Comparison of the Six Item Cognitive Impairment Test (6CIT) to Commonly-Used Short Cognitive Screening Instruments in a Memory Clinic Population
Abstract
Background:
Short cognitive screening instruments (CSI) are required to identify cognitive impairment in busy outpatient clinics. While the Six Item Cognitive Impairment Test (6CIT) is commonly used, its accuracy in those with mild cognitive impairment (MCI) and subjective cognitive decline (SCD) and against more widely-used CSIs is less well established.
Objective:
To examine the diagnostic accuracy of the 6CIT against the Montreal Cognitive Assessment (MoCA) and Quick Mild Cognitive Impairment (Qmci) screen across the cognitive spectrum in a memory clinic population.
Methods:
In total, 142 paired assessments were available (21 with SCD, 32 MCI, and 89 with dementia). Consecutive patients underwent a comprehensive assessment and were screened using the 6CIT, Qmci, and MoCA. Accuracy was determined from the area under receiver operating characteristic curves (AUC).
Results:
The median age of patients was 76 (±11) years; 68% were female. The median 6CIT score was 10/28 (±14). The 6CIT was strongly, negatively, and statistically significantly correlated with the Qmci (r = –0.84) and MoCA (r = –0.86). The 6CIT had good accuracy for separating cognitive impairment (MCI or dementia) from SCD, (AUC:0.88; 0.82–0.94), similar to the MoCA (AUC:0.92; 0.87–0.97, p = 0.308), but statistically lower than the Qmci (AUC:0.96; 0.94–0.99, p = 0.01). The 6CIT was faster to administer, median time 2.05 minutes versus 4.38 and 9.5 for the Qmci and MoCA, respectively.
Conclusion:
While the Qmci was more accurate than the 6CIT, the shorter administration time of the 6CIT, suggests it may be useful when assessing or monitoring cognitive impairment in busy memory clinics, though larger samples are required to evaluate.
INTRODUCTION
The prevalence of cognitive impairment (CI) including dementia is expected to increase worldwide to over 150 million people by 2050 [1], generating additional workload in already busy primary and secondary care (outpatient) clinics, as systems work towards better and earlier diagnosis [2]. Guidelines and recommendations support the need to provide diagnostic services close to patients in the community, but several structural barriers exist aggravated by staff shortages and inadequate training [3]. Screening of symptomatic patients is further limited by a lack of suitably sensitive and specific instruments [4–6]. While a wide variety of performance-based instruments are available, they vary in their administration times, cognitive domains assessed and ability to differentiate between normal cognition, mild cognitive impairment (MCI), and dementia [7], though it is suggested that no single instrument will likely suit all situations [7]. These are also influenced by the well-documented time-accuracy trade off such that short screens tend to lower levels of diagnostic accuracy [8].
The Six-Item Cognitive Impairment Test (6CIT) [9], is a commonly-used short cognitive screening instrument (CSI) and is reported to have high diagnostic accuracy for dementia in an older hospital population (90% sensitivity and 96% specificity at a cut-off of ≥8/28 for dementia) [10]. There are, however, relatively few studies examining its performance in outpatient (secondary care) settings [11] and especially in persons with MCI, before the onset of functional decline and in those with subjective cognitive decline (SCD), where individuals are symptomatic but do not have evidence of cognitive impairment on testing. A recent validation in a memory clinic population showed a sensitivity of 88% for dementia and 66% for MCI [11] when compared to the Mini-Mental State Examination (MMSE). Further, few studies have compared the 6CIT to other short screens and none to those useful in identifying early cognitive decline [12]. A recent systematic review in 2018 suggests that additional robust validation studies are needed, comparing the 6CIT to a wide range of short cognitive assessments [12].
Given these points, this study aims to compare the diagnostic accuracy for cognitive impairment of the 6CIT against two other short CSIs, the Quick Mild Cognitive Impairment (Qmci) screen [13–16], and Montreal Cognitive Assessment (MoCA) [17].
METHODS
Participants
This cross-sectional study was conducted parallel to a study evaluating the Qmci screen in an Irish memory clinic population and the methods have been reported elsewhere [16]. In summary, patients with cognitive symptoms referred to a university hospital geriatric medicine memory clinic in Ireland between January 2013 and December 2014 were included. Alzheimer’s type dementia and vascular dementia were classified using the DSM-R (4th-edition) [18]. Severity was correlated with the Reisberg Functional Assessment Staging (FAST scale) [19]. Amnestic type MCI was diagnosed using Petersen’s criteria according to the National Institute on Aging-Alzheimer’s Association workgroup diagnostic guidelines [20]. SCD was defined as subjective non-progressive memory loss in patients without objective cognitive deficits or functional decline, scoring ‘poor’ or ‘fair’ on a five-point Likert scale in response to the question “how is your memory?” [21]. In this sub-analysis, those with active depression (n = 23), delirium (n = 2), aged <45 years (n = 22), declining consent (n = 3), with an unclear diagnosis (n = 21) and unable to communicate in English (n = 2) were excluded. Depression was excluded clinically and screened with the GDS short-form [22] using a cut-off of ≥7 to optimize specificity [23].
Measures
Six-Item Cognitive Impairment Test
The 6CIT is a short (usually administration time between 2–3 minutes), CSI including seven questions covering three domains (orientation, episodic memory, and attention) [9]. The 6CIT is scored out of 28 points with higher scores suggesting cognitive impairment. Initially designed to detect dementia using a cut-off of ≥8/28, it is also validated in MCI [11] though sensitivity (66%) and specificity (70%) are low. A cut-off of ≥8/28 for dementia was used for the 6CIT [10] but more recently a cut-off of 9 or 10 is suggested based on more recent studies [12]. It does not have established cut-off scores for cognitive impairment.
Quick Mild Cognitive Impairment screen
The Qmci screen is a brief and effective test for detecting MCI. The Qmci screen has six subtests: Orientation, five registration items, a clock drawing test, a recall section, a test of verbal fluency and a logical memory test [13, 14]. It takes 3–5 minutes to complete [14]. The optimal Qmci screen cut-off score for cognitive impairment (MCI or dementia), based upon cut-off analysis is <62/100 [15, 16]. Unlike the 6CIT, lower Qmci screen scores suggest more impaired cognition.
Montreal Cognitive Assessment
The MoCA is a short CSI with seven subtests covering five cognitive domains including visuospatial, attention, processing speed, language, memory, and cognitive control scored out of 30 points with lower points indicating cognitive impairment. For screening in primary care, where high sensitivity is required, the established MoCA threshold of <26/30 is suggested [17]. Again, lower scores suggest more impaired cognition.
Data collection
Consecutive referrals underwent a comprehensive work-up for memory loss including history, physical examination, laboratory testing and neuroimaging. A short neuropsychological battery including the standardized MMSE (SMMSE) [24, 25] and two informant-rated assessments, the AD8 questionnaire [26] and IQCODE Short Form [27] were conducted by a consultant geriatrician, blind to the results of the CSIs, to inform the diagnosis. Cognitive screening was performed in a random counterbalanced order, approximately one hour before consultant review, by two independent trained raters, blind to each other and the final diagnosis. Alternate validated versions of verbal fluency (semantic for categories, e.g., animals) and logical memory (immediate verbal recall of a short story) were used for the Qmci screen and MoCA to reduce learning effects [28]. All groups underwent the same comprehensive review. The study adhered to the tenets of the Declaration of Helsinki. Ethics approval was obtained from the Clinical Research Ethics Committee of the Cork Teaching Hospitals (reference number: ECM 4 (aa) 03/04/12) and subjects provided informed consent. Assent was obtained from individuals who were felt to lack capacity.
Analysis
Data were analyzed using R version 3.5.0 (2018-04-23) -“Joy in Playing” (R Core Team, 2018) and SPSS V24 (Chicago, IL, USA). The Shapiro– Wilk test was used to test normality and found that the majority of data were non-parametric. These were compared using the Mann– Whitney U test. The Kruskal-Wallis test was used for comparisons between more than two groups. The Chi-squared test or Fishers exact test (applied where expected values were <5) was used to compare frequencies from contingency tables for categorical variables (e.g., sex). Spearman’s correlation coefficient (r) tested agreement between instruments. Diagnostic accuracy was assessed from the area under receiver operating characteristic (ROC) curves (AUC), compared using the DeLong method [29]. Covariate adjusted ROC curves were generated to assess for the effects of age and education. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) and false positive and false negative values were calculated for all tests at optimal cut-off points as determined by Youden’s index i.e. (J = Sensitivity + Specificity –1) [30].
RESULTS
In all, results for 142 patients with complete data were available and included in this analysis. The characteristics of participants according to each of the three diagnostic categories are presented in Table 1. The median age of these was 76 years, interquartile range (IQR)±11 (Q3–Q1 = 81–70), and the majority, 68%, were female. The median number of years in education was 12±4 years. Most (i.e., 63%, n = 89) had dementia with the majority of these having mild stage disease based on the FAST classification (77%). The remainder had MCI (22.5%, n = 32) and SCD (14.5%, n = 21). While those with MCI and dementia were more likely to be female (p = 0.024), there were no statistically significant differences in median age or education levels attained between patient groups. The median SMMSE score for the sample was 25/30. The median 6CIT score for the total sample was 10±14 points with statistically significant differences between scores for those with SCD (median 2), MCI (median 5), and dementia (median 17), such that these clearly separated diagnostic categories. The 6CIT had similarly strong, negative, and statistically significant correlations with the Qmci screen score (r = –0.84) and MoCA (r = –0.86). These correlations are presented in Table 2. Median administration times were 2.05 minutes for the 6CIT, 4.38 minutes for the Qmci, and 9.5 minutes for the MoCA.
Table 1
Characteristics of symptomatic patients (n = 142) screened for cognitive impairment in a geriatric memory clinic comparing those with subjective memory decline (SCD), mild cognitive impairment (MCI) and dementia
| Characteristics | Total | SCD | MCI | Dementia | p for |
| (n = 142) | (n = 21) | (n = 32) | (n = 89) | Difference* | |
| Median±IQR | Median±IQR | Median±IQR | Median±IQR | ||
| (Q3–Q1) or % | (Q3–Q1) or % | (Q3–Q1) or % | (Q3–Q1) or % | ||
| Age (y) | 76 | 71 | 77 | 77 | 0.156 |
| (81–70 =±11) | (75–71 =±4) | (80–71 =±9) | (82–71 =±11) | ||
| Sex (Female) | 68% | 43% | 75% | 72% | 0.024 |
| Education (y) | 12 | 11 | 12 | 11 | 0.06 |
| (14–10 =±4) | (13–10 =±3) | (16–11 =±5) | (13–10 =±3) | ||
| SMMSE | 25 | 29 | 28 | 21 | <0.001 |
| (28–20 =±8) | (30–29 =±1) | (29–26 =±3) | (25–16 =±9) | ||
| 6CIT | 10 | 2 | 5 | 17 | <0.001 |
| (18–4 =±14) | (2–0 =±2) | (9–2 =±7) | (21–10 =±11) | ||
| Qmci screen | 44 | 72 | 60 | 33 | <0.001 |
| (60–27 =±33) | (73–65 =±8) | (63–52 =±11) | (42–20 =±22) | ||
| MoCA | 17 | 25 | 21 | 13 | <0.001 |
| (22–10 =±12) | (27–3 =±4) | (23–20 =±3) | (16–7 =±9) |
6CIT, Six-Item Cognitive Impairment Test; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; Q, Quartile; Qmci screen, Quick Mild Cognitive Impairment screen; SMMSE, Standardized Mini-Mental State Examination. *Comparison between those with SCD, MCI and dementia using the Kruskal-Wallis test.
Table 2
Correlations (r) between cognitive screening instruments in a geriatric memory clinic population (n = 142) with 95% confidence intervals (CI)
| Cognitive screen | 6CIT | Qmci screen | MoCA |
| 6CIT | – | –0.84* | –0.86* |
| (CI: –0.88, –0.74) | (CI: –0.90, –0.81) | ||
| Qmci screen | –0.84* | – | 0.90* |
| (CI: –0.88, –0.74) | (CI: 0.89, 0.94) | ||
| MoCA | –0.86* | 0.90* | – |
| (CI: –0.90, –0.81) | (CI: 0.89, 0.94) |
6CIT, Six-Item Cognitive Impairment Test; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; Qmci screen, Quick Mild Cognitive Impairment screen. *p≤0.001.
Table 3 provides the optimal cut-off scores according to Youden’s Index compared with previously published values along with the psychometric properties for each of the three CSIs for identifying cognitive impairment (MCI or dementia) at these points. In this sample, the optimal cut-off for the 6CIT was ≥7, which provided a sensitivity of 77% and specificity of 90% for cognitive impairment. At its established cut-off (9/10) [12], the 6CIT had lower and relatively poor sensitivity for cognitive impairment (68%), albeit with high specificity (95%). In this sample, the Qmci screen [15, 16] and MoCA [17] had excellent sensitivity at their recommended cut-offs (89% and 98%, respectively), although the MoCA had markedly lower specificity (only 43%) compared to the Qmci screen (90%). Using Youden’s Index, both the Qmci screen and MoCA had lower cut-offs than their established values, which provided reduced sensitivity but better specificity for cognitive impairment. All three instruments had a high PPV, an important characteristic of any screening test.
Table 3
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive (PLR) and negative likelihood ratio (NLR) with 95% confidence intervals (CI), for the Six-Item Cognitive Impairment Test (6CIT), Quick Mild Cognitive Impairment (Qmci) screen and Montreal Cognitive Assessment (MoCA) in their ability to identify cognitive impairment (MCI or dementia)
| Cut-off | Youden’s Index | Sensitivity | Specificity | PPV | NPV | False Positive | False Negative | PLR† | NLR† |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| 6CIT | |||||||||
| ≥6 | 0.667 | 0.81 | 0.86 | 0.97 | 0.44 | 0.03 | 0.56 | 32.7 | 1.3 |
| (0.73–0.87) | (0.63–0.96) | (0.91–0.99) | (0.29–0.60) | (0.01–0.09) | (0.40–0.71) | (10.7–99.6) | (0.92–1.78) | ||
| ≥7∧ | 0.673 | 0.77 | 0.90 | 0.98 | 0.40 | 0.02 | 0.60 | 46.5 | 1.5 |
| (0.68–0.84) | (0.68–0.98) | (0.92–0.99) | (0.27–0.56) | (0.0–0.08) | (0.44–0.73) | (11.8–183) | (1.1–2.0) | ||
| ≥8 | 0.633 | 0.73 | 0.90 | 0.98 | 0.37 | 0.02 | 0.63 | 44 | 1.7 |
| (0.64–0.80) | (0.68–0.98) | (0.91–0.99) | (0.24–0.51) | (0.0–0.09) | (0.49–0.76) | (11.2–173) | (1.3–2.3) | ||
| ≥9* | 0.630 | 0.68 | 0.95 | 0.99 | 0.34 | 0.01 | 0.67 | 82 | 1.95 |
| (0.59–0.76) | (0.74–0.99) | (0.93–0.99) | (0.22–0.47) | (0.0–0.07) | (0.53–0.78) | (11.7–575) | (1.5–2.5) | ||
| ≥10 | 0.622 | 0.67 | 0.95 | 0.99 | 0.33 | 0.01 | 0.67 | 81 | 2 |
| (0.58–0.75) | (0.74–1.00) | (0.92–1.00) | (0.22–0.47) | (0.0–0.075) | (0.53–0.78) | (11.5–568) | (1.57–2.55) | ||
| Qmci screen | |||||||||
| <62* | 0.774 | 0.89 | 0.90 | 0.98 | 0.59 | 0.02 | 0.41 | 54 | 0.68 |
| (0.82–0.94) | (0.68–0.94) | (0.93–0.99) | (0.41–0.76) | (0.0–0.07) | (0.24–0.59) | (13.7–213) | (0.43–1.1) | ||
| <61 | 0.797 | 0.88 | 0.90 | 0.98 | 0.56 | 0.02 | 0.44 | 53 | 0.79 |
| (0.80–0.93) | (0.68–0.98) | (0.93–1.0) | (0.38–0.72) | (0.0–0.07) | (0.28–0.62) | (13.4–209) | (0.52–1.2) | ||
| <60 | 0.781 | 0.86 | 0.95 | 0.99 | 0.54 | 0.01 | 0.46 | 104 | 0.85 |
| (0.78–0.91) | (0.74–0.99) | (0.94–1.0) | (0.37–0.70) | (0.0–0.06) | (0.30–0.63) | (14.8–731) | (0.57–1.26) | ||
| <59 | 0.812 | 0.84 | 0.95 | 0.99 | 0.51 | 0.01 | 0.49 | 102 | 0.95 |
| (0.76–0.90) | (0.74–0.99) | (0.94–1.0) | (0.35–0.61) | (0.0–0.06) | (0.33–0.65) | (14.5–712) | (0.66–1.4) | ||
| <58 | 0.795 | 0.83 | 0.95 | 0.99 | 0.50 | 0.01 | 0.50 | 101 | 1 |
| (0.75–0.89) | (0.74–0.99) | (0.94–1.0) | (0.34–0.66) | (0.0–0.06) | (0.34–0.66) | (14.4–710) | (0.7–1.4) | ||
| <57 | 0.787 | 0.83 | 1.0 | 1.0 | 0.50 | 0.0 | 0.50 | NA | 1 |
| (0.74–0.89) | (0.81–1.0) | (0.95–1.0) | (0.34–0.66) | (0.0–0.05) | (0.34–0.66) | (NA) | (0.7–1.4) | ||
| <56∧ | 0.826 | 0.81 | 1.0 | 1.0 | 0.48 | 0.0 | 0.52 | NA | 1.1 |
| (73–87) | (0.81–1.0) | (0.95–1.0) | (0.33–0.63) | (0.0–0.05) | (0.37–0.67) | (NA) | (0.79–1.5) | ||
| MoCA | |||||||||
| <26* | 0.269 | 0.98 | 0.43 | 0.91 | 0.75 | 0.09 | 0.25 | 9.83 | 0.33 |
| (92–99) | (0.23–0.66) | (0.84–0.95) | (0.43–0.93) | (0.05–0.16) | (0.07–0.57) | (5.7–16.9) | (0.12–0.94) | ||
| <25 | 0.404 | 0.95 | 0.57 | 0.93 | 0.67 | 0.07 | 0.33 | 12.8 | 0.5 |
| (89–98) | (0.34–0.77) | (0.86–0.96) | (0.41–0.87) | (0.04–0.14) | (0.14–0.59) | (6.8–24) | (0.25–1) | ||
| <24 | 0.522 | 0.93 | 0.57 | 0.93 | 0.57 | 0.07 | 0.43 | 12.4 | 0.75 |
| (0.86–0.96) | (0.35–0.77) | (0.86–0.96) | (0.34–0.77) | (0.04–0.14) | (0.23–0.66) | (6.6–23.4) | (0.43–1.32) | ||
| <23 | 0.497 | 0.89 | 0.76 | 0.96 | 0.55 | 0.04 | 0.49 | 21.6 | 0.81 |
| (0.82–0.94) | (0.52–0.91) | (0.89–0.98) | (0.36–73) | (0.02–0.11) | (0.27–0.64) | (9.2–51) | (0.51–1.3) | ||
| <22 | 0.654 | 0.84 | 0.86 | 0.97 | 0.49 | 0.03 | 0.51 | 34 | 1.1 |
| (0.76–0.90) | (0.63–0.96) | (0.91–0.99) | (0.32–0.65) | (0.01–0.09) | (0.35–0.68) | (11.1–104) | (0.73–1.5) | ||
| <21∧ | 0.700 | 0.79 | 0.86 | 0.97 | 0.41 | 0.03 | 0.59 | 31.7 | 1.4 |
| (0.70–0.85) | (0.63–0.96) | (0.91–0.99) | (0.27–0.57) | (0.01–0.09) | (0.43–0.73) | (10.4–96.5) | (1.1–2.0) |
*Recommended cut-off score; ∧Optimal cut-off based on Youden’s Index; †Weighted for Prevalence (unweighted likelihood ratios are presented in the Supplementary Material); NA, not available.
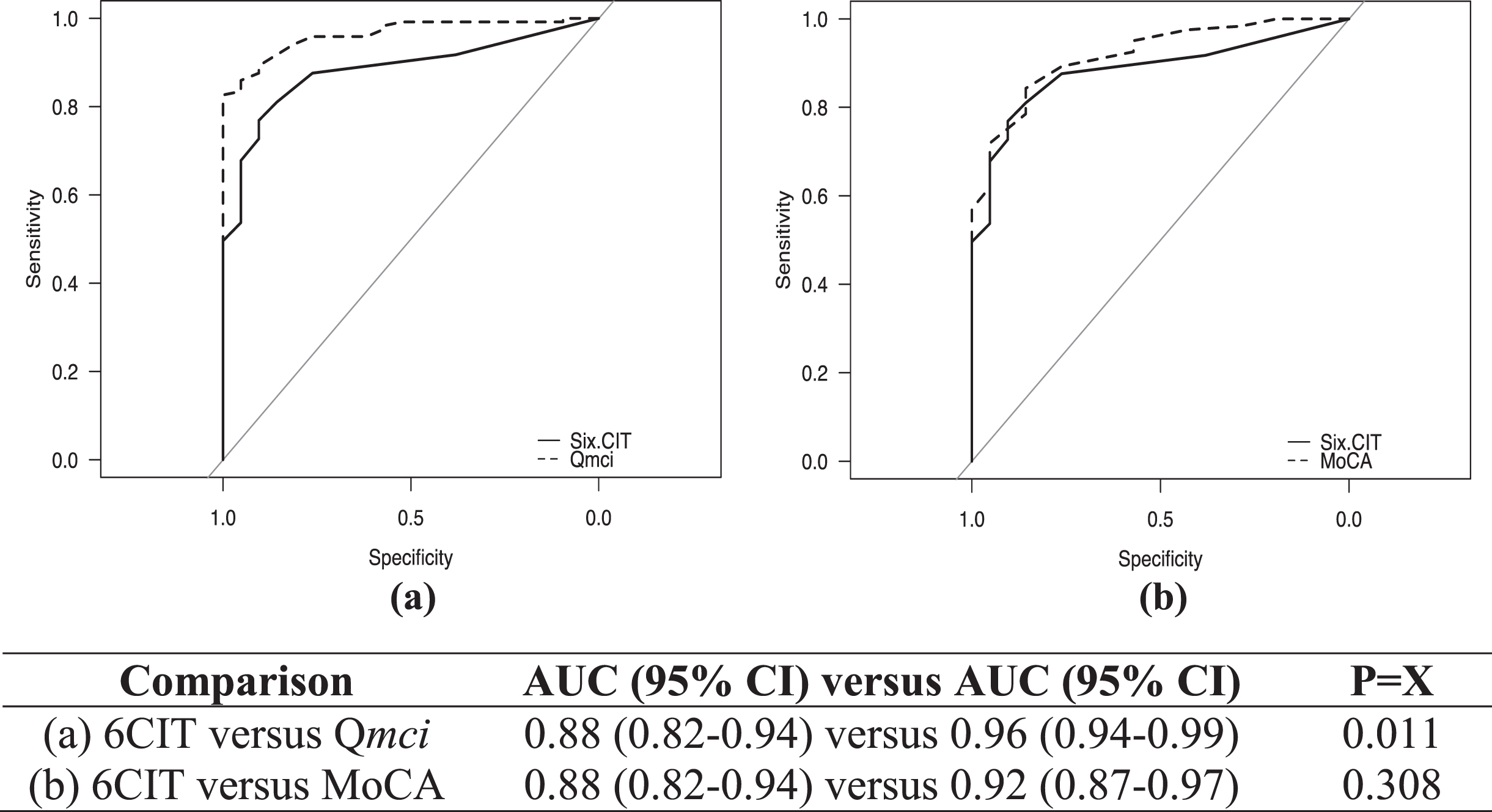
ROC curves showing the diagnostic accuracy of the CSIs for cognitive impairment are presented in Fig. 1, comparing the 6CIT to both the Qmci screen and MoCA. The 6CIT had good accuracy (AUC 0.88), although this was lower both the Qmci screen (AUC 0.96) and MoCA (AUC 0.92). This difference was not significant for the comparison to the MoCA (p = 0.308), but the Qmci screen was statistically significantly more accurate than the 6CIT (p = 0.011). There were no statistically significant differences between the Qmci screen and MoCA (p = 0.07). Adjusting the ROC curves for the effects of age and education showed no statistically significant change for the 6CIT (covariate-adjusted ROC curve: 0.878, 95% CI: 0.81–0.93, p = 0.43), Qmci screen (0.944, 95% CI: 0.90–0.97, p = 0.23) or MoCA, albeit the latter was reduced (0.894, 95% CI: 0.83, 0.94, p = 0.15). Comparing the ability of the CSIs to differentiate MCI from dementia, the Qmci screen (AUC: 0.97, p = 0.002) but not the MoCA (AUC: 0.92, p = 0.08) was statistically significantly more accurate than the 6CIT (AUC: 0.87). For separating SCD from MCI, the accuracy based on the AUC was lower for all instruments but there was again no significant difference between the 6CIT (AUC: 0.71) and the MoCA (AUC: 0.76), p = 0.56. While the Qmci screen was significantly more accurate (AUC: 0.87), it was of borderline significance (p = 0.049).
Fig. 1
Receiver operating characteristic curves with area under the curve (AUC) scores with 95% confidence intervals (CI), showing the accuracy of the Six-Item Cognitive Impairment Test (6CIT) compared with the (a) Quick Mild Cognitive Impairment (Qmci) screen and (b) Montreal Cognitive Assessment (MoCA) in their ability to identify cognitive impairment (MCI or dementia).
DISCUSSION
This study provides a psychometric evaluation of the 6CIT, validating it against two commonly-used short CSIs in a geriatric memory clinic population, where it showed strong and statistically significant correlation to two “MCI-specific” short CSI, the Qmci screen, and MoCA [16]. This is, to our knowledge, the first study to examine the 6CIT in this setting and shows that it has good diagnostic accuracy for separating those with cognitive impairment (MCI or dementia) compared to those with SCD. While it was less accurate than the Qmci screen in separating those with MCI from dementia, it was similar to the MoCA and all three instruments had similar (fair to good accuracy) for differentiating SCD from MCI. However, the Qmci screen was statistically significantly more accurate than the 6CIT in distinguishing cognitive impairment, irrespective of the comparison.
The accuracy of all three instruments remained after adjustment for the effects of age and education. The difference in diagnostic accuracy between the Qmci screen and the 6CIT, albeit clinically small, may be related to the design of the instruments and where they were originally intended for use. Designed for primary care, the 6CIT is more weighted towards verbal skills and lacks tests of higher cognitive function compared to the Qmci, designed for secondary care, which includes more cognitive domains. The 6CIT however, had a markedly shorter administration time than both the Qmci screen and MoCA, suggesting it may be useful as a short screen when assessing patients in busy clinical settings such as an emergency department (ED) [12, 31], particularly where there is a high index of suspicion for dementia or where monitoring progression of dementia over time is important. In this sense, it may be more useful as a quick screen to confirm dementia, particularly in settings such as hospitals [12].
In this study, the optimal cut-off for the 6CIT was ≥7/28, yielding a good sensitivity of 77% and excellent specificity of 90% for cognitive impairment. This is lower than its more established cut-off of ≥8 [10] and the more recently-recommended cut-off of 9 or 10 out of 28 points [12]. The 6CIT, however, had low and relatively poor sensitivity for cognitive impairment at 9/10 (67–68%), albeit with high specificity (95%). In this sample, the MoCA and Qmci screen both had excellent sensitivity at their recommended cut-offs (89% and 98%, respectively), although as seen in other studies [32], the MoCA had markedly lower specificity at a cut-off of <26 for cognitive impairment (only 43% versus 90% for the Qmci screen). Applying Youden’s Index, both had lower cut-offs than have been published before, likely reflecting the small sample size. In this study, all three CSI had a high PPV, an important characteristic of any screening test [33].
This study has a number of limitations. The prevalence of cognitive impairment was high in this sample with only 21 patients with SCD and 32 with MCI being available for analysis, potentially resulting in in spectrum bias. Further, the sample size was small and likely underpowered the study to show superiority of one CSI over another. This study was conducted at a single center, with a homogenous population, potentially reducing the generalizability of the findings. While alternative versions of the MoCA can reduce learning effects, these may differ in their severity level, potentially influencing the accuracy of scores [34]. Further study with a larger sample size is now required to examine the diagnostic performance of the 6CIT in MCI and particularly in differentiating those with MCI and SCD.
In conclusion, while the 6CIT was statistically significantly less accurate for detecting cognitive impairment (either MCI or dementia) and in separating MCI from dementia and MCI from SCD, when compared with the Qmci screen, its diagnostic accuracy was nevertheless good. The 6CIT was also statistically similar to the more widely-used MoCA. The short administration time of the 6CIT, however, suggests that it may be useful when assessing cognitive impairment in busy clinical settings including memory clinics and especially when monitoring change in cognition over time. Further studies with larger samples is now required to confirm the findings of this study. In the same sense, the 6CIT may be particularly useful in clinical settings where time is more limited such as in EDs, acute medical assessment units, hospital wards or in primary care and therefore, studies should also be conducted examining its performance compared with CSIs such as the MoCA and Qmci screen in these settings. In summary, although the sample size was small, this study suggests that the 6CIT is useful in identifying cognitive impairment in an outpatient (geriatric) memory clinic setting and may be particularly useful as a quick screen to confirm dementia.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
Dr. Rónán O’Caoimh and Prof. D. William Molloy are co-copyright holders of the Quick Mild Cognitive Impairment screen.
The authors report no other conflict of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-220117.
REFERENCES
[1] | GBD 2019 Dementia Forecasting Collaborators ((2022) ) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7: , e105–e125. |
[2] | Revez A , Timmons S , Fox S , Murphy A , O’Shea E ((2018) ) Dementia Diagnostic Services for Ireland: A literature review. Tullamore: National Dementia Office. Available at:https://dementiapathways.ie/_filecache/8a6/ba2/1116-dementia-diagnostic-services-for-ireland-literature-review-2018-.pdf, Last accessed December 29, 2022. |
[3] | Bernstein Sideman A , Al-Rousan T , Tsoy E , Piña Escudero SD , Pintado-Caipa M , Kanjanapong S , Mbakile-Mahlanza L , Okada de Oliveira M , De la Cruz-Puebla M , Zygouris S , Ashour Mohamed A , Ibrahim H , Goode CA , Miller BL , Valcour V , Possin KL ((2022) ) Facilitators and barriers to dementia assessment and diagnosis: Perspectives from dementia experts within a global health context. Front Neurol 13: , 769360. |
[4] | Lin JS , O’Connor E , Rossom RC , Perdue LA , Eckstrom E ((2013) ) Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 159: , 601–612. |
[5] | Canadian Task Force on Preventive Health Care , Pottie K , Rahal R , Jaramillo A , Birtwhistle R , Thombs BD , Singh H , Gorber SC , Dunfield L , Shane A , Bacchus M , Bell N , Tonelli M ((2016) ) Recommendations on screening for cognitive impairment in older adults. CMAJ 188: , 37–46. |
[6] | US Preventive Services Task Force , Owens DK , Davidson KW , Krist AH , Barry MJ , Cabana M , Caughey AB , Doubeni CA , Epling JW Jr , Kubik M , Landefeld CS , Mangione CM , Pbert L , Silverstein M , Simon MA , Tseng CW , Wong JB ((2020) ) Screening for cognitive impairment in older adults: US Preventive Services Task Force Recommendation Statement. JAMA 323: , 757–763. |
[7] | Cullen B , O’Neill B , Evans JJ , Coen RF , Lawlor BA ((2007) ) A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry 78: , 790–799. |
[8] | Larner AJ ((2015) ) Performance-based cognitive screening instruments: An extended analysis of the time versus accuracy trade-off. Diagnostics 5: , 504–512. |
[9] | Brooke P , Bullock R ((1999) ) Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry 14: , 936–940. |
[10] | Tuijl JP , Scholte EM , de Craen AJ , van der Mast RC ((2012) ) Screening for cognitive impairment in older general hospital patients: Comparison of the Six-Item Cognitive Impairment Test with the Mini-Mental State Examination. Int J Geriatr Psychiatry 27: , 755–762. |
[11] | Abdel-Aziz K , Larner AJ ((2015) ) Six-item Cognitive Impairment Test (6CIT): Pragmatic diagnostic accuracy study for dementia and MCI. Int Psychogeriatr 27: , 991–997. |
[12] | O’Sullivan D , O‘Regan N , Timmons S ((2016) ) Validity and reliability of the 6-Item Cognitive Impairment Test for screening cognitive impairment: A review. Dementia Geriatr Cogn Disord 42: , 42–49. |
[13] | O’Caoimh R , Gao Y , McGlade C , Healy L , Gallagher P , Timmons S , Molloy DW ((2012) ) Comparison of the Quick Mild Cognitive Impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 41: , 624–629. |
[14] | O’Caoimh R , Gao Y , Gallagher P , Eustace J , McGlade C , Molloy DW ((2013) ) Which part of the Quick mild cognitive impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing 42: , 324–330. |
[15] | O’Caoimh R , Gao Y , Svendovski A , Gallagher P , Eustace J , Molloy DW ((2017) ) Comparing approaches to optimize cut-off scores for short cognitive screening instruments in mild cognitive impairment and dementia. J Alzheimers Dis 57: , 123–133. |
[16] | O’Caoimh R , Timmons S , Molloy DW ((2016) ) Screening for mild cognitiveimpairment: Comparison of “MCI specific” screening instruments. J Alzheimers Dis 51: , 619–629. |
[17] | Nasreddine ZS , Phillips NA , Bedirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[18] | American Psychiatric Association ((1994) ) Diagnostic and Statistical Manual of Mental Disorders, 4th edition. American Psychiatric Association, Washington, DC. |
[19] | Reisberg B ((1988) ) Functional Assessment Staging (FAST). Psychopharmacol Bull 24: , 653–659. |
[20] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[21] | Paradise MB , Glozier NS , Naismith SL , Davenport TA , Hickie IB ((2011) ) Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: A cross-sectional study. BMC Psychiatr 11: , 108. |
[22] | Yesavage JA ((1988) ) Geriatric depression scale. Psychopharmacol Bull 24: , 709–711. |
[23] | Marc LG , Raue PJ , Bruce ML ((2008) ) Screening performance of the Geriatric Depression Scale (GDS-15) in a diverse elderly home care population. Am J Geriatr Psychiatry 16: , 914–921. |
[24] | Molloy DW , Alemayehu E , Roberts R ((1991) ) Reliability of a standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry 148: , 102–105. |
[25] | Molloy DW , Standish TIM ((1997) ) A guide to the standardized mini-mental state examination. Int Psychogeriatr 9: , 87–94. |
[26] | Galvin JE , Rose CM , Powlishta KK , Coats MA , Muich SJ , Grant E , Miller JP , Storandt M , Morris JC ((2005) ) The AD8. A brief informant interview to detect dementia. Neurology 65: , 559–564. |
[27] | Jorm AF ((1994) ) A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychol Med 24: , 145–153. |
[28] | Cunje A , Molloy DW , Standish TI , Lewis DL ((2007) ) Alternative forms of logical memory and verbal fluency tasks for repeated testing in early cognitive changes. Int Psychogeriatr 19: , 65–65. |
[29] | DeLong ER , DeLong DM , Clarke-Pearson DL ((1988) ) Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: , 837–845. |
[30] | Youden WJ ((1950) ) Index for rating diagnostic tests. Cancer 3: , 32–35. |
[31] | O’Sullivan D , Brady N , Manning E , O’Shea E , O’Grady S , O‘Regan N , Timmons S ((2018) ) Validation of the 6-Item Cognitive Impairment Test and the 4AT test for combined delirium and dementia screening in older emergency department attendees. Age Ageing 47: , 61–68. |
[32] | Thomann AE , Berres M , Goettel N , Steiner LA , Monsch AU ((2020) ) Enhanced diagnostic accuracy for neurocognitive disorders: A revised cut-off approach for the Montreal Cognitive Assessment. Alzheimers Res Ther 12: , 39. |
[33] | Zimmerman M ((2022) ) Positive predictive value: A clinician’s guide to avoid misinterpreting the results of screening tests. J Clin Psychiatry 83: , 22com14513. |
[34] | Lebedeva E , Huang M , Koski L ((2016) ) Comparison of alternate and original items on the Montreal Cognitive Assessment. Can Geriatr J 19: , 15–18. |




