Episodic Memory in Amnestic Behavioral Frontotemporal Dementia and Alzheimer’s Disease
Abstract
Behavioral frontotemporal dementia (bvFTD) may present with episodic memory deficits. In 38 patients with bvFTD and 61 with Alzheimer’s disease (AD) specific measures of verbal memory (learning curves and serial position effects) were studied through the Rey Auditory Verbal Learning test. Forty-two percent of bvFTD showed deficits of delayed recall memory similar to that found in AD including the serial position effects. Amnestic bvFTD had more severe atrophy in the left mesial temporal lobe than non-amnestic bvFTD. AD-like memory deficits are not infrequent in bvFTD and may be in part related to mesial temporal lobe atrophy.
INTRODUCTION
Cognitive profile of behavioral variant frontotemporal dementia (bvFTD) is characterized by executive deficits and a relative sparing of episodic memory respect to executive dysfunction [1]. However, some patients with probable bvFTD may present with memory complaints and lower scores in episodic memory tests [2, 3]. A recent meta-analysis showed that memory functioning in bvFTD is at an intermediate level between controls and patients with dementia due to Alzheimer’s disease (AD) [3]. Bertoux and colleagues reported that 43% of patients with bvFTD presented amnesia similar to that found in mild AD, as well as atrophy of the rostral prefrontal cortex and hippocampal-perihippocampal regions [4]. Decreased memory performance in bvFTD may result from an impairment of hippocampal-based consolidation of information, and/or from executive deficits that hinder the use of encoding and retrieval strategies [5, 6]. In favor of a primary memory deficit, previous studies showed that performances in executive tasks and in memory are not interdependent in bvFTD, while they are strongly associated in healthy subjects [7] and in conditions where medial temporal lobe regions are relatively preserved [8].
Recently Kloth et al. observed that a few patients with bvFTD had measures of free recall rate at the Rey Auditory Verbal Learning test (RAVLT) similar to those found in AD [9]. However, studying the serial positions effects, the amnestic bvFTD (a-bvFTD) group showed better performances than AD in the recency position of the immediate recall trial and proposed this index as discriminator between AD and bvFTD even when the overall memory impairment is similar. On the other way round, other measures of serial position effects, such as the ratio between immediate and delayed performance at recency position, have been proposed as diagnostic marker of AD-related neurodegeneration and as marker of progression to dementia in patients with mild cognitive impairment [10, 11].
The hypothesis of this study was that some patients with bvFTD may have objective episodic memory deficits similar to those found in AD, but with subtle differences which could be detected by analyzing serial position effects. To this purpose, immediate and delayed free recall at the episodic verbal memory task, different measures of serial position effects and learning curve performances were studied in bvFTD and AD patients.
METHODS
Patients
A total of 125 subjects participated in this retrospective study: 38 with bvFTD (mean age: 66.61±8.95 years, female/male: 15/23), 61 with AD (mean age: 71.30±8.08 years, female/male: 30/31), and 27 age-matched healthy elderly (mean age: 72.56±3.72 years, female/male: 20/7). Diagnoses of probable bvFTD and AD were made according to current diagnostic criteria [1, 12]. The inclusion criteria were: Mini-Mental State Examination (MMSE) score≥15 and lack of severe language deficit or behavioral disturbances interfering with neuropsychological assessment. Exclusion criteria were: previous diagnoses of primary psychiatric disorders; systemic or brain diseases responsible for behavioral/cognitive alterations; a past history of head injury; a diagnosis of another neurodegenerative disease; and/or evidence of substantial concomitant cerebrovascular disease defined by a history of stroke temporally related to the disease onset or presence of multiple or extensive infarcts or severe white matter hyperintensity burden assessed with MRI or CT scan; comorbid AD-pathology in a-bvFTD cases.
Patients with bvFTD underwent a diagnostic work-up including Neuropsychiatric Inventory (NPI) questionnaire, brain MRI and FDG-PET, and genetic testing for pathogenic mutations associated with FTD. Visual rating scales of regional brain atrophy and vascular load were applied to individual MRI scans of bvFTD patients by two readers (SM and AC). Medial temporal lobe atrophy (MTA) was assessed using a standardized scale evaluating atrophy on the base of width of coroid fissure, enlargement of the temporal horn of lateral ventricle and height of hippocampus, on the coronal T1-weighted plane images. The right and left sides were rated separately. Possible scores ranged from 0 (no atrophy) to 4 (severe atrophy) [13, 14]. Small vessel ischemia was assessed using the Fazekas scale. It evaluated white matter hyperintense signal abnormalities surrounding the ventricles and in the deep white matter [15]. Hyperintensity was graded depending on the size and confluence of lesions from 0 (absent) to 3 (confluent areas/extending into the deep white matter).
Lumbar puncture was performed to obtain CSF for detection of amyloid-β 1-42, total tau, and p-tau levels in bvFTD patients. All a-bvFTD patients had negative CSF biomarkers for AD, excluding AD-related co-pathology. Healthy elderly individuals served as control group (HC) and were recruited from participants to the community services among subjects with MMSE≥27. This study was completed in accordance with Helsinki declaration and was approved by the Ethical Committee of Padova Hospital (n. 0038879).
Neuropsychological assessment
Participants underwent neuropsychological assessment including: the MMSE [16] for the evaluation of global cognitive performance; the digit cancellation test [17] and the Trail Making Test-A [18] for the evaluation of visual attention; the digit span forward and backward tests [19] for short-term memory; the prose memory immediate and delayed recall tests for long-term memory; the letter fluency test for the assessment of word generation and executive functioning; the clock drawing test [16] for visuo-constructional abilities and executive functions; the Rey-Osterrieth Complex Figure (ROCF) test for the assessment of visuo-constructional abilities (ROCF copy) and long-term visual-spatial memory (ROCF delayed recall) [20].
The RAVLT was administered to each participant following standard procedure [21]. This test consists of 15 semantically unrelated nouns read aloud by the examiner, with a 1-s interval between each word, for five consecutive trials (trials 1 to 5), always in the same order. Immediately following each trial, subjects are asked to recall as many words as possible not considering the order in which they were read. There is no time constraint for recall. Following 15 min delay, each subject is again required to recall the word list (trial 6). The raw score obtained from summing the number of correctly recalled items in each trial (trials 1 + 2+3 + 4+5) represents the immediate recall score (IR). The delayed recall score (DR) is calculated from the number of items correctly recalled in trial 6. The effects of word serial position were assessed by parsing the full list into regions: the first 4 words represent the primacy region (P4) and the last 4 words represent the recency region (R4). We also calculated short primacy and recency by including only the first and last two words, thus creating P2 and R2, respectively, as recently proposed [9, 21]. Patients with bvFTD were divided into amnestic or non-amnestic subgroups according to individual values of DR performance as previously described in the paper of Kloth [9] and using cut-off level of normal DR based on Italian normative data.
From the RAVLT measures, two indices were calculated: the recency ratio (Rr) [10, 11] and the recency dominance (RD) [9]. The Rr is the ratio between the first 4 items in the recency region (R4) in both immediate recall and delayed recall, with the formula: R4 + 1 (trial 1 or trial 5) divided by R4 + 1 in delayed recall (trial 6). The RD is obtained by subtracting the number of recalled items in the primacy region (first 2 or 4 words) from the number of recalled items on recency positions (last 2 or 4 words), divided by the overall number of items recalled in that trial 1 [9]. RD scores of 0 indicate equal recency and primacy effects; positive RD values means poorer recall of primacy compared to recency; and finally, negative RD values indicate poorer recency compared to primacy. Both Rr and RD scores were calculated using either 4 and 2 recalled items of recency regions.
Statistical analysis
Continuous variables were tested for normality of distribution, and visual inspection of histograms and qi-plots were performed. Categorical variables were analyzed using chi-squared test, while continuous variables with Kruskal-Wallis test or ANCOVA when appropriate. The results were controlled for multiple comparison using FDR correction. Post-hoc analysis was performed with a Mann-Whitney U or independent t-test when appropriate and results were controlled for multiple comparison. The significance level used in the study was α=0.05. Age, sex, MMSE, and education were used as nuisance factors. Spearman correlation test was applied to investigate relation between specific memory measures and other cognitive variables.
RESULTS
Clinical and cognitive features of a-bvFTD
Sixteen over 38 bvFTD patients had delayed recall memory scores below cut-off values of normality and were therefore classified as a-bvFTD, while the remaining patients were classified as non-amnestic (na-bvFTD). Demographic, clinical, and cognitive characteristics of a-bvFTD, na-bvFTD, and AD are reported in Table 1.
Table 1
Demographic and cognitive data of AD, a-bvFTD, and na-bvFTD subgroups
| AD (n = 61) | a-bvFTD (n = 16) | na-bvFTD (n = 22) | AD versus a-bvFTD | AD versus na-bvFTD | a-bvFTD versus na-bvFTD | |||
| Mean/median± | Mean/median± | Mean/median± | Statistical | p | p | p | P | |
| SD/IQR | SD/IQR | SD/IQR | analysis | P | ||||
| Age (y) | 71.30±8.08 | 63.88±8.31 | 68.59±9.05 | F(2,99)=5.19 | <0.01 | 0.01 | 0.68 | 0.32 |
| Education (y) | 11.48±4.65 | 8.75±4.19 | 8.32±3.06 | F(2,96)=5.71 | <0.01 | 0.10 | <0.01 | 1.00 |
| Female [n] (%) | 30 (49.18) | 6 (37.5) | 9 (40.91) | χ2 = 0.93 | 0.62 | |||
| Disease duration (y) | 3.1±1.86 | 2.69±1.5 | 2.73±1.3 | 0.65 | ||||
| Global cognition | ||||||||
| MMSE score | 24.61±3.22 | 24±2.99 | 26.81±2.75 | F(2,95)=5.71 | <0.01 | 1.00 | 0.01 | 0.02 |
| Attention and memory span | ||||||||
| Digit cancellation | 41.18±11.99 | 40.93±12.10 | 39.70±9.08 | F(2,82)=4.05 | 0.02 | 1.00 | 0.03 | 0.08 |
| Digit span forward | 5.15±0.77 | 4.14±1.66 | 4.45±0.60 | F(2,81)=10.61 | <0.001* | <0.01 | <0.01 | 1.00 |
| TMT-A (s) | 97.32±65.67 | 120.31±83.38 | 78.78±35.48 | F(2,86)=0.72 | 0.48 | |||
| Executive functions | ||||||||
| Digit span backward | 3.55±0.89 | 2.69±1.49 | 3.19±0.75 | F(2,79)=4.97 | 0.01 | 0.02 | 0.16 | 1.00 |
| Phonemic fluency | 25.02±10.96 | 14.47±10.93 | 24.50±11.74 | F(2,81)=4.37 | 0.02 | 0.01 | 1.00 | 0.09 |
| Semantic fluency | 26.18±12.13 | 18.86±9.48 | 28.11±11.55 | F(2,77)=2.55 | 0.08 | |||
| Visual abilities | ||||||||
| Copy of ROCF | 24.08±9.90 | 23.71±8.77 | 22.62±7.72 | F(2,78)=1.29 | 0.28 | |||
| Clock drawing test | 6.16±3.21 | 6.14±3.38 | 6.05±3.67 | F(2,82)=0.14 | 0.86 | |||
| Episodic and Visual Memory | ||||||||
| RAVLT total IR | 24.23±8.73 | 21.00±7.33 | 29.77±7.86 | F(2,91)=0.66 | 0.51 | |||
| RAVLT total DR | 1±2 | 2±2 | 6±2 | χ2(99)=34.64 | <0.001* | 1.00 | <0.001 | <0.001 |
| Prose memory IR | 7.20±4.42 | 6.83±4.28 | 8.59±3.26 | F(2,74)=0.37 | 0.69 | |||
| Prose memory DR | 6.22±5.03 | 7.83±5.39 | 9.53±3.48 | F(2,73)=0.15 | 0.85 | |||
| Recall of ROCF | 6.23±5.52 | 7.23±5.71 | 9.67±7.19 | F(2,76)=0.14 | 0.86 | |||
*Represents significances that survive after correction for multiple comparison (13 variables). MMSE, Mini-Mental State Examination; TMT-A, Trail Making Test-A; ROCF, Rey-Osterrieth Complex Figure; RAVLT, Rey Auditory Verbal Learning task; IR, immediate recall; DR, delayed recall; AD, Alzheimer’s disease; a-bvFTD, amnestic variant of behavioral frontotemporal dementia; na-bvFTD, non-amnestic variant of behavioral frontotemporal dementia.
Patients with a-bvFTD and AD were comparable a part from a younger age of a-bvFTD. a-bvFTD performed worse than AD in short-term memory (digit span test) and phonemic fluency.
Amnestic-bvFTD had similar age and lower MMSE score than na-bvFTD. They had also a similar profile of cognitive impairment and similar burden of behavioral changes (mean NPI total score = a-bvFTD: 36±18; na-bvFTD: 30±17, p = 0,4). Analysis of MRI data from visual rating scales showed greater atrophy in the left medial temporal lobe in a-bvFTD respect to na-bvFTD (median±IQR=2±2 and 0±1 respectively, W = 93, punc = 0.01) while other measures of atrophy (GCA and right MTA) or vascular load (Fazekas scale) were similar. One patient with a-bvFTD tested positive for progranulin mutation, while 4/22 of na-bvFTD were positive for c9orf72 expansion.
Demographic and cognitive data of the whole bvFTD group, AD and HC are shown in Supplementary Table 1.
Verbal memory in a-bvFTD versus AD dementia
a-bvFTD had lower mean scores than AD and na-bvFTD in the last trial of the immediate recall of RAVLT (a-bvFTD=4.53±1.73; AD = 6.13±2.39; na-bvFTD=7.27±2.80; p = 0.01) (Fig. 1) and this finding correlated with worse working memory (digit span forward: r = 0.62, p = 0.02) and attention (digit cancellation: r = 0.69, p = 0.008). Generalized mixed effect model was used controlling for sex, age and MMSE. We found a significant group effect (p < 0.001). Post-hoc analysis revealed significant worse performances for a-bvFTD respect to both AD (t = 3.78, p < 0.001) and na-bvFTD (t = 4.79, p < 0.001).
Fig. 1
Number of words recalled in the immediate recall trials 1, 2, 3, 4, and 5 of the RAVLT in AD, a-bvFTD, na-bvFTD patients, and HC. Mean value of words recalled at trial 5 in the a-bvFTD is lower than AD (p = 0.010) and na-bvFTD (p = 0.013).
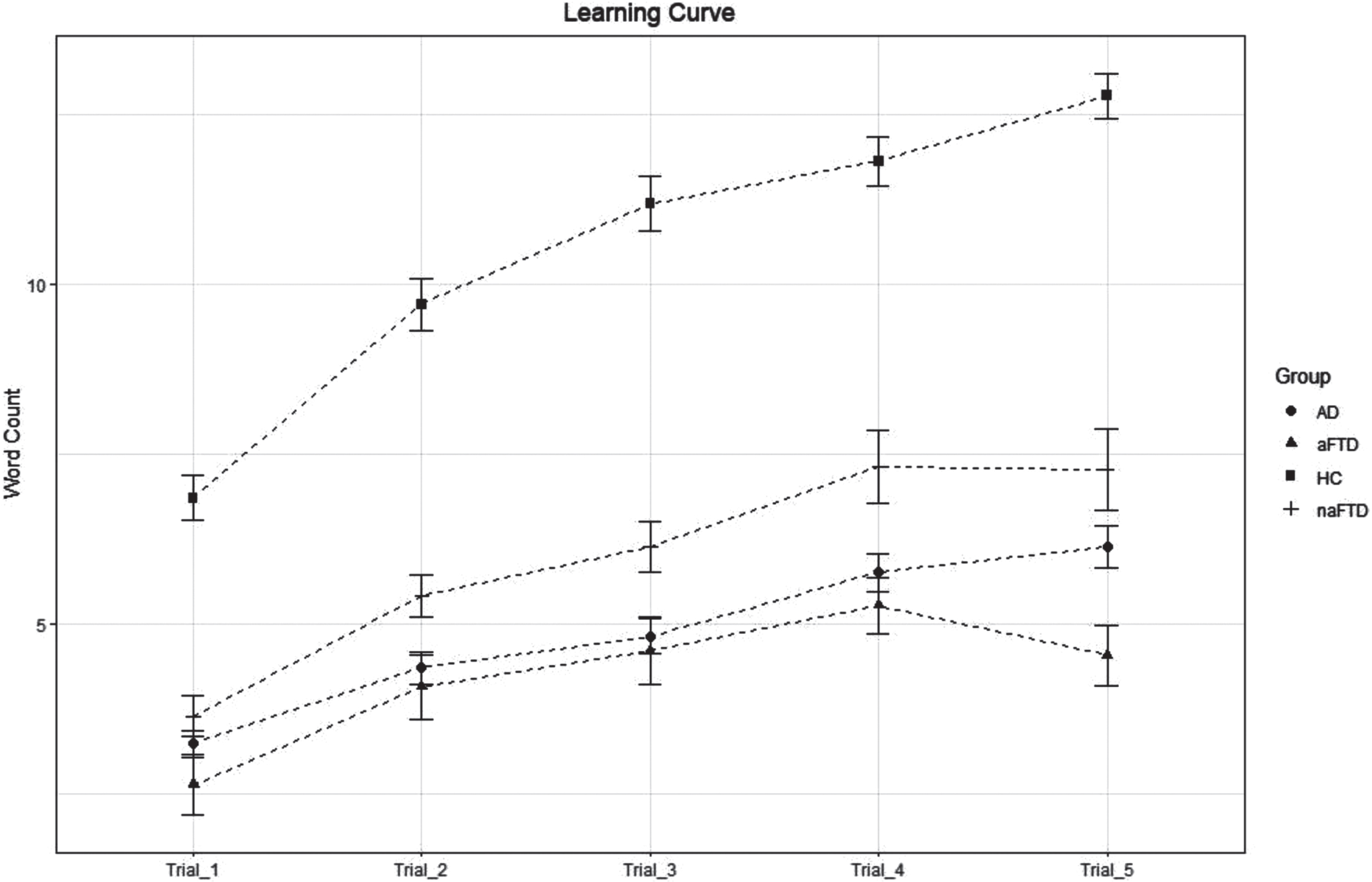
No intergroups differences were found in serial position effects, except for lower P2 scores in a-bvFTD than in AD group only in trial 5, that did not survive multiple comparison analysis (Table 2). The Rr at both trial 1 and 5 and RD indices were not significantly different between a-bvFTD and AD, while lower values of Rr were detected in na-bvFTD compared to AD.
Table 2
Serial position effects with the RAVLT of AD, a-bvFTD, and na-bvFTD subgroups
| RAVLT | AD | a-bvFTD | na-bvFTD | AD | AD | a-bvFTD | ||
| Serial position | (n = 61) | (n = 16) | (n = 22) | versus | versus | versus | ||
| effects | a-bvFTD | na-bvFTD | na-bvFTD | |||||
| Mean/median± | Mean/median± | Mean/median± | Statistical | p | p | p | P | |
| SD/IQR | SD/IQR | SD/IQR | P | |||||
| Primacy (4) Trial 1 | 1±1 | 0±1.25 | 1±1 | χ2(99)=1.53 | 0.46 | |||
| Primacy (2) Trial 1 | 0±1 | 0±1 | 1±1 | χ2(99)=1.84 | 0.40 | |||
| Primacy (4) Trial 5 | 2.08±1.17 | 1.38±1.15 | 2.23±1.11 | F(2,91)=3.04 | 0.052 | |||
| Primacy (2) Trial 5 | 1±1 | 0±1 | 1±1 | χ2(99)=8.89 | 0.01 | 0.02 | 1.00 | 0.11 |
| Recency (4) Trial 1 | 1.95±1.22 | 1.56±1.31 | 2.14±1.04 | F(2,91)=0.49 | 0.70 | |||
| Recency (2) Trial 1 | 1.25±0.83 | 0.94±0.77 | 1.50±0.51 | F(2,91)=1.27 | 0.29 | |||
| Recency (4) Trial 5 | 2.23±1.23 | 2.19±1.42 | 2.27±1.24 | F(2,91)=0.40 | 0.66 | |||
| Recency (2) Trial 5 | 1.23±0.80 | 1.38±0.81 | 1.14±0.77 | F(2,91)=0.11 | 0.9 | |||
| Rr index (4) Trial 1 | 2.29±1.17 | 2.03±1.47 | 1.40±0.90 | F(2,91)=3.58 | 0.03 | 1.00 | 0.03 | 0.24 |
| Rr index (4) Trial 5 | 2.50±1.22 | 2.42±1.51 | 1.37±0.58 | F(2,91)=3.1 | 0.052 | |||
| RD index (2) Trial 1 | 1±2 | 0.5±1 | 1±1.75 | χ2(99)=0.77 | 0.68 | |||
| RD index (4) Trial 1 | 1±3 | 1±2.5 | 1±2 | χ2(99)=0.39 | 0.82 |
RR, recency index; RD, recency dominance; (4), region of four items; (2), region of two items. RAVLT– IR, Rey’s Auditory Verbal Learning Test– Immediate Recall; AD, Alzheimer’s disease; a-bvFTD, amnestic variant of behavioral frontotemporal dementia; na-bvFTD, non-amnestic variant of behavioral frontotemporal dementia.
DISCUSSION
In this study 42% of bvFTD patients had deficits of delayed recall verbal memory. Although clinical diagnostic criteria of bvFTD [1] state that delayed memory is usually relatively preserved, we confirm the results of previous studies describing an impairment of episodic memory in a non-negligible subset of bvFTD patients [9, 22–25]. In general, however, patients with bvFTD perform better than AD when remembering details of a story [26, 27].
Very few studies investigated clinical and cognitive differences of a-bvFTD respect to na-bvFTD. Available literature gives mixed results with some studies showing worse lexical and semantic fluency in a-bvFTD [7] while others describing no differences in the extra-memory cognitive domains [25]. We did not find significant differences in the profile of cognitive impairment in a-bvFTD compared to na-bvFTD.
Decreased learning abilities in the immediate recall task is the only memory measure distinguishing a-bvFTD from AD. Other specific metrices of verbal memory such as serial effects, recency ratio and recency dominance did not differentiate the two groups except for a more evident short primacy at trial 5 that disappeared considering the whole primacy (with 4 positions). These data did not confirm previous results from Kloth and collaborators that found a reduced recall of primacy items in a-bvFTD and a maintained recall of recency items in AD [9]. They suggested that an index including these two measures, the recency dominance, could be useful in the differential diagnosis between a-bvFTD and AD. Differences with previous results may be due to different characteristics of recruited AD patients (higher MMSE and older age in our study) and low sample size of a-bvFTD in the previous study.
Amnesia in some bvFTD patients may be caused by both defective learning strategies due to frontal lobe dysfunction and impaired consolidation processes due to temporal lobe atrophy. It has been shown that atrophy and hypometabolism of mesial temporal structures may play a role in determining memory deficit in bvFTD [25]. Interestingly, we found higher mesial temporal lobe atrophy on visual rating scale in a-bvFTD than na-bvFTD suggesting a possible role of hippocampal atrophy in the genesis of memory deficits in a-bvFTD. However, a limitation of this study is that our findings could not disentangle the relative contribution of decreased encoding function from alteration of hippocampal-related memory processes. We could also not define whether memory deficits appear in the more advance disease stage in the course of bvFTD since a-bvFTD had greater global cognitive impairment than a-bvFTD, or whether is a distinct phenotype independent from disease severity.
In summary, patients with a-bvFTD may have memory impairment similar to that found in AD that may be caused by a different degree of both defective learning strategies due to frontal lobe dysfunction and impaired consolidation processes due to temporal lobe atrophy.
ACKNOWLEDGMENTS
We thank Davide Bruno for his helpful comments.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. CB received a grant by the Associazione Italiana Ricerca Alzheimer Onlus (AIRAlzh) funded by Consorzio Nazionale delle Cooperative di Consumatori (COOP ITALIA S.C.).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data are available on request to the senior author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-230015.
REFERENCES
[1] | Rascovsky K , Hodges JR , Knopman D , Mendez MF , Kramer JH , Neuhaus J , van Swieten JC , Seelaar H , Dopper EGP , Onyike CU , Hillis AE , Josephs KA , Boeve BF , Kertesz A , Seeley WW , Rankin KP , Johnson JK , Gorno-Tempini M-L , Rosen H , Prioleau-Latham CE , Lee A , Kipps CM , Lillo P , Piguet O , Rohrer JD , Rossor MN , Warren JD , Fox NC , Galasko D , Salmon DP , Black SE , Mesulam M , Weintraub S , Dickerson BC , Diehl-Schmid J , Pasquier F , Deramecourt V , Lebert F , Pijnenburg Y , Chow TW , Manes F , Grafman J , Cappa SF , Freedman M , Grossman M , Miller BL ((2011) ) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134: , 2456–2477. |
[2] | Poos JM , Jiskoot LC , Papma JM , Van Swieten JC , Van Den Berg E ((2018) ) Meta-analytic review of memory impairment in behavioral variant frontotemporal dementia. J Int Neuropsychol Soc 24: , 593–605. |
[3] | Turchetta CS , De Simone MS , Perri R , Fadda L , Caruso G , De Tollis M , Caltagirone C , Carlesimo GA ((2020) ) Forgetting rates on the recency portion of a word list predict conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis 73: , 1295–1304. |
[4] | Bertoux M , Flanagan EC , Hobbs M , Ruiz-Tagle A , Delgado C , Miranda M , Ibáñez A , Slachevsky A , Hornberger M ((2018) ) Structural anatomical investigation of long-term memory deficit in behavioral frontotemporal dementia. J Alzheimers Dis 62: , 1887–1900. |
[5] | Lemos R , Duro D , Simões MR , Santana I ((2014) ) The free and cued selective reminding test distinguishes frontotemporal dementia from Alzheimer’s disease. Arch Clin Neuropsychol 29: , 670–679. |
[6] | Miller BL , Cummings JL ((2007) ) The Human Frontal Lobes, Second Edition: Functions and Disorders, Guilford Publications, United Kingdom. |
[7] | Bertoux M , Ramanan S , Slachevsky A , Wong S , Henriquez F , Musa G , Delgado C , Flanagan E , Bottlaender M , Sarazin M , Hornberger M , Dubois B ((2016) ) So close yet so far: Executive contribution to memory processing in behavioral variant frontotemporal dementia. J Alzheimers Dis 54: , 1005–1014. |
[8] | Dobbins IG , Foley H , Schacter DL , Wagner AD ((2002) ) Executive control during episodic retrieval. Neuron 35: , 989–996. |
[9] | Kloth N , Lemke J , Wiendl H , Meuth SG , Duning T , Johnen A ((2020) ) Serial position effects rapidly distinguish Alzheimer’s from frontotemporal dementia. J Neurol 267: , 975–983. |
[10] | Bruno D , Reichert C , Pomara N ((2016) ) The recency ratio as an index of cognitive performance and decline in elderly individuals. J Clin Exp Neuropsychol 38: , 967–973. |
[11] | Bruno D , Gleason CE , Koscik RL , Pomara N , Zetterberg H , Blennow K , Johnson SC ((2019) ) The recency ratio is related to CSF amyloid beta 1-42 levels in MCI-AD. Int J Geriatr Psychiatry 34: , 415–419. |
[12] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[13] | Pasquier F , Leys D , Weerts JGE , Mounier-Vehier F , Barkhof F , Scheltens P ((1996) ) Inter-and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 36: , 268–272. |
[14] | Scheltens P , Launer LJ , Barkhof F , Weinstein HC , Gool WA ((1995) ) Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: Interobserver reliability. J Neurol 242: , 557–560. |
[15] | Fazekas F , Chawluk J , Alavi A , Hurtig H , Zimmerman R ((1987) ) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 149: , 351–356. |
[16] | Magni E , Binetti G , Bianchetti A , Rozzini R , Trabucchi M ((1996) ) Mini-Mental State Examination: A normative study in Italian elderly population. Eur J Neurol 3: , 198–202. |
[17] | Sala S Della , Laiacona M , Spinnler H , Ubezio C ((1992) ) A cancellation test: Its reliability in assessing attentional deficits in Alzheimer’s disease. Psychol Med 22: , 885–901. |
[18] | Mondini , S. Mapelli D , Vestri A , Arcara G , Bisiacchi PS ((2011) ) Esame Neuropsicologico Breve 2, ENB-2. Raffaello Cortina Editore. |
[19] | Monaco M , Costa A , Caltagirone C , Carlesimo GA ((2013) ) Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol Sci 34: , 749–754. |
[20] | Caffarra P , Vezzadini G , Dieci F , Zonato F , Venneri A ((2002) ) Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol Sci 22: , 443–447. |
[21] | Carlesimo GA , Caltagirone C , Gainotti G , Fadda L , Gallassi R , Lorusso S , Marfia G , Marra C , Nocentini U , Parnetti L ((1996) ) The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol 36: , 378–384. |
[22] | Bertoux M , De Souza LC , Corlier F , Lamari F , Bottlaender M , Dubois B , Sarazin M ((2014) ) Two distinct amnesic profiles in behavioral variant frontotemporal dementia. Biol Psychiatry 75: , 582–588. |
[23] | Hornberger M , Piguet O , Graham AJ , Nestor PJ , Hodges JR ((2010) ) How preserved is episodic memory in behavioral variant frontotemporal dementia?. Neurology 74: , 472–479. |
[24] | Ricci M , Graef S , Blundo C , Miller LA ((2012) ) Using the Rey Auditory Verbal Learning Test (RAVLT) to differentiate Alzheimer’s dementia and behavioural variant fronto-temporal dementia. Clin Neuropsychol 26: , 926–941. |
[25] | Hornberger M , Piguet O ((2012) ) Episodic memory in frontotemporal dementia: A critical review. Brain 135: , 678–692. |
[26] | Perri R , Fadda L , Caltagirone C , Carlesimo GA ((2013) ) Word list and story recall elicit different patterns of memory deficit in patients with Alzheimer’s disease, frontotemporal dementia, subcortical ischemic vascular disease, and Lewy body dementia. J Alzheimers Dis 37: , 99–107. |
[27] | Wicklund AH , Johnson N , Rademaker A , Weitner BB , Weintraub S ((2006) ) Word list versus story memory in Alzheimer disease and frontotemporal dementia. Alzheimer Dis Assoc Disord 20: , 86–92. |




