Depression and Anxiety in Older Adults with Dementia During the COVID-19 Pandemic
Abstract
This study examined the longitudinal association between dementia, activity participation, the coronavirus disease 2019 pandemic period, and 1-year mental health changes. We obtained data from the National Health and Aging Trends Study in the United States. We included 4,548 older adult participants of two or more survey rounds between 2018 and 2021. We identified baseline dementia status, and assessed depressive symptoms and anxiety at baseline and follow-up. Dementia and poor activity participation were independently associated with an increased prevalence of depressive symptoms and anxiety. Dementia care and support should address emotional and social needs under continued public health restrictions.
1INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has posed challenges to the health and lives of older adults with dementia. People with this condition are at particular risk of infection and poor outcomes, including hospitalization, severe pneumonia, and high mortality [1–3]. Therefore, governments have imposed strict physical distancing measures to protect vulnerable groups since the onset of the COVID-19 pandemic. However, such social measures increase the risk of social isolation among people with dementia [4]. The negative impact of COVID-19-related restrictions on people with dementia includes decreased well-being, mental health deterioration, and functional impairment [5]. Even before the pandemic, people with dementia had depression and anxiety—these neuropsychiatric symptoms commonly accompany dementia [3]. Notably, neuropsychiatric symptoms reflect unmet needs; therefore, we should prioritize psychosocial interventions.
Participation in meaningful activities benefits older adults’ emotional and physical well-being [6, 7], including those with dementia [1–3], providing a sense of continuity, quality of life, and self-identity [9]. Physical distancing measures during the pandemic could impede activity participation. Although quantitative data show that people with dementia experienced worsening mental health following the first lockdown [10–12] and the second wave of the pandemic [13, 14], few studies have included older adults without dementia as a control group and information on the level of activity participation. Understanding the longitudinal association between activity participation and mental health can inform the implications of promoting the mental health of people with dementia during long-term restrictions.
This study investigated the longitudinal association between activity participation and mental health in individuals with dementia during the COVID-19 pandemic.
2MATERIALS AND METHODS
2.1Design and setting
We used a retrospective longitudinal study design. Westat collected data from the National Health and Trends Study (NHATS), led by the Johns Hopkins University Bloomberg School of Public Health and the University of Michigan’s Institute for Social Research. Moreover, the National Institute on Aging provides support for the NHATS. The Johns Hopkins Medicine Institutional Review Board approved the study.
2.2Participants
We drew participants from the NHATS—an ongoing nationally representative population-based study of Medicare beneficiaries aged≥65 years in the United States [15].
NHATS enrolled 8,245 participants at baseline in 2011 and followed up with participants or proxy respondents using annual in-person surveys. In 2015, a new sample was introduced to restore the sample to the original size by age and race groups. Details of the design and protocol of the NHATS are available elsewhere [16].
We included older adults who participated in the 2018 and 2019 rounds of the NHATS surveys and excluded those identified as decedents in the 2018 or 2019 rounds of the NHATS surveys. Therefore, our sample included 4,548 participants who attended two or more rounds of the NHATS surveys between 2018 and 2021. We combined data from two consecutive rounds of surveys (2018 and 2019, 2019 and 2020, and 2020 and 2021) to establish panel data in which each case had (a) baseline dementia status, demographics, functional impairment, and mental health outcomes, and (b) 1-year follow-up activity participation and mental health outcomes.
2.3Measurements
We identified dementia status in the NHATS survey based on the validated algorithm for surveys [17]: reports of physician diagnosis, proxy responses to the AD8 dementia screening interview [18], and cognitive testing. NHATS participants are classified into three groups based on the algorithm: probable dementia, possible dementia, and no dementia. A report by either the participant or a proxy respondent that a doctor told the sample person that they had dementia or Alzheimer’s disease was used to classify persons as probable dementia. Proxy respondents not reporting a diagnosis who gave answers to the AD8 that met the criteria for likely dementia (a score of 2 or higher) also were classified as probable dementia. Score cutpoints applied to cognitive tests were used for all others with cognitive test information. In this study, having dementia was defined as having probable dementia.
We used the year of assessment at follow-up (2019, 2020, or 2021) as a period indicator in the analysis. Additionally, we did not use a dichotomous classification (before and during the pandemic) because the exact date on which the NHATS interviews were conducted was unavailable from 2020 onwards. Moreover, we defined 1-year change in mental health as that before (2018–2019), during (2019–2020), and after the onset of the COVID-19 pandemic (2020–2021).
Mental health outcomes included depressive symptoms and anxiety. We assessed depressive symptoms using the Patient Health Questionnaire-2 [19] with scores ranging from 0 –6; high scores indicated more severe depressive symptoms than low scores. Scores≥3 indicated the presence of depressive symptoms [20]. Furthermore, we assessed anxiety using the Generalized Anxiety Disorder-2 scale [21] with scores ranging from 0–6; high scores indicated high anxiety levels, and scores≥3 defined the presence of anxiety [22].
Moreover, we measured activity participation for two physical and five social activities in the past month [15]. We coded all activity items using dichotomous variables (1 = yes; 0 = no). Additionally, we calculated sum scores for the physical and social activity items, with high scores indicating participation in more activities within the respective domain.
We defined covariates based on the literature [6, 8, 23] and included participants’ age, sex, race or ethnicity, education, living alone, functional impairment, proxy response, and place of residence. Functional impairment comprised the activity of daily living (ADL) and instrumental activity of daily living (IADL) impairments. We assessed ADL impairment based on difficulty or help required for bathing, eating, dressing, toileting, getting around inside the home, or leaving home. Moreover, we assessed IADL impairment based on difficulty or help required in cooking, managing finances, managing medications, shopping, and doing laundry [24]. We categorized ADL and IADL impairments as “no,” “moderate,” or “severe” based on the reported impairments (0, 1–2, and≥3, respectively). Lastly, we assessed living alone, functional impairment, proxy response, and place of residence in each NHATS round and treated them as time-varying variables in the analyses.
2.4Statistical analysis
We calculated the proportion and 95% confidence intervals (CIs) of mental health outcomes across the baseline dementia status. Moreover, we calculated the mean and 95% CIs of activity participation based on the baseline dementia status.
Furthermore, we performed multivariable binomial logistic regression analysis to test for differences in depression and anxiety across dementia statuses at baseline and year of follow-up assessment. The model used each outcome measure at follow-up as the dependent variable and dementia status at baseline as the independent variable. Additionally, the model included baseline demographics and functional impairment as independent variables. Next, we added activity participation at the follow-up to the model, as an independent variable. In these analyses, each case had a time variable (follow-up in 2019, 2020, or 2021) and variables at baseline and follow-up. These models accounted for the clustering of outcome measures among older adults. Since the 1-year mental health changes were explicit between 2019 and 2020, we examined the interaction between dementia status and year of follow-up using dummy variables as independent variables: (a) dementia×2020 and (b) dementia×2021, in addition to the main effects of dementia and year of follow-up.
The regression analyses used full information maximum likelihood to handle missing data [25]. Since our concerns were controlling for within-cluster correlation rather than the clustering level, we used a sandwich estimator instead of modeling random effects [26]. Lastly, we performed all analyses using Mplus for Windows, version 8.8 (Muthén & Muthén, Los Angeles, California, USA). p < 0.05 indicated statistical significance.
3RESULTS
Supplementary Table 1 summarizes the participants’ characteristics in 2018. Overall, 525 participants (11.8%) had probable dementia. Of the 4,548 individuals who participated in the 2018 and 2019 surveys, 4,038 participated in the 2020 round. Additionally, of these 4,038 participants, 3,452 completed the 2021 round.
Figure 1 shows the proportion of mental health outcomes and the mean value of activity participation according to baseline dementia status. Depressive symptoms appeared between 2019 and 2020 and decreased between 2020 and 2021. Furthermore, the trajectory of social activity coincided with that of depression, decreasing between 2019 and 2020 and increasing between 2020 and 2021. Individuals with dementia consistently had a higher proportion of depression and anxiety and lower means of social and physical activity than those without dementia (Fig. 1).
Fig. 1
Mental health and activity participation by dementia status per year of assessment. A) Proportion of depressive symptoms. B) Proportion of anxiety. C) Mean score of social activity. D) Mean score of physical activity. The dot shows the proportion or mean score, and the bar shows the range of 95% confidence intervals. The blue dots and bars refer to older adults with dementia, and the pale blue dots and bars refer to those without dementia. Dementia status in 2018 refers to the assessment in the NHATS 2018 survey. Dementia status in 2019 and afterward refers to the assessment in the previous survey. NHATS, National Health and Aging Trends Study.
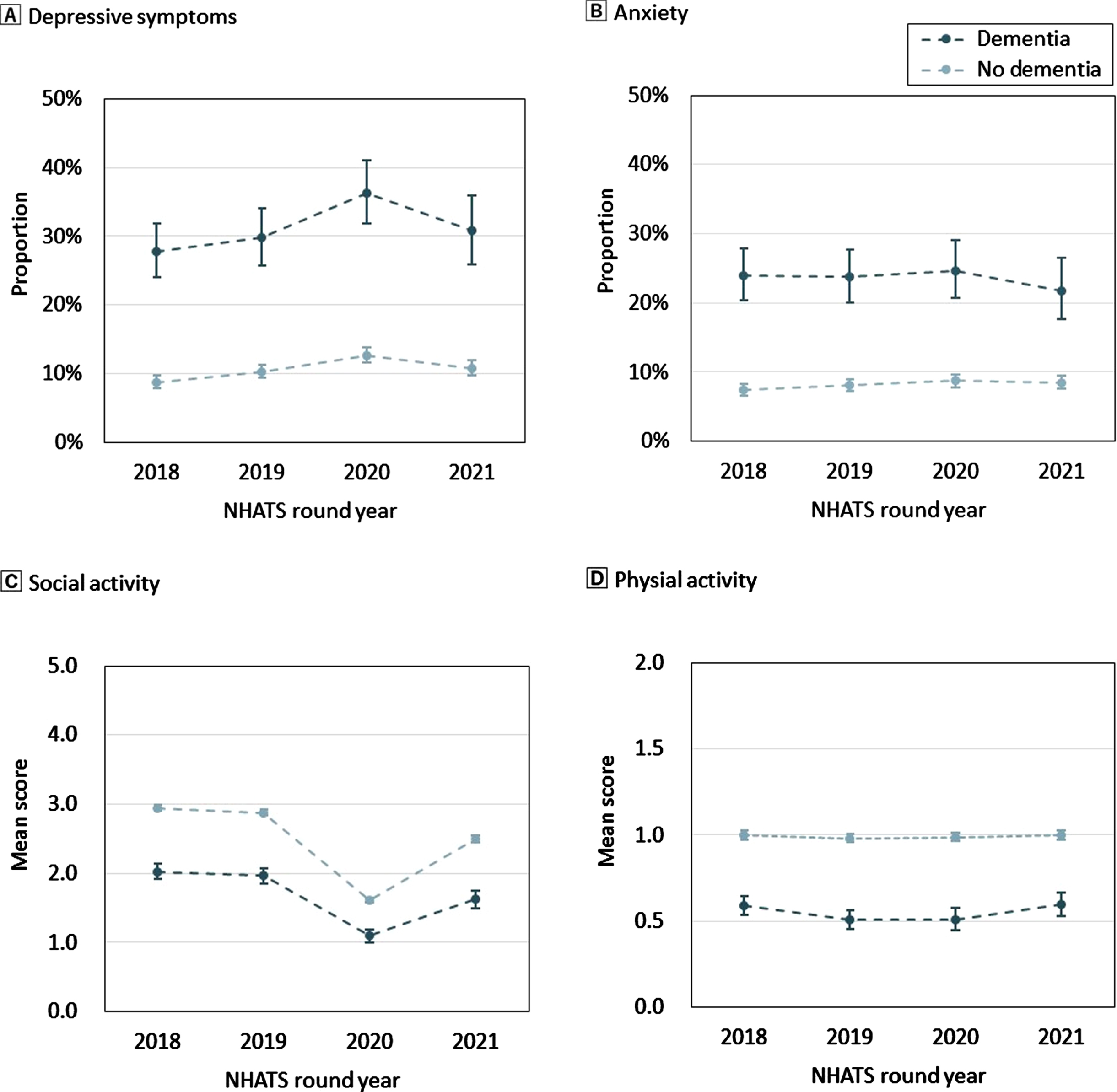
Figure 2 shows the adjusted odds ratio (OR) and 95% CI of 1-year change in mental health outcomes by dementia status and year of follow-up assessment. After adjusting for baseline demographics and functional impairment, an increased risk of depressive symptoms was associated with dementia at baseline and follow-up assessments in 2020. Additionally, the increased risk between 2019 and 2020 was insignificant after adjusting for activity participation. However, the association between the increased risk of mental health deterioration and baseline dementia remained significant. Furthermore, participation in social and physical activities was associated with a lower risk of depressive symptoms.
Fig. 2
Odds ratios and 95% confidence intervals for 1-year change in mental health by baseline dementia status, follow-up activity participation, and year of follow-up assessment. A) Proportion of depressive symptoms. B) Proportion of anxiety. The dot shows the adjusted odds ratio, and the bar shows the range of 95% confidence intervals of 1-year change in mental health outcomes. Blue dots and bars refer to odds ratios adjusted for baseline demographics and functional impairment, and pale blue dots and bars refer to those adjusted for baseline activity participation, demographics, and functional impairment.
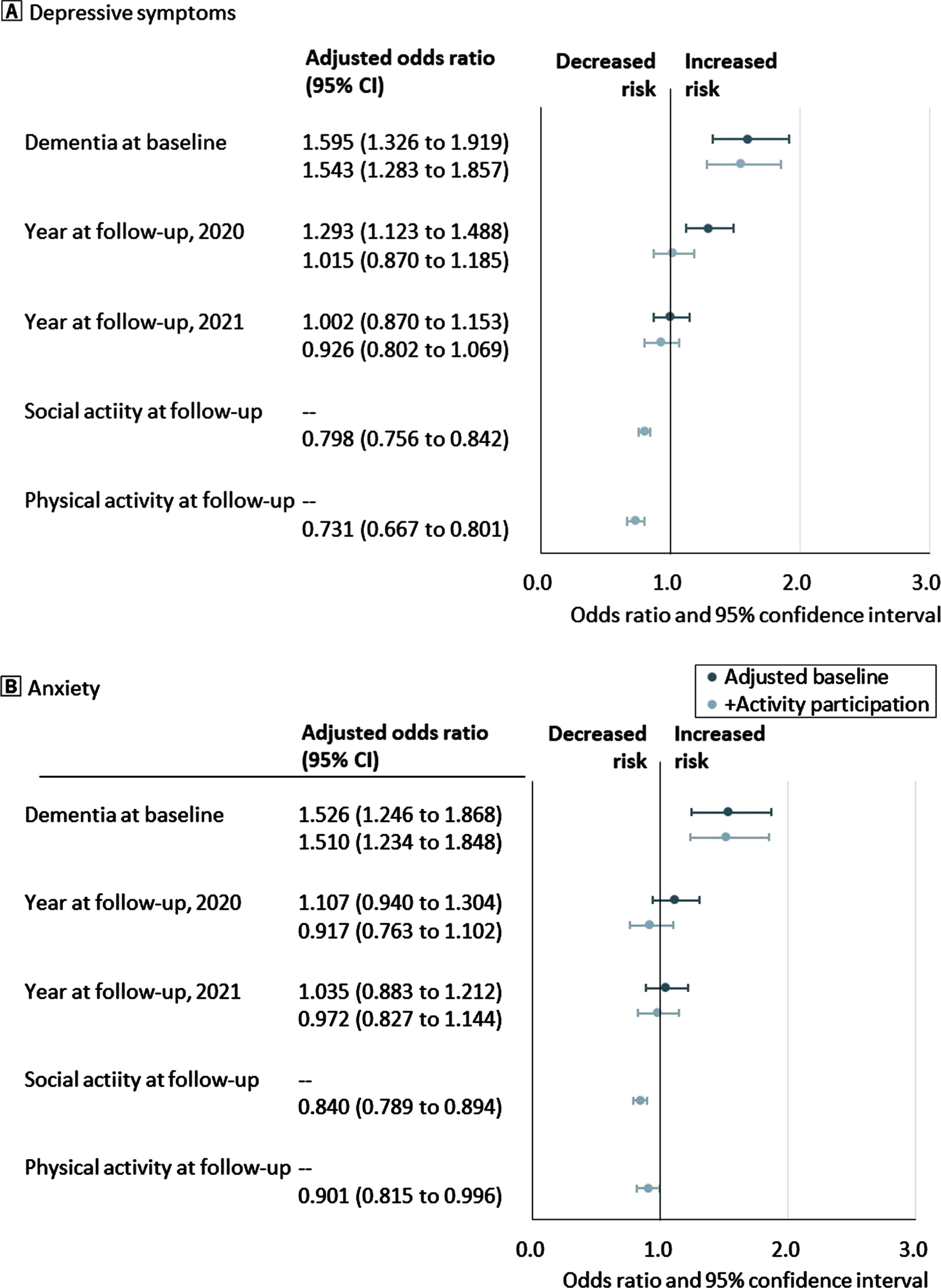
Moreover, baseline dementia was associated with an increased risk of anxiety, even after adjusting for activity participation. Participation in social and physical activities was associated with a low risk of anxiety. Lastly, the year of follow-up assessment was not associated with the risk of anxiety.
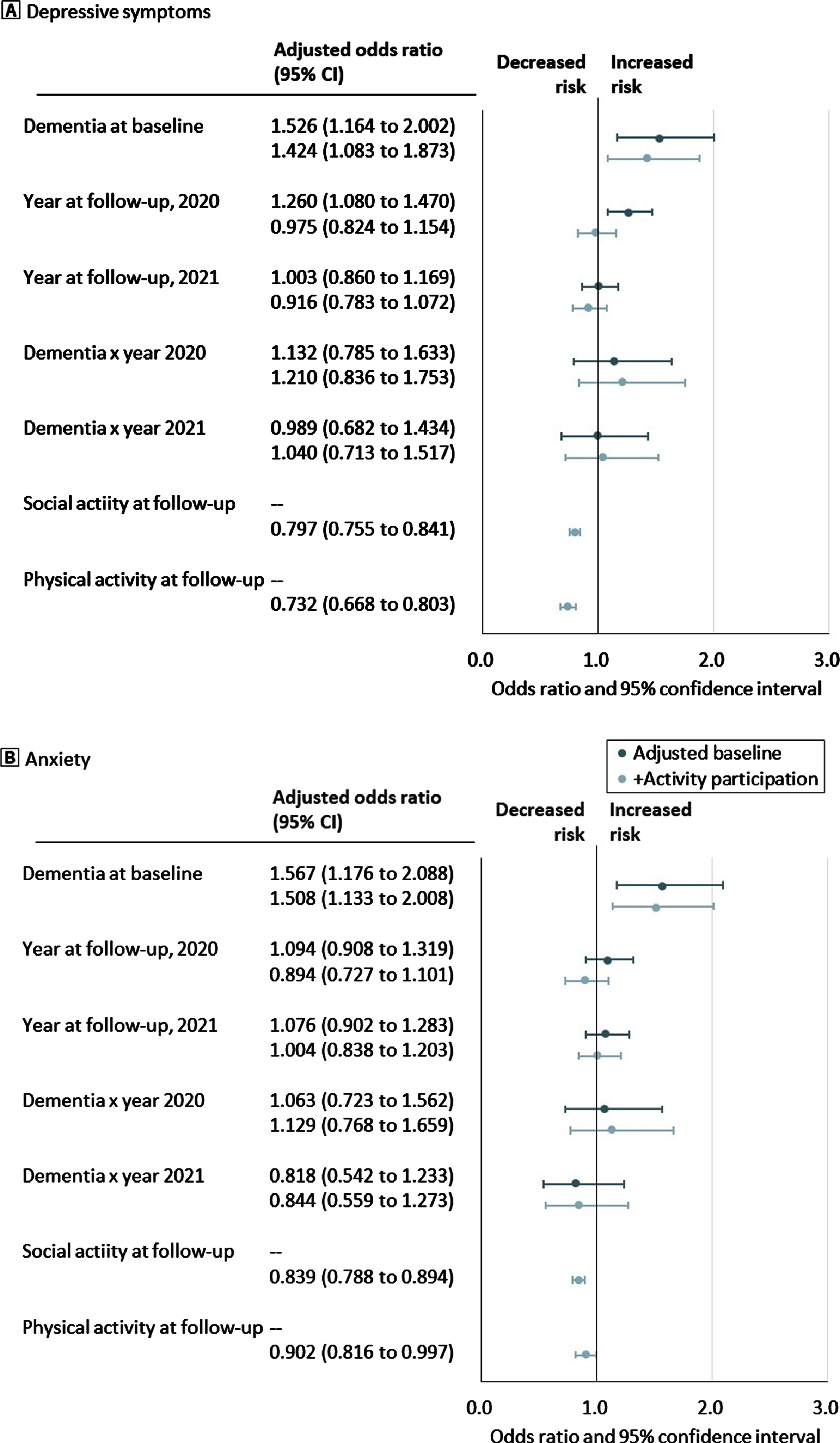
Figure 3 shows the adjusted OR and 95% CI of 1-year change in mental health outcomes by the interaction between dementia status and year of follow-up assessment. After adjusting for activity participation, baseline demographics, and functional impairment, the interaction between dementia and the 2019 and 2020 follow-up assessment years was not significant for depressive symptoms and anxiety. Participation in social and physical activities was associated with a low risk of depressive symptoms and anxiety.
Fig. 3
Odds ratios and 95% confidence intervals for 1-year change in mental health by baseline dementia status, year of follow-up assessment, the interaction effect between dementia and year of follow-up, and follow-up activity participation. A) Proportion of depressive symptoms. B) Proportion of anxiety. The dot shows the adjusted odds ratio, and the bar shows the range of 95% confidence intervals of 1-year change in mental health outcomes. Blue dots and bars refer to odds ratios adjusted for baseline demographics and functional impairment, and pale blue dots and bars refer to those adjusted for baseline activity participation, demographics, and functional impairment.
4DISCUSSION
4.1Main findings of this study
Older adults with dementia had an increased risk of depressive symptoms and anxiety before and during the COVID-19 pandemic. The overall level of depressive symptoms increased between 2019 and 2020 because of the onset of the pandemic and lifestyle changes caused by several restrictions. However, the association between an increased risk of depressive symptoms and period was insignificant after adjusting for activity participation. Poor activity participation was associated with a high risk of depressive symptoms and anxiety. Furthermore, the association between dementia and worse mental health outcomes remained significant even after controlling for activity participation. However, there was no interaction effect between dementia and year of follow-up for depressive symptoms and anxiety.
Worse mental health outcomes among people with dementia were consistent with suggestions from the qualitative data collected during the COVID-19 pandemic [10–12]. Our findings further present the quantitative evidence, hence providing a significant contribution to the existing literature. In particular, our observation was based on data from a large population-based cohort, with most participants living in private homes. Thus, the results illuminate the fact that widespread mental health challenges exist in the community-dwelling population. Even before the pandemic, depressive symptoms and anxiety were common (a prevalence of 11% and 9% in 2018, respectively) among older adults with dementia [27, 28]. Depressive symptoms are associated with excess mortality in older adults [29], especially in combination with dementia [30]. Therefore, the dementia-specific increased risk of depressive symptoms and anxiety during the onset of the COVID-19 pandemic could have further increased mortality in this population. We partially attribute this elevated risk to the reduced level of activity participation during this period; however, the dementia-specific risk remained even after adjusting for activity participation. One possible explanation is that older adults with dementia may experience chronic psychological distress because of their independence to care in daily life stimulated by rapid lifestyle changes and reduced social contact in 2020. People with dementia may be more vulnerable to reduced contact than those without dementia since loneliness and social isolation are linked to an increased risk of dementia [31]. Although several states in the United States have eased restrictions (for example, relaxing physical distancing requirements and reopening businesses) since 2021 [32], reduced policy support for dementia-friendly initiatives may have caused limited funding and resources needed to sustain the activity participation of persons with dementia [33]. Nevertheless, dementia care and support should address emotional and social needs using accessible means under long-term restrictions. For example, person-centered technology, such as service delivery using videoconferencing platforms, would provide feelings of connectedness and improve the resilience and well-being of people with dementia [34].
4.2Strengths and limitations
The strength of this study lies in the use of nationwide representative cohort data from the United States. The data enabled us to test the longitudinal association between baseline dementia status and 1-year mental health changes. Furthermore, the NHATS provided data from multiple periods, including before and after the onset of the COVID-19 pandemic. The results highlight that mental health deterioration was more prominent during the pandemic onset than in other 1-year periods. However, this study has some limitations. First, since the response date was unavailable in the NHATS data, we could not distinguish between participants before and after the onset of the pandemic in 2020. Additionally, the level of social and physical activities in early 2020 may have been higher than that in April 2020 and afterward, when the government imposed stay-at-home quarantine and other restrictions. Moreover, Medicare claim data was not consistent with the data from NHATS 2018 survey and afterward. Therefore, we could not confirm the diagnosis or healthcare use for dementia or depression in our sample. Lastly, data regarding the prescription of antidepressants, which is common among patients with dementia, was unavailable [35].
4.3Conclusions
Despite these limitations, this is the first study to indicate that having dementia and a reduced level of activity participation independently affected the risk of depressive symptoms and anxiety during the COVID-19 pandemic. These results highlight the emotional and social needs of people with dementia during public health crises. Therefore, dementia care and support should be developed to enable people with dementia to engage in meaningful activities and experience feelings of connectedness.
ACKNOWLEDGMENTS
National Health and Aging Trends Study. Produced and distributed by www.nhats.org with funding from the National Institute on Aging (grant number U01AG32947).
FUNDING
The Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant numbers JP21H03281, JP21H05171, JP21H05173, and JP21H05174]; and the Japan Agency for Medical Research and Development [Grant number 22579506] supported this work. The funders had no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The present research is based on deidentified data that are publicly available for registered users. Data and research materials are available at https://www.nhats.org/. We are not allowed to release the subsets of data used for the present analyses for NHATS due to their conditions of use.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230019.
REFERENCES
[1] | Brown EE , Kumar S , Rajji TK , Pollock BG , Mulsant BH ((2020) ) Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry 28: , 712–721. |
[2] | Mok VCT , Pendlebury S , Wong A , Alladi S , Au L , Bath PM , Biessels GJ , Chen C , Cordonnier C , Dichgans M , Dominguez J , Gorelick PB , Kim S , Kwok T , Greenberg SM , Jia J , Kalaria R , Kivipelto M , Naegandran K , Lam LCW , Lam BYK , Lee ATC , Markus HS , O’Brien J , Pai MC , Pantoni L , Sachdev P , Skoog I , Smith EE , Srikanth V , Suh GH , Wardlaw J , Ko H , Black SE , Scheltens P ((2020) ) Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID-19 pandemic, now and in the future. Alzheimers Dement 16: , 1571–1581. |
[3] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–446. |
[4] | Numbers K , Brodaty H ((2021) ) The effects of the COVID-19 pandemic on people with dementia. Nat Rev Neurol 17: , 69–70. |
[5] | Liu KY , Howard R , Banerjee S , Adelina CH , Joanne G , Martin K , Gill L , Jill M , John TO , Ross WP , Louise R , Martin R , James BR , David JS , Andrew S , Aida SG , Alistair B ((2021) ) Dementia wellbeing and COVID-19: Review and expert consensus on current research and knowledge gaps. Int J Geriatr Psychiatry 36: , 1597–1639. |
[6] | Hill NL , Mogle J , Bhargava S , Bratlee-Whitaker E , Wion RK , Sweeder L , Sliwinski M , Barnes LL ((2021) ) Within person associations among self-perceptions of memory, depressive symptoms, and activity participation in older adults. Gerontologist 61: , 1107–1117. |
[7] | Copeland M , Nowak GR , Liu H (2022) Social participation and self-reported depression during the COVID-19 pandemic among older adults. Aging Ment Health doi: 10.1080/13607863.2022.2126821. |
[8] | Oh A , Gan S , Boscardin WJ , Allison TA , Barnes DE , Covinsky KE , Smith AK ((2021) ) Engagement in meaningful activities among older adults with disability, dementia, and depression. JAMA Intern Med 181: , 560–562. |
[9] | Phinney A , Chaudhury H , O’Connor DL , ((2007) ) Doing as much as I can do: The meaning of activity for people with dementia. Aging Ment Health 11: , 384–393. |
[10] | El Haj M , Altintas E , Chapelet G , Kapogiannis D , Gallouj K ((2020) ) High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res 291: , 113294. |
[11] | Borg C , Rouch I , Pongan E , Getenet JC , Bachelet R , Herrmann M , Bohec AL , Laurent B ; COVCARE Group; Rey R , Dorey JM ((2021) ) Mental health of people with dementia during COVID-19 pandemic: What have we learned from the first wave? J Alzheimers Dis 82: , 1531–1541. |
[12] | Tsapanou A , Papatriantafyllou JD , Yiannopoulou K , Sali D , Kalligerou F , Ntanasi E , Zoi P , Margioti E , Kamtsadeli V , Hatzopoulou M , Koustimpi M , Zagka A , Papageorgiou SG , Sakka P ((2021) ) The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int J Geriatr Psychiatry 36: , 583–587. |
[13] | Clare L , Martyr A , Gamble LD , Pentecost C , Collins R , Dawson E , Hunt A , Parker S , Allan L , Burns A , Hillman A , Litherland R , Quinn C , Mattherws FE , Victor C ((2022) ) Impact of COVID-19 on ‘Living Well’ with mild-to-moderate dementia in the community: Findings from the IDEAL cohort. J Alzheimers Dis 85: , 925–940. |
[14] | Tsiakiri A , Vlotinou P , Terzoudi A , Heliopoulos I , Vadikolias K ((2022) ) Cognitive, functional, and emotional changes during the COVID-19 pandemic in Greek patients with neurocognitive disorders. J Alzheimers Dis 88: , 537–547. |
[15] | Freedman VA , Kasper JD ((2019) ) Cohort Profile: The National Health and Aging Trends Study (NHATS). Int J Epidemiol 48: , 1044–1045g. |
[16] | Freedman VA , Jennifer AS , Maureen ES , Judith DK (2022) National Health and Aging Trends Study User Guide: Rounds 1-11 Final Release, https://www.nhats.org/sites/default/files/2022-11/NHATS_User_Guide_R11_Final_Release.pdf, Posted 22 November 2022, Accessed 15 February 2023. |
[17] | Kasper JD , Freedman VA , Spillman B (2013) Classification of persons by dementia status in the National Health and Aging Trends Study. NHATS Technical Paper #5, https://www.nhats.org/sites/default/files/2022-09/NHATS%20Dementia%20Classification%20with%20Programming%20Statements_09232022.zip, Posted 1 August 2013, Accessed 15 February 2023. |
[18] | Galvin JE , Roe CM , Xiong C , Morris JC ((2006) ) Validity and reliability of the AD8 informant interview in dementia. Neurology 67: , 1942–1948. |
[19] | Kroenke K , Spitzer RL , Williams JB ((2003) ) The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med Care 41: , 1284–1292. |
[20] | Li C , Friedman B , Conwell Y , Fiscella K ((2007) ) Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc 55: , 596–602. |
[21] | Spitzer RL , Kroenke K , Williams JB , Löwe B ((2006) ) A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 166: , 1092–1097. |
[22] | Plummer F , Manea L , Trepel D , McMillan D ((2016) ) Screening for anxiety disorders with the GAD-7 and GAD-2: A systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry 39: , 24–31. |
[23] | Drazich BF , Li Q , Perrin NA , Szanton SL , Lee JW , Huang CM , Carlson MC , Samuel LJ , Regier NG , Rebok GW , Taylor JL ((2023) ) The relationship between older adults’ technology use, in-person engagement, and pandemic-related mental health. Aging Ment Health 27: , 156–165. |
[24] | Freedman VA , Kasper JD , Cornman JC , Agree EM , Bandeen-Roche K , Mor V , Spillman BC , Wallace R , Wolf DA ((2011) ) Validation of new measures of disability and functioning in the National Health and Aging Trends Study. J Gerontol A Biol Sci Med Sci 66: , 1013–1021. |
[25] | Cham H , Reshetnyak E , Rosenfeld B , Breitbart W ((2017) ) Full information maximum likelihood estimation for latent variable interactions with incomplete indicators. Multivariate Behav Res 52: , 12–30. |
[26] | McNeish D , Stapleton LM , Silverman RD ((2017) ) On the unnecessary ubiquity of hierarchical linear modeling. Psychol Methods 22: , 114–140. |
[27] | Teri L , Ferretti LE , Gibbons LE , Logsdon RG , McCurry SM , Kukull WA , McCormick WC , Bowen JD , Larson EB ((2015) ) Anxiety of Alzheimer’s disease: Prevalence, and comorbidity. J Gerontol A Biol Sci Med Sci 54: , M348–M352. |
[28] | Snowden MB , Atkins DC , Steinman LE , Bell JF , Bryant LL , Copeland C , Fitzpatrick AL ((2015) ) Longitudinal association of dementia and depression. Am J Geriatr Psychiatry 23: , 897–905. |
[29] | Schoevers RA , Geerlings MI , Deeg DJ , Holwerda TJ , Jonker C , Beekman AT ((2009) ) Depression and excess mortality: Evidence for a dose response relation in community living elderly. Int J Geriatr Psychiatry 24: , 1691–1676. |
[30] | Bellelli G , Frisoni GB , Turco R , Trabucchi M ((2008) ) Depressive symptoms combined with dementia affect 12-months survival in elderly patients after rehabilitation post-hip fracture surgery. Int J Geriatr Psychiatry 23: , 1073–1077. |
[31] | Salinas J , Beiser AS , Samra JK , O’Donnell A , DeCarli CS , Gonzales MM , Aparicio HJ , Seshadri S ((2022) ) Association of loneliness with 10-year dementia risk and early markers of vulnerability for neurocognitive decline. Neurology 98: , e1337–e1348. |
[32] | Lee JC , Mervosh S , Avila Y , Harvey B , Matthews AL (2021) See reopening plans and mask mandates for all 50 states, https://www.nytimes.com/interactive/2020/us/states-reopen-map-coronavirus.html, Posted 1 July 2021, Accessed 15 February 2023. |
[33] | Sun F , Opur FA , Kim HN , Prieto LR , Conyers C ((2022) ) Dementia-friendly initiatives within the context of COVID-19 pandemic: Challenges and strategies perceived by service professional stakeholders from the USA and China. Dementia (London) 21: , 1714–1733. |
[34] | Lai FH , Yan EW , Yu KK , Tsui WS , Chan DT , Yee BK ((2020) ) The protective impact of telemedicine on persons with dementia and their caregivers during the COVID-19 pandemic. Am J Geriatr Psychiatry 28: , 1175–1184. |
[35] | Enache D , Fereshtehnejad SM , Kåreholt I , Cermakova P , Garcia-Ptacek S , Johnell K , Religa D , Jelic V , Winblad B , Ballard C , Aarsland D , Fastbom J , Eriksdotter M ((2016) ) Antidepressants and mortality risk in a dementia cohort: Data from SveDem, the Swedish Dementia Registry. Acta Psychiatr Scand 134: , 430–440. |




