Tooth Loss Induces Memory Impairment and Glial Activation in Young Wild-Type Mice
Abstract
Background:
Tooth loss is closely associated with Alzheimer’s disease (AD). Previously, we reported that tooth loss induced memory impairment in amyloid precursor protein knock-in mice by decreasing neuronal activity and synaptic protein levels and increasing glial activation, neuroinflammation, and pyramidal neuronal cell loss without altering amyloid-β levels in the hippocampus. However, the effects of tooth loss in young wild-type mice have not been explored yet.
Objective:
We investigated the effects of tooth loss on memory impairment, neuronal activity, synaptic protein levels, glial activation, and pyramidal neuronal cell loss in young wild-type mice.
Methods:
Two-month-old wild-type mice were randomly divided into control and tooth loss groups. In the tooth loss group, maxillary molar teeth on both sides were extracted, whereas no teeth were extracted in the control group. Two months after tooth extraction, we performed a novel object recognition test to evaluate memory function. Glial activation, neuronal activity, synaptic protein levels, and the number of pyramidal neurons were evaluated using immunofluorescence staining, immunohistochemistry, and western blotting.
Results:
The tooth loss group exhibited memory impairment and decreased neuronal activity and the levels of synaptic proteins in both the hippocampus and cortex. Moreover, tooth loss increased the activation of phosphorylated c-Jun N-terminal kinase (JNK), heat shock protein 90 (HSP90), and glial activation and reduced the number of pyramidal neurons in the hippocampus.
Conclusion:
Tooth loss in the young wild-type mice will attenuate neuronal activity, decrease synaptic protein levels, and induce pyramidal neuronal loss, and eventually lead to memory impairment.
INTRODUCTION
Alzheimer’s disease (AD), the most common form of dementia, is associated with aging and has become a prominent health concern. The causes of AD are still unclear, and discovering the factors that accelerate AD prognosis is necessary for the development of effective treatments [1]. AD is a slow, irreversible, and progressive neurodegenerative disease that is pathologically characterized by the cardinal features of intracellular neurofibrillary tangles and extracellular amyloid plaques [2]. Additionally, various other factors, such as oxidative stress and neuroinflammation, are associated with AD [3, 4].
Tooth loss is a prevalent condition in the global population [5] and can negatively affect the quality of life by reducing functionality and causing aesthetic and social challenges [6, 7]. Dental caries (also known as tooth decay) and periodontitis can cause or exacerbate numerous disorders, such as diabetes, endocarditis, inflammatory diseases, and AD [8]. Several studies have shown that the most common cause of tooth loss among adults is dental caries, which affects 5–10 teeth per person [9–11]. Chronic periodontitis is the most prevalent cause of tooth loss in the elderly and is associated with a higher risk of impaired cognitive performance [12]. Other causes of tooth loss include planned orthodontic treatment, failure of endodontic treatment, fracture, and prosthetic treatment [13].
Human and animal research has uncovered a substantial link between mastication and cognitive function, with the number of functioning teeth likely to be relevant [14]. Masticatory dysfunction is caused by tooth loss and decreases hippocampal neurogenesis, resulting in hippocampal-dependent spatial memory and learning deficiencies [15, 16]. Mastication sends a large amount of sensory information to the brain, which aids in the hippocampus’s learning and memory processes [17]. In contrast, masticatory dysfunction and long-term deafferentation following tooth loss may impair cerebral blood circulation and neurotransmitter production, potentially leading to neurodegeneration [18].
Glial cell activation is a typical pathogenic characteristic in AD. In the AD brain, activated microglia and astrocytes are found around amyloid-β (Aβ) plaques and play beneficial and detrimental roles in disease progression [19, 20]. An increase in pro-inflammatory cytokines released from activated astrocytes and microglia ultimately leads to severe synaptic and neuronal losses [21, 22]. Masticatory impairment caused by tooth loss is a major source of chronic stress that may activate microglia [23], causing hippocampus-dependent cognitive impairment [24, 25].
Previously, we reported that tooth loss induces memory impairment in AppNL - G - F mice, which overexpress humanized Aβ with amyloid precursor protein (APP) mutations [Swedish (NL), Beyreuther/Iberian (F), and Arctic (G)], by activating stress-activated protein kinases and glial cells, decreasing synaptic protein levels and neuronal activity, and inducing pyramidal neuron loss without the altering of Aβ levels [26]. The main finding of this study was that tooth loss in AppNL - G - F mice activated glial cells, leading to an increase in pro-inflammatory cytokines, such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α). However, whether tooth loss can induce memory impairment and exacerbate AD-like pathologies in nonpathological conditions is still unclear. Furthermore, the extent to which tooth loss affects young animals remains unclear. Therefore, we investigated whether tooth loss in young wild-type (WT) mice affects cognitive function, neuronal activity, synaptic loss, glial activation, and expression of the stress-inducible proteins phosphorylated c-Jun N-terminal kinase (JNK), heat shock protein 90 (HSP90).
In this study, we extracted maxillary molar teeth from both sides of two-month-old WT mice. Two months after tooth extraction, we found that tooth loss in WT mice induced memory impairment and glial activation, attenuating neuronal activity, decreasing synaptic protein levels, and reducing the number of pyramidal neurons in the hippocampus.
MATERIALS AND METHODS
Experimental animals
Two-month-old C57BL/6J mice were obtained from Japan SLC, Inc. (Hamamatsu, Japan). The mice were divided into control (n = 7) and tooth loss (n = 7) groups. Under general anesthesia (5 mg/kg butorphanol, 4 mg/kg midazolam, 0.3 mg/kg medetomidine), maxillary molar teeth on both sides were removed in the tooth loss group. The maxillary molars on both sides were unaltered in the control group. All mice were given a powder diet after tooth extraction and housed in a 12-h light/dark cycle and had free access to food and water. Animal experiments and protocols were approved by the Animal Experiments of the Animal Experimentation Committee of Nagoya City University (21-029).
Novel object recognition test
The experimental procedure previously described [26], with some modifications, was used to conduct a novel object recognition test two months after tooth extraction to measure short-term memory. This test is based on the principle that mice have instinct to explore new objects. The experimental installation was made up three objects (A, B, and C), of which A was same to B, while the object of C was obviously diverse from A and B. The testing paradigm of the novel object recognition test consisted of three sessions: habituation, training, and retention. Each mouse was allowed to explore an empty box individually (40 cm×40 cm×40 cm) for three days (habituation session). On the fourth day, mice explore two familial objects (A and B) that were placed at opposite sides of the box and each mouse was placed midway between the two objects and allowed it to explore for 5 min (training session). Twenty four hours later, one (B) of the familial objects was replaced with a novel one (C) in the same position, and the mouse placed back to the box and allowed to explore for 5 min (retention session). The amount of time spent exploring each object was recorded. A discrimination index was calculated to represent the time spent exploring the novel object compared to the familiar objects as a proportion of the total exploration time of each mouse.
Immunohistochemistry
Following behavioral testing, the mice were put under anesthesia, perfused with cold phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.5 mM KH2PO4; pH 7.4) transcardially, and had their brains removed immediately. One hemisphere of each brain was postfixed overnight at 4°C with 4% paraformaldehyde solution in PBS and the fixed brains were cryoprotected with increasing concentrations of sucrose (20% and 30%) in 0.1 M phosphate buffer at 4°C for 2–3 days. Serial sagittal sections (40μm thickness) were cut using a vibratome (Leica Microsystems) and stored at –20°C until histological analysis. Immunofluorescence staining was performed as previously described [26]. Sections were washed in PBS and blocked in 5% normal goat serum in PBS-T (0.25% Triton X in PBS) for 1 h at room temperature (RT) following antigen retrieval. Brain sections were incubated with primary antibodies overnight at 4°C. The primary antibodies used were rabbit polyclonal anti-Iba1 (1/100, Wako Pure Chemical Industries, Osaka, Japan), mouse monoclonal anti-GFAP (1/100, Sigma-Aldrich, St. Louis, MO, USA), mouse monoclonal anti-c-Fos (1/100, EnCor Biotechnology, Gainesville, FL, USA), and mouse monoclonal anti-NeuN (1/100, Sigma-Aldrich). After washing with PBS-T, the sections were incubated for 1 h at RT with secondary antibodies. The secondary antibodies used were goat anti-rabbit Alexa Fluor 555 antibody (1/2,000, Thermo Fisher Scientific, Rockford, IL, USA) and goat anti-mouse Alexa Fluor 488 antibody (1/2,000, Thermo Fisher Scientific). Nuclei were stained with DAPI (Vector Laboratories Inc, Burlingame, CA, USA). Fluorescence signals were detected with A SpinSR10 confocal fluorescence microscope (Olympus, Southall, UK). Quantification of fluorescence signal in brain sample images was carried out using ImageJ software (NIH, Bethesda, MD, USA). To quantify Iba1-, GFAP-, and c-Fos-positive cells, we randomly quantified 2 fields (1 mm2 area per field) of the hippocampus and 4 fields (1 mm2 area per field) of the cortex and the means were used for statistical analysis. Measurements were taken from sections from each mouse and immunostaining analysis was performed blinded.
Western blot analysis
For biochemical analysis, cortical and hippocampal tissues from the other hemisphere of each brain were dissected, frozen in liquid nitrogen, and stored at –80°C. Hippocampal and cortical tissues (n = 7 per group) were homogenized in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS; pH 7.6) containing protease and phosphatase inhibitor cocktails (Wako Pure Chemical Industries). The resulting lysates were incubated for 30 min on ice and centrifuged at 12,000 rpm for 30 min at 4°C. The protein concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific). The same amounts of protein were subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) and separated proteins were immunoblotted on polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA), followed by incubation with primary antibodies overnight at 4°C after blocking with 5% skim milk in tris-HCl-buffered saline with 0.1% Tween 20 (TBS-T) at RT for 1 h. The primary antibodies used were anti-Iba1 (1/1000, Wako Pure Chemical Industries), anti-GFAP (1/1000, Sigma-Aldrich), anti-synaptophysin (SYP, 1/20,000, Abcam, Cambridge, UK), anti-PSD95 (1/1000, Cell Signaling, Danvers, MA, USA), anti-phospho (p) JNK [p-SAPK/JNK (T183/Y185), 1/1,000, Cell Signaling], anti-JNK (1/1,000, SAPK/JNK, Cell Signaling), anti-HSP90 (1/1,000, Cell Signaling), and anti-actin (Proteintech Group, Tokyo, Japan). The membranes were the washed with TBS-T and incubated with secondary antibodies conjugated to horseradish peroxidase. ImmunoStar Zeta or ImmunoStar LD (Wako Pure Chemical Industries) was used to visualize immunoreactive bands, which were analyzed with an Amersham Imager 680 (GE Healthcare Life Science, Marlborough, MA, USA). Signal intensity was quantified using ImageJ program (Bethesda, MD, USA).
2.5Statistical analysis
Statistical analysis was carried out using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Data are presented as the mean±standard deviation (SD). Significant differences between groups were assessed by a two-tailed unpaired Student’s t-test. Data were considered significant at p < 0.05.
RESULTS
Tooth loss induces memory impairment
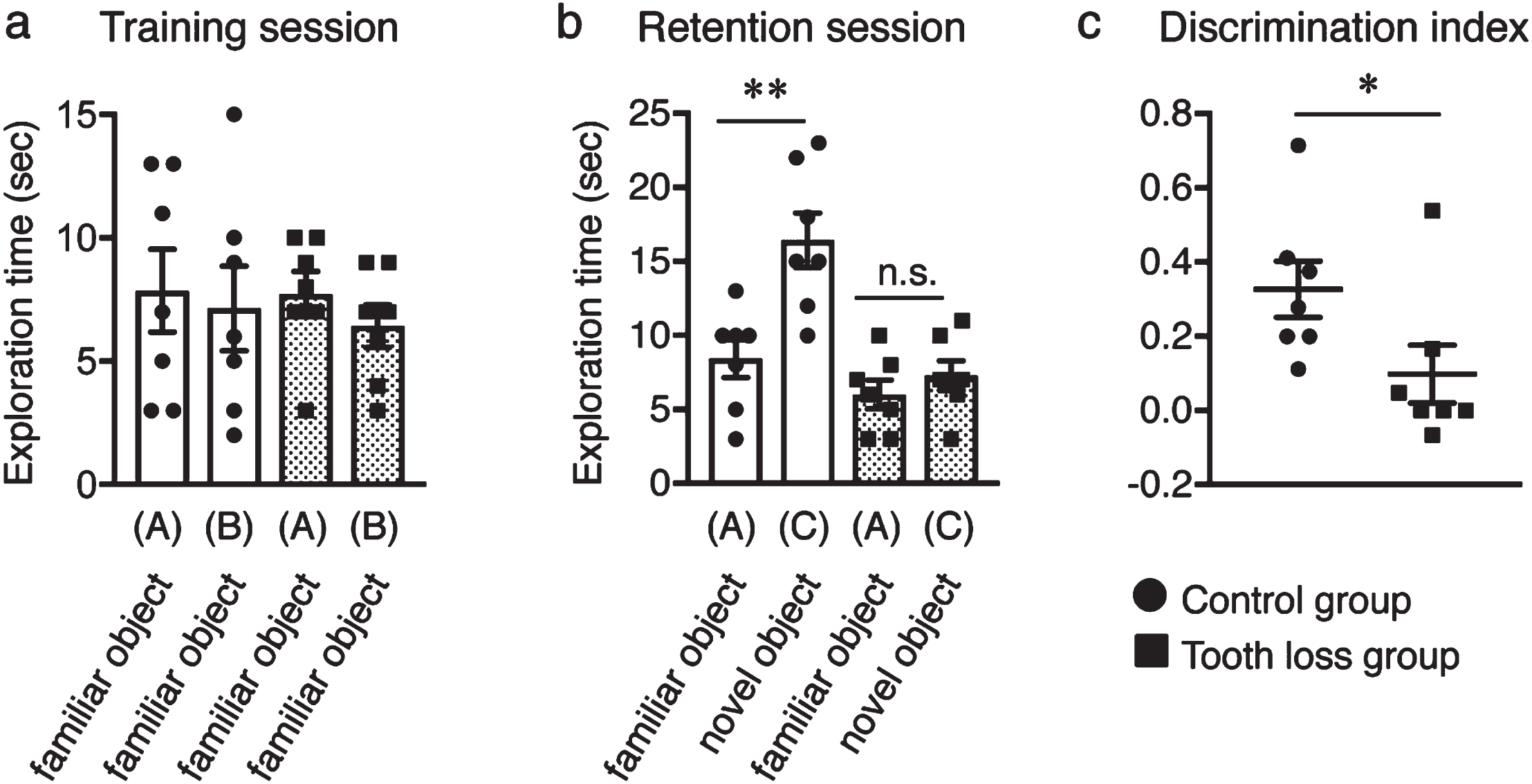
Tooth loss has been identified as a risk factor for AD. To evaluate the effect of tooth loss on visual recognition skills and short-term memory, we subjected WT mice to the novel object recognition test after extracting their upper molar teeth (the tooth loss group). WT mice with all teeth intact were used as the control group. During the training session, control and tooth loss mice spent almost the same amount of time exploring two familiar objects (Fig. 1a), suggesting that both groups had equal degrees of curiosity, motivation, and interest in exploring familiar objects. During the retention session, however, tooth loss mice spent similar amounts of time exploring both the familiar and novel objects, whereas control mice spent significantly more time exploring the novel object compared to the familiar object (Fig. 1b). Moreover, the discrimination index was higher in the control group than in the tooth loss group (Fig. 1c). This result indicates that tooth loss in WT mice induces visual recognition memory impairment, which is consistent with the results of our previous study in AppNL - G - F mice [26].
Fig. 1
Effect of tooth loss on memory impairment in wild-type mice. a) In the training session, both control and tooth loss mice exhibited the same exploratory time between the two familiar objects (A and B). b) In the retention session, the control mice spent a longer time exploring the novel object (C) than the familiar object (A), whereas tooth loss mice spent the same amount of time exploring the familiar object (A) and the novel object (C). c) Discrimination index (DI) of the novel object recognition test. Data are represented as the mean±SD. n = 7 per group. n.s., no significant difference; *p < 0.05, **p < 0.01 versus control group, as determined using Student’s t-test.
Tooth loss decreases neuronal activity in the hippocampus and cortex
The relationships between oral health, masticatory function, and cognitive function have been studied in animals and humans. During mastication, several regions of the central nervous system (CNS) are stimulated or activated. Previous studies have reported a clear correlation between lower neural activity and synapse formation as a result of diminished mastication [15]. Therefore, the number of activated neurons in the hippocampus and cortex of WT mice was measured via immunostaining of sagittal brain sections using the anti-c-Fos antibody as a neuronal activity marker. We found that the number of c-Fos+ neurons was significantly lower in the hippocampal dentate gyrus (DG), cornu ammonis (CA) 1, and CA3 regions (Fig. 2a) and cortex (Fig. 2b) of tooth loss mice than those in the control mice. Previously, it was reported that masticatory dysfunction is linked to a reduction in neural activity, which leads to a decrease in synaptic density [27]. Therefore, we quantified synaptic protein levels in the hippocampus and cortex using western blot analysis. Our results showed that the protein levels of synaptophysin (SYP, presynaptic protein) and postsynaptic density protein 95 (PSD95) were significantly decreased in the hippocampus and cortex of tooth loss mice compared to that of the control mice (Fig. 3a, b). These results indicate that tooth loss decreases neuronal activity and may contribute to synaptic dysfunction.
Fig. 2
Tooth loss decreases neuronal activity in the hippocampus and cortex. c-Fos immunostaining in the hippocampus (CA1, CA3, and DG regions) (a) and cortex (b). Sagittal brain sections were stained with an anti-c-Fos antibody (red) and DAPI nuclear counter stain (blue). The number of c-Fos+ cells in CA1, CA3, and DG in (a) and in the cortex in (b). CA, cornu ammonis; DG, dentate gyrus. Data are represented as the mean±SD. n = 6 per group. **p < 0.01 versus control group, as determined using Student’s t-test. Scale bars in (a): 500μm and in (b): 100μm.
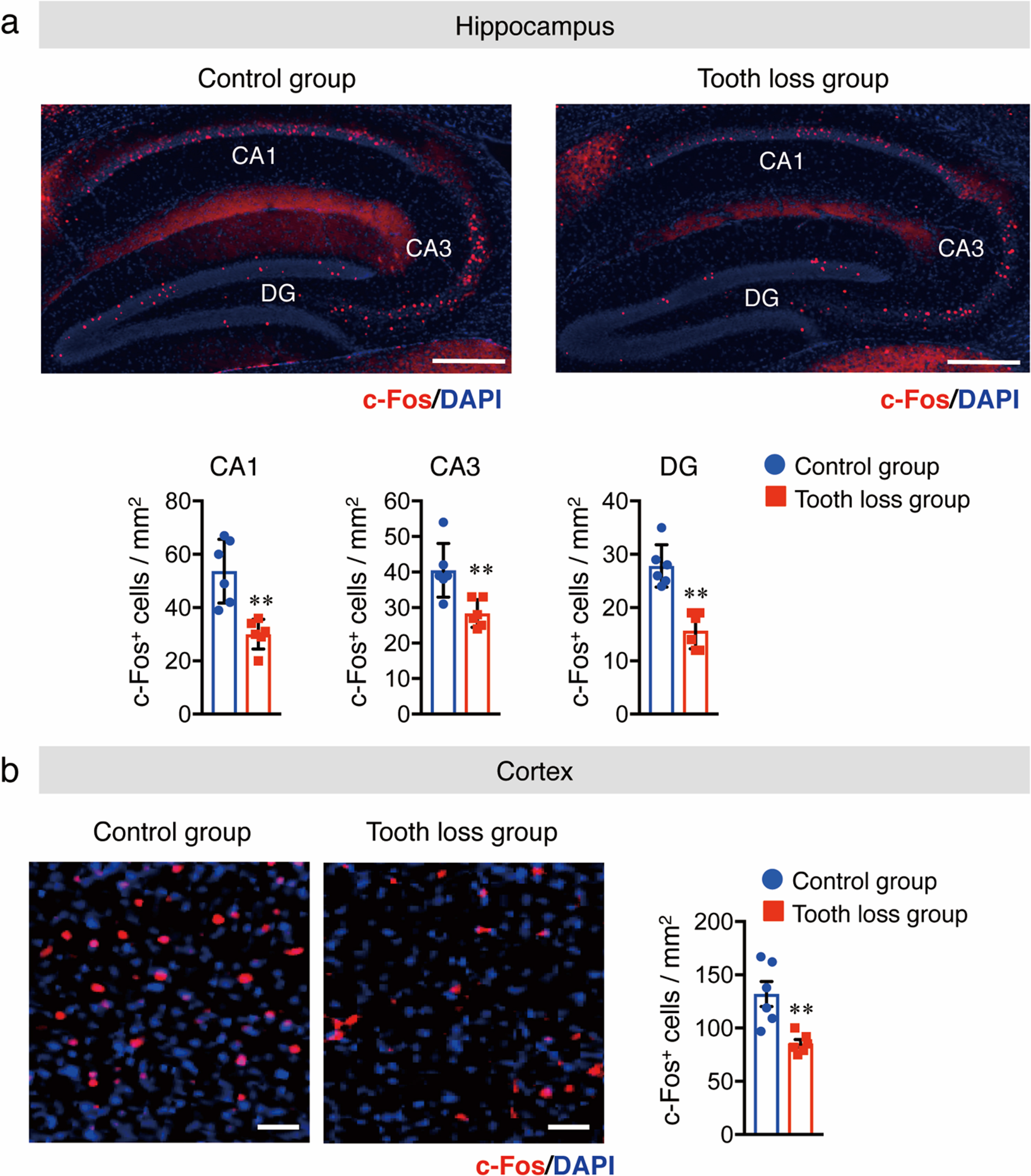
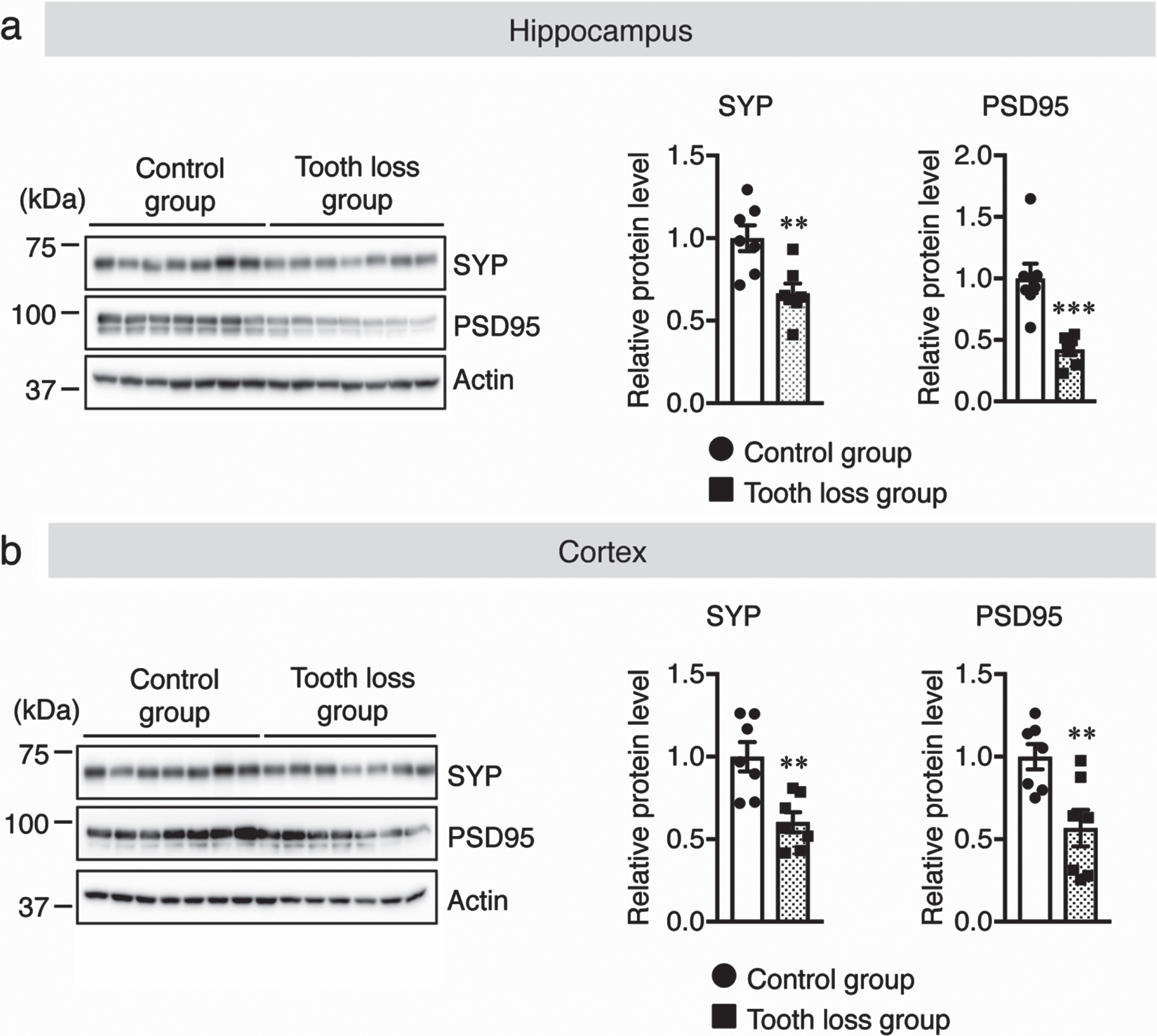
Fig. 3
Tooth loss decreases synaptic protein levels in the hippocampus and cortex. Western blot analysis of synaptophysin (SYP), PSD95, and actin in the hippocampus (a) and cortex (b). Protein levels were quantified by densitometry, normalized to the actin level, and expressed as the relative protein level. Data are represented as the mean±SD. n = 7 per group. **p < 0.01, ***p < 0.001 versus control group, as determined using Student’s t-test.
3.3Tooth loss increases protein levels of pJNK and HSP90 in the hippocampus
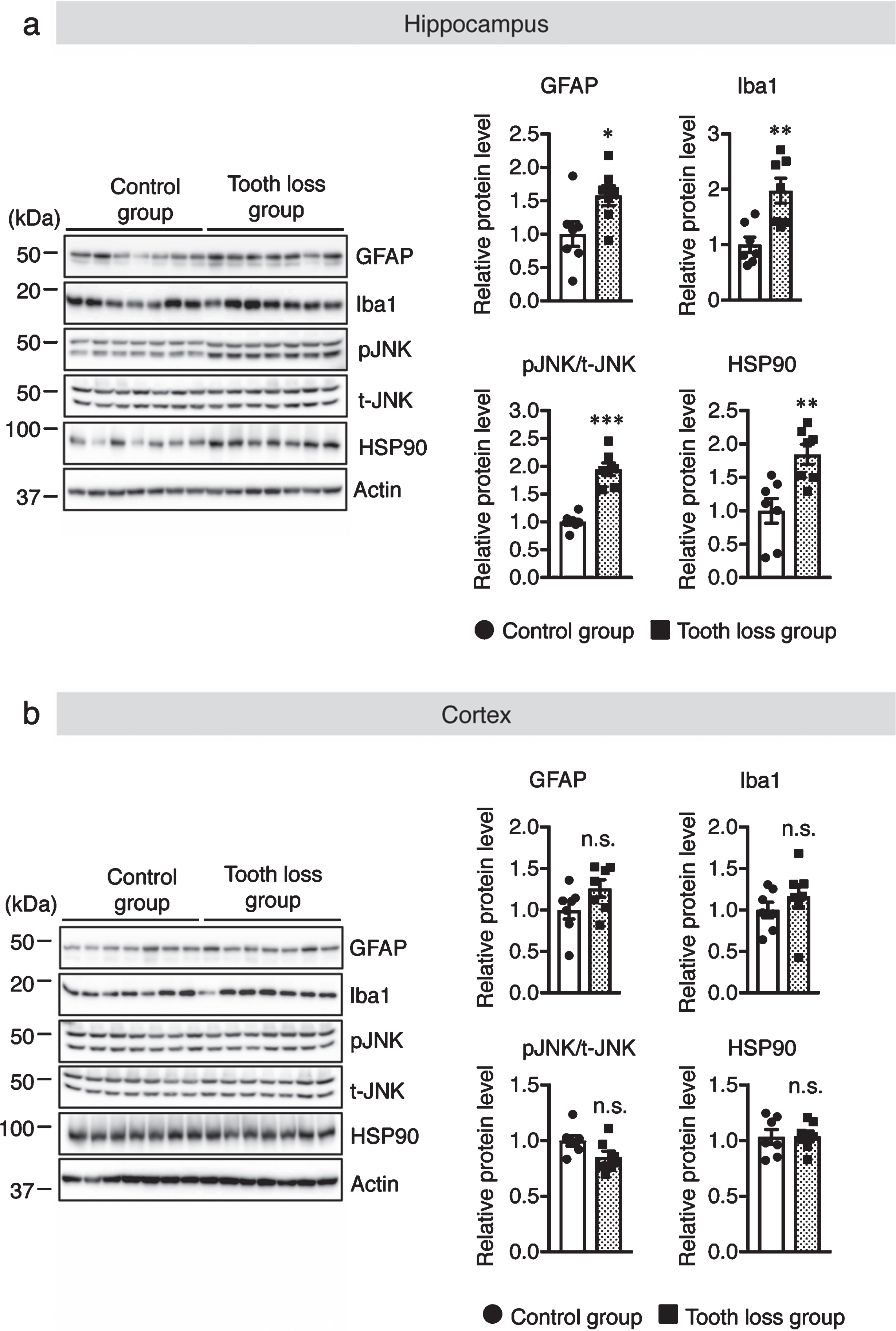
Masticatory dysfunction can also lead to chronic stress. A previous study showed that chronic stress caused by tooth loss impairs memory and learning functions [24]. Some proteins, such as heat shock protein 90 (HSP90) and the protein kinase JNK, are activated by chronic stress [28, 29]. Therefore, we measured the protein levels of HSP90 and phosphorylated JNK in the hippocampus and the cortex. Our western blot results showed that the levels of both proteins were significantly increased in the hippocampus, but not in the cortex, of tooth loss mice compared to that of the control mice (Fig. 4a, b). This result indicates that tooth loss induces chronic stress in the hippocampus.
Fig. 4
Tooth loss increases the protein levels of GFAP, Iba1, phosphorylated JNK, and HSP90 in the hippocampus. Western blot analysis of GFAP, Iba1, phosphorylated (p-) JNK, total (t-) JNK, HSP90, and actin in the hippocampus (a) and cortex (b). Protein levels were quantified by densitometry. GFAP and Iba1 protein levels were normalized to actin. p-JNK protein levels were normalized to t-JNK protein levels and expressed as values relative to the control. Data are represented as the mean±SD. n = 7 per group. n.s., no significant difference; *p < 0.05, **p < 0.01, ***p < 0.001 versus control group, as determined using Student’s t-test.
3.4Tooth loss activates glial cells in the hippocampus
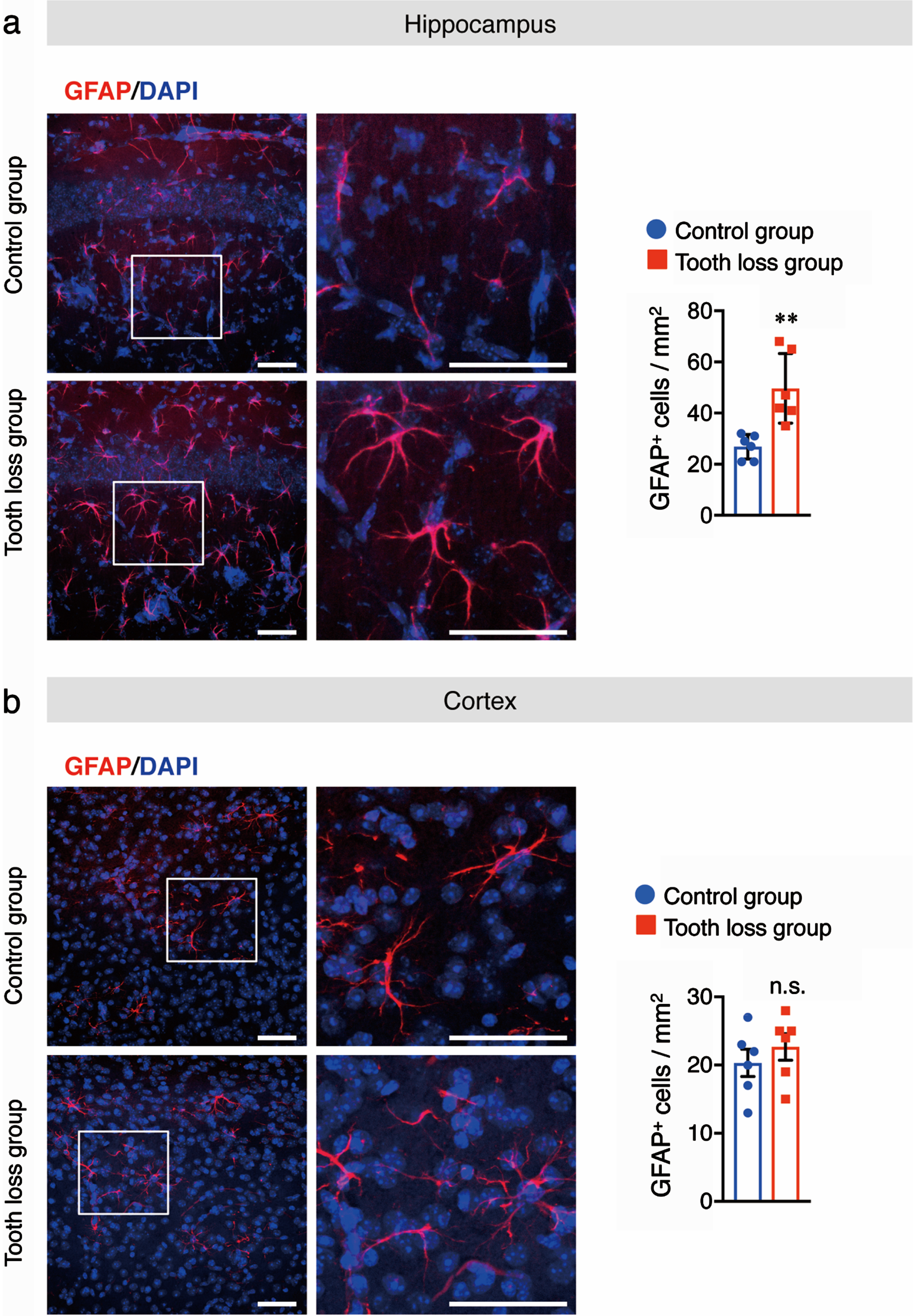
Chronic stress can alter the morphology and density of microglia [30]. Reactive microglia and astrocytes are involved in the progression of AD through the release of pro-inflammatory cytokines, which leads to neuronal cell death. To determine the effect of tooth loss on glia in WT mice, we investigated the levels of activated microglia and astrocytes in the brain. Western blotting was used to quantify the protein levels of Iba1, a microglia marker, and GFAP, an astrocyte marker, in hippocampal and cortical tissues. We found that the levels of both Iba1 and GFAP were higher in the hippocampus of tooth loss mice than in that of control mice (Fig. 4a). No significant difference between treatment groups was observed for either marker in the cortex (Fig. 4b). Next, we performed immunofluorescence staining using anti-Iba1 and anti-GFAP antibodies to confirm the results of our western blot analysis. As expected, we observed a significant increase in the number of Iba1+ (Fig. 5a) and GFAP+ cells (Fig. 6a) in the hippocampus of tooth loss mice compared to that in the control mice. Consistent with the western blotting results, the number of Iba1+ and GFAP+ cells was not significantly different in the cortex between treatment groups; however, we did note a tendency for both markers to be more abundant in tooth loss mice than that in the control mice (Fig. 5b, 6b). These data indicate that tooth loss in WT mice may activate astrocytes and microglia in the hippocampus.
Fig. 5
Tooth loss enhances microglial activation in the hippocampus. Sagittal brain sections of mice were immunostained with the anti-Iba1 antibody (red) and cell nuclei were stained with DAPI (blue). Representative images of Iba1+ cells in the hippocampus (a) and cortex (b) are shown. Numbers of Iba1+ cells in the hippocampus (a, right panel) and cortex (b, right panel) are presented. Boxed regions in the left panel images are magnified in the right panels. Data are represented as the mean±SD. n = 6 per group. n.s., no significant difference; **p < 0.01 versus control group, as determined using Student’s t-test. Scale bars: 100μm.
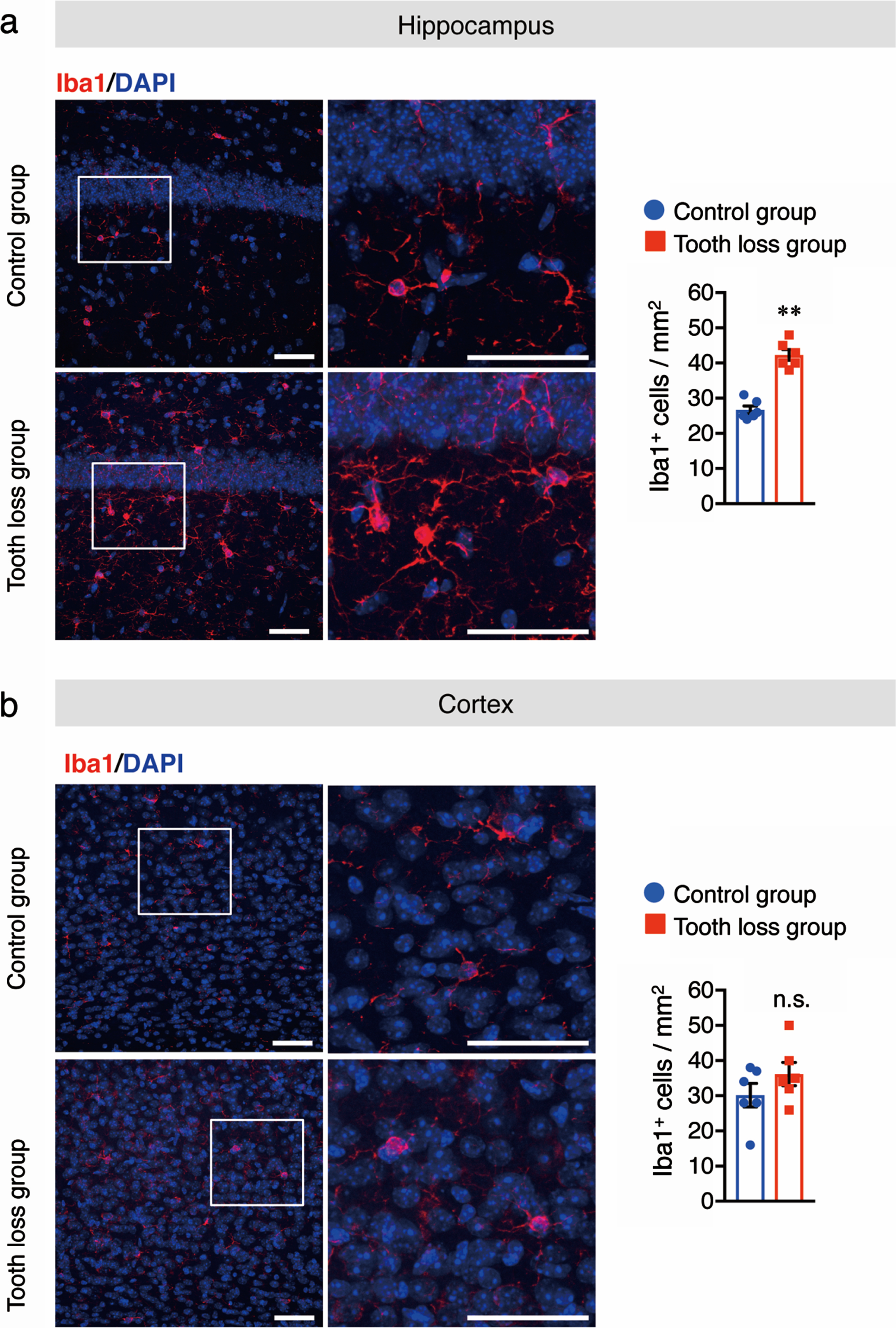
Fig. 6
Tooth loss enhances astrocyte activation in the hippocampus. Sagittal brain sections of mice were immunostained with the anti-GFAP antibody (red) and cell nuclei were stained with DAPI (blue). Representative images of GFAP+ cells in the hippocampus (a) and cortex (b) are shown. Numbers of GFAP+ cells in the hippocampus (a, right panel) and cortex (b, right panel) are presented. Boxed regions in the left panel images are magnified in the right panels. Data are represented as the mean±SD. n = 6 per group. n.s., no significant difference; **p < 0.01 versus control group, as determined using Student’s t-test. Scale bars: 100μm.
3.5The number of pyramidal neurons in the hippocampus is reduced as a result of tooth loss
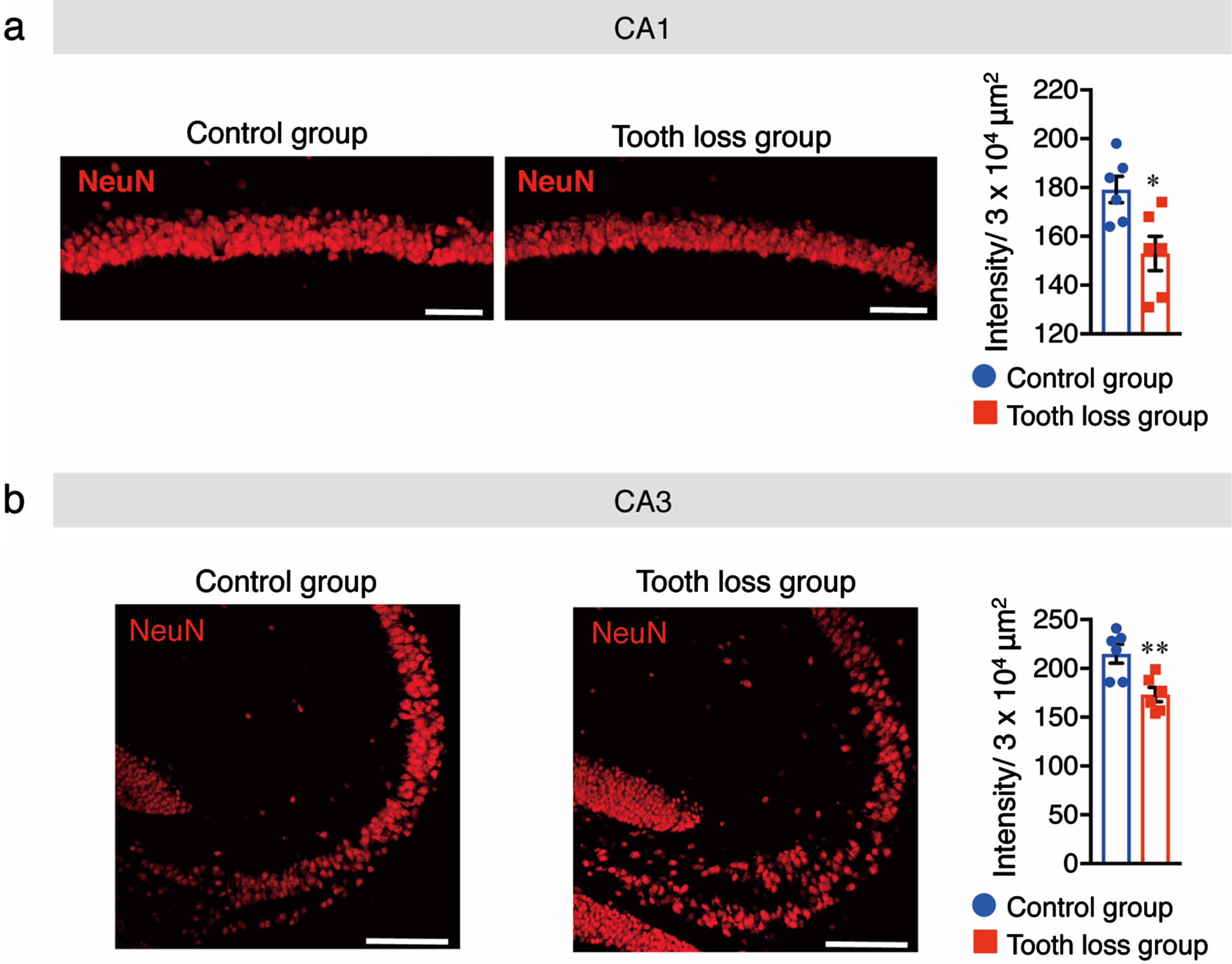
Previous studies have suggested that tooth loss and microglial activation induce pyramidal neuronal loss. Indeed, our previous findings showed that tooth loss in AppNL - G - F mice induced pyramidal neuronal loss in the CA1 and CA3 regions of the hippocampus. Therefore, we investigated the number of pyramidal neurons in CA1 and CA3 regions of the hippocampus following tooth loss in WT mice. Sagittal brain sections were immunostained with an anti-NeuN antibody to mark neuronal cells and the fluorescence intensity of NeuN+ cells was quantified. In the CA1 and CA3 regions of the hippocampus, fluorescence intensity was significantly lower in tooth loss mice than in control mice (Fig. 7); no difference was observed in the cortex between treatment groups (data not shown). These findings suggest that tooth loss in WT mice may induce pyramidal neuronal loss in the CA1 and CA3 regions of the hippocampus through tooth loss-induced glial activation.
Fig. 7
Tooth loss induces pyramidal neuronal loss in the hippocampus. Sagittal brain sections of mice were immunostained with the anti-NeuN antibody. Representative images of NeuN fluorescence staining in the CA1 (a) and CA3 (b) regions of the hippocampus are shown, along with quantification of the fluorescence intensity of NeuN+ cells. Data are represented as the mean±SD. n = 6 per group. *p < 0.05, **p < 0.01 versus control group, as determined using Student’s t-test. Scale bars: 50μm.
DISCUSSION
Recently, several studies by our group and others have shown that tooth extraction in elderly animals and AD-like mouse models aggravates AD-like pathologies, which eventually lead to the impairment of learning and memory functions [24, 26, 31–33]. In this study, we aimed to expand on the findings of these previous studies by investigating the effects of tooth loss in young WT mice. This work discovered that tooth loss attenuated neuronal activity, decreased synaptic protein levels, and induced pyramidal neuronal loss even in young WT mice, ultimately leading to memory impairment.
Several clinical and animal studies have demonstrated that masticatory stimulation or chewing plays an important role in transferring sensory information to many areas of the CNS and preserving hippocampus-dependent cognitive function [17]. c-Fos, an immediate early gene, is activated in response to neuronal activity and modulates synaptic plasticity, and its expression is positively correlated with cognitive function [34]. We speculate that the reduced neuronal activity (as indicated by the number of c-Fos+ neurons) observed in the hippocampal CA1 and CA3 regions and cortex of molarless mice may be related to changes in the motor neuron activity that controls the masticatory muscles [35]. These findings are consistent with our previous findings that tooth loss in AppNL - G - F mice reduces the number of c-Fos+ cells in the hippocampus and cortex. Furthermore, this reduction in neuronal activity may explain the reduction in synaptic proteins observed following tooth loss. Neuronal activity is important for the regulation of synaptic strength and plasticity [36, 37]. Synaptic dysfunction and impaired synaptic plasticity are implicated in several neurodegenerative diseases, including the early stages of AD [38]. In AD, soluble Aβ oligomers can bind to synaptic proteins, resulting in synaptic dysfunction associated with cognitive decline [39]. Furthermore, impaired mastication caused by tooth loss reduces sensory input from sensory receptors, which in turn reduces synaptic density in the cerebral cortex [40]. Thus, we speculate that the reduced expression of pre- and postsynaptic proteins in the hippocampus and cortex following tooth loss in WT mice is a direct result of the decreased neuronal activity observed in these regions.
Glial activation is involved in several neurodegenerative diseases, including AD. It has been reported that cooperative activity between glial cells and neurons results in the modulation of cognitive function [41]. Recent studies have demonstrated that early synaptic and neuronal losses occur due to the release of neuroinflammatory cytokines by reactive glial cells [42–44]. Indeed, we observed an increase in the number of activated Iba1+ and GFAP+ glial cells in the hippocampus following tooth loss, which coincided with the loss of pyramidal neurons in this region. Thus, we hypothesize that tooth loss-activated glial cells in the hippocampus may secrete proinflammatory cytokines, which cause pyramidal neuron cell death. However, we were unable to measure the mRNA levels of proinflammatory and anti-inflammatory cytokines due to limited brain samples; thus, further studies are required to measure these cytokine levels to fully elucidate the underlying mechanisms.
Chronic stress is widely recognized as a predisposing and promoting factor for some neurodegenerative diseases, including AD [45]. Chronic stress activates the hypothalamic-pituitary-adrenal axis, resulting in an increase in glucocorticoid hormones released from the adrenal cortex, which reduces neuronal activity and increases microglial activation [46]. Occlusal disharmony and reduced mastication due to tooth loss act as chronic stressors that impair learning and memory function [24, 27]. Moreover, occlusal disharmony increases plasma glucocorticoid levels, leading to a decrease in the number of neurons in the hippocampus, further contributing to memory impairment [47]. Interestingly, we found that tooth loss elevated the levels of the stress biomarkers, pJNK and HSP90, in the hippocampus but not in the cortex. This result is consistent with the increase in glial activation we observed in the hippocampus, suggesting that chronic stress caused by reduced mastication may be responsible for this glial phenotype. However, it is unclear why tooth loss induces chronic stress in the hippocampus but not in the cortex. One possible explanation is that the hippocampus is one of the first brain regions that is functionally and structurally modified by stress [48]. Further work will be required to understand the different stress responses of the hippocampus and cortex to tooth loss. Taken together, tooth loss in WT mice induces chronic stress in the hippocampus, leading to glial activation, which may eventually contribute to memory impairment in young WT mice.
Consistent with our previous finding that tooth loss in AppNL - G - F mice induced memory impairment and exacerbated AD-related pathologies, similar detrimental effects were also exerted in young mice by tooth loss, as demonstrated by memory impairment, attenuating neuronal activity, decreasing synaptic protein levels, and reducing the number of pyramidal neurons in the hippocampus. Thus, observations in the present study suggest that treating diseased teeth, orthodontic treatment and preserving them from a young age are very important for the acquisition of memory and learning ability.
Conclusion
In the present study, we investigated the effects of tooth loss on cognitive function, neuronal activity, synaptic protein levels, glial activation, and pyramidal neuronal cell loss in in young WT mice. We found that tooth loss induced memory impairment by reducing neuronal activity and synaptic density and increasing pJNK and HSP90 levels, which in turn increased glial activation in the hippocampus. From our study and others, it is becoming increasingly apparent that oral health is linked to general health status. Our study also suggests that treating diseased teeth and preserving them from a young age may lower the risk of future memory impairment.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported by JSPS KAKENHI Grant Numbers 15K15712 (to M.M.) and 20K07762 (to C-G. J.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also supported by AMED under Grant Numbers JP20dk0207050h001 and JP20de010702 (to M.M.).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Burns A , Iliffe S ((2009) ) Alzheimer’s disease. BMJ 338: , b158. |
[2] | Selkoe DJ ((2001) ) Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev 81: , 741–766. |
[3] | Heneka MT , Carson MJ , El Khoury J , Landreth GE , Brosseron F , Feinstein DL , Jacobs AH , Wyss-Coray T , Vitorica J , Ransohoff RM , Herrup K , Frautschy SA , Finsen B , Brown GC , Verkhratsky A , Yamanaka K , Koistinaho J , Latz E , Halle A , Petzold GC , Town T , Morgan D , Shinohara ML , Perry VH , Holmes C , Bazan NG , Brooks DJ , Hunot S , Joseph B , Deigendesch N , Garaschuk O , Boddeke E , Dinarello CA , Breitner JC , Cole GM , Golenbock DT , Kummer MP ((2015) ) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14: , 388–405. |
[4] | Tonnies E , Trushina E ((2017) ) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57: , 1105–1121. |
[5] | Marcenes W , Kassebaum NJ , Bernabe E , Flaxman A , Naghavi M , Lopez A , Murray CJ ((2013) ) Global burden of oral conditions in 1990–2010: A systematic analysis. J Dent Res 92: , 592–597. |
[6] | Batista MJ , Lawrence HP , de Sousa Mda L ((2014) ) Impact of tooth loss related to number and position on oral health quality of life among adults. Health Qual Life Outcomes 12: , 165. |
[7] | Gerritsen AE , Allen PF , Witter DJ , Bronkhorst EM , Creugers NH ((2010) ) Tooth loss and oral health-related quality of life: A systematic review and meta-analysis. Health Qual Life Outcomes 8: , 126. |
[8] | Brennan DS , Singh KA ((2011) ) General health and oral health self-ratings, and impact of oral problems among older adults. Eur J Oral Sci 119: , 469–473. |
[9] | Papas A , Joshi A , Giunta J ((1992) ) Prevalence and intraoral distribution of coronal and root caries in middle-aged and older adults. Caries Res 26: , 459–465. |
[10] | Slade GD , Spencer AJ ((1997) ) Distribution of coronal and root caries experience among persons aged 60+ in South Australia. Aust Dent J 42: , 178–184. |
[11] | Winn DM , Brunelle JA , Selwitz RH , Kaste LM , Oldakowski RJ , Kingman A , Brown LJ ((1996) ) Coronal and root caries in the dentition of adults in the United States, 1988–1991. J Dent Res 75 Spec No: , 642–651. |
[12] | Kaye EK , Valencia A , Baba N , Spiro A , 3rd Dietrich T , Garcia RI ((2010) ) Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc 58: , 713–718. |
[13] | Ayna B , Ayna E , Celenk S ((2010) ) Endodontic and prosthetic treatment of teeth with periapical lesions in a 16-year-old-girl. J Appl Oral Sci 18: , 201–206. |
[14] | Elsig F , Schimmel M , Duvernay E , Giannelli SV , Graf CE , Carlier S , Herrmann FR , Michel JP , Gold G , Zekry D , Muller F ((2015) ) Tooth loss, chewing efficiency and cognitive impairment in geriatric patients. Gerodontology 32: , 149–156. |
[15] | Fukushima-Nakayama Y , Ono T , Hayashi M , Inoue M , Wake H , Ono T , Nakashima T ((2017) ) Reduced mastication impairs memory function. J Dent Res 96: , 1058–1066. |
[16] | Oue H , Miyamoto Y , Okada S , Koretake K , Jung CG , Michikawa M , Akagawa Y ((2013) ) Tooth loss induces memory impairment and neuronal cell loss in APP transgenic mice. Behav Brain Res 252: , 318–325. |
[17] | Miyake S , Wada-Takahashi S , Honda H , Takahashi SS , Sasaguri K , Sato S , Lee MC ((2012) ) Stress and chewing affect blood flow and oxygen levels in the rat brain. Arch Oral Biol 57: , 1491–1497. |
[18] | Lin CS ((2018) ) Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr 18: , 5. |
[19] | Dickson DW , Farlo J , Davies P , Crystal H , Fuld P , Yen SH ((1988) ) Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am J Pathol 132: , 86–101. |
[20] | Saito T , Matsuba Y , Mihira N , Takano J , Nilsson P , Itohara S , Iwata N , Saido TC ((2014) ) Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci 17: , 661–663. |
[21] | Gaur S , Agnihotri R ((2015) ) Alzheimer’s disease and chronic periodontitis: Is there an association? Geriatr Gerontol Int 15: , 391–404. |
[22] | Holmes C , Cotterell D ((2009) ) Role of infection in the pathogenesis of Alzheimer’s disease: Implications for treatment. CNS Drugs 23: , 993–1002. |
[23] | de Pablos RM , Herrera AJ , Espinosa-Oliva AM , Sarmiento M , Munoz MF , Machado A , Venero JL ((2014) ) Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation 11: , 34. |
[24] | Kubo KY , Iwaku F , Watanabe K , Fujita M , Onozuka M ((2005) ) Molarless-induced changes of spines in hippocampal region of SAMP8 mice. Brain Res 1057: , 191–195. |
[25] | Kubo KY , Kojo A , Yamamoto T , Onozuka M ((2008) ) The bite-raised condition in aged SAMP8 mice induces dendritic spine changes in the hippocampal region. Neurosci Lett 441: , 141–144. |
[26] | Taslima F , Jung CG , Zhou C , Abdelhamid M , Abdullah M , Goto T , Saito T , Saido TC , Michikawa M ((2021) ) Tooth loss induces memory impairment and gliosis in App knock-in mouse models of Alzheimer’s disease. J Alzheimers Dis 80: , 1687–1704. |
[27] | Onozuka M , Watanabe K , Nagasaki S , Jiang Y , Ozono S , Nishiyama K , Kawase T , Karasawa N , Nagatsu I ((2000) ) Impairment of spatial memory and changes in astroglial responsiveness following loss of molar teeth in aged SAMP8 mice. Behav Brain Res 108: , 145–155. |
[28] | Han B , Yu L , Geng Y , Shen L , Wang H , Wang Y , Wang J , Wang M ((2016) ) Chronic stress aggravates cognitive impairment and suppresses insulin associated signaling pathway in APP/PS1 mice. J Alzheimers Dis 53: , 1539–1552. |
[29] | Jolly C , Morimoto RI ((2000) ) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92: , 1564–1572. |
[30] | Tynan RJ , Naicker S , Hinwood M , Nalivaiko E , Buller KM , Pow DV , Day TA , Walker FR ((2010) ) Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun 24: , 1058–1068. |
[31] | Kato T , Usami T , Noda Y , Hasegawa M , Ueda M , Nabeshima T ((1997) ) The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behav Brain Res 83: , 239–242. |
[32] | Onozuka M , Watanabe K , Fujita M , Tomida M , Ozono S ((2002) ) Changes in the septohippocampal cholinergic system following removal of molar teeth in the aged SAMP8 mouse. Behav Brain Res 133: , 197–204. |
[33] | Oue H , Miyamoto Y , Koretake K , Okada S , Doi K , Jung CG , Michikawa M , Akagawa Y ((2016) ) Tooth loss might not alter molecular pathogenesis in an aged transgenic Alzheimer’s disease model mouse. Gerodontology 33: , 308–314. |
[34] | Gallo FT , Katche C , Morici JF , Medina JH , Weisstaub NV ((2018) ) Immediate early genes, memory and psychiatricdisorders: Focus on c-Fos, Egr1 and Arc. Front Behav Neurosci 12: , 79. |
[35] | Shimazaki Y , Soh I , Saito T , Yamashita Y , Koga T , Miyazaki H , Takehara T ((2001) ) Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res 80: , 340–345. |
[36] | Chaudhury S , Sharma V , Kumar V , Nag TC , Wadhwa S ((2016) ) Activity-dependent synaptic plasticity modulates the critical phase of brain development. Brain Dev 38: , 355–363. |
[37] | Walmsley B , Berntson A , Leao RN , Fyffe RE ((2006) ) Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways. J Physiol 572: , 313–321. |
[38] | Mango D , Saidi A , Cisale GY , Feligioni M , Corbo M , Nisticò R ((2019) ) Targeting synaptic plasticity in experimental models of Alzheimer’s disease. Front Pharmacol 10: , 778. |
[39] | Benarroch EE ((2018) ) Glutamatergic synaptic plasticity and dysfunction in Alzheimer disease: Emerging mechanisms. Neurology 91: , 125–132. |
[40] | Yamamoto T , Hirayama A ((2001) ) Effects of soft-diet feeding on synaptic density in the hippocampus and parietal cortex of senescence-accelerated mice. Brain Res 902: , 255–263. |
[41] | Perea G , Araque A ((2010) ) GLIA modulates synaptic transmission. Brain Res Rev 63: , 93–102. |
[42] | Dejanovic B , Huntley MA , De Maziere A , Meilandt WJ , Wu T , Srinivasan K , Jiang Z , Gandham V , Friedman BA , Ngu H , Foreman O , Carano RAD , Chih B , Klumperman J , Bakalarski C , Hanson JE , Sheng M ((2018) ) Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron 100: , 1322–1336 e1327. |
[43] | Hong S , Beja-Glasser VF , Nfonoyim BM , Frouin A , Li S , Ramakrishnan S , Merry KM , Shi Q , Rosenthal A , Barres BA , Lemere CA , Selkoe DJ , Stevens B ((2016) ) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352: , 712–716. |
[44] | Shi Q , Chowdhury S , Ma R , Le KX , Hong S , Caldarone BJ , Stevens B , Lemere CA ((2017) ) Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med 9: , eaaf6295. |
[45] | Sharma VK , Singh TG , Garg N , Dhiman S , Gupta S , Rahman MH , Najda A , Walasek-Janusz M , Kamel M , Albadrani GM , Akhtar MF , Saleem A , Altyar AE , Abdel-Daim MM ((2021) ) Dysbiosis and Alzheimer’s disease: A role for chronic stress? Biomolecules 11: , 678. |
[46] | Nair A , Bonneau RH ((2006) ) Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol 171: , 72–85. |
[47] | Kubo KY , Yamada Y , Iinuma M , Iwaku F , Tamura Y , Watanabe K , Nakamura H , Onozuka M ((2007) ) Occlusal disharmony induces spatial memory impairment and hippocampal neuron degeneration via stress in SAMP8 mice. Neurosci Lett 414: , 188–191. |
[48] | McEwen BS ((2000) ) The neurobiology of stress: From serendipity to clinical relevance. Brain Res 886: , 172–189. |




