Lower Cerebrospinal Fluid Amyloid-β42 Predicts Sooner Time to Antipsychotic Use in Alzheimer’s Disease
Abstract
Background:
Cerebrospinal fluid (CSF) biomarkers of amyloid-β42 (Aβ42) and phosphorylated-tau help clinicians accurately diagnose Alzheimer’s disease (AD). Whether biomarkers help prognosticate behavioral and psychological symptoms of dementia (BPSD) is unclear.
Objective:
Determine whether CSF biomarker levels aid prognostication of BPSD in AD.
Methods:
This retrospective cohort study included patients over 65 with a diagnosis of AD based on CSF biomarkers. We measured time from CSF testing to the first antipsychotic use in the following months. We then analyzed time to antipsychotic (AP) use with respect to Aβ42, total tau, phosphorylated tau, and amyloid-to-tau index using a survival analysis approach.
Results:
Of 86 AD patients (average 72±5 years, 46.5% male), 11 patients (12.7%) received APs following CSF testing. Patients with Aβ42 below the median had sooner time-to-AP use. This was significant on a log-rank test (p = 0.04). There was no difference in time-to-AP use if the group was stratified by levels of total tau, phosphorylated tau, or amyloid-to-tau index.
Conclusion:
These results suggest a relationship between lower CSF Aβ42 levels and sooner AP use. This supports prior reports suggesting a correlation between BPSD and Aβ deposition on PET. These results highlight the need for further prospective studies on Aβ levels and BPSD.
INTRODUCTION
Behavioral and psychological symptoms of dementia (BPSD) are a common feature of Alzheimer’s disease (AD). BPSD is a broad term encompassing the spectra of agitation, psychosis, and mood disorders that often complicate dementia. These symptoms can be chronic or, in the case of delirium, acute [1]. They occur in 90% of dementia patients over five years, cause significant patient and caregiver burden, and are a frequent precipitant of institutionalization [2]. First-line interventions depend on the phenotype of BPSD but are typically non-pharmacologic, given the lack of effective treatment options and potential for serious side effects, as outlined in the recent Delphi consensus guidelines. They recommend antipsychotics (AP) as first-line pharmacotherapy for psychosis and second-line for agitation without psychosis [3]. AP medications are commonly prescribed despite their well-publicized risks of increased mortality and cardiovascular events in the elderly, as outlined in the FDA black box warning, in addition to other side effects such as extrapyramidal symptoms and drowsiness.
A majority of patients with AD will eventually develop psychosis as part of their dementia, and the psychosis portends more severe dementia with more rapid decline [4]. Non-pharmacologic measures are often inadequate alone, or the potential for physical harm due to BPSD may be great, prompting the need for medications. Due to the high incidence of BPSD and lack of treatment alternatives, many patients are prescribed an antipsychotic at some point in their disease. A 2017 meta-analysis found a pooled prevalence of antipsychotic use to be 27.3% in individuals with dementia [5].
Frequent use of APs and their potential for serious adverse events make this class of medications a critical consideration when counseling patients about BPSD and AD prognosis. Correspondingly, AP use is an important clinical outcome that may serve as a proxy for particularly challenging cases of BPSD in retrospective studies seeking to guide prognosis.
Prognostication has been historically elusive in AD. While AD biomarker testing has revolutionized the in-vivo diagnosis of AD through measurements of amyloid-β42 (Aβ42) and tau, guiding prognosis with biomarkers is only in nascent stages. As such, the relationship between AD biomarkers and antipsychotic use is relatively unexplored. Investigators have examined the relationship between AD pathology and BPSD by measuring depression, apathy, psychosis, and agitation. These studies had small sample sizes and primarily utilized PET tracers [6–9], which provide valuable data on cortical Aβ42 and tau but are not used regularly in clinical practice [10–12].
CSF measurements of amyloid and tau are a common in vivo diagnostic approach to AD. However, a PubMed search on January 24, 2022 using medical subject headings (MeSH) “((“Cerebrospinal Fluid” [MeSH]) AND “Alzheimer Disease” [MeSH]) AND (“Behavioral Symptoms” [MeSH] OR “Depression” [MeSH] OR “Depressive Disorder” [MeSH] OR “Aggression” [MeSH] OR “Depressive Disorder, Major” [MeSH] OR “Psychomotor Agitation” [MeSH] OR “Delirium” [MeSH] OR “Apathy” [MeSH] OR “Psychotic Disorders” [MeSH] OR “Hallucinations” [MeSH])” only revealed 5 papers, none of which were relevant to clinical prognosis. Through further review, we found one paper looking at the longitudinal change in CSF following administration of risperidone versus galantamine found that, compared to baseline, levels of CSF Aβ42 were lower following administration of risperidone [13].
There appears to be a gap in the literature regarding CSF biomarkers of AD and BPSD. Filling this gap may help expand our use of CSF AD biomarkers beyond diagnosis and into personalized prognosis for AD. This retrospective analysis attempts to explore the relationship between CSF biomarkers of AD and antipsychotic use in patients with a definitive AD diagnosis through a survival analysis approach. Based on the link between tau deposition, clinical symptoms, and structural changes in the brain [10, 11, 14], along with widespread reports of normal cognition in the setting of amyloid deposition [11, 15, 16], we hypothesized that elevated Tau would predict sooner time to antipsychotic use.
MATERIALS AND METHODS
Our cohort was generated from an existing database of all patients over 65 with a chart diagnosis of Alzheimer’s type dementia (as defined previously in a large validated database) [17], seen at Northwestern Memorial HeathCare in Chicago, IL, USA between May 2005 and March 2018. This database was developed by the study authors to understand local patterns of antipsychotic use and was created through a query of Northwestern’s enterprise data warehouse (EDW). This retrospective study was approved by the Northwestern University Institutional Review Board through an expedited review and was exempt from individual patient consent requirements. We excluded patients with a diagnosis of schizophrenia, schizotypal disorder, bi-polar disorder, or psychotic illness due to other primary psychiatric diseases, patients enrolled in palliative care, patients with tracheostomy or PEG-tube, or those with a pervasive developmental disorder. From this database, we focused on the subsection of patients with a biomarker diagnosis of AD on CSF as part of their clinical care.
AD biomarker testing at our hospital is performed using the ADMark® sandwich ELISA assay for phosphorylated tau (P-tau 181), total tau (T-tau), and Aβ42 performed by Athena Diagnostics (Worcester, MA, USA). This assay has been well validated in research and clinical settings [18]. The results reported by the laboratory also include an index of the ratio of Aβ42 to P-tau, called the amyloid-to-tau index (ATI) [18]. All CSF biomarkers were tested during routine clinical practice by cognitive neurologists at Northwestern Memorial Hospital’s Neurobehavior and Memory clinic. The samples are stored in polypropylene tubes, frozen, and shipped to Athena diagnostics soon after collection. At the laboratory, samples are thawed and processed using separate ELISA antibodies for each test component. The ELISA is run a second time if the referral laboratory notes quality control issues or aberrant results. This is rare and, to our knowledge, was not required for any of the samples reported in our final analysis.
Based on CSF results, we excluded patients whose biomarkers were negative for the diagnosis of AD, despite a diagnosis of AD recorded in the electronic medical record (which may reflect an error in the record, so-called “chart lore”). Patients with borderline or indeterminate CSF values were included, given that all patients were diagnosed in consultation with a behavioral neurologist using their best clinical judgment.
After identifying this cohort of confirmed AD patients, we conducted a chart review to record patients’ Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam (MMSE) performed in the clinic before CSF testing. These values were not easily obtained through an EDW query and thus required chart review. To assist in the retrospective comparison of initial cognitive function between patients, MoCA scores were converted to the equivalent MMSE score, based on the ADNI study [19].
We compared the values of Aβ42, P-Tau, T-Tau, and ATI between patients who received an antipsychotic at any point following their lumbar puncture using a Mann-Whitney U test of statistical significance. However, we need to account for variable follow-up in our retrospective study by performing a survival analysis with censoring. We did this by stratifying our cohort by each CSF laboratory result to perform four separate survival analyses. We stratified patients by median split for Aβ42, T-tau, P-tau, or ATI. A median split was chosen because separating the cohort by interquartile ranges was limited by the small number of endpoint events in each group. For the survival analysis, we used Kaplan-Meier plots to compare the time to first antipsychotic use following CSF testing. The data of each survival curve was tested for statistical significance using a log-rank test.
If the log-rank test was statistically significant, we looked to see if baseline characteristics could explain the difference. We compared nominal features using a Chi-square test and quantitative data using a Mann-Whitney U or linear regression tests to determine statistical significance. Due to our results on the log-rank tests, this was performed only for our cohort as grouped by Aβ42 level. We used an alpha of 0.05 as our threshold for statistical significance for all analyses.
For statistical analysis, we used open-source packages for Python 3.7.3. Survival analysis was performed using Lifelines v0.25.7 [20]. Other tests of statistical significance were performed using SciPy v1.5.4 [21].
RESULTS
We identified 108 patients that received CSF testing for AD with a chart diagnosis of AD throughout our entire hospital system between 2012 and 2018. After excluding patients with negative CSF biomarkers, we identified 86 AD patients for our retrospective analysis.
Of those patients, 11 received an antipsychotic prescription in the months following their procedure. Of these 11 patients, the average time from CSF testing to antipsychotic prescription was 397±493 days. The average MMSE score and standard deviation at the time of testing were 24±4.5 indicating that the population overall had a mild degree of impairment. The average levels of CSF biomarkers for the entire group are given below in Table 1. Looking at the entire cohort, it appeared that antipsychotic use was associated with lower Aβ42 and correspondingly lower ATI. However, as noted previously in the methods, this does not account for variable follow-up with censoring, so a survival analysis was needed to confirm these results.
Table 1
Levels of CSF AD Biomarkers, Entire Group, and Antipsychotic (AP) versus No AP
| CFS Component Level (pg,±SD) | Entire Cohort (n = 86) | No AP (n = 75) | AP (n = 11) | p (AP versus no AP) |
| Aβ42 | 383±148 | 401±150 | 296±108 | 0.013 |
| P-Tau | 97±38 | 97±35 | 110±55 | 0.302 |
| T-Tau | 668±316 | 665±311 | 764±361 | 0.215 |
| ATI | 0.42±0.19 | 0.43±0.20 | 0.30±0.16 | 0.025 |
If our cohort was stratified by the level of P-tau, T-tau, or ATI levels (using a median split), there was no significant difference in time to antipsychotic use by a log-rank test. Only when patients were stratified by levels of Aβ42 was there a statistically significant difference (Table 2). With a median split for CSF levels of Aβ42, the lower group had an average Aβ42 level of 254±74 pg. The group with higher levels of Aβ42 had an average level of 504±83 pg. The survival curve comparing these two groups is found in Fig. 1. To see if other available variables could explain time to antipsychotic use, we compared demographic data and MMSE scores between the two groups. No statistically significant difference in these variables was found (Table 3).
Table 2
Results of a Log-rank Test for Time to Antipsychotic Use Following Testing
| CSF Biomarker Level | p |
| Aβ42 | 0.04 |
| P-Tau | 0.70 |
| T-Tau | 0.54 |
| ATI | 0.21 |
Fig. 1
Kaplan-Meier survival analysis of time-to-antipsychotic use following testing of AD biomarkers. The group with the lower Aβ42 is in blue, while the higher-Aβ42 group is in orange. The shaded regions represent the 95% confidence intervals. Patients in the lower Aβ42 group had sooner time-to-AP use which was confirmed with a log-rank test (p = 0.04).
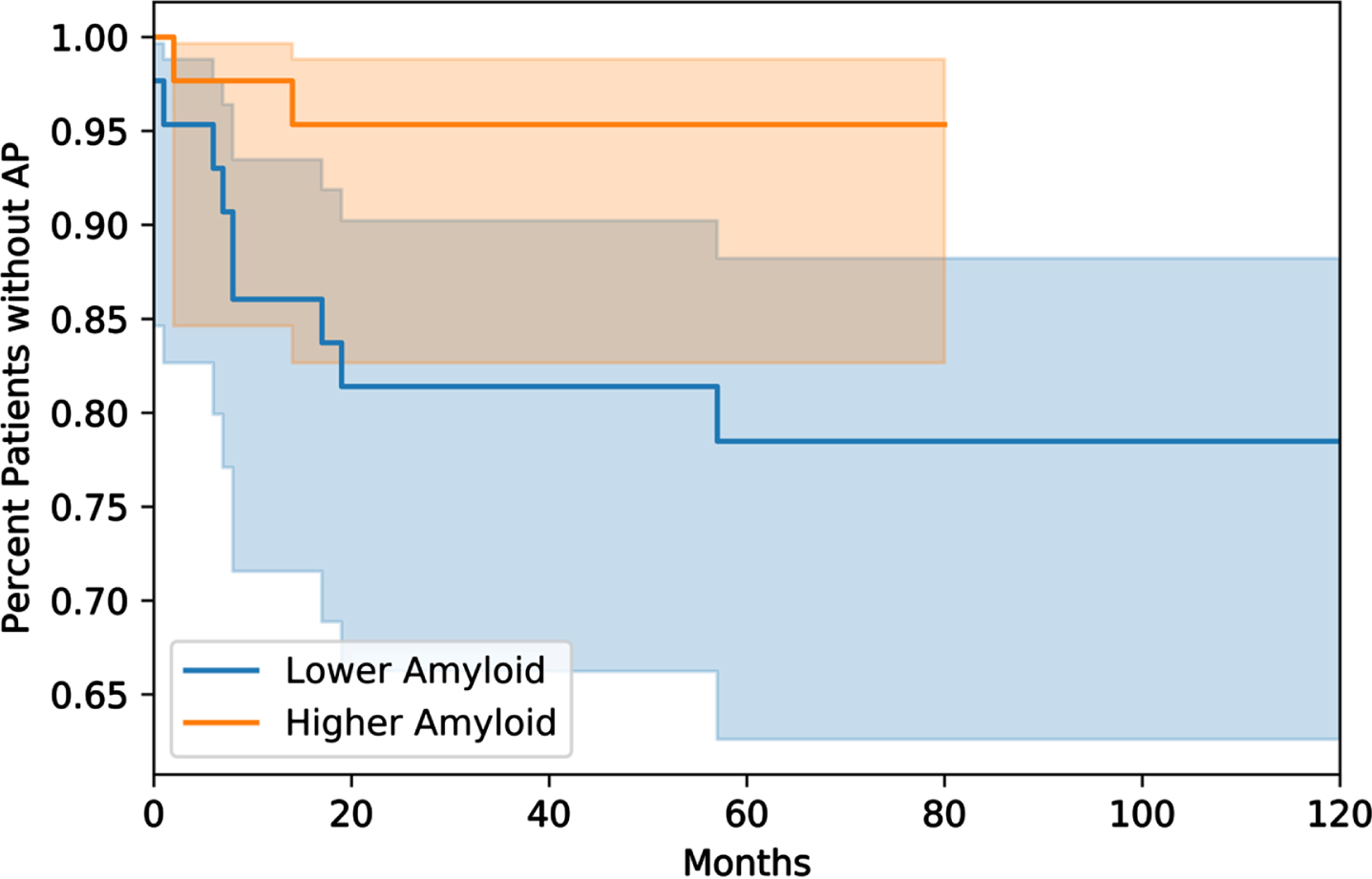
Table 3
Demographic Characteristics of the Lower Aβ42 versus Higher Aβ42 Group
| Aβ42 Low (n = 43) | Aβ42 High (n = 43) | p | |
| % M | 51.20% | 41.86% | 0.20 |
| % Non-white | 16.3% | 25.5% | 0.51 |
| Avg Age at CSF | 72.0±5.9 | 73.1±4.4 | 0.76 |
| MMSE | 24.1±4.3 | 23.9±4.9 | 0.35 |
| CCIDX | 3.09±2.55 | 3.09±2.36 | 0.49 |
| % On Cognitive Enhancer | 97.7% | 95.3% | 0.83 |
| No MMSE | 4 | 2 | 0.71 |
Patient Characteristics by Aβ42 Level (Median Split). MMSE, Mini-Mental Status Exam; CCIDX, Charlson Comorbidity Index.
In total, 9 patients in the group with lower Aβ42 received an antipsychotic, but only 2 in the higher group. Of those patients who received an antipsychotic, the average time to prescription was 428±538 days in the lower Aβ42 group. In the higher Aβ42 group, the time to antipsychotic use was 256±244 days. While these results may seem to contradict the results of the log-rank test, these numbers only reflect those patients who received an antipsychotic and not the likelihood of the group, as a whole, to receive an antipsychotic over time. The large standard deviation for time to antipsychotic use is accounted for by a skewed distribution of time to prescription with a long rightward tail.
DISCUSSION
Our findings add to a growing literature on BPSD and its relationship to biomarkers of Alzheimer’s disease. Initially, we hypothesized that tau would most closely associate with symptoms, given existing models of disease [10, 16, 22, 23]. This theory was supported by an earlier 2008 study by Skogseth et al. [24], showing an association between higher levels of tau in CSF and apathy. Another study showed CSF tau levels were associated with neuropsychiatric symptoms [23]. We were surprised by our conclusions, but other investigators have observed a relationship between Aβ42 and neuropsychiatric symptoms [6–9].
Though our results add to evidence of a connection between Aβ42 and neuropsychiatric symptoms, our study differed from previous research on BPSD and biomarkers in several ways. First, we looked only at patients with confirmed AD and did not use disease stage in our inclusion criteria (as opposed to MCI, pre-symptomatic AD, or patients with biomarker-proven Aβ42 deposition without pathologic levels of phosphorylated-tau). However, based on average MMSE scores, our population appeared to be in the milder stages of disease. Secondly, most other studies utilized amyloid PET imaging, not CSF, which, without confirmation of neurofibrillary tangle pathology, precludes a definitive diagnosis of AD.
Our study most closely approximates the 2008 Skogseth et al. paper [24], which looked at CSF biomarker levels in 32 patients with confirmed AD but, unlike tau as mentioned earlier, did not find an association between Aβ42 levels and neuropsychiatric symptoms. However, the authors looked for an association between symptoms and biomarker levels at a single point in time, while our paper used a survival analysis approach to assist in prognostication. Also, their CSF assay differed from the commercial Athena assay used in our retrospective study. At the time of the 2008 study, CSF AD biomarkers were in their nascent stages, only utilized in research and not available for routine clinical use.
We believe the distinction between CSF and PET biomarker studies is important. While the current cutoffs for CSF Aβ42 correlate well with positivity on Aβ42 PET [25] and Aβ42 deposition on autopsy, the precise level of Aβ42 in CSF may reflect individual physiology of CSF flow/Aβ42 clearance or an equilibrium between serum and CSF Aβ42 levels independent of plaque deposition [26]. Thus, unlike Aβ42 PET, low Aβ42 on CSF is not a direct measurement of cortical amyloid plaque burden and instead represents a measurement of the dynamic, multifaceted process of CSF Aβ42 clearance.
Limitations
Our approach had several limitations, however. First, because this is a retrospective analysis, we cannot control for confounding variables such as APOE status or more detailed assessments of psychiatric status at the time of antipsychotic use. Though we excluded patients with premorbid borderline personality disorder, schizophrenia, or psychosis due to primary medical illness, we could not control for the use of other psychiatric medications, which were not included in our query. We focused on antipsychotics because their use suggests more disruptive, challenging to treat agitated or psychotic behaviors, which we feel has more prognostic utility.
Furthermore, in our cohort, the number of patients who subsequently used an antipsychotic was small, limiting our ability to do subgroup analysis. The small number of endpoint events likely reflects that all patients included in this study were followed at a subspecialty cognitive neurology practice where prescribers are sensitive to the risks of antipsychotics, and social strategies are considered first-line. Also, CSF biomarkers do not provide anatomic information. Our institution makes regular clinical use of CSF biomarkers and is thus a good target for data mining of local electronic medical records.
Conclusions
Our study found that AD patients with lower CSF amyloid may have sooner time to antipsychotic use, suggesting a more aggressive course of BPSD. However, we cannot comment on the influence of location and degree of Aβ42 plaque deposition. With that in mind, the results of this study more accurately suggest that the physiology of amyloid clearance into CSF may play a role in the development of BPSD.
Besides a greater understanding of the pathophysiology of AD, further study of these biomarkers may also help clinicians provide a personalized medicine approach to prognostication, but more extensive prospective studies are needed. Expanding use of AD biomarkers beyond diagnosis is in early stages but may also help with tracking treatment responses, and increase the economic value of testing [27]. With CSF physiology in mind, future studies looking at fluid biomarkers of AD may also benefit from tandem investigation of processes essential to amyloid clearance such as sleep, glymphatic function, APOE status, blood-brain barrier integrity, and anti-amyloid medications. With the recent approval of aducanumab and lecanemab by the FDA, this research also highlights the importance of following behavioral outcomes in clinical trials targeting amyloid.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was funded by the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program.
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to report.
DATA AVAILABILITY
Due to HIPAA and the sensitivity of the electronic medical records data used our dataset is not shared publicly.
REFERENCES
[1] | Bellelli G , Morandi A , Di Santo SG , Mazzone A , Cherubini A , Mossello E , Bo M , Bianchetti A , Rozzini R , Zanetti E , Musicco M , Ferrari A , Ferrara N , Trabucchi M ((2016) ) “Delirium Day”: A nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med 14: , 106. |
[2] | Ballard CG , Gauthier S , Cummings JL , Brodaty H , Grossberg GT , Robert P , Lyketsos CG ((2009) ) Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 5: , 245–255. |
[3] | Kales HC , Lyketsos CG , Miller EM , Ballard C ((2019) ) Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. Int Psychogeriatr 31: , 83–90. |
[4] | Ballard C , Kales HC , Lyketsos C , Aarsland D , Creese B , Mills R , Williams H , Sweet RA ((2020) ) Psychosis in Alzheimer’s disease. Curr Neurol Neurosci Rep 20: , 57. |
[5] | Kirkham J , Sherman C , Velkers C , Maxwell C , Gill S , Rochon P , Seitz D ((2017) ) Antipsychotic use in dementia: Is there a problem and are there solutions? Can J Psychiatry 62: , 170–181. |
[6] | Krell-Roesch J , Lowe VJ , Neureiter J , Pink A , Roberts RO , Mielke MM , Vemuri P , Stokin GB , Christianson TJ , Jack CR , Knopman DS , Boeve BF , Kremers WK , Petersen RC , Geda YE ((2018) ) Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: The Mayo Clinic Study of Aging. Int Psychogeriatr 30: , 245–251. |
[7] | Idland A-V , Wyller TB , Støen R , Eri LM , Frihagen F , Ræder J , Chaudhry FA , Hansson O , Zetterberg H , Blennow K , Bogdanovic N , Brækhus A , Watne LO ((2016) ) Preclinical amyloid-β and axonal degeneration pathology in delirium. J Alzheimers Dis 55: , 371–379. |
[8] | Holmes SE , Esterlis I , Mazure CM , Lim YY , Ames D , Rainey-Smith S , Martins RN , Salvado O , Dore V , Villemagne VL , Rowe CC , Laws SM , Masters CL , Maruff P , Pietrzak RH ((2016) ) β-amyloid, APOE and BDNF genotype, and depressive and anxiety symptoms in cognitively normal older women and men. Am J Geriatr Psychiatry 24: , 1191–1195. |
[9] | Donovan NJ , Locascio JJ , Marshall GA , Gatchel J , Hanseeuw BJ , Rentz DM , Johnson KA , Sperling RA , Harvard Aging Brain Study ((2018) ) Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry 175: , 530–537. |
[10] | Jack CR , Knopman DS , Jagust WJ , Petersen RC , Weiner MW , Aisen PS , Shaw LM , Vemuri P , Wiste HJ , Weigand SD , Lesnick TG , Pankratz VS , Donohue MC , Trojanowski JQ ((2013) ) Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12: , 207–216. |
[11] | Petersen C , Nolan AL , de Paula França Resende E , Miller Z , Ehrenberg AJ , Gorno-Tempini ML , Rosen HJ , Kramer JH , Spina S , Rabinovici GD , Miller BL , Seeley WW , Heinsen H , Grinberg LT ((2019) ) Alzheimer’s disease clinical variants show distinct regionalpatterns of neurofibrillary tangle accumulation. ActaNeuropathol (Berl) 138: , 597–612. |
[12] | Wilde A , Maurik IS , Kunneman M , Bouwman F , Zwan M , Willemse EAJ , Biessels GJ , Minkman M , Pel R , Schoonenboom NSM , Smets EMA , Wattjes MP , Barkhof F , Stephens A , Lier EJ , Batrla-Utermann R , Scheltens P , Teunissen CE , Berckel BNM , Flier WM ((2017) ) Alzheimer’s biomarkers in daily practice (ABIDE) project: Rationale and design. Alzheimers Dement (Amst) 6: , 143–151. |
[13] | Bloniecki V , Aarsland D , Blennow K , Cummings J , Falahati F , Winblad B , Freund-Levi Y ((2017) ) Effects of risperidone and galantamine treatment on Alzheimer’s disease biomarker levels in cerebrospinal fluid. J Alzheimers Dis 57: , 387–393. |
[14] | Brier MR , Gordon B , Friedrichsen K , McCarthy J , Stern A , Christensen J , Owen C , Aldea P , Su Y , Hassenstab J , Cairns NJ , Holtzman DM , Fagan AM , Morris JC , Benzinger TLS , Ances BM ((2016) ) Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 8: , 10. |
[15] | Wallin AK , Blennow K , Zetterberg H , Londos E , Minthon L , Hansson O ((2010) ) CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 74: , 1531–1537. |
[16] | Jansen WJ , Ossenkoppele R , Knol DL , Tijms BM , Scheltens P , Verhey FRJ , Visser PJ , Aalten P , Aarsland D , Alcolea D , Alexander M , Almdahl IS , Arnold SE , Baldeiras I , Barthel H , van Berckel BNM , Bibeau K , Blennow K , Brooks DJ , van Buchem MA , Camus V , Cavedo E , Chen K , Chetelat G , Cohen AD , Drzezga A , Engelborghs S , Fagan AM , Fladby T , Fleisher AS , van der Flier WM , Ford L , Förster S , Fortea J , Foskett N , Frederiksen KS , Freund-Levi Y , Frisoni GB , Froelich L , Gabryelewicz T , Gill KD , Gkatzima O , Gómez-Tortosa E , Gordon MF , Grimmer T , Hampel H , Hausner L , Hellwig S , Herukka S-K , Hildebrandt H , Ishihara L , Ivanoiu A , Jagust WJ , Johannsen P , Kandimalla R , Kapaki E , Klimkowicz-Mrowiec A , Klunk WE , Köhler S , Koglin N , Kornhuber J , Kramberger MG , Van Laere K , Landau SM , Lee DY , de Leon M , Lisetti V , Lleó A , Madsen K , Maier W , Marcusson J , Mattsson N , de Mendonça A , Meulenbroek O , Meyer PT , Mintun MA , Mok V , Molinuevo JL , Møllergård HM , Morris JC , Mroczko B , Van der Mussele S , Na DL , Newberg A , Nordberg A , Nordlund A , Novak GP , Paraskevas GP , Parnetti L , Perera G , Peters O , Popp J , Prabhakar S , Rabinovici GD , Ramakers IHGB , Rami L , Resende de Oliveira C , Rinne JO , Rodrigue KM , Rodríguez-Rodríguez E , Roe CM , Rot U , Rowe CC , Rüther E , Sabri O , Sanchez-Juan P , Santana I , Sarazin M , Schröder J , Schütte C , Seo SW , Soetewey F , Soininen H , Spiru L , Struyfs H , Teunissen CE , Tsolaki M , Vandenberghe R , Verbeek MM , Villemagne VL , Vos SJB , van Waalwijk van Doorn LJC , Waldemar G , Wallin A , Wallin ÅK , Wiltfang J , Wolk DA , Zboch M , Zetterberg H ((2015) ) Prevalence of cerebral amyloid pathology inpersons without dementia: A meta-analysis. JAMA 313: , 1924. |
[17] | Jaakkimainen RL , Bronskill SE , Tierney MC , Herrmann N , Green D , Young J , Ivers N , Butt D , Widdifield J , Tu K ((2016) ) Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: A validation study using family physicians’ electronic medical records. J Alzheimers Dis 54: , 337–349. |
[18] | Tariciotti L , Casadei M , Honig LS , Teich AF , McKhann II GM , Tosto G , Mayeux R ((2018) ) Clinical experience with cerebrospinal fluid Aβ42, total and phosphorylated tau in the evaluation of 1,016 individuals for suspected dementia. J Alzheimers Dis 65: , 1417–1425. |
[19] | Helmi L , Meagher D , O’Mahony E , O’Neill D , Mulligan O , Murthy S , McCarthy G , Adamis D ((2016) ) Agreement and conversion formula between mini-mental state examination and montreal cognitive assessment in an outpatient sample. World J Psychiatry 6: , 358. |
[20] | Davidson-Pilon C , Kalderstam J , Jacobson N , Reed S , Kuhn B , Zivich P , Williamson M , Abdeali JK , Datta D , Fiore-Gartland A , Parij A , WIlson D , Gabriel , Moneda L , Moncada-Torres A , Stark K , Gadgil H , Jona , Singaravelan K , Besson L , Peña MS , Anton S , Klintberg A , Growth Jeff , Noorbakhsh J , Begun M , Kumar R , Hussey S , Seabold S , Golland D (2020) Lifelines: v0.25.7. |
[21] | SciPy 1.0 Contributors, Virtanen P , Gommers R , Oliphant TE , Haberland M , Reddy T , Cournapeau D , Burovski E , Peterson P , Weckesser W , Bright J , van der Walt SJ , Brett M , Wilson J , Millman KJ , Mayorov N , Nelson ARJ , Jones E , Kern R , Larson E , Carey CJ , Polat İ , Feng Y , Moore EW , VanderPlas J , Laxalde D , Perktold J , Cimrman R , Henriksen I , Quintero EA , Harris CR , Archibald AM , Ribeiro AH , Pedregosa F , van Mulbregt P ((2020) ) SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat Methods 17: , 261–272. |
[22] | Braak H , Alafuzoff I , Arzberger T , Kretzschmar H , Del Tredici K ((2006) ) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol (Berl) 112: , 389–404. |
[23] | Cotta Ramusino M , Perini G , Vaghi G , Dal Fabbro B , Capelli M , Picascia M , Franciotta D , Farina L , Ballante E , Costa A ((2021) ) Correlation of frontal atrophy and CSF tau levels with neuropsychiatric symptoms in patients with cognitive impairment: A memory clinic experience. Front Aging Neurosci 13: , 595758. |
[24] | Skogseth R , Mulugeta E , Ballard C , Rongve A , Nore S , Alves G , Aarsland D ((2008) ) Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord 25: , 559–563. |
[25] | Müller EG , Edwin TH , Stokke C , Navelsaker SS , Babovic A , Bogdanovic N , Knapskog AB , Revheim ME ((2019) ) Amyloid-β PET—Correlation with cerebrospinal fluid biomarkers and prediction of Alzheimer's disease diagnosis in a memory clinic. PLoS One 14: , e0221365. |
[26] | Strozyk D , Blennow K , White LR , Launer LJ , CSF Aβ 42 levels correlate with amyloid- neuropathology in a population-based autopsy study. Neurology 60: , 5. |
[27] | Cotta Ramusino M , Perini G , Altomare D , Barbarino P , Weidner W , Salvini Porro G , Barkhof F , Rabinovici GD , van der Flier WM , Frisoni GB , Garibotto V , Teipel S , Boccardi M ((2021) ) Outcomes of clinical utility in amyloid-PET studies: State of art and future perspectives. Eur J Nucl Med Mol Imaging 48: , 2157–2168. |




