Confirmed Synergy Between the ɛ4 Allele of Apolipoprotein E and the Variant K of Butyrylcholinesterase as a Risk Factor for Alzheimer’s Disease: A Systematic Review and Meta-Analysis
Abstract
Background:
Alzheimer’s disease (AD) has several risk factors. APOE4 is the main one, and it has been suggested that there may be a synergy between it and BCHE-K as a risk factor.
Objective:
To investigate the association between APOE4 and BCHE-K as a risk factor for AD.
Methods:
We searched PubMed, Web of Science, Embase, and Scopus on August 8, 2021 for studies that analyzed the association of APOE4 and BCHE-K with AD. The random effect model was performed in meta-analysis according to age group. A chi-square was performed with the meta-analysis data to verify if the effect found is not associated only with the E4 allele.
Results:
Twenty-one studies with 6,853 subjects (3,528 AD and 3,325 Controls) were included in the meta-analysis. The quality of the evidence is moderate. There is a positive E4-K association for subjects with AD as shown by the odds ratio of 3.43. The chi-square meta test, which measures the probability that the E4-K association is due to chance, has an odds ratio of 6.155, indicating that the E4-K association is not a random event. The odds ratio of an E4-K association in subjects with AD increases to OR 4.46 for the 65- to 75-year-old group and OR 4.15 for subjects older than 75 years. The probability that the E4-K association is due to chance is ruled out by chi-square meta test values of OR 8.638 and OR 9.558.
Conclusion:
The synergy between APOE4 and BCHE-K is a risk factor for late-onset AD.
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative and irreversible disease, characterized by the presence of senile plaques with extracellular deposits of amyloid- β (Aβ) protein, called amyloid plaques and fibrillar tangles, composed of the hyperphosphorylated tau protein, causing damage to neurons [1]. Such neuronal damage results in brain atrophy, memory loss, neuromotor impairment, general brain degradation, and death [2].
There are several factors that can lead to the establishment of the clinical case of AD, being considered a multifactorial disease [3]. Among these factors, the main ones include impaired lifestyle (sedentary lifestyle, alcoholism, smoking, unhealthy diet), exposure to pesticides, depression, mild cognitive impairment, and genetic mutations [1, 3, 4].
Mutations in APP, PSEN1, PSEN2, ABCA7, and SORL1 genes have been linked to early-onset and familial AD. The mutations in genes identified so far that are related to late-onset AD include: ABCA7, BIN1, CASS4, CD33, CD2AP, CELF1, CLU, CR1, DSG2, EPHA1, FERMT2, HLA-DRB5-HLA-DRB1, INPP5D, MEF2 C, MS4A6A/MS4A4E, NME8, PICALM, PTK2B, SLC24A4, SORL1, ZCWPW1, TREM2, and APOE [5]. Among these, the gene that encodes apolipoprotein E (apoE), located on chromosome 19, has an allele (E4) that has been the main one related to increased susceptibility to this disease [5–7].
A genetic association between APOE4 and the variant K of butyrylcholinesterase (BCHE-K) has been suggested as a possible cause of increased chance of establishing AD [8]. Butyrylcholinesterase (BChE) is an enzyme synthesized from the BCHE gene and acts on the degradation of acetylcholine in the synaptic cleft, with greater prevalence in damaged regions of the brain by reaction to the accumulation of Aβ [9–11]. This association hypothesis had as its initial proposition the interaction observed between the enzyme BChE and neurofibrillary tangles, added to evidence that the K variant has less activity than its wild-type form [12–14].
Several studies have confirmed this hypothesis of association and others have not [8, 15, 16], for this reason the purpose of this study was to develop a systematic review and meta-analysis guided to answer the following question: Is there an association between APOE4 and BCHE-K with AD?
Therefore, the PICO strategy is based on people with AD as a Population and for Intervention, it was the genotyping of the BCHE-K and APOE4 alleles. For Comparison, a corresponding control group was analyzed and for Outcome, the number of events with or without association between the analyzed genes was verified.
MATERIALS AND METHODS
Information sources
This meta-analysis was performed according to the guidelines proposed by the PRISMA statement and Meta-Analysis of Observational Studies in Epidemiology [17]. The research protocol of this systematic review was registered in PROSPERO (Supplementary Material). The PubMed (MedLine), Web of Science, Embase, and Scopus databases were last consulted on August 16, 2021 by two authors of this study. No tools were used at this stage, nor any type of external assistance beyond the research group.
Search strategy
The search strategies were built manually because when they were performed using only terms registered on the platforms, such as MeSH terms, a lack of synonyms was found, specifically the lack of the term “Apolipoprotein E” in the singular. Therefore, it was decided to build search strategies manually and individually for each platform referring to its database, in order to increase research efficiency. These strategies can be found in the Supplementary Material. The central terms for the construction of the search strategies were: “Alzheimer’s Disease”, “Apolipoprotein E” and “Butyrylcholinesterase K”. Studies in languages other than English were later excluded.
Inclusion and exclusion criteria
Inclusion criteria were: 1) people with probable or definitive diagnosis of AD, 2) compared with a group of cognitively healthy elderly, 3) genotyping of the APOE and BCHE genes in both groups; 4) articles in English. For exclusion: 1) systematic reviews, abstracts, meta-analyses, repeated studies; 2) not presenting the results of the genotyping by groups and only the association between both, 3) not presenting the ages or the compared groups have equidistant ages.
Selection process
The selection process was carried out by two independent reviewers, the first and second authors of this work. The duplicate removal step was assisted by the Mendeley reference manager (Version 1.19.4) [18] and the RAYYAN QCRI tool [19] was used to assist in the reading step by title and abstract. The conflicts between the reviewers that arose at this stage were resolved by the decision to include the article for full reading. The inclusion of studies that, after the integral reading stage, still had conflicts between the reviewers, was resolved from the decision of a third reviewer, the third author of this work (the list of decisions about each study can be found in the Supplementary Material).
Data extraction and bias risk assessment
The data extraction process was performed by the two independent reviewers, conflicts regarding data from the studies in this process were resolved in a meeting with the third reviewer from the research group. The following data were extracted: First author name, year of publication, type of study, ethnicity, genotyping, outcome, sample size of the experimental and control groups, age, gender, number of people carrying the alleles studied. All data were extracted in an integral way, that is, each carrier of the allelic combinations in both groups as a whole number.
To assess the risk of bias in the studies, the ROBINS-I tool [20] was used, which assesses the pre-intervention, intervention, and post-intervention criteria. The assessment for each item ranges from low risk to risk of critical bias. In the meta-analysis of this review, only studies with low risk and moderate risk of bias were included and can be found in the Supplementary Material.
Meta-analysis, statistical analysis, and quality of evidence
The meta-analysis and funnel plot was performed using the RevMan application [21]. Statistical heterogeneity between studies was assessed by the Cochran Q statistic (p values < 0.10 were considered indicative of statistically significant heterogeneity) and I2 statistic (I2 values less than 25% represented low heterogeneity, values between 50% represented moderate heterogeneity and values greater than 75% represented high heterogeneity) [22]. The random effect was used due to the different rates of heterogeneity found in the different forest plots. The chi-square test with Yates correction was used with meta-analysis data and presented in odds ratios (OR). The odds is the ratio of the probability that the event of interest occurs to the probability that it does not [23] and OR greater than 4 is very significant. The quality of evidence was evaluated using the GRADE tool [24].
Necessary adaptations performed
During the development of the work, exceptional needs were found that had to be circumvented due to some studies found to be old, before the 2000 s, with missing standardization.
Due to the non-standardized form of age presentation and to maintain the quality of the evidence, this work incorporated standards that caused other studies (Table 1) to be excluded in the secondary analyses, the incorporated standard was the separation of age groups: younger than 65 years (<65), between 65 to 75 years (65-75), and older than (>75).
Table 1
Characteristics of the included studies in meta-analysis
| Year/ Study | Country | Ethnicity | Sample Size AD | Sample Size control | Gender AD (M/W) | Gender Control (M/W) | Average age AD | Was there a separation by age group? | Test performed | Outcome of the association ɛ4, K, and AD |
| 1997/ Lehmann [8] | UK* | Caucasian | 110 | 172 | – | – | 65.9 and 81.4 | Yes | χ2 with Yates correction and OR | Increase risk |
| 1998/ Crawford [25] | USA | Probably Caucasian | 391 | 201 | 210/181 | 80/121 | 76.4 and 72.4** | No | χ2 and OR through logistic regression | Presence of K increase risk in non-APOE4 carriers |
| 1998/ Kehoe [26] | UK | Probably Caucasian | 184 | 71 | 73/108 | – | *** | No | Pearson χ2 and logistic regression | No association |
| 1998/ Singleton [27] | UK* | Probably Caucasian | 119 | 120 | – | – | 64.8 and 82.6 | Yes | χ2 | No association |
| 1999/ Grubber [28] | USA* | Caucasian | 245 | 241 | 103/142 | 116/125 | – | Yes | χ2 and OR | Presence of K decrease risk in APOE4 carriers |
| 1999/ Ki [29] | Korea* | Korean | 86 | 106 | 62/24 | 46/60 | 73.2 and 77.9 | Yes | χ2 and OR | No association |
| 1999/ Sodeyama [30, 31] | Japan* | Japanese | 36 | 86 | – | – | 87.5 | No | χ2 | No association |
| 1999/ Tilley [32] | UK* | Caucasian | 177 | 118 | 67/110 | 81/37 | 81.6 | No | χ2 and OR through logistic regression | Increase risk in people 75 years old and |
| 1999/ Wiebusch [15] | Canada | Caucasian | 135 | 70 | 63/72 | 45/25 | – | Yes | χ2 with Yates correction, Fisher’s exact test and OR | Increase risk |
| 1999/ Yamamoto [33] | Japan* | Probably Japanese | 476 | 684 | – | – | – | Yes | χ2 | No association |
| 2000/Alvarez-Arcaya [34] | Spain | Caucasian | 249 | 250 | 80/169 | 75/175 | 75.3 | Yes | OR and chi-square or Fisher exact test | K variant is a protective factor in woman |
| 2000/ Lee [35] | China | Chinese | 87 | 101 | 42/45 | 56/45 | 69.9 | Yes | χ2 with Yates correction and OR | No association |
| 2000/ Mattila [36] | Finland | Caucasian | 80 | 67 | 29/51 | 37/30 | 73.5 and 80.4 | No | χ2 and multinomial logistic regression | No association |
| 2000/ MciLroy [37] | Ireland | Probably Caucasian | 175 | 187 | 62/113 | 58/129 | 77.7 | Yes | χ2 with Yates correction and OR | Increase risk |
| 2004/ Raygani [38] | Iran* | Probably Persian | 105 | 129 | 45/60 | 45/84 | 75 | Yes | χ2, OR and logistic regression | Increase risk |
| 2005/ Beyer [39] | Spain* | Probably Caucasian | 206 | 181 | 77/129 | 61/120 | 72.6 | Yes | χ2, OR and logistic regression | Unclear association |
| 2007/ Piccardi [40] | Italy* | Probably Caucasian | 158 | 118 | 42/116 | 42/116 | 76.8 | No | χ2 | No association |
| 2010/ Bizarro [41] | Italy* | Probably Caucasian | 167 | 126 | 63/104 | 62/67 | 73.32 | No | χ2 with Yates correction | No association |
| 2013/ Johansson [42] | Sweden | Caucasian | 28 | 17 | 14/14 | 9/8 | 74 | No | Spearman rank order correlation test | Unclear association |
| 2016/ Vijayaraghavan [43] | Norway | Probably Caucasian | 97 | 80 | 31/76 | 42/44 | 74.6 | No | χ2 and Fisher exact test | No association but marked cognitive decline over the years |
| 2017/ Gabriel [44] | Portugal* | Probably Caucasian | 217 | 200 | 100/117 | 89/111 | 70.6 | No | χ2 | No association |
*Country of the study was not available, therefore it was deduced from the data shown in the text or at the authors’ address. **The study had two groups: clinic and community, both were used simultaneously in the general group (without age separation), this was the way found to include this article. ***The study presented several groups, we finally used the CAD group (confirmed Alzheimer’s disease) that the authors made available, we used the data only in general classifications (without age separation). – Data not shown.
The nomenclature adopted for this systematic review was: APOE4(+) when there is at least one E4 allele and APOE4(-), when not there, the same pattern was adopted for the K variant of the BCHE gene, with BCHE-K(+) being present and BCHE-K (-) when it is not [8].
In order to extract data from some studies, secondary calculations had to be made and the main one was the calculation of the frequency of the different BCHE allele multiplied by the frequency of APOE4 presence and absence, calculated with the total number of study participants, this was a reason that made our quality of evidence will be penalized.
There was still the possibility that this effect was found to be a “hitchhiking effect”, that is, the results obtained could only be the previously confirmed effect of the e4 allele in the establishment of AD [45] and to extinguish this possibility it was necessary to carry out more tests. These tests were all allelic combination Forests plots and the chi-square meta-test.
Data were compared in a chi-square meta-test (application of the chi-square test with the meta-analysis data) with Yates correction, assuming statistically significant data when p < 0.05. Normally this type of test is not used in meta-analyses, but this review makes its use possible due to: 1) need beyond the dichotomous analysis that is standardized in meta-analyses and 2) most of the studies used in this review used the chi-square with the presence of a negative reference, the Yates correction was applied due to the use by the first study on the subject (Table 1).
The negative reference in the chi-square test with Yates correction in this study is the use of a group with AD and the control, both without the presence of the E4 allele and the K variant, for comparison with the AD and Control groups, and the presence of alleles E4 and variant K occurs according to the calculation tested (Tables 2–5).
Table 2
Chi-square meta-test between allelic combinations of the APOE and BCHE genes of people with AD and control group in the general population
| APOE4 | BCHE-K | AD | Control | p | Odds | CI 95% | I2* |
| – | – | 1116 | 1954 | reference | reference | 68% | |
| – | + | 456 | 689 | 0.0415 | 1.159 | 1.007 to 1.330 | 63% |
| + | – | 1379 | 545 | <0.0001 | 4.427 | 3.913 to 5.008 | 64% |
| + | + | 580 | 165 | <0.0001 | 6.155 | 5.109 to 7.416 | 48% |
+=presence; –=absence; *heterogeneity referring to the allelic comparison of the Alzheimer compared to the control group.
Table 3
Chi-square meta-test between allelic combinations of APOE and BCHE genes in people with AD and control group in younger than 65 years
| APOE4 | BCHE-K | AD | Control | p | Odds | CI 95% | I2* |
| – | – | 94 | 176 | reference | reference | 0% | |
| – | + | 25 | 38 | 0.5619 | 1.232 | NS | 38% |
| + | – | 85 | 58 | <0.0001 | 2.744 | 1.797 to 4.165 | 0% |
| + | + | 26 | 19 | 0.0056 | 2.562 | 1.357 to 4.780 | 0% |
+=presence; –=absence; *heterogeneity referring to the allelic comparison of the Alzheimer compared to the control group.
Table 4
Chi-square meta-test between allelic combinations of APOE and BCHE genes in people with AD and control group with people aged 65 to 75 years
| APOE4 | BCHE-K | AD | Control | p | Odds | CI 95% | I2* |
| – | – | 229 | 602 | reference | reference | 32% | |
| – | + | 81 | 171 | 0.1831 | 1.245 | NS | 1% |
| + | – | 403 | 171 | <0.0001 | 6.195 | 4.878 to 7.834 | 53% |
| + | + | 138 | 42 | <0.0001 | 8.638 | 5.898 to 12.650 | 38% |
+=presence; –=absence; *heterogeneity referring to the allelic comparison of the Alzheimer compared to the group.
Table 5
Chi-square meta-test between allelic combinations of APOE and BCHE genes in people with AD and control group with people older than 75 years
| APOE4 | BCHE-K | AD | Control | p | Odds | CI 95% | I2* |
| – | – | 230 | 469 | reference | reference | 42% | |
| – | + | 151 | 153 | <0.0001 | 2.012 | 1.529 to 2.652 | 73% |
| + | – | 259 | 118 | <0.0001 | 4.476 | 3.427 to 5.863 | 75% |
| + | + | 150 | 32 | <0.0001 | 9.558 | 6.372 to 14.570 | 3% |
+=presence; –=absence; *heterogeneity referring to the allelic comparison of the Alzheimer compared to the control group.
The use of a negative reference enables a more accurate answer than the dichotomous analysis of the meta-analysis in a study of genetic synergism (that is why most of the included studies used this negative reference, see the studies in Table 1). In other words, the use of a negative reference allows a better comparison in this study: The meta-analysis allows a comparison between two categories of groups, the control group and the group with the disease under analysis, a dichotomous perspective (e.g., first group, affected by AD and APOE4/BCHE-K carriers compared with, second group, control non-APOE4/BCHE-K carriers), but this study requires the analysis of four categories of groups analyzed simultaneously made possible by the chi-square test in order to exclude the aforementioned “hitchhike effect” (e.g., first group, affected by AD and carriers APOE4/BCHE-K compared with, second group, affected by AD and APOE4/BCHE-K non-carriers, compared with, third group, APOE4/BCHE-K carrier controls and compared with, fourth group, APOE4/BCHE-K non-carrier controls).
RESULTS
In total, 490 studies were found using the proposed terms. After removing duplicates, 235 articles were read by title and abstract. Of these 235, 55 were selected for full reading of the publication, where 30 articles were excluded for not meeting the inclusion criteria, thus leaving 25 articles for inclusion. During the peer review process 1 previously excluded article was requested to be re-included. However, it was decided to keep only articles with low and moderate risk of bias in the meta-analysis, which led to the exclusion of 5 articles, leaving 21 articles in the general meta-analysis (Fig. 1). The quality of the evidence, performed with the GRADE tool [24], was considered moderate (Supplementary Material).
Fig. 1
Literature search flowchart.
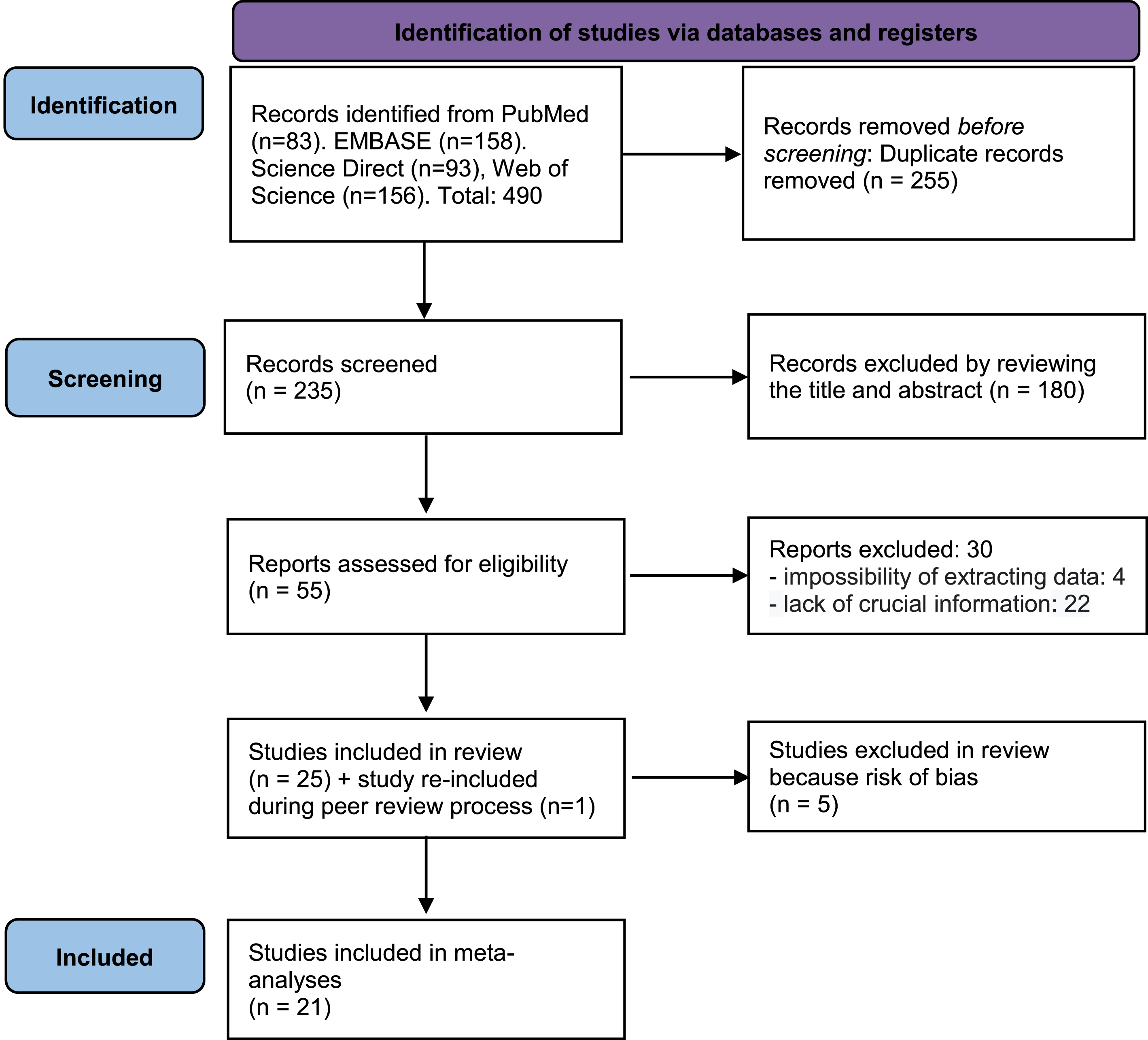
Dichotomous comparisons (AD compared to Control) without age separation of APOE4(+)/BCHE-K(+) resulted in OR = 3.43 with CI = 2.61-4.52 (p < 0.00001) and APOE4(+)/BCHE-K(-) resulted in OR = 3.20 with CI = 2.59-3.96 (p < 0.00001) (Figs. 2 and 3). Figures 4–6 show the Forest plot’s APOE4(+)/BCHE-K(+) in the AD group compared to control in the respective age groups: younger than 65 years old (<65), between 65 years old and 75 years old (65-75) and older than 75 years (>75). The other Forest plots and funnel plots can be found in the Supplementary Material.
Fig. 2
Forest plot of the comparison between people APOE4(+)/BCHE-K(+) with Alzheimer’s disease and control without age separation.
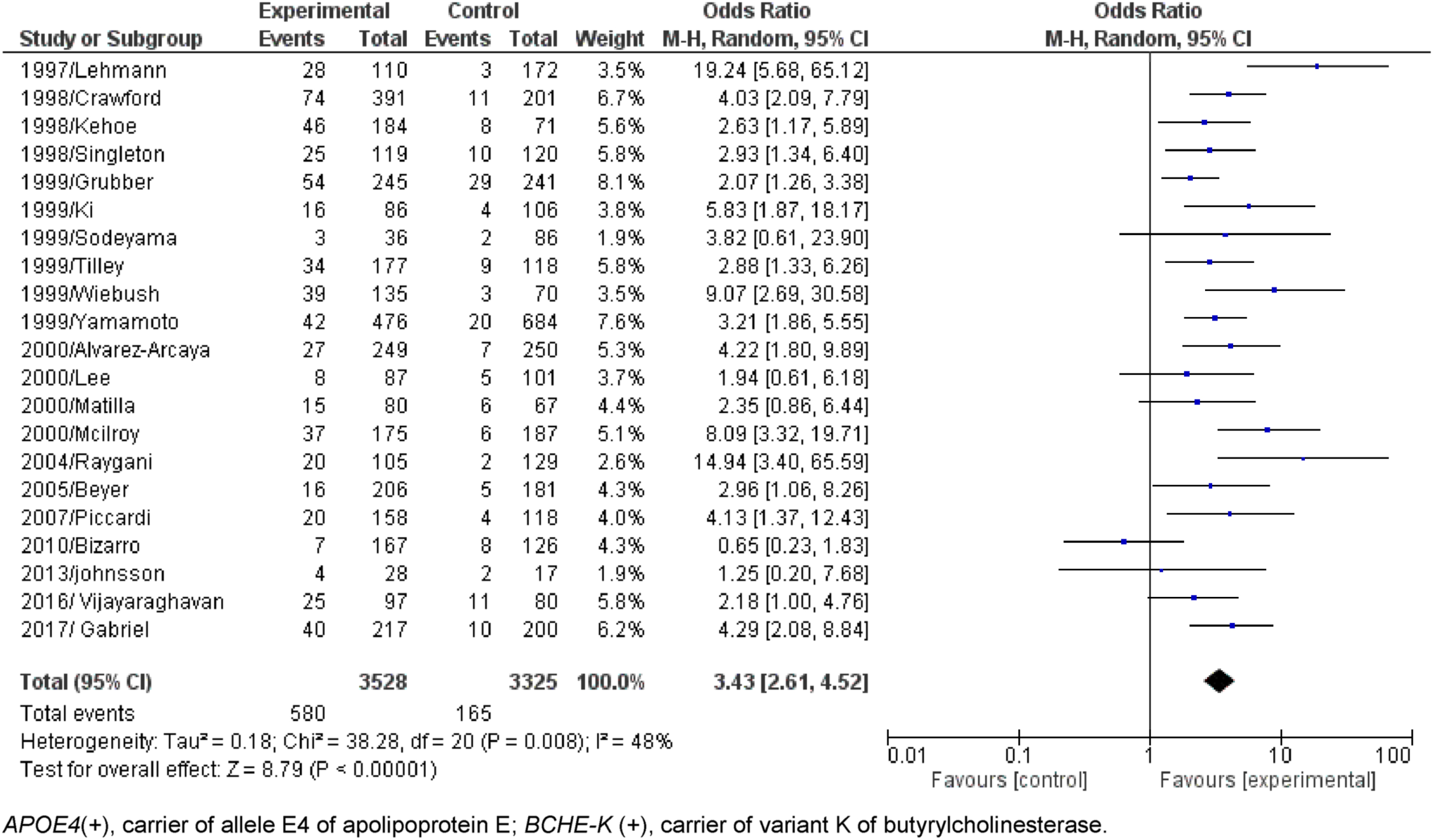
Fig. 3
Forest plot of the comparison between people APOE4(+)/BCHE-K(-) with Alzheimer’s disease and control without age separation.
Fig. 4
Forest plot of the comparison between people APOE4(+)/BCHE-K(+) with Alzheimer’s disease and control in the population younger than 65 years old.
Fig. 5
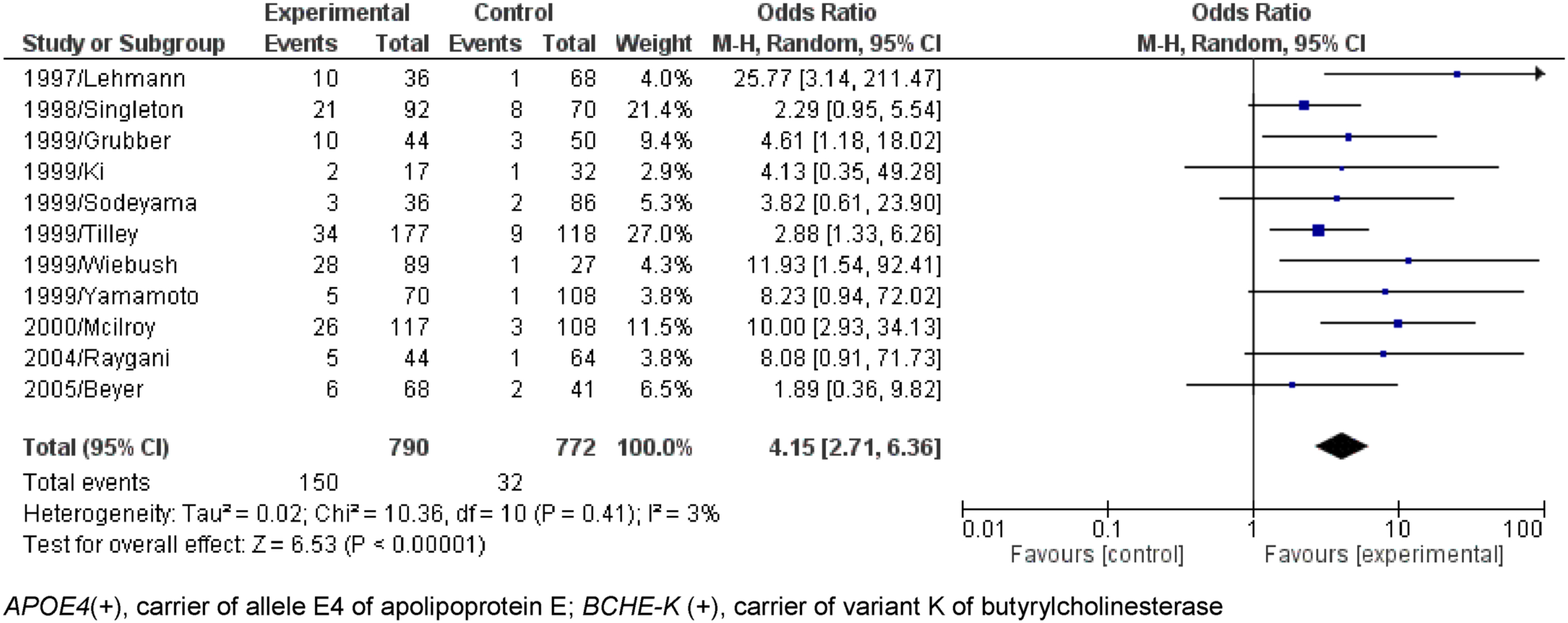
Forest plot of the comparison between people APOE4(+)/BCHE-K(+) with Alzheimer’s disease and control in population between 65 and 75 years old.
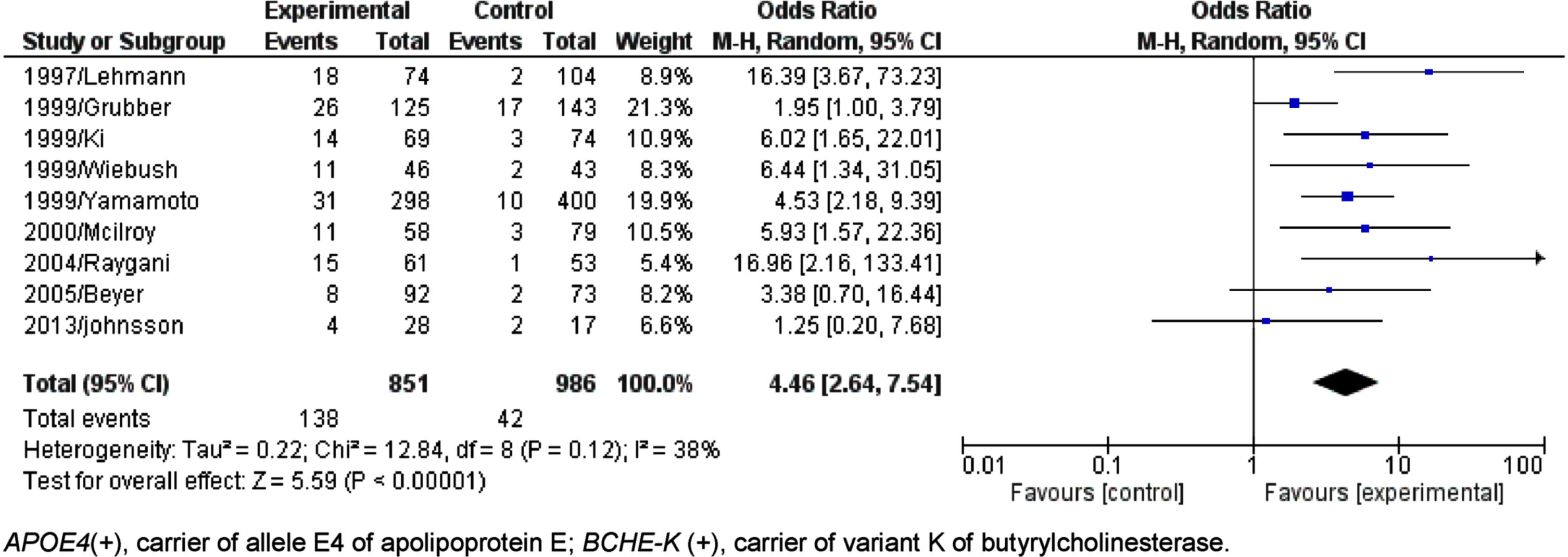
Fig. 6
Forest plot of the comparison between people APOE4(+)/BCHE-K(+) with Alzheimer’s disease and control in population older than 75 years.
Finally, the chi-square meta-tests with the data obtained, with Yates correction, comparing all the results, showed that the association APOE4 (+)/BCHE-K(+) is a risk factor, and is superior the only presence of the E4 allele, evidencing the pathogenic role of the K variant (Tables 2–5).
The results found are related to the presence or absence of at least one allele of each of the genes studied (i.e., APOE4/e-+BCHE-K/-), considering that few studies showed genotyping in homozygosity for APOE4, and also few presented data referring to APOE2, preventing the calculation of the effect value of the E2-K interaction in relation to AD.
Still on the E2-K interaction, only the study by Wiebush (1999) [15] clearly showed the genotypes referring to the E2 allele and the K variant, and despite the reported low number of the E2 allele (n = 19, where 11 were AD), it is noteworthy that of these 11 E2 individuals, 6 had the K variant, and of these, 5 had an ɛ4 allele together (E2/E4, K/- in heterozygosity) and the individual who did not have the E4 allele had the K allele in homozygosity (E2/E3,K/K).
At first, the result of the association between the presence of APOE4 and BCHE-K was positive for the general population, people aged between 65 and 75 years and older than 75 years (AD x Control - General population – Fig. 2: p < 0.00001, O.R.=3.43, 95% C.I.=2.61 to 4.52; 65 to 75 years – Fig. 5: p < 0.00001; O.R.=4.46, 95% C.I.=2.64 to 7.54, and; 75 Years or more – Fig. 6: p < 0.00001; O.R.=4.15 95% C.I.=2.71 to 6.36).
The chi-square meta-test used as a reference the data on the allelic combination both negative for E4 presence and K variant, confirmed that there is no hitchhiking effect and that the E4 allelic combination+K variant is 6.155 (O.R., 5.109 to 7.416) compared to 4.427 (O.R., 3.913 to 5.008) of the same combination without the K variant when compared to the benchmark in the general population (Table 2).
As expected, for early AD (younger than 65 years), the multifactorial risk alleles were not significant for AD origin, the OR was 2.562 (1.357 to 4.780) and was not significant (p = 0.056) with presence of the E4-K alleles compared to OR = 2.744 (1.797-4.165) when in the presence of only the E4 allele (Table 3).
The K variant allele synergistically with the ɛ4 allele evaluated in people aged 65 to 75 years has OR = 8.638 (5.898 to 12.650) compared to OR = 6.195 (4.878 to 7.834) when only the allele is present, E4 (Table 4). In the age group with people older than 75 years, the allele combination E4 and K has OR = 9.558 (6.372 to 14.570) compared to OR = 4.476 (3.427 to 5.863) in the presence of only the E4 allele (Table 5).
A secondary result was found to confirm that the K variant of the BCHE gene alone has an effect on the development of AD in the general population in relation to age (p = 0.0415, O.R.=1.159, 95% C.I.=1.007 to 1.330 – Table 2). Detailing this result in age groups, as expected it is not significant in people younger than 65 years (p = 0.5619 – Table 3) and is not significant in people aged between 65 and 75 years (p = 0.1831 – Table 4), but it is significant in people older than 75 years (p < 0.001, O.R.=2.012, 95% C.I.=1.529 to 2.652 – Table 5).
DISCUSSION
This study had a prevalence in the sample of Caucasian and Asian participants; none was carried out with a focus on African descents, Indigenous, and Latin patients, so the effects of the E4-K allelic combination for the previously cited ethnicities may be different from those found in this study due to ethnogenetic factors.
Studies on the possible association between APOE4 and BCHE-K with the development of AD have shown conflicting results (Table 1). Our results are important because they help to demonstrate a positive effect of the association between APOE4 and BCHE-K in LOAD, and that this association is not from a hitchhiking effect with the APOE4 allele, but rather that the two alleles act synergistically, greatly increasing the chance development of AD, especially in people over 75 years of age.
Furthermore, the results suggest that the K variant of the BCHE gene alone may be a risk factor for AD in people older than 75 years. No positive influence was found on the development of early-onset AD, which may hypothesize that the role of BCHE-K alone or associated with certain APOE alleles in people younger than 65 years may be protective, but this hypothesis needs further investigation.
BChE has several functions, among them the role in cholinergic pathways in the degradation of neurotransmitters, such as acetylcholine [9] and in fat catabolism via the hydrolysis of octanoyl ghrelin into desacyl ghrelin and octanoic acid [9, 46]. The activity of the BChE-K in blood plasma has been reported to be reduced by 30% when compared to the wild-type allele [13, 14].
It is known that with aging there is naturally cognitive impairment [47], sarcopenia [48] and fat gain [49]. It is likely that the K variant activity deficit [9, 40, 41] is acting in synergy with these and other aging risk factors for the onset of AD.
The possible mechanism behind the results regarding the E4-K pathological association is unclear, but indirect evidence and probably the sum of these BChE-K deficient factors [9, 13, 14, 50] are presented, along with the pathological role of E4 [5, 7, 51] which may provide an elucidation of these data obtained.
It has been found that APOE4 individuals have dysregulated apoE expression, possibly by pericytes in the blood-brain barrier [52] and perhaps this dysregulation is related to Wiebush’s initial evidence [15] in the E2-K interaction, where most AD individuals and with BCHE-K and APOE2, had an E4 allele together (E2/E4), indirectly suggesting, in an initial hypothesis, that the pathological role of the E4-K association is superior to the protective role of APOE2.
β-secretase cleaves amyloid-β protein precursor into Aβ peptide [53–55], increasing its concentration and activating secondary purification mechanisms. However, these mechanisms are deficient in E4 people [56–58], facilitating reactions such as neuroinflammation, which increases neurotoxicity.
The regions are compromised and when injured, the activity of butyrylcholinesterase increases in cholinergic degradation, replacing the primary action of acetylcholinesterase [9]. This dysregulation in the expression of E4 isoforms, accumulation of Aβ peptide and recruited BChE-K promotes the aggregation of these biochemical components in the Darreh-Shori hypothesis, which proposes the existence of a complex called BAβACs (BuChE/AChE-Aβ-ApoE Complex) [59, 60], corroborated later and reclassified into light, heavy, and ultra-heavy according to their molecular weight [60].
Our data contribute to the Darreh-Shorri theory [59–61], and it can be suggested that the pathological role of the K variant in this context is that its structural alteration in relation to wild type has a pathological synergy with E4 in the accumulation of BAβACs within the amyloid plaques by subtle molecular interaction.
This interaction may be in the sense of preventing the coupling of the E4 isoform in the initial form, since it was observed that people with AD and E4 have a lower accumulation of soluble BAβACs [59–61], explained by the inverse association between the genotype E4/E-, in which there is less accumulation of initial ApoE-Aβ complexes, resulting in high deposition of Aβ in the brain [60, 61] and there may be a positive E4-K interaction that facilitates the deposition of BAβACs within Aβ deposits.
This discrete molecular interaction is probably time-dependent, that is, over the years its effect is increasingly evident, gradually and silently interfering, mainly in the pathological accumulation of Aβ in the brain. This would explain why the E4-K association has a greater effect as a risk factor for sporadic AD in people older than 75 years than in people aged 65 to 75 years.
Conclusion
After more than two decades, the present study has brought evidence that contributes to a positive answer to the question “Is there an association between the ɛ4 allele of apolipoprotein E and the K variant of the BCHE gene with the development of AD?” raised by Lehmann, in 1997 [8], with the highest degree of scientific evidence available that the methodology of meta-analyses allows.
The authors of this study suggest that the next research involving this theme includes different ethnicities, since different statistical results in different ethnicities could indicate a possibility of investigation and, in the future, the chance of modulation of the effect of the E4-K association with AD.
If possible, there is also a suggestion to standardize the separation of groups into age groups (younger than 65 years old, between 65 to 75 and older than 75 years old), presentation of numbers between genders and APOE4 (+) classifications: carriers of E4 allele of apolipoprotein E; APOE4 (-): non-carriers of E4 allele of apolipoprotein E; BCHE-K (+): butyrylcholinesterase variant K carriers; BCHE-K (-): butyrylcholinesterase variant K non-carriers. The last suggestion would be to also investigate the E2 allele of apolipoprotein E with BCHE-K and AD.
ACKNOWLEDGMENTS
We are grateful for all the support provided by the Neurosciences and Behavior laboratory at UNICENTRO, PPGCF/UNICENTRO-UEPG and AEPAPA.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and its supplementary material.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-220084.
REFERENCES
[1] | Breijyeh Z , Karaman R ((2020) ) Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 25: , 5789. |
[2] | Sutphen CL , McCue L , Herries EM , Xiong C , Ladenson JH , Holtzman DM , Fagan AM; ADNI ((2018) ) Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimers Dement 14: , 869–879. |
[3] | Hersi M , Irvine B , Gupta P , Gomes J , Birkett N , Krewski D ((2017) ) Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 61: , 143–187. |
[4] | Suri S , Heise V , Trachtenberg AJ , Mackay CE ((2013) ) The forgotten APOE allele: A review of the evidence and suggested mechanisms forthe protective effect of APOE ɛ2. Neurosci Biobehav Rev 37: , 2878–2886. |
[5] | Kim JH ((2018) ) Genetics of Alzheimer’s disease. Dement Neurocogn Disord 17: , 131–136. |
[6] | Serrano-Pozo A , Das S , Hyman BT ((2021) ) APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol 20: , 68–80. |
[7] | Uddin M , Kabir M , Al Mamun A , Abdel-Daim MM , Barreto GE , Ashraf GM ((2019) ) APOE and Alzheimer’s disease: Evidence mounts that targeting APOE4 may combat Alzheimer’s pathogenesis. Mol Neurobiol 56: , 2450–2465. |
[8] | Lehmann DJ , Johnston C , Smith AD ((1997) ) Synergy between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset confirmed Alzheimer’s disease. Hum Mol Genet 6: , 1933–1936. |
[9] | Li Q , Yang H , Chen Y , Sun H ((2017) ) Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur J Med Chem 132: , 294–309. |
[10] | Darvesh S ((2016) ) Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer’s disease. Curr Alzheimer Res 13: , 1173–1177. |
[11] | Lockridge O ((2015) ) Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 148: , 34–46. |
[12] | Mesulam M , Geula C ((1994) ) Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann Neurol 36: , 722–727. |
[13] | Lockridge O , Duysen EG , Masson P ((2011) ) Butyrylcholinesterase: Overview, structure, and function. Anticholinesterase Pesticides 10: , 25–41. |
[14] | Bartels CF , Jensen FS , Lockridge O , Van der Spek AF , Rubinstein HM , Lubrano T , La Du BN ((1992) ) DNA mutation associated with the human butyrylcholinesterase K-variant and its linkage to the atypical variant mutation and other polymorphic sites. Am J Hum Genet 50: , 1086–1103. |
[15] | Wiebusch H , Poirier J , Sévigny P , Schappert K ((1999) ) Furtherevidence for a synergistic association between APOEɛ4and BCHE-K in confirmed Alzheimer’s disease. Hum Genet 104: , 158–163. |
[16] | Panegyres PK , Mamotte CD , Vasikaran SD , Wilton S , Fabian V , Kakulas BA ((1999) ) Butyrylcholinesterase K variant and Alzheimer’s disease. J Neurol 246: , 369–370. |
[17] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , Moher D ((2021) ) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surgery 88: , 105906. |
[18] | Reiswig J ((2010) ) Mendeley. J Med Libr Assoc 98: , 193–194. |
[19] | Ouzzani M , Hammady H , Fedorowicz Z , Elmagarmid A ((2016) ) Rayyan— a web and mobile app for systematic reviews. Syst Rev 5: , 210. |
[20] | Sterne JA , Hernán MA , Reeves BC , Savović J , Berkman ND , Viswanathan M , Henry D , Altman DG , Ansari MT , Boutron I , Carpenter JR , Chan AW , Churchill R , Deeks JJ , Hróbjartsson A , Kirkham J , Jüni P , Loke YK , Pigott TD , Ramsay CR , Regidor D , Rothstein HR , Sandhu L , Santaguida PL , Schünemann HJ , Shea B , Shrier I , Tugwell P , Turner L , Valentine JC , Waddington H , Waters E , Wells GA , Whiting PF , Higgins JP ((2016) ) ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355: , i4919. |
[21] | Schmidt L , Shokraneh F , Steinhausen K , Adams CE ((2019) ) Introducing RAPTOR: RevMan parsing tool for reviewers. Syst Rev 8: , 151. |
[22] | Melsen WG , Bootsma MCJ , Rovers MM , Bonten MJM ((2014) ) The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 20: , 123–129. |
[23] | Bland JM , Altman DG ((2000) ) The odds ratio. BMJ 320: , 1468. |
[24] | Schünemann H , Brożek J , Guyatt G , Oxman A. GRADE handbookfor grading quality of evidence and strength of recommendations. guidelinedevelopment.org/handbook. Updated October 2013. Accessed on May 15, 2023. |
[25] | Crawford F , Fallin D , Suo Z , Abdullah L , Gold M , Gauntlett A , Mullan M ((1998) ) The butyrylcholinesterase gene is neither independently nor synergistically associated with late-onset AD in clinic-and community-based populations. Neurosci Lett 249: , 115–118. |
[26] | Kehoe PG , Williams H , Holmans P , Wilcock G , Cairns NJ , Neal J , Owen MJ ((1998) ) The butyrylcholinesterase K variant and susceptibility to Alzheimer’s disease. J Med Genet 35: , 1034–1035. |
[27] | Singleton AB , Smith G , Gibson AM , Woodward R , Perry RH , Ince PG , Morris CM ((1998) ) No association between the K variant of the butyrylcholinesterase gene and pathologically confirmed Alzheimer’s disease. Hum Mol Genet 7: , 937–939. |
[28] | Grubber JM , Saunders AM , Crane-Gatherum AR , Scott WK , Martin ER , Haynes CS , Pericak-Vance MA ((1999) ) Analysis of association between Alzheimer disease and the K variant of butyrylcholinesterase (BCHE-K). Neurosci Lett 269: , 115–119. |
[29] | Ki CS , Na DL , Kim JW , Kim HJ , Kim DK , Yoon BK ((1999) ) No association between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset Alzheimer’s disease. Am J Med Genet 88: , 113–115. |
[30] | Sodeyama N , Yamada M , Mizusawa H , Itoh Y , Otomo E , Suematsu N , Matsushita M ((1999) ) Association between butyrylcholinesterase K variant and the Alzheimer type neuropathological changes in apolipoprotein E ɛ4 carriers older than 75 years. J Neurol Neurosurg Psychiatry 67: , 693–694. |
[31] | Yamada M , Sodeyama N , Itoh Y , Suematsu N , Otomo E , Matsushita M , Mizusawa H ((1998) ) Butyrylcholinesterase K variant and cerebral amyloid angiopathy. Stroke 29: , 2488–2490. |
[32] | Tilley L , Morgan K , Grainger J , Marsters P , Morgan L , Lowe J , Kalsheker N ((1999) ) Evaluation of polymorphisms in the presenilin-1 gene and the butyrylcholinesterase gene as risk factors in sporadic Alzheimer’s disease. Eur J Hum Genet 7: , 659–663. |
[33] | Yamamoto Y , Yasuda M , Mori E , Maeda K ((1999) ) Failure to confirm a synergistic effect between the K-variant of the butyrylcholinesterase gene and the ɛ4 allele of the apolipoprotein gene in Japanese patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 67: , 94–96. |
[34] | Combarros O , Llorca J , Sánchez-Guerra M , Berciano J , Fernández-Viadero C , Peña N ((2000) ) The butyrylcholinesterase K variant is a protective factor for sporadic Alzheimer’s disease in women. Acta Neurol Scand 102: , 350–353. |
[35] | Lee DW , Liu HC , Liu TY , Chi CW , Hong CJ ((2000) ) No association between butyrylcholinesterase K-variant and Alzheimer disease in Chinese. Am J Med Genet 96: , 167–169. |
[36] | Mattila KM , Rinne JO , Röyttä M , Laippala P , Pietilä T , Kalimo H , Lehtimäki T ((2000) ) Dipeptidyl carboxypeptidase 1 (DCP1) and butyrylcholinesterase (BCHE) gene interactions with the apolipoprotein E ɛ4 allele as risk factors in Alzheimer’s disease and in Parkinson’s disease with coexisting Alzheimer pathology. J Med Genet 37: , 766–770. |
[37] | McIlroy SP , Crawford VLS , Dynan KB , McGleenon BM , Vahidassr MD , Lawson JT , Passmore AP ((2000) ) Butyrylcholinesterase K variant is genetically associated with late onset Alzheimer’s disease in Northern Ireland. J Med Genet 37: , 182–185. |
[38] | Raygani AV , Zahrai M , Soltanzadeh A , Doosti M , Javadi E , Pourmotabbed T ((2004) ) Analysis of association between butyrylcholinesterase K variant and apolipoprotein E genotypes in Alzheimer’s disease. Neurosci Lett 371: , 142–146. |
[39] | Beyer K , Lao JI , Latorre P , Ariza A ((2005) ) Age at onset: An essential variable for the definition of genetic risk factors for sporadic Alzheimer’s disease. Ann N Y Acad Sci 1057: , 260–278. |
[40] | Piccardi M , Congiu D , Squassina A , Manconi F , Putzu PF , Mereu RM , Del Zompo M ((2007) ) Alzheimer’s disease: Case-control association study of polymorphisms in ACHE, CHAT, and BCHE genes in a Sardinian sample. Am J Med Genet B Neuropsychiatr Genet 144: , 895–899. |
[41] | Bizzarro A , Guglielmi V , Lomastro R , Valenza A , Lauria A , Marra C , Masullo C ((2010) ) BuChE K variant is decreased in Alzheimer’s disease not in fronto-temporal dementia. J Neural Transm (Vienna) 117: , 377–383. |
[42] | Johansson P , Almqvist EG , Johansson JO , Mattsson N , Andreasson U , Hansson O , Svensson J ((2013) ) Cerebrospinal fluid (CSF) 25-hydroxyvitamin D concentration and CSF acetylcholinesterase activity are reduced in patients with Alzheimer’s disease. PloS One 8: , 81989. |
[43] | Vijayaraghavan S , Darreh-Shori T , Rongve A , Berge G , Sando SB , White LR , Aarsland D ((2016) ) Association of butyrylcholinesterase-K alleleand apolipoprotein E ɛ4 allele with cognitive declinein dementia with Lewy bodies and Alzheimer’s disease. JAlzheimers Dis 50: , 567–576. |
[44] | Gabriel AJ , Almeida MR , Ribeiro MH , Durães J , Tábuas-Pereira M , Pinheiro AC , Baldeiras I ((2017) ) Association between butyrylcholinesterase and cerebrospinal fluid biomarkers in Alzheimer’s disease patients. Neurosci Lett 641: , 101–106. |
[45] | Crean S , Ward A , Mercaldi CJ , Collins JM , Cook MN , Baker NL , Arrighi HM ((2011) ) Apolipoprotein E ɛ4 prevalence in Alzheimer’s disease patients varies across global populations: A systematic literature review and meta-analysis. Dement Geriatr Cogn Disord 31: , 20–30. |
[46] | De Vriese C , Gregoire F , Lema-Kisoka R , Waelbroeck M , Robberecht P , Delporte C ((2004) ) Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology 145: , 4997–5005. |
[47] | Reddy PH , Manczak M , Mao P , Calkins MJ , Reddy AP , Shirendeb U ((2010) ) Amyloid-β and mitochondria in aging and Alzheimer’s disease: Implications for synaptic damage and cognitive decline. J Alzheimers Dis 20: , S499–S512. |
[48] | Wakabayashi H ((2014) ) Presbyphagia and spenic dysphagia: Association between aging, sarcopenia, and deglutition disorders. J Frailty Aging 3: , 97–103. |
[49] | Frasca D , Blomberg BB , Paganelli R ((2017) ) Aging, obesity, and inflammatory age-related diseases. Front Immunol 8: , 1745. |
[50] | Simão-Silva DP , Bertolucci PHF , Labio RW , Payão SLM , Furtado-Alle L , Souza RLR ((2013) ) Association analysis between K and– 116A variants of butyrylcholinesterase and Alzheimer’sdisease in a Brazilian population. Chem Biol Interact 203: , 358–360. |
[51] | Emrani S , Arain HA , DeMarshall C , Nuriel T ((2020) ) APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: A systematic review. Alzheimers Res Ther 12: , 141. |
[52] | Blanchard JW , Bula M , Davila-Velderrain J , Akay LA , Zhu L , Frank A , Tsai LH ((2020) ) Reconstruction of the human blood– brain barrier reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med 26: , 952–963. |
[53] | Lane RM , Farlow MR ((2005) ) Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer’s disease. J Lipid Res 46: , 949–968. |
[54] | Puglielli L , Tanzi RE , Kovacs DM ((2003) ) Alzheimer’s disease: The cholesterol connection. Nat Neurosci 6: , 345–351. |
[55] | Wang ZH , Xia Y , Liu P , Liu X , Edgington-Mitchell L , Lei K , Ye K ((2021) ) ApoE4 activates C/EBPβ/δ-secretase with 27-hydroxycholesterol, driving the pathogenesis of Alzheimer’s disease. Prog Neurobiol 202: , 102032. |
[56] | Huang YWA , Zhou B , Wernig M , Südhof TC ((2017) ) ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168: , 427–441. |
[57] | Koutsodendris N , Nelson MR , Rao A , Huang Y ((2022) ) Apolipoprotein E and Alzheimer’s disease: Findings, hypotheses, and potential mechanisms. Annu Rev Pathol 17: , 73–99. |
[58] | Mahley RW , Rall SC ((2000) ) Apolipoprotein E: Far more than a lipid transport protein. Annu Rev Genomics Hum Genet 1: , 507–537. |
[59] | Darreh-Shori T , Modiri N , Nordberg A ((2009) ) P1-163: Apolipoprotein E and Butyrylcholinesterase synergistically promote Ab peptides oligomerization. Alzheimers Dement 5: , 225. |
[60] | Lana E , Gellerbring A , Jung S , Nordberg A , Unger Lithner C , Darreh-Shori T ((2019) ) Homomeric and heteromeric Aβ species exist in human brain and csf regardless of Alzheimer’s disease status and risk genotype. Front Mol Neurosci 12: , 176. |
[61] | Darreh-Shori T , Forsberg A , Modiri N , Andreasen N , Blennow K , Kamil C , Nordberg A ((2011) ) Differential levels of apolipoprotein E and butyrylcholinesterase show strong association with pathological signs of Alzheimer’s disease in the brain in vivo. Neurobiol Aging 32: , 2320-e15–32. |




