Translation of Collaborative Dementia Care Management into Different Healthcare Settings: Study Protocol for a Multicenter Implementation Trial (DCM:IMPact)
Abstract
Background:
Collaborative care models for people living with dementia (PwD) have been developed and evaluated, demonstrating safety, efficacy, and cost-effectiveness. However, these studies are based on heterogeneous study populations and primary care settings, limiting the generalizability of the results. Therefore, this study aims to implement and evaluate collaborative care across various healthcare settings and patient populations.
Objective:
To describe the study design of this multicenter implementation trial.
Methods:
This single-arm, multicenter, longitudinal implementation study will be conducted in five different healthcare settings, including 1) physicians’ networks, 2) dementia networks, 3) counselling centers, 4) hospitals, and 5) ambulatory care services. Eligibility criteria are: having a formal dementia diagnosis or having been screened positive for dementia and living community-dwelling. The staff of each healthcare setting identifies patients, informs them about the study, and invites them to participate. Participants will receive a baseline assessment followed by collaborative individualized dementia care management, comprising proven safe, effective, and cost-effective modules. Over six months, specially-qualified nurses will assess patients’ unmet needs, transfer them to individualized care plans, and address them, cooperating with various healthcare providers. A follow-up assessment is conducted six months after baseline. Approximately 60–100 PwD per setting per year are expected to participate. Differences across settings will be assessed regarding acceptability, demand, implementation success and barriers, efficacy, and cost-effectiveness.
Results:
We expect that acceptability, demand, implementation success and barriers, efficacy, and cost-effectiveness will vary by patients’ sociodemographic and clinical characteristics and unmet needs in each setting.
Conclusion:
The results will provide evidence highlighting differences in the implementation of collaborative care in various healthcare settings and demonstrating the settings with the highest need, best conditions for a successful implementation, and highest (cost-)effectiveness, as well as the population group that benefits most from collaborative care.
Trial registration:
German Clinical Trials Register: DRKS00025074. Registered 16 April 2021-retrospectively registered.
INTRODUCTION
Due to demographic changes, the older population is rapidly increasing and thus, the prevalence of age-associated diseases, such as dementia. The number of persons living with dementia (PwD) is predicted to increase from 57 million to 153 million worldwide by 2050 [1]. Additionally, to the substantial prevalence, considering the costs of dementia further underlines the health priority of dementia diseases. The global costs of dementia are estimated to be over $1 trillion and could double by 2030 [2]. In Germany, the total annual costs of PwD from the payers’ perspective are approximately € 34 billion and could reach € 90 billion by 2060 [3].
Without a cure, early identification and diagnostic and appropriate post-diagnostic support and care could delay the progression of this disease [4, 5]. However, previous studies revealed that only 39% of PwD were detected in primary care [6]. Only 30% and 36% receive recommended and evidence-based dementia-specific medication with antidementia drugs and non-drug treatments, respectively [7–9]. Additionally, 22% of PwD receive potentially inappropriate medications [10]. The resulting drug-related problems, in particular, are associated with adverse health-related outcomes and increasing healthcare costs [11]. Therefore, almost all community-dwelling PwD (99%) and their caregivers (97%) have unmet care needs, representing a prevailing inappropriate treatment and care situation [12].
Several countries introduced collaborative, multi-professional, and intersectoral dementia care management (DCM) programs to overcome the challenges of timely diagnosis with guideline-related post-diagnostic support and improve treatment and care in dementia diseases [13, 14]. These programs are defined as interventions provided in the community to coordinate the treatment and care for PwD and meet their individual needs according to evidence-based guidelines [15, 16]. There is already some evidence on the effectiveness of such approaches [17]. Previous studies revealed that DCM could delay patients’ institutionalization, reduce behavioral disturbances and depression, and reduce the caregivers’ burden [17]. The DelpHi-MV trial (Dementia: Life- and Person-Centered Help) [18] conducted in the primary care setting confirmed the findings of previous trials, demonstrating significant improvements in patients’ pharmaceutical treatment, neuropsychiatric symptoms, and the caregivers’ burden, but also patients’ health-related quality of life and overall cost-effectiveness [19, 20]. In particular, subgroups such as those living alone and with high comorbidity benefit most from DCM [21, 22].
Despite these previous findings, the evidence is limited to specific study populations (e.g., mildly cognitively impaired patients) and healthcare settings (e.g., primary care) where collaborative care was implemented, limiting the results’ generalizability. For implementation into the primary care system, there is still a lack of knowledge about different acceptance rates, implementation barriers, and success, as well as the quantitative demand for such collaborative models of dementia care. The DCM concept must be implemented and evaluated across different primary care settings to assess differences in acceptance, efficacy, and cost-effectiveness to reveal which setting would be most suitable for implementing collaborative dementia care and how benefits can be optimized.
MATERIALS AND METHODS
Trial objective
The Dementia Care Management Implementation Study (DCM:IMPact) aims to assess the acceptance, implementation barriers, and success as well as the efficacy and cost-effectiveness of collaborative dementia care across various healthcare settings.
Study design and setting
The DCM:IMPact study is a single-arm, multicenter, longitudinal implementation study of collaborative care that is implemented across the following different German healthcare settings: 1) physicians’ network, 2) ambulatory care service, 3) regional dementia care network, 4) counselling care center, and 5) hospital.
Nurses will receive an equipped workplace in the respective setting and must pass a dementia-specific qualification during the first six months. The curriculum elaborated and evaluated within DelpHi-MV comprises theoretical and practical elements amounting to 610 hours. Requirements and qualification contents are described elsewhere [23, 24].
Collaborative DCM will be implemented in these five healthcare settings for 18 months as a subsidiary health service complementing existing routine care structures, including a baseline and six-month follow-up assessment.
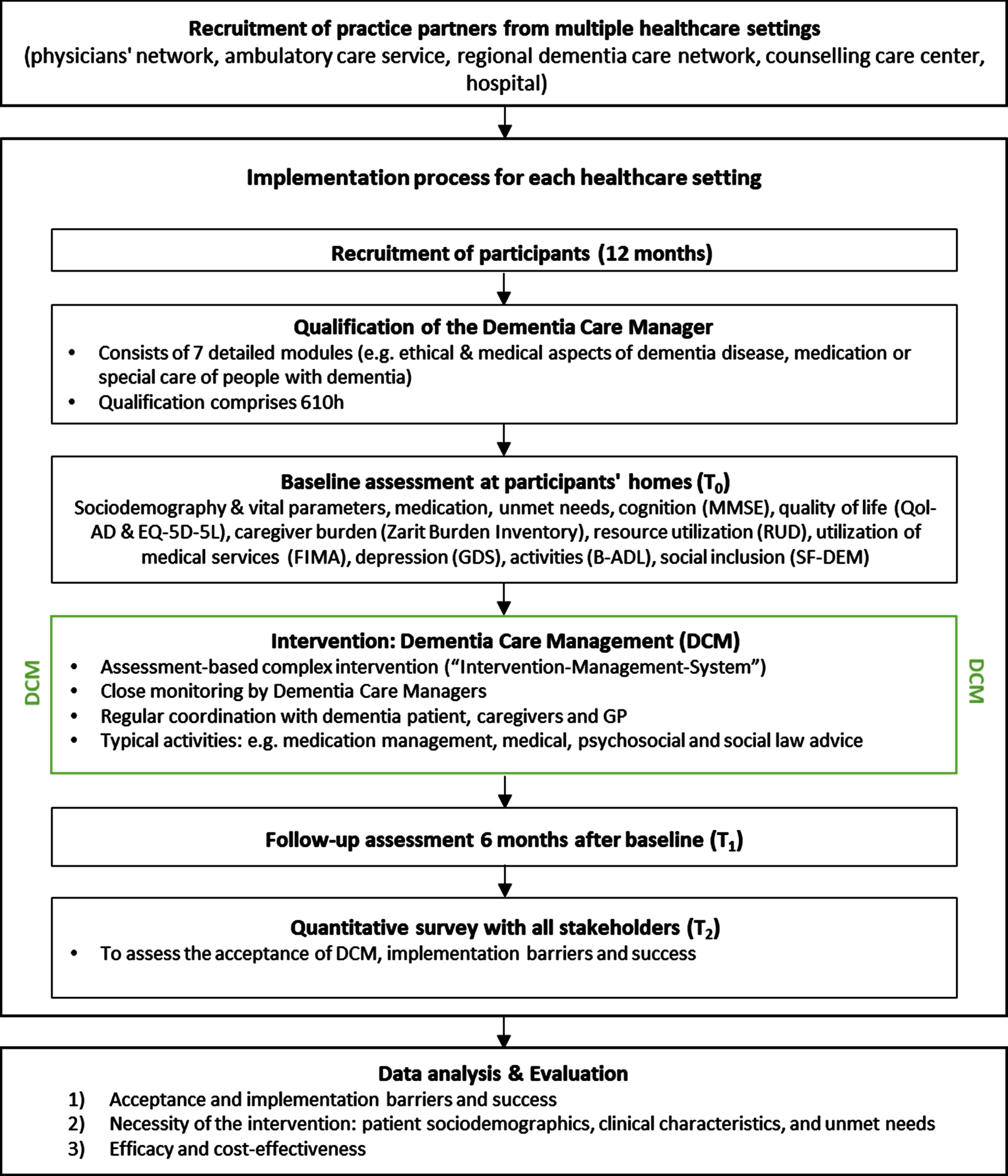
The DCM will be provided for six months for each PwD following the intervention of the DelpHi trial, which was proven to be safe, effective, and cost-effective [19, 20, 25]. Figure 1 illustrates the study design.
Fig. 1
DCM:IMPact study design.
Eligibility criteria
Patients are eligible if they meet the following criteria: 1) having a formal dementia diagnosis or having been screened positive for dementia using the DemTect procedure [26], 2) residing in the community, 3) being able to read and understand the German language, and 4) provide written informed consent (IC) for participation in the study. PwD living in nursing homes or institutionalized care settings will not be included in the study. In addition, caregivers of PwD could also be included in the study. While for the study enrolment of the PwD it is not required to have an informal caregiver, the eligibility criteria for caregivers are: 1) the caregiver has been designated as a family caregiver by the study participant, and 2) has provided written IC.
Recruitment
Recruitment proceeds on two levels. 1) The first level includes cooperation with practice partners. “Memoranda of Understanding” are concluded with suitable cooperation partners representing one of the targeted settings. The following goals guided the preselection of the respective healthcare settings: i) patients should be approached early as possible and ii) the settings in their entirety should represent the principal access to healthcare in Germany. 2) At the patient level, the collaborative partners will identify potential participants in their respective settings. Recruitment is adapted to each setting. In the physician networks, the respective GPs or specialists, like neurologists and psychiatrists, will recruit patients and potential family caregivers according to the eligibility criteria. In nursing services, regional dementia care networks, and counselling centers, qualified staff members will take on this task, inform eligible patients about the study and invite them and their informal caregivers (if applicable) to participate. Obtaining written IC for the study will be done as routine care in each setting.
Study timeline
This implementation study will consist of three phases and will be conducted over nine years in five settings. 1) The first year serves as the preparatory phase. 2) After one year, the next phase starts with the first patients being enrolled in the first setting. Within each setting, the intervention will be carried out over 18 months. Subsequently, enrollment into the second setting will start after two and a half years. Following this pattern, the last patient will be enrolled in the final setting after eight years, receiving the intervention for six months. Thus, for each setting, patients can be recruited within a time frame of 12 months. After completing the baseline assessment (T0), the intervention will be carried out for six months for each PwD. Over six months, each care plan is implemented over six months in collaboration with various healthcare providers, including monitoring and reviewing outcomes. A follow-up assessment (T1) will be conducted six months after baseline. 3) The last six months of the study are dedicated to the scientific evaluation according to the mentioned outcomes.
Ethical approval
The study will be conducted following the criteria of the Helsinki Declaration, the ICH Guidelines for Good Clinical Practice, the Memorandum for the Preservation of Good Scientific Practice (DFG), the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS/WHO), the CONSORT criteria, and the Guidelines to Develop and Evaluate Complex Interventions (Medical Research Council). The Ethical Committee of the Chamber of Physicians of Mecklenburg-Western Pomerania approved the study protocol and documents for written IC (registry number BB 001/19).
Only individual interviews and questionnaire surveys will be conducted in this study. Patients or family caregivers can withdraw their consent to participate in the study. According to current knowledge, there are no risks or complications for patients due to the assessment of the intervention. No medications or medical devices will be used as part of the intervention. The intervention was already implemented and tested in the DelpHi-MV study. Therefore, no harmful side effects to PwD or caregivers are expected. Nevertheless, health parameters are regularly collected to intervene if necessary.
Intervention
Explanation for the choice of comparators
Collaborative DCM will be implemented successively for two years in five different German healthcare settings and evaluated in terms of acceptability, implementation barriers and success, efficacy, and cost-effectiveness, which means that differences in these outcomes will be assessed by comparing the five different settings. As previously mentioned, recruitment will be conducted differently by the respective stakeholders in each setting. Either primary care physicians, specialists, or selected staff will identify patients, inform them about the study, and invite them to participate. Once enrolled, PwD will automatically be assigned to the intervention that dementia-specifically qualified nurses (dementia care managers) carry out. The intervention, procedures, and measures are similar to the randomized, controlled DelpHi-MV trial. The control group of this study will also be used for sub-group analyses testing the efficacy and cost-effectiveness of the intervention groups compared to usual care (control group of the DelpHi-MV trial).
Intervention description
The collaborative DCM intervention starts with a comprehensive assessment of the PwD and caregivers’ medical, pharmaceutical, social, psycho-social, and care needs. The assessed needs are transferred into an individualized list of intervention tasks needed to address the identified unmet needs. The intervention plan will be subsequently discussed and validated with the treating GP and carried out in close cooperation with various healthcare providers of the specific setting within six months [25]. After the assessment, PwD (and their family caregivers, if existing and participating) receive the intervention according to the DelpHi-Standard [25]. The intervention addresses the following three main pillars: 1) medication management, 2) treatment and care management, and 3) caregiver support. These pillars comprise eight different action fields that each include several foci. Each focus is associated with specific intervention modules containing algorithms and trigger points based on the initial assessment results. This intervention procedure is supported by a computer-based intervention management system (IMS). The IMS is an interactive system that supports the dementia care manager in identifying patients’ unmet needs and generating adequate interventions to address these needs. Following predefined filter functions and algorithms, possible interventions are selected and listed in a preliminary treatment and care plan. The intervention is described in more detail elsewhere [25].
Strategies to improve adherence to interventions
During the six-month time frame of the intervention, the dementia care manager monitors whether the indicated interventions to address a specific need of the patient as much as possible are successfully implemented [18, 25]. If not, the nurse will actively try to support the implementation of these tasks. The personal and regular contact between the dementia care manager and the patients also helps to strengthen adherence to all interventions that require the active participation of patients and caregivers.
Adverse event reporting and harms
Since the DCM aims to provide existing routine care resources and services to PwD according to their needs, adverse events or harms due to the intervention or study implementation are generally not expected. However, if adverse events or harms occur, they will be documented. All processes within the study will be conducted in close collaboration with primary care physicians and other healthcare providers in the respective settings.
Outcomes
The following outcomes will be assessed to evaluate the differences in the implementation of collaborative dementia care management between the five healthcare settings:
(1) Acceptance and implementation barriers and success
Implementation success will be assessed by the number of recruited and treated PwD and caregivers during the implementation period of two years in each setting. We hypothesize that the higher the recruitment and care load of care management (i.e., identified unmet needs), the lower the implementation barriers and the higher the success. Although we cannot predict the number of patients for each setting, we assume it differs depending on their sociodemographic and clinical characteristics and unmet needs. Furthermore, we will assess the acceptance, intervention barriers and success using self-designed quantitative surveys administered at the end of each implementation period. Patients, caregivers and participating and/or affected healthcare providers will be asked to state their subjectively perceived benefits from the collaborative model of care and whether or not the intervention was supportive for patients and caregivers. Additionally, we will evaluate whether healthcare providers state that the IMS was supportive.
(2) Necessity of the intervention: patient sociodemographics, clinical characteristics, and unmet needs
Patients’ and caregivers’ socio-demographics (age, sex, living situation: alone versus not alone) and clinical differences (cognitive and functional impairment, comorbidities) will be assessed. The patient’s cognitive and functional impairment will be assessed using the Mini-Mental State-Examination (MMSE) [27] and the Bayer Activities of Daily Living Scale [28], respectively. Patient characteristics will be used to identify at what stage of the disease patients were allocated to the intervention. Previous studies indicated that collaborative care management programs are more effective in the early stages of diseases [19–21]. Furthermore, the number of unmet needs will be assessed across the various healthcare settings. Unmet needs cover medical, psychosocial, social-legal, medication, and nursing needs and will be assessed by using the IMS [29]. The higher the number of unmet needs, the higher the demand for such programs, and the higher the likelihood of achieving a higher efficacy and cost-effectiveness.
(3) Efficacy and cost-effectiveness
Since DCM implementation depends on the particular patients’ and caregivers’ needs, the healthcare services initiated and their utilization, intervention efficacy, and cost-effectiveness are likely to vary by setting. To evaluate the effectiveness of the intervention, we will also assess the change in neuropsychiatric symptoms over time using the Neuropsychiatric Inventory (NPI) [30] and the change in the quality of life over time using the Quality of Life in Alzheimer’s Disease (QoL-AD) [31] as a self-and proxy-rating.
Furthermore, personnel and running costs to implement the intervention will be recorded. Different workloads of dementia care managers also result in different costs for the intervention per PwD. Therefore, implementation costs will be assessed to the number of PwD treated per setting and compared across settings. We will assess the pattern and intensity of the intervention in each setting by evaluating the number of services provided by the dementia care managers as well as through the number of home visits, physician visits, telephone calls and the number of contacts with the different healthcare providers involved in the care of PwD. Within the cost-effectiveness analyses, effects are assessed by the change in health-related quality of life over the six months using the EQ-5D-5 L [32, 33] as a patient self- and caregiver proxy-rating.
For further subgroup analyses, data from the control group of the DelpHi-MV study will be available to assess efficacy and cost-effectiveness compared to usual care. The eligibility criteria were in both studies the same (formally diagnosed or suspected dementia and living at home). However, in the DelpHi-MV trial, age was defined as ≥70 years, which will also be considered in the statistical analyses adjusted for patients’ sociodemographic and clinical characteristics to ensure comparability.
Sample size
Experience from the DelpHi-MV study and the systematic review by Somme et al. indicate that a caseload of approximately 60 PwD per year and care manager is needed to provide intensive care management for PwD [14, 18]. Therefore, after qualifying the nurses of each setting within the first six months of each implementation period of two years, we expect that 90 dyads, consisting of PwD and their caregivers, can be recruited, resulting in a total of 450 PwD across the five different settings.
Data collection and management
Plans for assessment and collection of outcomes
This study examines differences in implementing collaborative DCM across health settings. Before starting the intervention, the care manager will conduct a computer-assisted baseline assessment at the participant’s home. The assessment is based on primary data from participants (PwD and their caregivers) and collected by dementia care managers in face-to-face interviews. A similar assessment will be conducted six months after completing the baseline assessment, i.e., after six months of intervention. The following instruments will be used within the baseline and follow-up assessment:
• health-related quality of life (HRQoL) will be measured using the Quality of Life in Alzheimer’s Disease (QOL-AD) [31] and the EQ-5D-5 L [32, 33];
• the activities of daily living (ADL) will be assessed using the Bayer Activities of Daily Living Scale (B-ADL) [28];
• depression will be assessed according to the Geriatric Depression Scale (GDS) [34];
• the social functioning in dementia (SF-DEM) [35] will measure social inclusion;
• caregiver burden will be measured using the Zarit burden interview (ZBI) [36];
• utilization of healthcare resources among elderly individuals will be assessed by the FIMA questionnaire [37];
• the cognitive impairment will be assessed by the MMSE [27];
• informal care, as well as caregiver’s productivity losses, will be evaluated using the Resource Utilization in Dementia Instrument (RUD) [38].
At the end of the implementation procedure in the specific setting, i.e., after two years, self-designed quantitative questionnaires will be conducted with all stakeholders to assess the acceptance, implementation barriers, and success.
Plans to promote participant retention and complete follow-up
The individual staff in the setting will pre-select suitable patients who are known to them and are already receiving services. In addition, the intervention is provided by qualified study personnel who are also part of the staff of the respective setting. Consequently, it can be assumed that stakeholders are vested in ensuring that the collaborative dementia care program implementation achieves at least the same level of success as other routine care services, including participant retention and complete follow-up. Study discontinuation is documented by the study staff in the participant’s data. If only the consent is withdrawn, the research data collected so far remain in the study database, are anonymized and analyzed. If further data use is also prohibited, all research data collected up to that point will be deleted from the research database.
Data management
All data will be entered and stored by the dementia care managers into the IMS using a mobile touchscreen tablet PC specially developed for this purpose. The database is located on a central server located in a closed network. Since healthcare settings across Germany can participate, the data transfer between the mobile IT systems and the server takes place via secured VPN connections. Personal identifying data, such as a patient’s name and address, is transferred from the study server to the tablet PC during home visits. After the home visit and the transfer of the collected data to the central server, the personal data is deleted so that no more patient data is stored on the tablet PC. The entered data are then only available to the study team for quality and completeness control and analysis. During the preparation of the evaluation data set, the person-identifying data are removed from the data set and are pseudonymized with random numbers. Only the pseudonymized patient IDs are available to the study personnel outside of the visits. The completed paper-based interview forms of the participants and family caregivers are stored in separately locked filing cabinets according to the guidelines and recommendations for ensuring good epidemiological practice (GEP) [39]. Data will be stored for ten years following current data security and privacy standards as documented in the Institute for Community Medicine Institutional Privacy Policy (September 2019 version).
Data confidentiality
After eligible patients have been identified and informed about the study, the IC is required to share personal information with the study team, such as contact information. If participants agree, they will sign an IC in which they (a) agree to the disclosure of personal address information and (b) release the staff of the respective setting from their duty of confidentiality to the study team and (c) allow the study team to contact them.
Data collection, transfer, and maintenance are separated throughout the study regarding personnel and functions. Documents containing personal data are accessible only to authorized study team members. The dementia care managers receive personal data, such as a participant’s address, only during home visits for that particular day. The scientific evaluations are performed pseudonymously. Only anonymized and aggregated data are published, not allowing identification about the individual participants.
Statistical methods
The samples will be separated concerning PwD demographics, cognitive and functional status (mild versus moderate to severe), comorbidities, as well as living situation (living alone versus not alone) to identify who benefits most from the DCM and for which subgroup the highest efficacy and cost-effectiveness could be achieved across the settings.
Further, descriptive statistics will be conducted to assess the acceptance and the implementation barriers and success and assess the necessity of the intervention by describing the patient’s socio-demographic and clinical characteristics and the type and number of unmet needs across settings.
Univariate analyses (Fisher’s exact test, t-test) and multivariate linear and logistic regression models will be used to identify differences between settings. Differences in the patient’s neuropsychiatric symptoms and HRQoL will be assessed as a delta between the baseline (T0) and six-month follow-up assessment (T1). Multivariate regression models will assess the setting-specific effectiveness concerning these outcomes.
Intervention costs will be assessed by dividing the total cost for the intervention by the number of recruited patients and carried-out interventions per setting. Average healthcare costs per patient will be calculated using recorded healthcare resource utilization (assessed by the FIMA questionnaire) added by published unit costs in Euros (€) [40, 41]. The incremental cost-effectiveness ratios (ICER) will be calculated using the incremental cost per quality-adjusted life-year gained in one setting compared with all other settings [42]. For further subgroup analyses, the control group who received care as usual in the DelpHi-MV trial will also be used as a comparator for cost-effectiveness analyses. Because of the highly skewed cost distribution, standard errors and confidence intervals will be estimated by bootstrapping (2000 replications) [43]. To handle sampling uncertainty in the ICER, we will use nonparametric bootstrapping, creating 1000 resamples stratified for the cluster and group distribution [44]. The probability of DCM cost-effectiveness is calculated using these resamples and different willingness-to-pay (WTP) margins [45, 46].
The number of missing data will be identified and declared for the univariate analyses and multivariate regressions. Consistent with previous studies, multiple imputations via chained equation (MICE) will be used for multivariate regression analyses [19, 20, 47–49].
Access to data and code is available upon request from the authors in compliance with ethical provisions and applicable data protection regulations. The protocol is accessible via the German Clinical Trials Register (DRKS00025074).
Dissemination plans
It is intended to publish the results in peer-reviewed scientific journals. To make the scientific findings accessible to a broader audience, the existing public relations channels of all stakeholders involved, such as funders or healthcare providers, will be used.
RESULTS
In principle, the examined outcomes will differ by patients’ sociodemographic and clinical characteristics and identified unmet needs in each setting. Furthermore, we expect the higher the number of recruited PwD and the care load of the respective dementia care manager (i.e., identified unmet needs), the lower the implementation barriers and the higher the success. An increased number of identified unmet needs will also indicate a greater demand and, thus, an increased likelihood of achieving greater efficacy and cost-effectiveness. Additionally, we assume that the intervention will be more effective in the early dementia stages. The acceptance will vary depending on the stakeholders’ role (i.e., PwD, family caregiver, physician, or nurse).
DISCUSSION
The DCM:IMPact study aims to implement collaborative dementia care as a dementia-specific care service in different settings and to examine differences in patients’ socio-demographic and clinical characteristics, unmet needs as well as acceptance, implementation barriers and implementation success, and the efficacy and cost-effectiveness of the DCM across five healthcare settings in Germany. This is of vital importance to identify the setting where the highest benefits could be achieved by implementing collaborative dementia care. This study is expected to expand evidence on the target population of PwD to better understand their needs depending on the setting and subsequently meet them most effectively. In this context, a particular group of patients may receive optimal care in a specific setting. Therefore, it would make sense to efficiently use scarce healthcare resources by implementing collaborative care in settings where patients’ and caregivers’ needs and acceptance in the setting are greatest and implementation barriers low so that the intervention could have the highest impact. For this reason, the results of this study also serve as decision-making aids for implementing such models of collaborative care in routine care in Germany and worldwide.
In addition, the study will identify specific needs beyond outpatient care for PwD. Based on the findings, both the intervention and the qualification content of prospective dementia care managers can be modified according to the needs of the particular setting and its stakeholders. For this purpose, the subjective experiences of all stakeholders will also be included. The ethical, legal, political, and economic frameworks of healthcare systems worldwide are pretty universal. However, Germany’s federal structure allows it to address local circumstances, resulting in regional variations in supply and demand for healthcare services. Since the DCM will be implemented across regions and healthcare sectors in Germany, the present study will also improve the generalizability of the findings.
The findings gained from DCM:IMPact can improve the quality of life and care for PwD and their caregivers in different settings. By involving all stakeholders and settings and analyzing the respective implementation barriers, transaction costs can be minimized during prospective implementation. In addition, the results will show where the greatest need is and who benefits most from utilizing collaborative care.
TRIAL STATUS
Enrolment in the study started on 1 February 2019. Under the current recruitment plan, the last patient is expected to be enrolled on 1 March 2026.
ACKNOWLEDGMENTS
The authors acknowledge Agnes Meyer, Mandy Freimark, and Kerstin Albuerne. They thank all participating patients, their caregivers, the healthcare providers, and employees of the respective setting.
FUNDING
The German Dementia Aid funds this trial (DZNE Foundation for Research and Innovation at the Donors’ Association for the Promotion of Sciences and Humanities in Germany). The funders have not influenced the conceptualization and conduct of the study and will not have any role in the data analysis and publication of the results.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | GBD 2019 Dementia Forecasting Collaborators ((2022) ) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7: , e105–e125. |
[2] | Wimo A , Guerchet M , Ali GC , Wu YT , Prina AM , Winblad B , Jönsson L , Liu Z , Prince M ((2017) ) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13: , 1–7. |
[3] | Michalowsky B , Kaczynski A , Hoffmann W ((2019) ) Ökonomische und gesellschaftliche Herausforderungen der Demenz in Deutschland –Eine Metaanalyse. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 62: , 981–992. |
[4] | Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin e.V. (DEGAM) (2008) DEGAM – Leitlinie Nr. 12 Demenz. |
[5] | Deutsche Gesellschaft für Psychiatrie Psychotherapie und Nervenheilkunde und Deutsche Gesellschaft für Neurologie ((2017) ) S3-Leitlinie Demenzen, Springer-Verlag, Berlin, Heidelberg. |
[6] | Eichler T , Thyrian JR , Hertel J , Köhler L , Wucherer D , Dreier A , Michalowsky B , Teipel S , Hoffmann W ((2014) ) Rates of formal diagnosis in people screened positive for dementia in primary care: Results of the DelpHi-Trial. J Alzheimers Dis 42: , 451–458. |
[7] | Michalowsky B , Flessa S , Eichler T , Hertel J , Dreier A , Zwingmann I , Wucherer D , Rau H , Thyrian JR , Hoffmann W ((2018) ) Healthcare utilization and costs in primary care patients with dementia: Baseline results of the DelpHi-trial. Eur J Health Econ 19: , 87–102. |
[8] | Wübbeler M , Thyrian JR , Michalowsky B , Hertel J , Laporte Uribe F , Wolf-Ostermann K , Schäfer-Walkmann S , Hoffmann W ((2015) ) Nonpharmacological therapies and provision of aids in outpatient dementia networks in Germany: Utilization rates and associated factors. J Multidiscip Healthc 8: , 229–236. |
[9] | Wucherer D , Eichler T , Kilimann I , Hertel J , Michalowsky B , Thyrian JR , Teipel S , Hoffmann W ((2015) ) Antidementia drug treatment in people screened positive for dementia in primary care. J Alzheimers Dis 44: , 1015–1021. |
[10] | Wucherer D , Eichler T , Hertel J , Kilimann I , Richter S , Michalowsky B , Thyrian JR , Teipel S , Hoffmann W ((2017) ) Potentially inappropriate medication in community-dwelling primary care patients who were screened positive for dementia. J Alzheimers Dis 55: , 691–701. |
[11] | Wohlgemuth A , Michalowsky B , Wucherer D , Eichler T , Thyrian JR , Zwingmann I , Rädke A , Hoffmann W ((2020) ) Drug-related problems increase healthcare costs for people living with dementia. J Alzheimers Dis 73: , 791–799. |
[12] | Black BS , Johnston D , Rabins PV , Morrison A , Lyketsos C , Samus QM ((2013) ) Unmet needs of community-residing persons with dementia and their informal caregivers: Findings from the maximizing independence at home study. J Am Geriatr Soc 61: , 2087–2095. |
[13] | Prince M , Comas-Herrera A , Knapp M , Guerchet M , Karagiannidou M (2016) World Alzheimer Report 2016. Improving healthcare for people living with dementia. Coverage, Quality and Costs now and in the Future. Alzheimer’s Disease International, London. |
[14] | Somme D , Trouve H , Drame M , Gagnon D , Couturier Y , Saint-Jean O ((2012) ) Analysis of case management programs for patients with dementia: A systematic review. Alzheimers Dement 8: , 426–436. |
[15] | Applebaum R , Phillips P ((1990) ) Assuring the quality of in-home care: The “other” challenge for long-term care. Gerontologist 30: , 444–450. |
[16] | Eloniemi-Sulkava U , Notkola IL , Hentinen M , Kivelä SL , Sivenius J , Sulkava R ((2001) ) Effects of supporting community-living demented patients and their caregivers: A randomized trial. J Am Geriatr Soc 49: , 1282–1287. |
[17] | Reilly S , Miranda-Castillo C , Malouf R , Hoe J , Toot S , Challis D , Orrell M ((2015) ) Case management approaches to home support for people with dementia. , CD. Cochrane Database Syst Rev 1: , 008345. |
[18] | Thyrian JR , Fiß T , Dreier A , Böwing G , Angelow A , Lueke S , Teipel S , Fleßa S , Grabe HJ , Freyberger HJ , Hoffmann W ((2012) ) Life- and person-centred help in Mecklenburg-Western Pomerania, Germany (DelpHi): Study protocol for a randomised controlled trial. Trials 13: , 56. |
[19] | Michalowsky B , Xie F , Eichler T , Hertel J , Kaczynski A , Kilimann I , Teipel S , Wucherer D , Zwingmann I , Thyrian JR , Hoffmann W ((2019) ) Cost-effectiveness of a collaborative dementia caremanagement-Results of a cluster-randomized controlled trial. Alzheimers Dement 15: , 1296–1308. |
[20] | Thyrian JR , Hertel J , Wucherer D , Eichler T , Michalowsky B , Dreier-Wolfgramm A , Zwingmann I , Kilimann I , Teipel S , Hoffmann W ((2017) ) Effectiveness and safety of dementia care management in primary care: A randomized clinical trial. JAMA Psychiatry 74: , 996–1004. |
[21] | Rädke A , Michalowsky B , Thyrian JR , Eichler T , Xie F , Hoffmann W ((2020) ) Who benefits most from collaborative dementia care from a patient and payer perspective? A subgroup cost-effectiveness analysis. J Alzheimers Dis 74: , 449–462. |
[22] | Thyrian JR , Eichler T , Pooch A , Albuerne K , Dreier A , Michalowsky B , Wucherer D , Hoffmann W ((2016) ) Systematic, early identification of dementia and dementia care management are highly appreciated by general physicians in primary care - results within a cluster-randomized-controlled trial (DelpHi). J Multidiscip Healthc 9: , 183–190. |
[23] | Dreier A , Hoffmann W ((2013) ) [Dementia Care Manager for patients with dementia. Determination of the requirements and qualifications contents for nurses in the DelpHi-MV study]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 56: , 1398–1409. |
[24] | Dreier A , Thyrian JR , Eichler T , Hoffmann W ((2016) ) Qualifications for nurses for the care of patients with dementia and support to their caregivers: A pilot evaluation of the dementia care management curriculum. Nurse Educ Today 36: , 310–317. |
[25] | Eichler T , Thyrian JR , Dreier A , Wucherer D , Köhler L , Fiß T , Böwing G , Michalowsky B , Hoffmann W ((2014) ) Dementia care management: Going new ways in ambulant dementia care within a GP-based randomized controlled intervention trial. Int Psychogeriatr 26: , 247–256. |
[26] | Calabrese P , Kessler J ((2000) ) Screening for cognitive impairment in dementia —the DemTect procedure. Eur Neuropsychopharmacol 10: , 369. |
[27] | Kessler J , Markowitsch H , Denzler P (1990) Mini-Mental-Status-Test (MMST) [German Version], Beltz Test GmbH, Göttingen. |
[28] | Hindmarch I , Lehfeld H , de Jongh P , Erzigkeit H ((1998) ) The Bayer Activities of Daily Living Scale (B-ADL). Dement Geriatr Cogn Disord 9 Suppl 2: , 20–26. |
[29] | Eichler T , Thyrian JR , Fredrich D , Köhler L , Wucherer D , Michalowsky B , Dreier A , Hoffmann W ((2014) ) The benefits of implementing a computerized intervention-management-system (IMS) on delivering integrated dementia care in the primary care setting. Int Psychogeriatr 26: , 1377–1385. |
[30] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: , S10–16. |
[31] | Logsdon RG , Gibbons LE , McCurry SM , Teri L ((2002) ) Assessing quality of life in older adults with cognitive impairment. Psychosom Med 64: , 510–519. |
[32] | ((1990) ) EuroQol–a new facility for the measurement of health-related quality of life. Health Policy 16: , 199–208. |
[33] | Rabin R , de Charro F ((2001) ) EQ-5D: A measure of health status from the EuroQol Group. Ann Med 33: , 337–343. |
[34] | Gauggel S , Birkner B ((1999) ) Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS). Z Klin Psychol Psychother 28: , 18–27. |
[35] | Sommerlad A , Singleton D , Jones R , Banerjee S , Livingston G ((2017) ) Development of an instrument to assess social functioning in dementia: The Social Functioning in Dementia scale (SF-DEM). Alzheimers Dement (Amst) 7: , 88–98. |
[36] | Zarit SH , Orr NK , Zarit JM ((1985) ) The hidden victims of Alzheimer’s disease: Families under stress, New York University Press, New York. |
[37] | Seidl H , Bowles D , Bock JO , Brettschneider C , Greiner W , König HH , Holle R ((2015) ) FIMA –Fragebogen zur Erhebung von Gesundheitsleistungen im Alter: Entwicklung und Pilotstudie. Gesundheitswesen 77: , 46–52. |
[38] | Wimo A , Nordberg G ((2007) ) Validity and reliability of assessments of time. Comparisons of direct observations and estimates of time by the use of the resource utilization in dementia (RUD)-instrument. Arch Gerontol Geriatr 44: , 71–81. |
[39] | Hoffmann W , Latza U , Terschüren C ((2005) ) [Guidelines and recommendations for ensuring Good Epidemiological Practice (GEP) –revised version after evaluation]. Gesundheitswesen 67: , 217–225. |
[40] | Bock JO , Brettschneider C , Seidl H , Bowles D , Holle R , Greiner W , König HH ((2015) ) [Calculation of standardised unit costs from a societal perspective for health economic evaluation]. Gesundheitswesen 77: , 53–61. |
[41] | Byford S , Torgerson DJ , Raftery J ((2000) ) Economic note: Cost of illness studies. BMJ 320: , 1335. |
[42] | Briggs AH , Gray AM ((1999) ) Handling uncertainty in economic evaluations of healthcare interventions. BMJ 319: , 635–638. |
[43] | Desgagne A , Castilloux AM , Angers JF , LeLorier J ((1998) ) The use of the bootstrap statistical method for the pharmacoeconomic cost analysis of skewed data. Pharmacoeconomics 13: , 487–497. |
[44] | Obenchain RL ((1999) ) Resampling and multiplicity in cost-effectiveness inference. J Biopharm Stat 9: , 563–582. |
[45] | Grosse SD ((2008) ) Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 8: , 165–178. |
[46] | Neumann PJ , Cohen JT , Weinstein MC ((2014) ) Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 371: , 796–797. |
[47] | Rubin DB , Schenker N ((1991) ) Multiple imputation in health-care databases: An overview and some applications. Stat Med 10: , 585–598. |
[48] | Sterne JA , White IR , Carlin JB , Spratt M , Royston P , Kenward MG , Wood AM , Carpenter JR ((2009) ) Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338: , b2393. |
[49] | White IR , Royston P , Wood AM ((2011) ) Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: , 377–399. |




