The Effects of SARS-CoV-2 Infection on the Cognitive Functioning of Patients with Pre-Existing Dementia
Abstract
Background:
Cognitive postscripts of COVID-19, codenamed as ‘cognitive COVID’ or ‘brain fog,’ characterized by multidomain cognitive impairments, are now being reckoned as the most devastating sequelae of COVID-19. However, the impact on the already demented brain has not been studied.
Objective:
We aimed to assess the cognitive functioning and neuroimaging following SARS-CoV-2 infection in patients with pre-existing dementia.
Methods:
Fourteen COVID-19 survivors with pre-existing dementia (four with Alzheimer’s disease, five with vascular dementia, three with Parkinson’s disease dementia, and two with the behavioral variant of frontotemporal dementia) were recruited. All these patients had detailed cognitive and neuroimaging evaluations within three months before suffering from COVID-19 and one year later.
Results:
Of the 14 patients, ten required hospitalization. All developed or increased white matter hyperintensities that mimicked multiple sclerosis and small vessel disease. There was a significant increase in fatigue (p = 0.001) and depression (p = 0.016) scores following COVID-19. The mean Frontal Assessment Battery (p < 0.001) and Addenbrooke’s Cognitive Examination (p = 0.001) scores also significantly worsened.
Conclusion:
The rapid progression of dementia, the addition of further impairments/deterioration of cognitive abilities, and the increase or new appearance of white matter lesion burden suggest that previously compromised brains have little defense to withstand a new insult (i.e., ‘second hit’ like infection/dysregulated immune response, and inflammation). ‘Brain fog’ is an ambiguous terminology without specific attribution to the spectrum of post-COVID-19 cognitive sequelae. We propose a new codename, i.e. ‘FADE-IN MEMORY’ (i.e., Fatigue, decreased Fluency, Attention deficit, Depression, Executive dysfunction, slowed INformation processing speed, and subcortical MEMORY impairment).
INTRODUCTION
Cognitive problems after COVID-19, codenamed as ‘cognitive COVID’ or ‘brain fog’, characterized by multidomain cognitive impairments, are now being reckoned as the most devastating sequelae of this disease [1–4]. Studies so far showed decreased attention and concentration, executive dysfunction, memory impairment, and delay in information processing speed dominate this clinical scenario [5–7]. Most cognitive post-COVID-19 studies have been performed on previously healthy individuals without any cognitive impairment prior to the COVID-19 infection [8–14]. Senescence, ‘long COVID’, prolonged hospital stay, need for ventilator support, high-flow oxygen therapy, and cytokine storm have been considered potential risk factors in isolation or combination for post-COVID-19 cognitive impairment across different studies [15–26]. Multifaceted plausible biological mechanisms underlying these cognitive symptoms have been proposed, i.e. immune dysregulation, ongoing systemic inflammation stemming from autoimmunity, direct viral invasion through the disrupted blood-brain barrier, and cerebral micro-hemorrhages [15–24]. Social stigma, isolation, loneliness, fear, panic, and inactivity contribute to post-COVID-19 cognitive impairment [4, 17, 27–31].
Several studies unraveled fatigue and cognitive impairment as the two most important post-COVID-19 neurological aftermath [18–21, 27, 28, 32]. Studies so far are consistent with white matter intensity changes in patients with post-COVID-19 cognitive impairment [33–37]. Numerous proposals are in the pipeline; however, the primary mechanism(s) of post-COVID-19 fatigue is/are still enigmatic. Researchers have mostly pointed toward cytokine and endocrine influences without much objective evidence [15–32]. Depression, albeit a primary psychiatric disease, is a post-COVID-19 burden associated with cognitive impairment, forcing researchers to rethink possible common neurobiological basis intertwining depression and post-COVID-19 cognitive impairment [15–32].
Post-COVID-19 rapid deterioration of cognitive abilities has been observed in previously cognitively intact people [8–14]. However, the impact on the already demented brain has not been studied. We aimed 1) to assess the cognitive functioning and neuroimaging following SARS-CoV-2 infection in patients with pre-existing dementia; 2) to search for underlying risk factors, pathophysiological basis, and possible central nervous system localization-related neurobiological association; and 3) to search for the missing thread unifying post-COVID-19 depression and fatigue with ensuing cognitive impairment.
MATERIALS AND METHODS
Out of a total of 550 patients with dementia who attended the wards of the Burdwan Medical College and Hospital (Neurology Superspecialty and Internal Medicine Wings), Bangur Institute of Neurosciences, and private cognitive specialty clinics, in West Bengal, India, between May 2013 and September 2022, we had the opportunity to recruit 14 COVID-19 survivors (nine men and five women) with a detailed neuropsychological and neuroimaging assessment within three months before suffering from COVID-19 and one year later. Four of them were previously diagnosed with Alzheimer’s disease [38, 39], five with vascular dementia (defined as dementia in conjunction with signs of focal neurological signs on clinical examination, evidence of relevant cerebrovascular disease on brain imaging, and either of onset of dementia within three months of a documented stroke or abrupt onset of cognitive impairment or step-wise/fluctuating deterioration of cognitive impairment) [40], three with Parkinson’s disease dementia [41], and two with the behavioral variant of frontotemporal dementia [42].
Cognitive functioning was performed through Addenbrooke’s Cognitive Examination III (ACE-III) [43], Frontal Assessment Battery [44], and the Trail Making Test Part B [45]. Attention, language, memory, visuospatial and fluency scores according to ACE-III [43] were taken thoroughly and analyzed to compare individual domain scores pre-COVID-19 and post-COVID-19. A gross comparative assessment of cognitive domain scores was calculated using the percentage of reduction of individual scores of each domain total score of ACE-III assessment pre- and post-COVID-19. The clinical dementia rating (CDR) scale [46] was also applied to our cohort pre and post-COVID-19. We used the fatigue severity scale [47] and two first items of the nine-item Patient Health Questionnaire [48] to measure fatigue and depression, respectively.
Brain magnetic resonance (MRI) (acquired with a Siemens Verio 1.5-T MRI scanner) was performed in all patients before COVID-19 and one year later. MRI was interpreted meticulously by three consultant radiologists with a comparison of pre and post-COVID-19 imaging findings. The Fazekas scale was used to quantify the amount of white matter T2 hyperintense lesions [49], and the Global Cortical Atrophy scale for cortical atrophy [50].
Statistical analyses
Routine descriptive statistics summarized data, namely mean and standard deviation (SD) for numerical variables that were normally distributed, the median and inter-quartile range for skewed numerical variables, and counts and percentages for categorical variables. Numerical variables were compared between the same groups at different times by paired t-test. McNemar test [51] was employed for comparisons of nominal data, and the Wilcoxon rank sum test [52] for ordinal data of the same group at different times. Analyses were two-tailed, and a statistical significance level was set at p < 0.05 for all comparisons. Statistical analyses were performed in SPSS Version 25.0 (SPSS, Inc., Chicago, IL).
RESULTS
The results are summarized in Tables 1 to 3. During COVID-19, ten of the 14 patients required hospitalization, but none had a stroke. The mean age of our cohort was 65.6±5.02 years (mean±SD). The mean age of patients with pre-existing Alzheimer’s disease, Parkinson’s disease dementia, frontotemporal dementia, and vascular dementia were 66.5±2.89 years, 68.0±6.00 years, 56.5±2.12 years, and 67.2±2.59 years respectively. There was a significant increase in fatigue (p = 0.001) and depression (p = 0.016) scores following COVID-19. The mean Frontal Assessment Battery (p < 0.001), ACE-III (p = 0.001), and CDR scale (p = 0.002) scores significantly worsened. Specifically, the worsening was seen in attention (p = 0.019), memory (p < 0.001), fluency (p = 0.006), language (p < 0.001), and visuospatial (p < 0.001) domains. Executive functions assessed by the Clock-Drawing test (p = 0.021) and the Trail-Making Test Part B (p = 0.016) also deteriorated significantly. There were a significant increase of periventricular white matter (p = 0.002) and deep white matter hyperintensities (p = 0.001), and global cortical atrophy (p = 0.025) on neuroimaging following COVID-19 infection in the follow-up period.
Table 1
Baseline demographic and clinical profile prior to COVID-19
| Whole cohort (n = 14) | Alzheimer’s disease (n = 4) | Parkinson’s disease dementia (n = 3) | Behavioral variant of frontotemporal dementia (n = 2) | Vascular dementia (n = 5) | |
| Sex (Men: Women) | 9 : 5 | 3 : 1 | 1 : 2 | 1 : 1 | 4 : 1 |
| Age (y) | 65.6±5.02 | 66.5±2.89 | 68.0±6.00 | 56.5±2.12 | 67.2±2.59 |
| Presence of vascular risk factors | 57.1% | 25.0% | 33.3% | 50.0% | 100% |
Table 2
Characteristics of COVID-19
| Whole cohort (n = 14) | Alzheimer’s disease (n = 4) | Parkinson’s disease dementia (n = 3) | Behavioral variant of frontotemporal dementia (n = 2) | Vascular dementia (n = 5) | |
| 1. Requirement of hospitalization | 71.4% | 75.0% | 100% | 50.0% | 60.0% |
| Mean duration of stay among hospitalized patients (days) | 11.7±7.01 | 6.7±2.89 | 18.0±6.25 | 8.0±0.0 | 11.7±8.33 |
| 2. Requirement of mechanical ventilation | 21.4% | 0.0% | 66.7% | 0.0% | 20.0% |
| 3. Requirement of high-flow oxygen | 50.0% | 25.0% | 100% | 50.0% | 40.0% |
| 4. Occurrence of cytokine storm | 42.9% | 25.0% | 100% | 0.0% | 40.0% |
| 5. Occurrence of stroke | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
Table 3
Cognitive profile and neuroimaging features before and following COVID-19
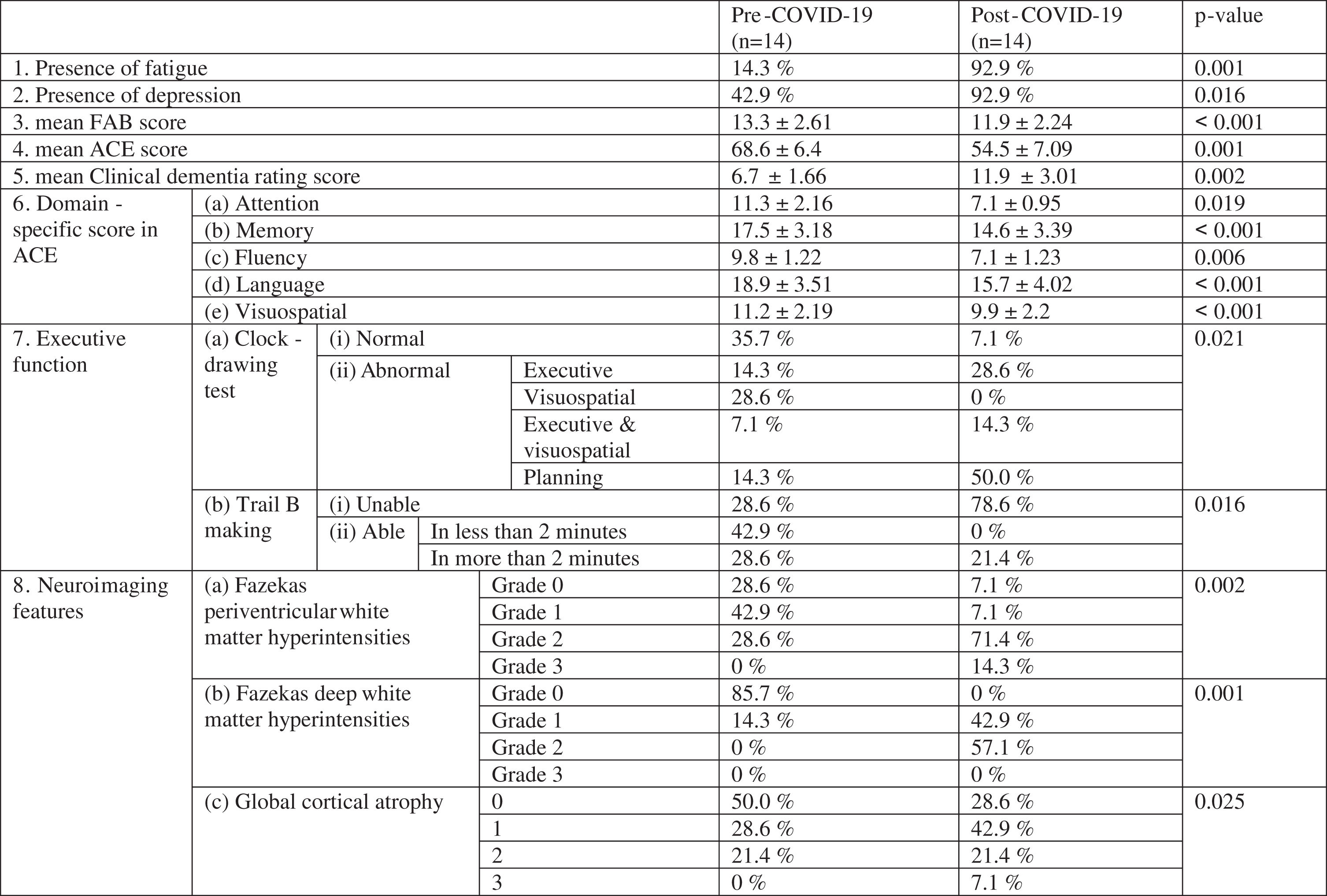 |
DISCUSSION
The most noteworthy observation was that all 14 (i.e.,100%) patients, one year after SARS-CoV-2 infection, had fatigue, depression, objective attention/concentration difficulties, executive dysfunctions, slowed information processing speed, and sub-cortical type memory impairments, irrespective of their previous cognitive status. Patients with a previous deficit in those domains scored poorer in post-COVID-19 assessment than in other domains. Fluency deteriorated significantly following COVID-19. Slowly progressive dementias like Alzheimer’s disease and vascular dementia, which usually have a fluctuating course, showed relatively unusual significant, relentless, and rapid progression in terms of deterioration of total ACE-III score at one year post-COVID-19.
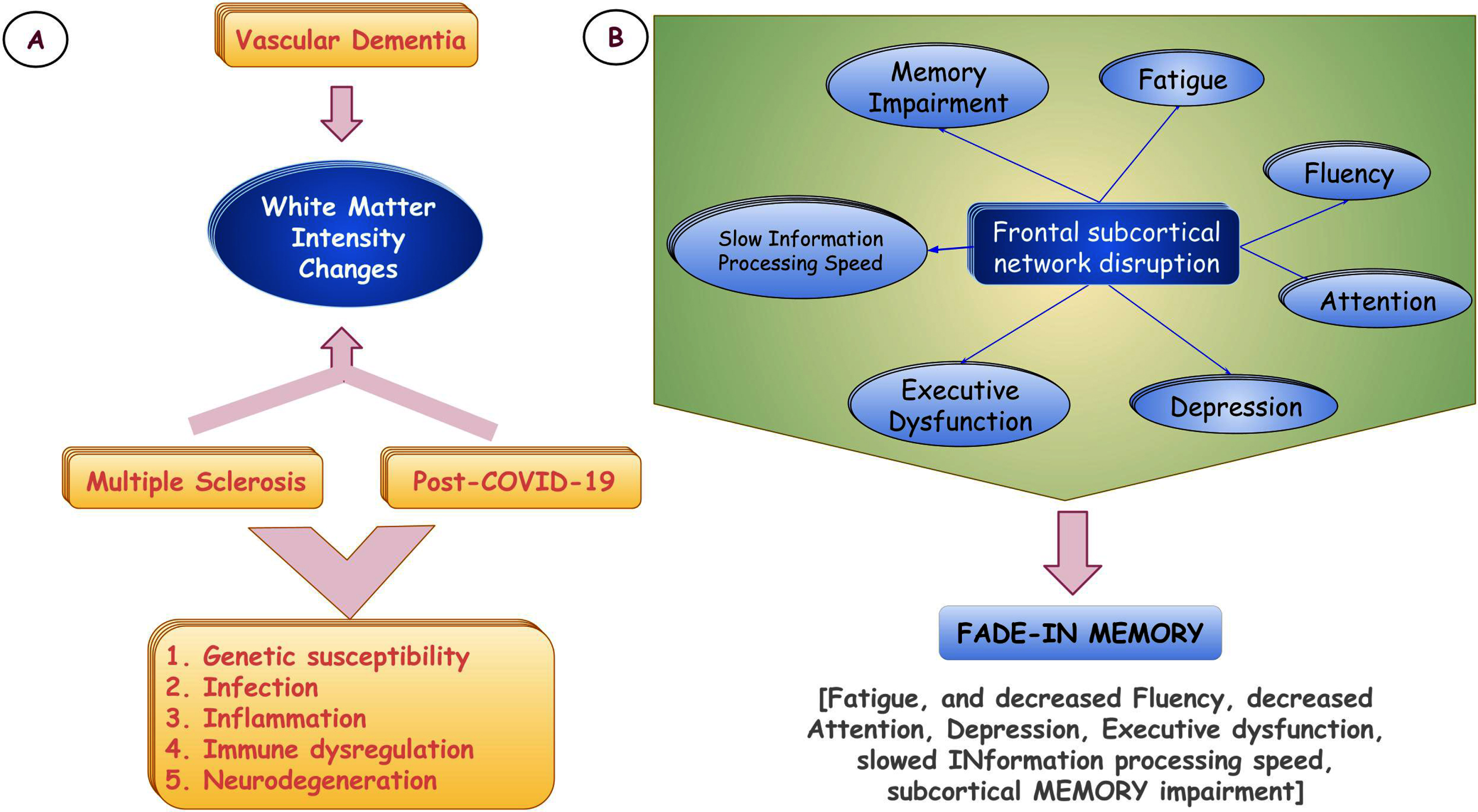
The spectrum of cognitive domain involvement followed a specific pattern, which indicates underlying disruption of frontal sub-cortical networks/connections. Fatigability/fatigue is a predominant symptom in multiple sclerosis (MS) and has been conjectured as a relatively new symptom in dementia [53, 54]. The striking presence of post-COVID-19 fatigue [21, 55–59] might indicate either shared pathomechanisms or similar lesion locations between MS and COVID-19 (Fig. 1A). The pattern of decreased attention, executive dysfunctions, delayed information procession speed, mood changes, and memory impairment retrieved with cues commonly seen in vascular dementia [60–62], which is almost similar to post-COVID-19 cognitive impairment [1–16, 63]. A similar pattern of cognitive impairments is also seen in MS patients [64–71]. Notably, cerebral lesions in MS [72–76], vascular dementia [77–80], and post-COVID-19 [5, 36, 37, 81–84] predominantly and characteristically disrupt the same frontal sub-cortical circuits. Henceforth, it might be held accountable for similar kinds of cognitive impairment.
Fig. 1
The possible common pathomechanisms linking multiple sclerosis and post-COVID-19 brain involvement (A). Proposal of a new codename regarding post-COVID-19 cognitive sequelae (B).
Similarly, we observed that all patients had white matter hyperintensities on MRI, involving periventricular deep white matter, juxta-cortical white matter, and superficial white matter mimicking lesions seen in MS and small vessel disease leading to vascular dementia [72–84]. Another striking observation has been the presence of fatigue and depression. Depression is very common in dementia and may precede, coincide or follow cognitive symptoms in all types of dementia, especially in Alzheimer’s disease and vascular dementia [85–89]. Depression is one important associate of post-COVID-19 cognitive impairment [10, 21, 90]. Disruption of frontal subcortical circuitry or involvement of the cortico-striato-thalamo-cortical loop is thought to be intricately twinning [91–94]. However, environmental factors like loss, loneliness, financial burden, and uncertainty are key players in depression and have been found across different studies [4, 17, 95, 96].
Fatigue is multifactorial. Muscle disease and organ failures are commonly considered in evaluating patients with fatigue. In the post-COVID-19 situation, the pathobiological conundrum has been extended, and the hypothalamic-pituitary-adrenal axis will draw attention in this regard [97–99]. Many studies hold responsible the indiscriminate steroid use during COVID-19 time [100] and modulation of the hypothalamic-pituitary-adrenal axis [101].
Both pathobiological and lesion location-wise, the brain model of MS has numerous similarities with the “post-COVID-19 brain” in terms of inflammation and immune dysregulation triggered by genetic susceptibility and infection, leading to white matter changes, subcortical cognitive impairments, fatigue, and neurodegeneration [102–113]. Researchers may argue against this, as vascular dementia also has similar lesion locations involving white matter tracts; however, patients with vascular dementia usually do not have unusual fatigue. The author’s view in this regard is that it is not only the white matter burden, which is responsible for fatigue, but the pathobiological process underneath that has an important role to play. Thus, inflammation in the brain and resultant white matter abnormalities and subsequent neurodegeneration have intriguing relationships underpinning fatigue that authors have proposed, which needs further translational research and substantiation. The presence of cognitive fatigue in all of our patients (which was not present before they suffered from COVID-19), irrespective of the types of dementia, further strengthened the hypothesis proposed.
Post-COVID-19 dementia research will be challenging based on the evidence in this study. There will be more chance of getting a mixed cognitive pattern in the evaluation. In our small but detailed study, all these patients with dementia (irrespective of their types) had obvious addition to pre-existing T2-weighted imaging and T2-FLAIR-weighted intensity changes, and involvement of cognitive domains changed with an added burden of frontal subcortical cognitive impairment, giving them a difficult-to-evaluate mixed picture of dementia. More importantly, it has also been observed that the so-called relatively slowly progressive course of Alzheimer’s disease and fluctuations in vascular dementia changed, and a rapid and relentlessly progressive nature emerged. White matter intensity changes (i.e., T2/T2-FLAIR burden) were seen in all patients, albeit a small number of patients in our cohort, had pre-existing conventional vascular risk factors. This has left ample room to consider factors responsible for increasing the white matter burden. Different studies have established multifactorial causation for white matter burden and intensity changes. Direct viral invasion, immune dysregulation, persistent inflammation, and coagulopathies have been considered, proposed, and studied previously without any definitive conclusion [104–111].
Interestingly, we saw that rapid progression of dementia, the addition of further impairments/deterioration of cognitive abilities (mostly subcortical type), and increase or new appearance of white matter lesion burden were common in our patients, irrespective of dementia type, the severity of COVID-19, presence of vascular risk factors, oxygen or ventilator support. This probably inculcates the notion that previously compromised brains have little defense to withstand a new insult (i.e., ‘second hit’ like infection/dysregulated immune response and inflammation), which usually heralds severe cognitive consequences. Moreover, depression, loneliness, uncertainty, loss, fear, and other psychological perspectives on the surge during the COVID-19 pandemic need further attention and be studied to assess their impact on cognitive abilities and white matter tract.
Proposal
‘Brain fog’ is an ambiguous terminology without specific attribution to the spectrum of post-COVID-19 cognitive sequelae. Based on the progression of cognitive deficits and the association with white-matter intensity changes, we propose a new codename, i.e. ‘FADE-IN MEMORY’ (i.e., Fatigue, and decreased Fluency, Attention deficit, Depression, Executive dysfunction, slowed INformation processing speed, and subcortical MEMORY impairment) (Fig. 1B). The authors presume it would be more appropriate, befitting, and scientifically apropos with specific attribution of domains involved and associated neuroimaging changes.
The intricate and complex interplay of infection (trigger), immune dysregulation and persistent inflammation, coagulopathies to microangiopathy, demyelination, axonal dropouts, and finally, neurodegeneration leading to predominant subcortical type cognitive impairment, including fatigue and depression, suggests there must have some common pathomechanisms between MS and post-COVID-19 brain involvement (Fig. 1A).
Finally, yet importantly, the authors also put “fatigue” as the single most important ‘cognitive biomarker’ in their proposal. This cognitive biomarker, i.e. cognitive fatigue, might fill the gaps in our understanding of the post-COVID-19 brain and might be a potential missing dovetail (along with depression and subcortical type of memory impairment) splicing “multiple sclerosis-brain” and “post-COVID-19-brain”.
ACKNOWLEDGMENTS
The authors have no acknowledgements to report.
FUNDING
J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD— platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Guo P , Benito Ballesteros A , Yeung SP , Liu R , Saha A , Curtis L , Kaser M , Haggard MP , Cheke LG ((2022) ) COVCOG 1: Factors predicting physical, neurological and cognitive symptoms in long COVID in a community sample. A first publication from the COVID and Cognition Study. Front Aging Neurosci 14: , 804922. |
[2] | Guo P , Benito Ballesteros A , Yeung SP , Liu R , Saha A , Curtis L , Kaser M , Haggard MP , Cheke LG ((2022) ) COVCOG 2: Cognitive and memory deficits in long COVID: A second publication from the COVID and Cognition Study. Front Aging Neurosci 14: , 804937. |
[3] | Asadi-Pooya AA , Akbari A , Emami A , Lotfi M , Rostamihosseinkhani M , Nemati H , Barzegar Z , Kabiri M , Zeraatpisheh Z , Farjoud-Kouhanjani M , Jafari A , Sasannia S , Ashrafi S , Nazeri M , Nasiri S , Shahisavandi M ((2022) ) Long COVID syndrome-associated brain fog. J Med Virol 94: , 979–984. |
[4] | Dubey S , Dubey MJ , Ghosh R , Mitchell AJ , Chatterjee S , Das S , Pandit A , Ray BK , Das G , Benito-León J ((2022) ) The cognitive basis of psychosocial impact in COVID-19 pandemic. Does it encircle the default mode network of the brain? A pragmatic proposal. Med Res Arch 10: , 10.18103/mra.v10i3.2707. |
[5] | Douaud G , Lee S , Alfaro-Almagro F , Arthofer C , Wang C , McCarthy P , Lange F , Andersson JLR , Griffanti L , Duff E , Jbabdi S , Taschler B , Keating P , Winkler AM , Collins R , Matthews PM , Allen N , Miller KL , Nichols TE , Smith SM ((2022) ) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604: , 697–707. |
[6] | Henneghan AM , Lewis KA , Gill E , Kesler SR ((2022) ) Cognitive impairment in non-critical, mild-to-moderate COVID-19 survivors. Front Psychol 13: , 770459. |
[7] | Tavares-Júnior JWL , de Souza ACC , Borges JWP , Oliveira DN , Siqueira-Neto JI , Sobreira-Neto MA , Braga-Neto P ((2022) ) COVID-19 associated cognitive impairment: A systematic review. Cortex 152: , 77–97. |
[8] | Bertuccelli M , Ciringione L , Rubega M , Bisiacchi P , Masiero S , Del Felice A ((2022) ) Cognitive impairment in people with previous COVID-19 infection: A scoping review. Cortex 154: , 212–230. |
[9] | Houben S , Bonnechère B ((2022) ) The impact of COVID-19 infection on cognitive function and the implication for rehabilitation: A systematic review and meta-analysis. Int J Environ Res Public Health 19: , 7748. |
[10] | Hampshire A , Trender W , Chamberlain SR , Jolly AE , Grant JE , Patrick F , Mazibuko N , Williams SC , Barnby JM , Hellyer P , Mehta MA ((2021) ) Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 39: , 101044. |
[11] | Liu YH , Wang YR , Wang QH , Chen Y , Chen X , Li Y , Cen Y , Xu C , Hu T , Liu XD , Yang LL , Li SJ , Liu XF , Liu CM , Zhu J , Li W , Zhang LL , Liu J , Wang YJ ((2021) ) Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener 16: , 48. |
[12] | Crivelli L , Palmer K , Calandri I , Guekht A , Beghi E , Carroll W , Frontera J , García-Azorín D , Westenberg E , Winkler AS , Mangialasche F , Allegri RF , Kivipelto M ((2022) ) Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement 18: , 1047–1066. |
[13] | Stavem K , Einvik G , Tholin B , Ghanima W , Hessen E , Lundqvist C ((2022) ) Cognitive function in non-hospitalized patients 8-13 months after acute COVID-19 infection: A cohort study in Norway. PLoS One 17: , e0273352. |
[14] | García Cena C , Costa MC , Saltarén Pazmiño R , Santos CP , Gómez-Andrés D , Benito-León J ((2022) ) Eye movement alterations in post-COVID-19 condition: A proof-of-concept study. Sensors (Basel) 22: , 1481. |
[15] | Liu YH , Chen Y , Wang QH , Wang LR , Jiang L , Yang Y , Chen X , Li Y , Cen Y , Xu C , Zhu J , Li W , Wang YR , Zhang LL , Liu J , Xu ZQ , Wang YJ ((2022) ) One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: A longitudinal cohort study. JAMA Neurol 79,: , 509–517. |
[16] | Alonso-Lana S , Marquié M , Ruiz A , Boada M ((2020) ) Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front Aging Neurosci 12: , 588872. |
[17] | Dubey S , Sengupta S , Ghosh R , Dubey MJ , Chatterjee S , Das G , Roy D , Ray BK , Benito-León J ((2021) ) COVID-19 pandemic, personality and geriatric population: Proposed pragmatism. J Patient Exp 8: , 23743735211059051. |
[18] | Proal AD , VanElzakker MB ((2021) ) Long COVID or Post-acute Sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12: , 698169. |
[19] | Perrotta F , Corbi G , Mazzeo G , Boccia M , Aronne L , D’Agnano V , Komici K , Mazzarella G , Parrella R , Bianco A ((2020) ) COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin Exp Res 32: , 1599-1608. Erratum in: Aging Clin Exp Res 32, 1909. |
[20] | Mohamed MS , Johansson A , Jonsson J , Schiöth HB ((2022) ) Dissecting the molecular mechanisms surrounding post-COVID-19 syndrome and neurological features. Int J Mol Sci 23: , 4275. |
[21] | Ceban F , Ling S , Lui LMW , Lee Y , Gill H , Teopiz KM , Rodrigues NB , Subramaniapillai M , Di Vincenzo JD , Cao B , Lin K , Mansur RB , Ho RC , Rosenblat JD , Miskowiak KW , Vinberg M , Maletic V , McIntyre RS ((2022) ) Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain Behav Immun 101: , 93–135. |
[22] | Lamontagne SJ , Winters MF , Pizzagalli DA , Olmstead MC ((2021) ) Post-acute sequelae of COVID-19: Evidence of mood & cognitive impairment. Brain Behav Immun Health 17: , 100347. |
[23] | Lechner-Scott J , Levy M , Hawkes C , Yeh A , Giovannoni G ((2021) ) Long COVID or post COVID-19 syndrome. Mult Scler Relat Disord 55: , 103268. |
[24] | Graham EL , Clark JR , Orban ZS , Lim PH , Szymanski AL , Taylor C , DiBiase RM , Jia DT , Balabanov R , Ho SU , Batra A , Liotta EM , Koralnik IJ ((2021) ) Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers. Ann Clin Transl Neurol 8: , 1073–1085. |
[25] | Miners S , Kehoe PG , Love S ((2020) ) Cognitive impact of COVID-19: Looking beyond the short term. Alzheimers Res Ther 12: , 170. |
[26] | Daroische R , Hemminghyth MS , Eilertsen TH , Breitve MH , Chwiszczuk LJ ((2021) ) Cognitive impairment after COVID-19-A review on objective test data. Front Neurol 12: , 699582. |
[27] | Dubey S , Biswas P , Ghosh R , Chatterjee S , Dubey MJ , Chatterjee S , Lahiri D , Lavie CJ ((2020) ) Psychosocial impact of COVID-19. Diabetes Metab Syndr 14: , 779–788. |
[28] | Ghosh R , Biswas P , Chatterjee S , Sengupta S , Dubey MJ , Ray BY , Dubey S ((2021) ) Love and emotions at the time of COVID-19. Minerva Psychiatry 62: , 156–163. |
[29] | Lampraki C , Hoffman A , Roquet A , Jopp DS ((2022) ) Loneliness during COVID-19: Development and influencing factors. PLoS One 17: , e0265900. |
[30] | Pietrabissa G , Simpson SG ((2020) ) Psychological consequences of social isolation during COVID-19 outbreak. Front Psychol 11: , 2201. |
[31] | Sepúlveda-Loyola W , Rodríguez-Sánchez I , Pérez-Rodríguez P , Ganz F , Torralba R , Oliveira DV , Rodríguez-Mañas L ((2020) ) Impact of social isolation due to COVID-19 on health in older people: Mental and physical effects and recommendations. J Nutr Health Aging 24: , 938–947. |
[32] | Ramakrishnan RK , Kashour T , Hamid Q , Halwani R , Tleyjeh IM ((2021) ) Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol 12: , 686029. |
[33] | Huang S , Zhou Z , Yang D , Zhao W , Zeng M , Xie X , Du Y , Jiang Y , Zhou X , Yang W , Guo H , Sun H , Liu P , Liu J , Luo H , Liu J ((2022) ) Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain 145: , 1830–1838. |
[34] | Williamson BJ , Vagal AS ((2022) ) Beyond the: White matter microstructural differences detectable in patients recovered from COVID-19 at 1-year follow-up. AJR Am J Roentgenol 219: , 685. |
[35] | Pelizzari L , Cazzoli M , Lipari S , Laganà MM , Cabinio M , Isernia S , Pirastru A , Clerici M , Baglio F ((2022) ) Mid-term MRI evaluation reveals microstructural white matter alterations in COVID-19 fully recovered subjects with anosmia presentation. Ther Adv Neurol Disord 15,: , 17562864221111995. |
[36] | Lu Y , Li X , Geng D , Mei N , Wu PY , Huang CC , Jia T , Zhao Y , Wang D , Xiao A , Yin B ((2020) ) Cerebral micro-structural changes in COVID-19 patients – An MRI-based 3-month follow-up study. EClinicalMedicine 25: , 100484. |
[37] | Khodanovich MY , Kamaeva DA , Naumova AV ((2022) ) Role of demyelination in the persistence of neurological and mental impairments after COVID-19. Int J Mol Sci 23: , 11291. |
[38] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[39] | Dubois B , Feldman HH , Jacova C , Dekosky ST , Barberger-Gateau P , Cummings J , Delacourte A , Galasko D , Gauthier S , Jicha G , Meguro K , O’brien J , Pasquier F , Robert P , Rossor M , Salloway S , Stern Y , Visser PJ , Scheltens P ((2007) ) Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol 6: , 734–746. |
[40] | Erkinjuntti T ((1994) ) Clinical criteria for vascular dementia: The NINDS-AIREN criteria. Dementia 5: , 189–192. |
[41] | Vasconcellos LF , Pereira JS ((2015) ) Parkinson’s disease dementia: Diagnostic criteria and risk factor review. J Clin Exp Neuropsychol 37: , 988–993. |
[42] | Rascovsky K , Hodges JR , Kipps CM , Johnson JK , Seeley WW , Mendez MF , Knopman D , Kertesz A , Mesulam M , Salmon DP , Galasko D , Chow TW , Decarli C , Hillis A , Josephs K , Kramer JH , Weintraub S , Grossman M , Gorno-Tempini ML , Miller BM ((2007) ) Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer Dis Assoc Disord 21: , S14–18. |
[43] | Hodges JR , Larner AJ ((2017) ) Addenbrooke’s Cognitive Examinations: ACE, ACE-R, ACE-III, ACEapp, and M-ACE. In Cognitive Screening Instruments: A Practical Approach, Second Edition, LarnerAJ, ed. Springer, Berlin, Alemania, pp. 109–137. |
[44] | Dubois B , Slachevsky A , Litvan I , Pillon B ((2000) ) The FAB: A Frontal Assessment Battery at bedside. Neurology 55: , 1621–1626. |
[45] | Correia S , Ahern DC , Rabinowitz AR , Farrer TJ , Smith Watts AK , Salloway S , Malloy PF , Deoni SC ((2015) ) Lowering the floor on Trail Making Test Part B: Psychometric evidence for a new scoring metric. Arch Clin Neuropsychol 30: , 643–656. |
[46] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[47] | Krupp LB , LaRocca NG , Muir-Nash J , Steinberg AD ((1989) ) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46: , 1121–1123. |
[48] | Maurer DM , Raymond TJ , Davis BN ((2018) ) Depression: Screening and diagnosis. Am Fam Physician 98: , 508–515. |
[49] | Andere A , Jindal G , Molino J , Collins S , Merck D , Burton T , Stretz C , Yaghi S , Sacchetti DC , Jamal SE , Reznik ME , Furie K , Cutting S ((2022) ) Volumetric white matter hyperintensity ranges correspond to Fazekas scores on brain MRI. J Stroke Cerebrovasc Dis 31: , 106333. |
[50] | Kaushik S , Vani K , Chumber S , Anand KS , Dhamija RK ((2021) ) Evaluation of MR visual rating scales in major forms of dementia. J Neurosci Rural Pract 2: , 16–23. |
[51] | Xiang JX ((2016) ) On two-sample McNemar test. J Biopharm Stat 26: , 217–226. |
[52] | Hilton JF ((1996) ) The appropriateness of the Wilcoxon test in ordinal data. Stat Med 15: , 631–645. |
[53] | Braley TJ , Chervin RD ((2010) ) Fatigue in multiple sclerosis: Mechanisms, evaluation, and treatment. Sleep 33: , 1061–1067. |
[54] | Daumas L , Manera V , Robert P , Zory R ((2021) ) Associations between apathy and fatigue in patients with neurocognitive disorders. Alzheimers Dement 17: , e057472. |
[55] | El Sayed S , Shokry D , Gomaa SM ((2021) ) Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol Rep 41: , 50–55. |
[56] | Wostyn P ((2021) ) COVID-19 and chronic fatigue syndrome: IS the worst yet to come? Med Hypotheses 146: , 110469. |
[57] | Joli J , Buck P , Zipfel S , Stengel A ((2022) ) Post-COVID-19 fatigue: A systematic review. Front Psychiatry 13: , 947973. |
[58] | Rudroff T , Fietsam AC , Deters JR , Bryant AD , Kamholz J ((2020) ) Post-COVID-19 fatigue: Potential contributing factors. Brain Sci 10: , 1012. |
[59] | Ortelli P , Ferrazzoli D , Sebastianelli L , Engl M , Romanello R , Nardone R , Bonini I , Koch G , Saltuari L , Quartarone A , Oliviero A , Kofler M , Versace V ((2021) ) Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J Neurol Sci 420: , 117271. |
[60] | Paul R , Moser D , Cohen R , Browndyke J , Zawacki T , Gordon N ((2001) ) Dementia severity and pattern of cognitive performance in vascular dementia. Appl Neuropsychol 8: , 211–217. |
[61] | Sengupta P , Ganguly J , Pal S , Ghosal M ((2019) ) Pattern of cognitive deficits in vascular dementia. Indian J Med Res 149: , 503–507. |
[62] | Bhat A , Biswas A , Das G , Lahiri D , Dubey S , Mukherjee A ((2021) ) Behavioral variations among vascular cognitive impairment subtypes - A comparative study. Appl Neuropsychol Adult. doi: 10.1080/23279095.2021.1954002. |
[63] | Vannorsdall TD , Brigham E , Fawzy A , Raju S , Gorgone A , Pletnikova A , Lyketsos CG , Parker AM , Oh ES ((2022) ) Cognitive dysfunction, psychiatric distress, and functional decline after COVID-19. J Acad Consult Liaison Psychiatry 63: , 133–143. |
[64] | De Meo E , Portaccio E , Giorgio A , Ruano L , Goretti B , Niccolai C , Patti F , Chisari CG , Gallo P , Grossi P , Ghezzi A , Roscio M , Mattioli F , Stampatori C , Simone M , Viterbo RG , Bonacchi R , Rocca MA , De Stefano N , Filippi M , Amato MP ((2021) ) Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 78: , 414–425. |
[65] | Benedict RHB , Amato MP , DeLuca J , Geurts JJG ((2020) ) Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol 19: , 860–871. |
[66] | Achiron A , Polliack M , Rao SM , Barak Y , Lavie M , Appelboim N , Harel Y ((2005) ) Cognitive patterns and progression in multiple sclerosis: Construction and validation of percentile curves. J Neurol Neurosurg Psychiatry 76: , 744–749. |
[67] | Lovera J , Kovner B ((2012) ) Cognitive impairment in multiple sclerosis. Curr Neurol Neurosci Rep 12: , 618–627. |
[68] | Kumar S , Gangopadhyay G , Biswas A , Dubey S , Pandit A , Das S , Ray BK ((2021) ) A comparison of cognitive performances between neuromyelitis optica spectrum disorder and multiple sclerosis patients in Indian context. Egypt J Neurol Psychiatry Neurosurg 57: , 97. |
[69] | Dagenais E , Rouleau I , Tremblay A , Demers M , Roger É , Jobin C , Duquette P ((2016) ) Prospective memory in multiple sclerosis: The impact of cue distinctiveness and executive functioning. Brain Cogn 109: , 66–74. |
[70] | Olazarán J , Cruz I , Benito-León J , Morales JM , Duque P , Rivera-Navarro J ((2009) ) Cognitive dysfunction in multiple sclerosis: Methods and prevalence from the GEDMA Study. Eur Neurol 61: , 87–93. |
[71] | DeLuca GC , Yates RL , Beale H , Morrow SA ((2015) ) Cognitive impairment in multiple sclerosis: Clinical, radiologic and pathologic insights. Brain Pathol 25: , 79–98. |
[72] | Arnett PA , Rao SM , Bernardin L , Grafman J , Yetkin FZ , Lobeck L ((1994) ) Relationship between frontal lobe lesions and Wisconsin Card Sorting Test performance in patients with multiple sclerosis. Neurology 44: , 420–425. |
[73] | Aldrete Cortez VR , Duriez-Sotelo E , Carrillo-Mora P , Pérez-Zuno JA ((2013) ) Correlación entre las lesiones desmielinizantes y el deterioro de las funciones ejecutivas en una muestra de pacientes Mexicanos con esclerosis múltiple [Correlation between demyelinating lesions and executive function decline in a sample of Mexican patients with multiple sclerosis]. Neurologia 28: , 394–399. |
[74] | Klawiter EC ((2013) ) Current and new directions in MRI in multiple sclerosis. Continuum (Minneap Minn) 19: , 1058–1073. |
[75] | Gaetano L , Magnusson B , Kindalova P , Tomic D , Silva D , Altermatt A , Magon S , Müller-Lenke N , Radue EW , Leppert D , Kappos L , Wuerfel J , Häring DA , Sprenger T ((2020) ) White matter lesion location correlates with disability in relapsing multiple sclerosis. Mult Scler J Exp Transl Clin 6: , 2055217320906844. |
[76] | Charil A , Zijdenbos AP , Taylor J , Boelman C , Worsley KJ , Evans AC , Dagher A ((2003) ) Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: Application to 452 patient data sets. Neuroimage 19: , 532–544. |
[77] | Duering M , Zieren N , Hervé D , Jouvent E , Reyes S , Peters N , Pachai C , Opherk C , Chabriat H , Dichgans M ((2011) ) Strategic role of frontal white matter tracts in vascular cognitive impairment: A voxel-based lesion-symptom mapping study in CADASIL. Brain 13: , 2366–2375. |
[78] | Alber J , Alladi S , Bae HJ , Barton DA , Beckett LA , Bell JM , Berman SE , Biessels GJ , Black SE , Bos I , Bowman GL , Brai E , Brickman AM , Callahan BL , Corriveau RA , Fossati S , Gottesman RF , Gustafson DR , Hachinski V , Hayden KM , Helman AM , Hughes TM , Isaacs JD , Jefferson AL , Johnson SC , Kapasi A , Kern S , Kwon JC , Kukolja J , Lee A , Lockhart SN , Murray A , Osborn KE , Power MC , Price BR , Rhodius-Meester HFM , Rondeau JA , Rosen AC , Rosene DL , Schneider JA , Scholtzova H , Shaaban CE , Silva NCBS , Snyder HM , Swardfager W , Troen AM , van Veluw SJ , Vemuri P , Wallin A , Wellington C , Wilcock DM , Xie SX , Hainsworth AH ((2019) ) White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y) 5: , 107–117. |
[79] | Hainsworth AH , Minett T , Andoh J , Forster G , Bhide I , Barrick TR , Elderfield K , Jeevahan J , Markus HS , Bridges LR ((2017) ) Neuropathology of white matter lesions, blood-brain barrier dysfunction, and dementia. Stroke 48: , 2799–2804. |
[80] | Prins ND , van Dijk EJ , den Heijer T , Vermeer SE , Koudstaal PJ , Oudkerk M , Hofman A , Breteler MM ((2004) ) Cerebral white matter lesions and the risk of dementia. Arch Neurol 61: , 1531–1534. |
[81] | Duan K , Premi E , Pilotto A , Cristillo V , Benussi A , Libri I , Giunta M , Bockholt HJ , Liu J , Campora R , Pezzini A , Gasparotti R , Magoni M , Padovani A , Calhoun VD ((2021) ) Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol Stress 14: , 100326. |
[82] | Najt P , Richards HL , Fortune DG ((2021) ) Brain imaging in patients with COVID-19: A systematic review. Brain Behav Immun Health 16: , 100290. |
[83] | Roy D , Ghosh R , Dubey S , Dubey MJ , Benito-León J , Kanti Ray B ((2021) ) Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can J Neurol Sci 48: , 9–24. |
[84] | Kim PH , Kim M , Suh CH , Chung SR , Park JE , Kim SC , Choi YJ , Lee JH , Kim HS , Baek JH , Choi CG , Kim SJ ((2021) ) Neuroimaging findings in patients with COVID-19: A systematic review and meta-analysis. Korean J Radiol 22: , 1875–1885. |
[85] | Kitching D ((2015) ) Depression in dementia. Aust Prescr 38: , 209–2011. |
[86] | Agüera-Ortiz L , García-Ramos R , Grandas Pérez FJ , López-Álvarez J , Montes Rodríguez JM , Olazarán Rodríguez FJ , Olivera Pueyo J , Pelegrin Valero C , Porta-Etessam J ((2021) ) Depression in Alzheimer’s disease: A Delphi Consensus on etiology, risk factors, and clinical management. Front Psychiatry 12: , 638651. |
[87] | Bennett S , Thomas AJ ((2014) ) Depression and dementia: Cause, consequence or coincidence? Maturitas 79: , 184–190. |
[88] | Diniz BS , Butters MA , Albert SM , Dew MA , Reynolds CF 3rd ((2013) ) Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 202: , 329–335. |
[89] | Dubey S , Dubey MJ , Ghosh R , Mukherjee D , Pandit A , Benito-León J ((2022) ) Behavioral and psychological symptoms in neurodegenerative dementias: Harbinger, follower, or constant collateral? Egypt J Neurol Psychiatr Neurosurg 58: , 102. |
[90] | Hartung TJ , Neumann C , Bahmer T , Chaplinskaya-Sobol I , Endres M , Geritz J , Haeusler KG , Heuschmann PU , Hildesheim H , Hinz A , Hopff S , Horn A , Krawczak M , Krist L , Kudelka J , Lieb W , Maetzler C , Mehnert-Theuerkauf A , Montellano FA , Morbach C , Schmidt S , Schreiber S , Steigerwald F , Störk S , Maetzler W , Finke C ((2022) ) Fatigue and cognitive impairment after COVID-19: A prospective multicentre study. EClinicalMedicine 53: , 101651. |
[91] | Newhouse A , Kritzer MD , Eryilmaz H , Praschan N , Camprodon JA , Fricchione G , Chemali Z ((2022) ) Neurocircuitry hypothesis and clinical experience in treating neuropsychiatric symptoms of postacute sequelae of severe acute respiratory syndrome coronavirus 2. J Acad Consult Liaison Psychiatry 63: , 619–627. |
[92] | Fusunyan M , Praschan N , Fricchione G , Beach S ((2021) ) Akinetic mutism and coronavirus disease 2019: A narrative review. J Acad Consult Liaison Psychiatry 62: , 625–633. |
[93] | Peters SK , Dunlop K , Downar J ((2016) ) Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Front Syst Neurosci 10: , 104. |
[94] | Li B , Mody M ((2016) ) Cortico-striato-thalamo-cortical circuitry, working memory, and obsessive-compulsive disorder. Front Psychiatry 7: , 78. |
[95] | Sujan MSH , Tasnim R , Islam MS , Ferdous MZ , Haghighathoseini A , Koly KN , Pardhan S ((2022) ) Financial hardship and mental health conditions in people with underlying health conditions during the COVID-19 pandemic in Bangladesh. Heliyon 8: , e10499. |
[96] | Dubey MJ , Ghosh R , Chatterjee S , Biswas P , Chatterjee S , Dubey S ((2020) ) COVID-19 and addiction. Diabetes Metab Syndr 14: , 817–823. |
[97] | Chatterjee S , Ghosh R , Biswas P , Dubey S , Guria RT , Sharma CB , Kalra S ((2020) ) COVID-19: The endocrine opportunity in a pandemic. Minerva Endocrinol 45: , 204–227. |
[98] | Ghosh R , Roy D , Roy D , Mandal A , Dutta A , Naga D , Benito-León J ((2021) ) A rare case of SARS-CoV-2 infection associated with pituitary apoplexy without comorbidities. J Endocr Soc 5: , bvaa203. |
[99] | Ghosh R , Ray A , Roy D , Das S , Dubey S , Benito-León J ((2022) ) Parkinsonism with akinetic mutism following osmotic demyelination syndrome in a SARS-CoV-2 infected elderly diabetic woman: A case report. Neurologia 37: , 706–708. |
[100] | Ghosh R , Roy D , Benito-León J ((2022) ) Mucormycosis in COVID-19: The Indian scenario. J Mycol Med 32: , 101275. |
[101] | Chatterjee S , Ghosh R , Vardhan B , Ojha UK , Kalra S ((2022) ) An epidemic of iatrogenic Cushing’s syndrome in anticipation in post-COVID era. J Family Med Prim Care 11: , 412–413. |
[102] | Manjaly ZM , Harrison NA , Critchley HD , Do CT , Stefanics G , Wenderoth N , Lutterotti A , Müller A , Stephan KE ((2019) ) Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry 90: , 642–651. |
[103] | Bertoli M , Tecchio F ((2020) ) Fatigue in multiple sclerosis: Does the functional or structural damage prevail? Mult Scler 26: , 1809–1815. |
[104] | Bellucci G , Rinaldi V , Buscarinu MC , Reniè R , Bigi R , Pellicciari G , Morena E , Romano C , Marrone A , Mechelli R , Salvetti M , Ristori G ((2021) ) Multiple sclerosis and SARS-CoV-2: Has the interplay started? Front Immunol 12: , 755333. |
[105] | Dziedzic A , Saluk-Bijak J , Miller E , Niemcewicz M , Bijak M ((2021) ) The impact of SARS-CoV-2 infection on the development of neurodegeneration in multiple sclerosis. Int J Mol Sci 22: , 1804. |
[106] | Ferini-Strambi L , Salsone M ((2021) ) COVID-19 and neurological disorders: Are neurodegenerative or neuroimmunological diseases more vulnerable? J Neurol 268: , 409–419. |
[107] | Lingor P , Demleitner AF , Wolff AW , Feneberg E ((2022) ) SARS-CoV-2 and neurodegenerative diseases: What we know and what we don’t. J Neural Transm (Vienna) 129: , 1155–1167. |
[108] | Li C , Liu J , Lin J , Shang H ((2022) ) COVID-19 and risk of neurodegenerative disorders: A Mendelian randomization study. Transl Psychiatry 12: , 283. |
[109] | Michelena G , Casas M , Eizaguirre MB , Pita MC , Cohen L , Alonso R , Garcea O , Silva BA ((2022) ) Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult Scler Relat Disord 57: , 103368. |
[110] | Lima M , Aloizou AM , Siokas V , Bakirtzis C , Liampas I , Tsouris Z , Bogdanos DP , Baloyannis SJ , Dardiotis E ((2022) ) Coronaviruses and their relationship with multiple sclerosis: Is the prevalence of multiple sclerosis going to increase after the Covid-19 pandemia? Rev Neurosci 33: , 703–720. |
[111] | Shalaby NM , Shehata HS ((2021) ) Could SARS-CoV-2 herald a surge of multiple sclerosis? Egypt J Neurol Psychiatr Neurosurg 57: , 22. |




