17.6) | 0.084 |
| LTCI-certified, n (%) | 12 (36.4) | 15 (44.1) | 0.518 |
| LTCI level | |||
| Independent, n (%) | 21 (63.6) | 20 (58.8) | 0.804 |
| Support level 1, n (%) | 4 (12.1) | 4 (11.8) | |
| Support level 2, n (%) | 1 (3.0) | 0 (0.0) | |
| Care level 1, n (%) | 6 (18.2) | 8 (23.5) | |
| Care level 2, n (%) | 1 (3.0) | 2 (5.9) | |
| Social care service use, n (%) | 7 (21.2) | 13 (38.2) | 0.128 |
| Day care use hours/month, mean (SD) | 1.6 (3.7) | 3.0 (4.9) | 0.121 |
Data are presented as mean (SD) or n (%). Differences in parameters among groups were compared using the Mann– Whitney U test. The chi-squared test was used for categorical variables. MCI, mild cognitive impairment; AD, Alzheimer’s disease; GP, general physician; MMSE, Mini-Mental State Examination; LTCI, long-term care insurance; SD, standard deviation.
Attributes and care-related time allocation of family caregivers
Regarding the attributes of the family caregivers, no significant differences in age, gender, relationship, number of family members caring for the patient other than themselves, or percentage of contribution to care were found. In addition, about 70% of the family caregivers lived together with the patient; no significant differences were found between the two groups. No significant differences in the percentage of family caregivers with paid work, the amount of time worked, or the reasons that they stopped working were found. In addition, no significant differences in the numbers of hospital visits by family caregivers or comorbidities of them were observed between the two groups. In terms of the time spent with the patient, the family caregivers of patients with mild AD spent more time supporting IADLs and less time spent looking after the patient, but no significant differences were found between the two groups(Table 2).
Medical and care cost analysis
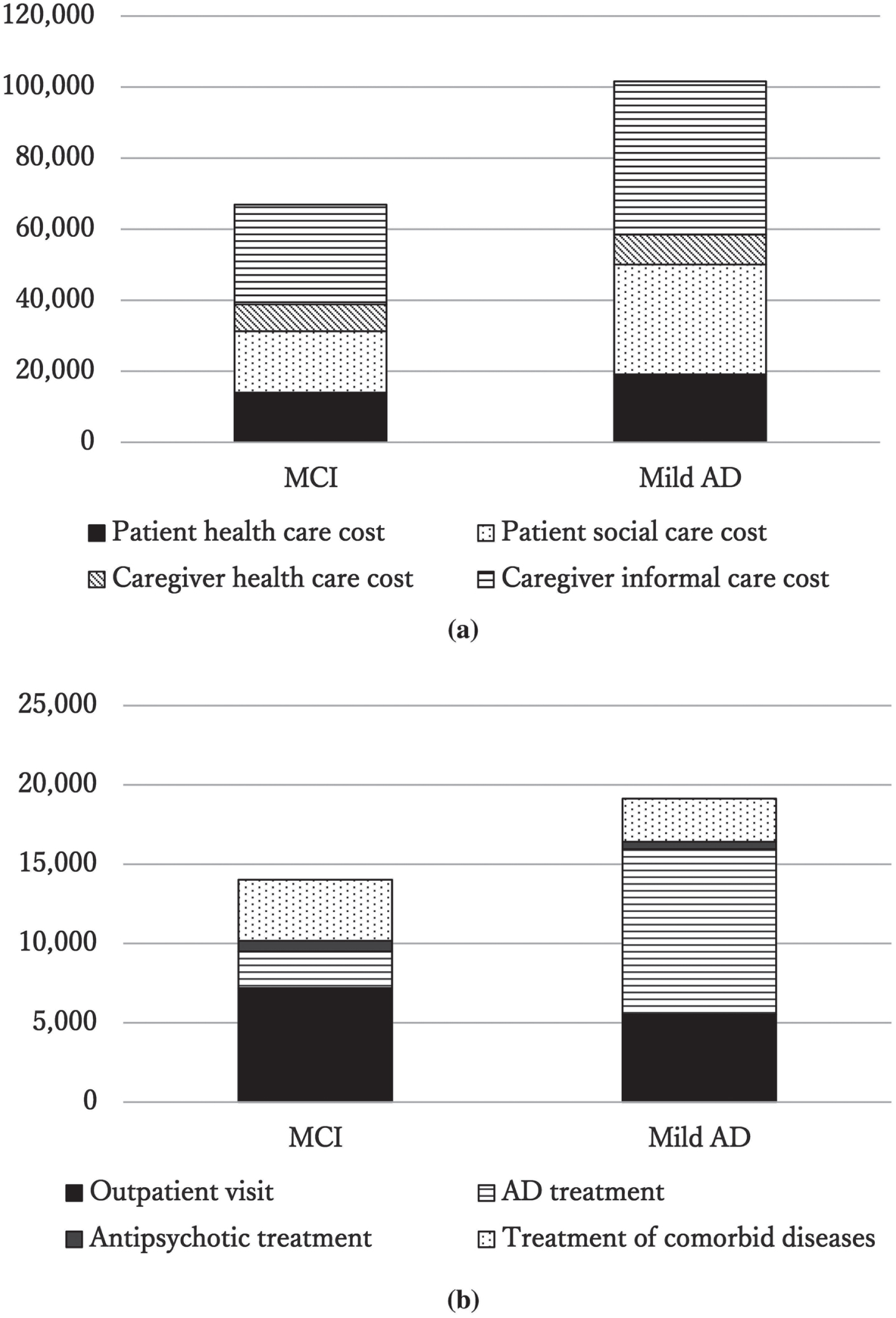
Based on the above data, patient medical costs, patient social care costs, family caregiver medical costs, family caregiver informal care costs, and total costs were calculated. The total costs, patient medical costs, patient social care costs, and family caregiver informal care costs were higher for the mild AD than for the MCI group; however, only patient medical expenses showed a significant difference between groups (Table 3, Fig. 1A). A more detailed assessment of patient medical costs revealed that anti-dementia drug treatment costs were significantly higher in the mild AD than in the MCI group. However, no significant difference in psychotropic medication or comorbidity treatment costs was observed (Table 3, Fig. 1B).
Table 2
Basic characteristics of the family caregivers of patients with MCI and mild AD
| MCI (n = 33) | Mild AD (n = 34) | p | |
| Age, mean (SD) | 64.9 (12.3) | 66.6 (12.0) | 0.581 |
| Female, n (%) | 25 (75.8) | 27 (79.4) | 0.72 |
| Caregiver’s relation to patient, n (%) | |||
| Spouse | 17 (51.5) | 16 (47.1) | 0.726 |
| Child | 14 (42.4) | 17 (50.0) | |
| Other | 2 (6.1) | 1 (2.9) | |
| Number of informal caregivers, n (%) | |||
| 0 | 18 (54.5) | 14 (41.2) | 0.241 |
| 1 | 9 (27.3) | 9 (26.5) | |
| 2 | 3 (9.1) | 9 (26.5) | |
| 3 | 3 (9.1) | 1 (2.9) | |
| 4+ | 0 (0.0) | 1 (2.9) | |
| Contribution to care, n (%) | |||
| 0– 20 | 1 (3.0) | 4 (11.8) | 0.308 |
| 20– 40 | 1 (3.0) | 2 (5.9) | |
| 40– 60 | 5 (15.2) | 3 (8.8) | |
| 60– 80 | 0 (0.0) | 2 (5.9) | |
| 80– 100 | 26 (78.8) | 23 (67.6) | |
| Living with patient, n (%) | 23 (69.7) | 24 (70.6) | 0.936 |
| Work for pay, n (%) | 13 (39.4) | 20 (58.8) | 0.112 |
| Working hours | 11.6 (16.8) | 15.5 (17.7) | 0.255 |
| Reason for stopping working, n (%) | |||
| Never worked | 2 (10.0) | 2 (14.3) | 0.942 |
| Early retirement | 1 (5.0) | 1 (7.1) | |
| Reached retirement age | 4 (20.0) | 4 (28.6) | |
| Own health problems | 4 (20.0) | 2 (14.3) | |
| Other | 9 (45.0) | 5 (35.7) | |
| Hospital/clinic visit, yes (%) | 28 (84.8) | 27 (79.4) | 0.562 |
| Number of comorbidities, mean (SD) | 1.2 (1.1) | 0.9 (0.9) | 0.344 |
| Time spent caring for the patient, mean (SD) | |||
| IADL support | 13.6 (26.2) | 21.2 (38.6) | 0.815 |
| Supervision | 12.8 (62.6) | 6.0 (12.0) | 0.781 |
Data are presented as mean (SD) or n (%). Differences in parameters among groups were compared using the Mann– Whitney U test. The chi-square test was used for categorical variables. MCI, mild cognitive impairment; AD, Alzheimer’s disease; IADL, instrumental activities of daily living; SD, standard deviation.
DISCUSSION
Pharmacological and non-pharmacological interventions in the earlier stages of dementia are becoming increasingly important, as is cost-effectiveness when considering such interventions. In the present study, we examined the costs associated with MCI and mild AD. As a result, compared with patients with MCI, those with mild AD had higher patient medical costs, patient social care costs, family caregiver informal care costs, and total costs, but only patient medical costs were significantly higher. When patient medical costs were compared between patients with MCI and those with mild AD, more anti-dementia medications were prescribed in mild AD, resulting in higher patient medical costs.
In previous European and Japanese studies on the costs of AD, family caregiver informal care costs were found to be the highest, and differences in patient social care costs and patient medical costs were observed between countries [21, 22]. However, both informal care costs for family caregivers and social care costs for patients increased with the severity of dementia [21, 22]. Direct patient medical costs increased with the severity of dementia in some countries, but little difference was observed by dementia severity in Japan [21, 22]. As the ages of patients and family caregivers were similar in the European and Japanese studies, the different medical and nursing care service systems in each country may have resulted in the different cost trends.
According to the present study, patient medical costs, patient social care costs, and family caregiver informal care costs were higher in mild AD than in MCI, and the total costs tended to be significantly higher. A recent study in the US found that family caregiver informal care costs and patient social care costs were significantly higher in mild AD than in MCI, but no significant difference in direct patient medical costs was observed [17]. The reason for this may be the differences between the medical and social care systems in Japan and the US, as well as the fact that both patients and family caregivers in the US study were about 10 years younger and had more comorbidities compared with the participants in the present study. In addition, the overall medical costs in the US are several-fold higher than those in Japan, which may have reduced the impact regarding the monetary cost of prescribing anti-dementia medications in patients with mild AD.
Table 3
Monthly mean cost for each of the cost components associated with MCI and mild AD
| □ | MCI (n = 33) Mean (SD) | mild AD (n = 34) Mean (SD) | p |
| Patient medical costs | 14,019.1 (8,548.5) | 19,131.2 (7,026.1) | 0.022 |
| Patient social care costs | 17,220.1 (39,147.3) | 30,879.6 (51,742.1) | 0.137 |
| Caregiver health care costs | 7,573.2 (6,324.5) | 8,399.8 (8,998.3) | 0.791 |
| Caregiver informal care costs | 28,100.9 (62,786.4) | 43,208.5 (78,489.6) | 0.895 |
| Total | 66,913.3 (85,481.3) | 101,619.2 (98,845.7) | 0.060 |
| Patient medical costs (detailed) | Mean (SD) | Mean (SD) | p |
| Outpatient visits | 7,184.6 (5,567.1) | 5,529.8 (6,071.1) | 0.361 |
| Anti-dementia treatment | 2,313.6 (4,530.6) | 10,416.2 (3,350.2) | <0.001 |
| Antipsychotic treatment | 676.4 (1,304.9) | 458.8 (1,316.3) | 0.115 |
| Treatment of comorbid diseases | 3,844.6 (3,722.0) | 2,726.5 (2,590.8) | 0.106 |
Data are presented as mean and SD. Differences in parameters among groups were compared using the Mann– Whitney U test. MCI, mild cognitive impairment; AD, Alzheimer’s disease; SD, standard deviation. Monthly mean costs are presented in Japanese yen. In 2019, the average exchange rate for 1 USD was 109.0 yen.
In the US, the informal care costs for family caregivers of patients with mild AD were more than double those of patients with MCI, but in the present study, the difference was only about 1.5 times [17]. The reason for this is thought to be the difference between the two countries in the level of dementia severity at which social care can be used. In Japan, at the stage of mild AD, it is conceivable that the need for supporting IADLs and the time spent looking after the patient were suppressed to a certain extent by entrusting patient care to long-term care insurance services, that is, patient social care [23].
In comparison with studies in Japan, our previous study on patient social care costs and another study using RUD showed nearly the same changes in patient social care costs and severity [21, 23]. In addition, the ratio and cost of patient medical costs, patient social care costs, and informal care costs for family caregivers of patients with mild AD in this study using RUD showed similar trends to a previous study using RUD [21], which was considered to indicate the validity of the present data. The results of the present study suggest that patient social care costs and family caregiver informal care costs may be lower for patients with MCI than for those with mild AD because the diagnostic criteria indicate that patients with MCI have a greater ability to carry out IADLs. However, no statistically significant differences in patient social care costs or family caregiver informal care costs were found between MCI and mild AD. We attributed this in part to the fact that the mean age of patients with MCI was about 80 years, and in addition to dementia, there may be cases where IADL support is required because of conditions such as frailty and disabilities caused by cerebrovascular and/or musculoskeletal diseases, thereby necessitating the use of long-term care insurance services. When calculating the social costs associated with dementia in the future, more sophisticated estimation methods may be necessary.
This study had some limitations. First, it was conducted at a single medical institution, so in consideration of regional differences, caution is required when generalizing the results. Second, the number of patients who could be interviewed during the survey period was smaller than the number calculated as statistically necessary. It is therefore desirable to increase the numbers of participants from various regions and medical institutions before conducting a future study.
In the present study, MCI was associated with higher costs than mild AD, but a significant difference was seen only for patient medical costs. Because total costs increase as dementia progresses to the moderate and severe levels, therapeutic interventions and risk reduction efforts in the early stages of AD are needed to reduce costs and resource use for people with dementia and foster a more sustainable aging society. The results of this study are expected to help provide more accurate estimates of the costs and to verify the cost-effectiveness of early interventions for AD.
Fig. 1
(a) Monthly mean cost for each of the four cost components associated with MCI and mild AD. In the bar graph, patient medical costs are filled in black, patient social care costs are indicated with dots, family caregiver medical costs are indicated with oblique lines, and family caregiver informal care costs are indicated with horizontal lines. The vertical axis is in Japanese yen. In 2019, the average exchange rate for 1 USD was 109.0 yen. (b) Monthly mean cost for each of the four cost components among the patient medical costs associated with MCI and mild AD. In the bar graph, anti-dementia drug treatment costs are indicated with horizontal lines, outpatient visits are filled in black, antipsychotic treatment costs are filled in gray, and treatment of comorbid diseases are indicated with dots. The vertical axis is in Japanese yen. In 2019, the average exchange rate for 1 USD was 109.0 yen.
ACKNOWLEDGMENTS
We thank Dr. Kaname Ueda of Eli Lilly Japan K.K. for her helpful discussion.
FUNDING
This research was conducted as a collaborative project with Eli Lilly Japan K.K. and was financially supported by Eli Lilly Japan K.K. This study was also supported by a grant for promoting research from Fujita Health University (to H.T.). The funders had no role in the data collection or analysis.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
REFERENCES
[1] | Prince M , Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International London, UK. |
[2] | Sado M , Ninomiya A , Shikimoto R , Ikeda B , Baba T , Yoshimura K , Mimura M ((2018) ) The estimated cost of dementia in Japan, the most aged society in the world. PLoS One 13: , e0206508. |
[3] | Montgomery W , Ueda K , Jorgensen M , Stathis S , Cheng Y , Nakamura T ((2018) ) Epidemiology, associated burden, and current clinical practice for the diagnosis and management of Alzheimer’s disease in Japan. Clinicoecon Outcomes Res 10: , 13–28. |
[4] | Wimo A , Jonsson L , Bond J , Prince M , Winblad B ((2013) ) The worldwide economic impact of dementia 2010. Alzheimers Dement 9: , 1-11.e13. |
[5] | Hurd MD , Martorell P , Delavande A , Mullen KJ , Langa KM ((2013) ) Monetary costs of dementia in the United States. N Engl J Med 368: , 1326–1334. |
[6] | Ku LJ , Pai MC , Shih PY ((2016) ) Economic impact of dementia by disease severity: Exploring the relationship between stage of dementia and cost of care in Taiwan. PLoS One 11: , e0148779. |
[7] | Nakabe T , Sasaki N , Uematsu H , Kunisawa S , Wimo A , Imanaka Y ((2018) ) The personal cost of dementia care in Japan: A comparative analysis of residence types. Int J Geriatr Psychiatry 33: , 1243–1252. |
[8] | Reed C , Happich M , Argimon JM , Haro JM , Wimo A , Bruno G , Dodel R , Jones RW , Vellas B , Belger M ((2017) ) What drives country differences in cost of Alzheimer’s disease? An explanation from resource use in the GERAS Study. J Alzheimers Dis 57: , 797–812. |
[9] | Schaller S , Mauskopf J , Kriza C , Wahlster P , Kolominsky-Rabas PL ((2015) ) The main cost drivers in dementia: A systematic review. Int J Geriatr Psychiatry 30: , 111–129. |
[10] | Wimo A , Jonsson L , Fratiglioni L , Sandman PO , Gustavsson A , Skoldunger A , Johansson L ((2016) ) The societal costs of dementia in Sweden 2012 - relevance and methodological challenges in valuing informal care. Alzheimers Res Ther 8: , 59. |
[11] | Ikeda S , Mimura M , Ikeda M , Wada-Isoe K , Azuma M , Inoue S , Tomita K ((2021) ) Economic burden of Alzheimer’s disease dementia in Japan. J Alzheimers Dis 81: , 309–319. |
[12] | Wimo A , Guerchet M , Ali GC , Wu YT , Prina AM , Winblad B , Jonsson L , Liu Z , Prince M ((2017) ) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13: , 1–7 . |
[13] | Kivipelto M , Mangialasche F , Snyder HM , Allegri R , Andrieu S , Arai H , Baker L , Belleville S , Brodaty H , Brucki SM , Calandri I , Caramelli P , Chen C , Chertkow H , Chew E , Choi SH , Chowdhary N , Crivelli L , Torre R , Du Y , Dua T , Espeland M , Feldman HH , Hartmanis M , Hartmann T , Heffernan M , Henry CJ , Hong CH , Håkansson K , Iwatsubo T , Jeong JH , Jimenez-Maggiora G , Koo EH , Launer LJ , Lehtisalo J , Lopera F , Martínez-Lage P , Martins R , Middleton L , Molinuevo JL , Montero-Odasso M , Moon SY , Morales-Pérez K , Nitrini R , Nygaard HB , Park YK , Peltonen M , Qiu C , Quiroz YT , Raman R , Rao N , Ravindranath V , Rosenberg A , Sakurai T , Salinas RM , Scheltens P , Sevlever G , Soininen H , Sosa AL , Suemoto CK , Tainta-Cuezva M , Velilla L , Wang Y , Whitmer R , Xu X , Bain LJ , Solomon A , Ngandu T , Carrillo MC ((2020) ) World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 16: , 1078–1094. |
[14] | Ngandu T , Lehtisalo J , Solomon A , Levalahti E , Ahtiluoto S , Antikainen R , Backman L , Hanninen T , Jula A , Laatikainen T , Lindstrom J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385: , 2255–2263. |
[15] | Prados MJ , Liu Y , Jun H , Lam J , Mattke S ((2022) ) Projecting the long-term societal value of a disease-modifying treatment for Alzheimer’s disease in the United States. Alzheimers Dement 18: , 142–151. |
[16] | van Dyck CH , Swanson CJ , Aisen P , Bateman RJ , Chen C , Gee M , Kanekiyo M , Li D , Reyderman L , Cohen S , Froelich L , Katayama S , Sabbagh M , Vellas B , Watson D , Dhadda S , Irizarry M , Kramer LD , Iwatsubo T ((2023) ) Lecanemab in early Alzheimer’s disease. N Engl J Med 388: , 9–21. |
[17] | Robinson RL , Rentz DM , Andrews JS , Zagar A , Kim Y , Bruemmer V , Schwartz RL , Ye W , Fillit HM ((2020) ) Costs of early stage Alzheimer’s disease in the United States: Cross-Sectional Analysis of a Prospective Cohort Study (GERAS-US). J Alzheimers Dis 75: , 437–450. |
[18] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[19] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Jr. , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[20] | Wimo A , Gustavsson A , Jönsson L , Winblad B , Hsu MA , Gannon B ((2013) ) Application of Resource Utilization in Dementia (RUD) instrument in a global setting. Alzheimers Dement 9: , 429-435 e417. |
[21] | Nakanishi M , Igarashi A , Ueda K , Brnabic AJM , Treuer T , Sato M , Kahle-Wrobleski K , Meguro K , Yamada M , Mimura M , Arai H ((2020) ) Costs and resource use associated with community-dwelling patients with Alzheimer’s disease in Japan: Baseline results from the prospective observational GERAS-J Study. J Alzheimers Dis 74: , 127–138. |
[22] | Wimo A , Reed CC , Dodel R , Belger M , Jones RW , Happich M , Argimon JM , Bruno G , Novick D , Vellas B , Haro JM ((2013) ) The GERAS Study: A prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries–study design and baseline findings. J Alzheimers Dis 36: , 385–399. |
[23] | Takechi H , Kokuryu A , Kuzuya A , Matsunaga S ((2019) ) Increase in direct social care costs of Alzheimer’s disease in Japan depending on dementia severity. Geriatr Gerontol Int 19: , 1023–1029. |




