A Case of Minimally Progressive Prodromal Alzheimer’s Disease
Abstract
Prodromal Alzheimer’s disease (AD) is a neurodegenerative condition typically progressing to dementia within 3 years. We describe a case of a mild cognitive impairment (MCI) patient with biomarker evidence for amyloidosis, tau, and neurodegeneration who had minimal changes in clinical phenotype during an 11-year period. AD biomarkers were obtained with cerebrospinal fluid analysis and amyloid PET imaging, both of which supported a biological diagnosis of AD. However, the patient’s neuropsychological profile remained stable over 11 years except for mild memory-retrieval changes. This case provides evidence that MCI with supportive AD biomarkers may have an atypically minimal progression.
INTRODUCTION
Incorporation of biomarkers into practice has enabled clinicians not only an unprecedented opportunity to confirm an Alzheimer’s disease (AD) diagnosis but also the ability to render a prognosis in mild cognitive impairment (MCI). Between 59–61% of patients with cognitive impairment and evidence of positive AD biomarkers progress to dementia within 3 years [1]. We report a patient diagnosed with prodromal AD based on history, neuropsychological assessment, and the presence of both positive amyloid PET and cerebrospinal fluid (CSF) biomarkers followed over an 11-year period who exhibited minimal evidence for clinically significant disease progression based on longitudinal functional status and neuropsychological assessment.
CASE PRESENTATION
A 73-year-old Caucasian male with a past medical history significant for atrial flutter and obstructive sleep apnea presented to neurology in October 2010 with one year of memory problems characterized by difficulty managing finances that resulted in bankruptcy. Furthermore, there were problems with repetitive statements, misplacing objects, forgetting appointments, and confusion related to turning on his computer. By history, he had otherwise intact instrumental activities of daily living (IADLs). Initial Montreal Cognitive Assessment (MoCA) was 27/30. Medications included rosuvastatin, tamsulosin, metoprolol, lisinopril, and escitalopram. Neurological examination was unremarkable. He was referred for formal cognitive testing in January 2011 that showed mild deficits (1–1.5 standard deviations from community norms) involving learning, memory, and attention/executive function. There was relatively intact memory storage on recognition testing. Brain MRI revealed atrophy on visual read affecting the posterior parietal region, inclusive of the precuneus and bilateral angular gyrus with sparing of the hippocampi. He was diagnosed with amnestic MCI and continued to be followed longitudinally in clinic. CSF biomarker studies (Athena diagnostics) showed Aβ= 435.15 pg/ml, t-tau, 452.8 pg/ml, and p-tau = 101.35 pg/ml in October 2013. Based upon the positive findings, he was diagnosed with prodromal AD. An August 2016 florbetapir amyloid PET scan confirmed increased uptake involving the prefrontal, lateral temporal, and parietal regions consistent with confirmed the presence of moderate to severe cerebral amyloid plaques. Genetic testing showed that the patient was an APOE ɛ3/ɛ4 carrier.
The patient was followed for a total of 11 years between October 2010 and October 2021. During this time, he engaged in an active lifestyle inclusive of routine exercise 3x/week, woodworking, and consumption of the Mediterranean diet. Longitudinal cognitive screening revealed MoCA scores that ranged from 25–29 with the most recent being 26/30 in March 2020. A total of five subsequent neuropsychological evaluations were performed between October 2013 and August 2021. The battery consisted of measures assessing processing speed, language, visuospatial functions, encoding, memory retrieval and recognition as well as executive functions (Table 1). Results showing neuropsychological test performance by cognitive domain are depicted in Fig. 1 over 5-year intervals including 2010, 2016, and 2021. T-scores for processing speed, language, visuospatial functions, encoding, and executive functioning remained stable (i.e., variability not greater than 1 standard deviation from baseline) over these three time periods (Fig. 1). In 2021, the patient’s memory retrieval T-scores declined to a minimal degree by 1.3 standard deviations compared to baseline; however, his memory-recognition scores remained constant between 2010 and 2021, reflecting a stable neuropsychological phenotype. Geriatric depression score-15 ranged from 0–3 during this time.
Fig. 1
Neuropsychological Test Performance by Cognitive Domain 2010–2021. *T-scores are standardized values calculated using normative data (i.e., mean, standard deviation) from test performance of healthy peers of similar age and/or education. Values <29 Impaired; 30–36 Borderline Impaired; 37–42 Low Average; 43–56 Average;>56 High Average or better. Scores shown here for each cognitive domain were obtained by averaging T-scores across individual tests. No clinically significant “outlier” values were identified during this process. Memory Recognition scores are not normally distributed.
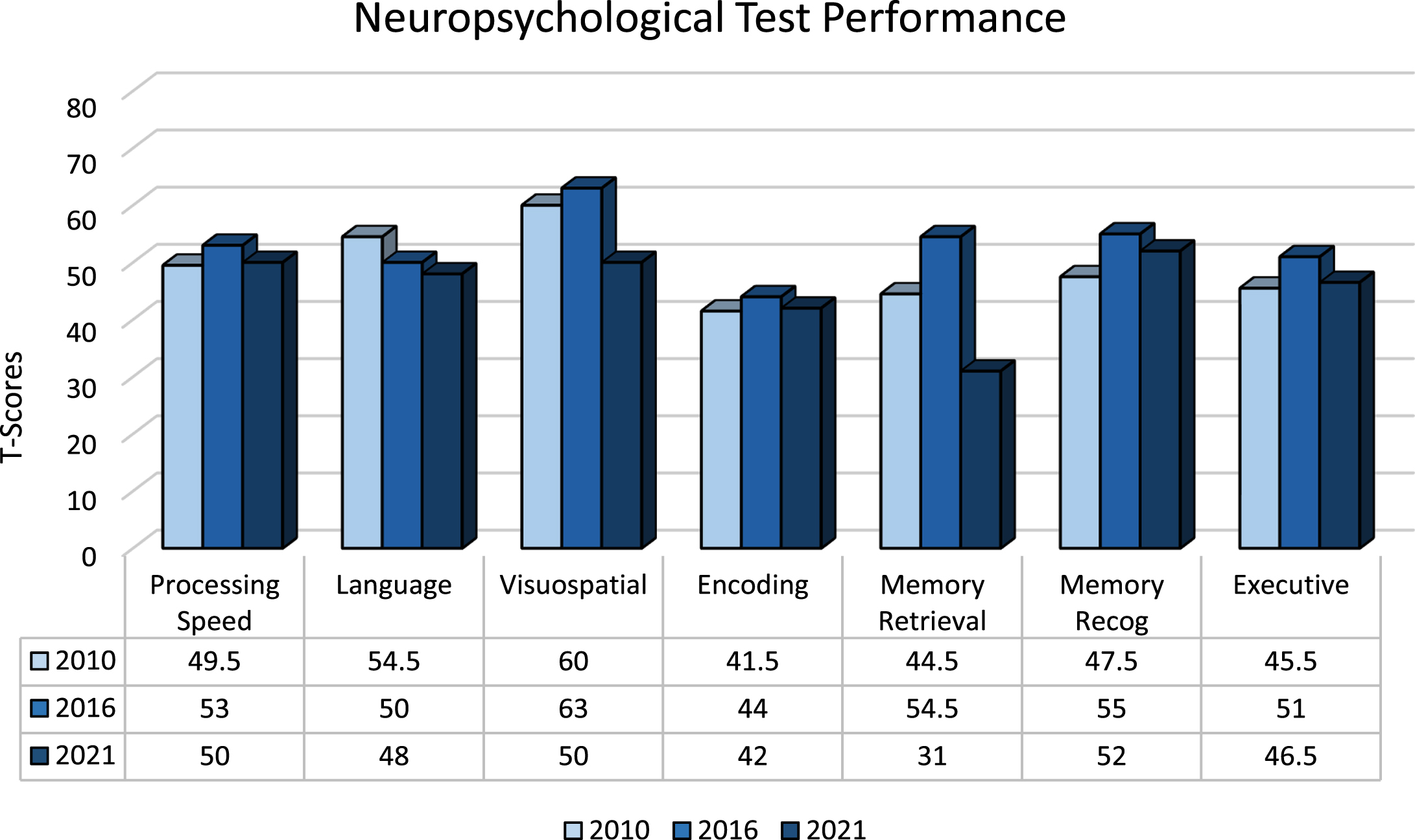
Table 1
Neuropsychological test battery
| Processing Speed | WAIS-IV Coding |
| Word Reading Condition, Delis-Kaplan Executive Function System (DKEFS) Color-Word Test | |
| Language | Boston Naming Test |
| Animal Naming | |
| Visuospatial | WAIS-IV Block Design |
| Encoding | Hopkins Verbal Learning Test – Revised (HVLT-R), Trials 1–3 |
| Brief Visuospatial Memory Test – Revised (BVMT-R), Trials 1–3 | |
| Memory Retrieval | HVLT-R Delayed Recall |
| BVMT-R Delayed Recall | |
| Memory Recognition | HVLT-R Recognition |
| BVMT-R Recognition | |
| Executive | Trailmaking Test, Part B |
| Controlled Oral Word Association Test (FAS) |
Throughout the course of his condition, the patient retained the ability to participate in most IADLs including woodworking, medication management, and meal preparation. It was recommended he stop driving due to inattention and impulsivity rather than an observed decline in his skill level according to standards during an occupational therapy behind-the-wheel assessment in 2014.
DISCUSSION
This case study describes a patient presenting with subjective and objective cognitive impairment who was found to have evidence for amyloidosis, tau phosphorylation, and neurodegeneration (A+/T+/N+) early in the course of his disease, thus meeting the NIA-AA research framework for AD, but failed to significantly progress from a cognitive or functional standpoint 12 years from symptom onset and 8 years following biological confirmation of AD [2]. This patient had evidence of neurodegeneration as represented by CSF t-tau. Although there was minimal decline in memory-retrieval over the course of longitudinal follow-up, the patient’s memory storage abilities remained relatively unchanged. Neuropsychological evaluation suggested the relative decline on tasks of memory-retrieval is a function of a dysexecutive process rather than a classical amnestic pattern of AD recognition memory dysfunction.
Understood as a continuum of disease, the entire process of AD may last between 15 and 25 years in its progression from pre-clinical (NIH stage 1-2) to advanced dementia (NIH stage 6 [3]. For an individual aged 70, the typical estimated duration of the prodromal phase is 4 years [3]. In addition, 59–61% of patients meeting International Working Group-2 and National Institute of Aging research criteria for prodromal AD progress to dementia within 3 years [1].
With a clinical phenotype that remained objectively stable for >10 years in the setting of early biological evidence for an AD process, our patient represents an outlier from the typical prodromal AD patient. We refer to this presentation as a “prodromal AD long-term non-progressor.” This phenomenon has been under described in the literature but may represent an extreme clinical phenotype that may benefit from a defining criteria (e.g., ATN positivity with stable neuropsychological testing and function >5 years from ATN positivity in the absence of other clinical factors contributing to cognitive impairment).
A potential explanation for the patient’s delayed progression may be related to cognitive reserve, a concept that relates to lifelong experiences such as education and occupation that may enable certain individuals to be more resilient to AD-related brain changes [4]. This individual had completed one year of graduate school and general intellectual funding was characterized by superior verbal comprehension and perceptual reasoning. Furthermore, his profile showed impairment predominantly affecting executive functions as compared to memory storage, thus suggesting the outside possibility of obstructive sleep apnea (OSA) driving the clinical symptomatology. However, CSF analysis performed in patients with untreated OSA have demonstrated biomarker changes resembling that found in AD [5]. In addition, our patient may have experienced a more benign neurodegenerative condition that has clinical symptomatic overlap with AD (e.g., amnestic dementia). For instance, patients with primary age-related tauopathy, a condition characterized by the presence of neurofibrillary tangles in the absence of amyloid pathology, may present as a continuum ranging from normal cognition to mild amnestic symptoms, rarely progressing to a profound dementia syndrome [6]. Furthermore, the recently described limbic-predominant, age-related TDP-43 encephalopathy may present with or without amyloid pathology and can be misdiagnosed with AD before neuropathology is obtained [7, 8] It should be noted that this latter condition typically affects patients aged ≥80 and has non-specific biomarker findings on imaging and CSF.
Longitudinal biomarker studies have shown variable conversion rates for MCI depending on whether the neurodegenerative process involves or spares the medial temporal lobes. FDG-PET studies in subjects with amnestic MCI reveal that patients with a more limbic-predominant pattern of hypometabolism have only a 7% dementia conversion rate over 8.20±3.30 years [9]. On the other hand, longitudinal biomarker studies of A+/N+ patients with FDG-PET hypometabolism, but lacking hippocampal atrophy (as noted in our patient), have shown that these individuals are less likely to progress to dementia at 9 years as compared to those with hippocampal atrophy [10]. Whether hippocampal atrophy deserves greater weight in terms of its role as a predictive biomarker for MCI conversion to dementia is a question that deserves further investigation.
Although this case study was limited by the lack of longitudinal biomarker data, we believe this case study provides insight into subjects who may have atypical longitudinal progression despite the presence of positive ATN biomarkers and further supports continued investigation into the predicative value of molecular PET imaging and CSF biomarkers in clinical practice.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Vos SJ , Verhey F , Frolich L , Kornhuber J , Wiltfang J , Maier W , Peters O , Ruther E , Nobili F , Morbelli S , Frisoni GB , Drzezga A , Didic M , van Berckel BN , Simmons A , Soininen H , Kloszewska I , Mecocci P , Tsolaki M , Vellas B , Lovestone S , Muscio C , Herukka SK , Salmon E , Bastin C , Wallin A , Nordlund A , de Mendonca A , Silva D , Santana I , Lemos R , Engelborghs S , Van der Mussele S , Alzheimer’s Disease Neuroimaging Initiative, Freund-Levi Y , Wallin AK , Hampel H , van der Flier W , Scheltens P , Visser PJ ((2015) ) Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138: , 1327–1338. |
[2] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R , Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[3] | Scheltens P , De Strooper B , Kivipelto M , Holstege H , Chetelat G , Teunissen CE , Cummings J , van der Flier WM ((2021) ) Alzheimer’s disease. Lancet 397: , 1577–1590. |
[4] | Stern Y ((2012) ) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11: , 1006–1012. |
[5] | Liguori C , Mercuri NB , Izzi F , Romigi A , Cordella A , Sancesario G , Placidi F ((2017) ) Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep 40: , doi: 10.1093/sleep/zsx011. |
[6] | Crary JF , Trojanowski JQ , Schneider JA , Abisambra JF , Abner EL , Alafuzoff I , Arnold SE , Attems J , Beach TG , Bigio EH , Cairns NJ , Dickson DW , Gearing M , Grinberg LT , Hof PR , Hyman BT , Jellinger K , Jicha GA , Kovacs GG , Knopman DS , Kofler J , Kukull WA , Mackenzie IR , Masliah E , McKee A , Montine TJ , Murray ME , Neltner JH , Santa-Maria I , Seeley WW , Serrano-Pozo A , Shelanski ML , Stein T , Takao M , Thal DR , Toledo JB , Troncoso JC , Vonsattel JP , White CL 3rd , Wisniewski T , Woltjer RL , Yamada M , Nelson PT ((2014) ) Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 128: , 755–766. |
[7] | Robinson JL , Porta S , Garrett FG , Zhang P , Xie SX , Suh E , Van Deerlin VM , Abner EL , Jicha GA , Barber JM , Lee VM , Lee EB , Trojanowski JQ , Nelson PT ((2020) ) Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain 143: , 2844–2857. |
[8] | Nelson PT , Dickson DW , Trojanowski JQ , Jack CR , Boyle PA , Arfanakis K , Rademakers R , Alafuzoff I , Attems J , Brayne C , Coyle-Gilchrist ITS , Chui HC , Fardo DW , Flanagan ME , Halliday G , Hokkanen SRK , Hunter S , Jicha GA , Katsumata Y , Kawas CH , Keene CD , Kovacs GG , Kukull WA , Levey AI , Makkinejad N , Montine TJ , Murayama S , Murray ME , Nag S , Rissman RA , Seeley WW , Sperling RA , White CL 3rd , Yu L , Schneider JA ((2019) ) Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 142: , 1503–1527. |
[9] | Tondo G , Carli G , Santangelo R , Mattoli MV , Presotto L , Filippi M , Magnani G , Iannaccone S , Cerami C , Perani D , Alzheimer’s Disease Neuroimaging Initiative ((2021) ) Biomarker-based stability in limbic-predominant amnestic mild cognitive impairment. Eur J Neurol 28: , 1123–1133. |
[10] | Ou YN , Xu W , Li JQ , Guo Y , Cui M , Chen KL , Huang YY , Dong Q , Tan L , Yu JT , Alzheimer’s Disease Neuroimaging Initiative ((2019) ) FDG-PET as an independent biomarker for Alzheimer’s biological diagnosis: A longitudinal study. Alzheimers Res Ther 11: , 57. |




