Patient with Corticobasal Syndrome Showing Disease-Associated Biomarkers of Dementia with Lewy Bodies: A Treviso Dementia (TREDEM) Registry Case Report
Abstract
Background:
An 82-year-old right-handed man, a retired teacher, reported the occurrence, three years earlier, of difficulties in moving his left arm and foot, tremor in his left hand, and gestures of the left upper limb that appeared to be independent of the patient’s will.
Objective:
We describe an unusual case of corticobasal syndrome (CBS) showing disease-associated biomarkers of dementia with Lewy bodies (DLB).
Methods:
Clinical, neuropsychological, imaging, and biomarker evaluations were conducted, including tau and amyloid-β levels in the cerebrospinal fluid (CSF) and a RT-QuIC assay for α-synuclein both in the CSF and olfactory mucosa (OM), as well as a QEEG assessment.
Results:
The patient presented resting tremor, mild extrapyramidal hypertonus, mild bradykinesia on the left side, and severe apraxia on the left upper limb. Brain MRI showed a diffuse right hemisphere atrophy which was prominent in the posterior parietal and temporal cortices, and moderate in the frontal cortex and the precuneus area. 18F-FDG PET imaging showed reduced glucose metabolism in the right lateral parietal, temporal, and frontal cortices with involvement of the right precuneus. The putamen did not appear to be pathological at DaTQUANT. Neuropsychological tests showed memory and visual-perceptual deficits. CSF tau and amyloid measurements did not show clear pathological values. RT-QuIC for α-synuclein in CSF and OM samples were positive. The QEEG analysis showed a pre-alpha dominant frequency in posterior derivations, typical of early stages of DLB.
Conclusion:
Although in the present patient the clinical diagnosis was of probable CBS, unexpectedly positive biomarkers for DLB suggested the co-presence of multiple pathologies.
INTRODUCTION
Corticobasal syndrome (CBS) is characterized by asymmetrical involuntary movements, rigidity, apraxia, tremor, myoclonus, dystonia, alien limb (AL) phenomena, and cortical sensory deficits. CBS is associated with several distinct histopathologies, which share the presence of tauopathy, including corticobasal degeneration, Alzheimer’s disease, and other forms of tau-related frontotemporal lobar degeneration, including progressive supranuclear palsy [1].
CBS was first described in 1967 and 1968, when Rebeiz et al. [2, 3] reported the clinical and neuropathological features of three patients with a syndrome they called “corticodentatonigral degeneration with neuronal acromasia". Gibb and Marsden coined the term corticobasal degeneration (CBD) in 1989 [4]. The clinical features described by Rebeiz et al. were associated with different neuropathological phenotypes. Consequently, two distinct terms were adopted: CBD, in which the pathological findings described by Rebeiz et al. are present, and CBS, which refers to the clinical phenotype independent of the underlying histopathology [1].
Armstrong et al. [5] proposed diagnostic criteria in which the basic motor characteristics of probable CBS consist of an asymmetrical presentation that includes two or more of the following signs: limb stiffness/akinesia, dystonia or myoclonus, as well as two or more of the following characteristics: orobuccofacial or limb apraxia, cortical sensory deficit or AL phenomena. Additional criteria based on associated motor, cognitive, and linguistic deficits were included [5]. Indeed, CBS, which was initially considered to be an entity with primary damage of basal ganglia and frontal parietal cortex, corresponding to parkinsonism and apraxia respectively, also shows variable involvement of frontal, parietal, and temporal cortices, resulting in a combination of parkinsonism and cognitive impairments. The main cortical features include apraxia, AL phenomena, cortical sensory loss, global cognitive impairment, behavioral alterations, and language disturbances [6].
AL phenomena are a heterogeneous group of behaviors in which one or more limbs of a patient, usually an arm, behave in a way that appears intentional but is independent of what the patient reports as being his/her movement intentions [7–11]. In 1992, Doody and Jankovich [8] proposed that AL should be “reserved for cases where the hand feels foreign along with observable involuntary movements". Marchetti and Della Sala in 1998 [12] specified that AL is linked to posterior lesions of the corpus callosum, probably involving the parietal cortex. Complex, purposeful hand movements which are performed against the patient’s will and which cannot be voluntarily inhibited should be identified instead with the term “Anarchic hand” [12] and are related to anterior lesions of the corpus callosum and the supplementary motor area contralateral to the rebel hand [12]. Scepkowski in 2003 [13] proposed three distinct variants within the AL spectrum: a) a posterior, “sensory” type, corresponding to AL in Marchetti and Della Sala’s description; b) an anterior, “frontal” type corresponding to the anarchic hand definition; c) a callosal type, which consists of actions that interfere with those performed by the contralateral, unaffected, hand [13].
Finally, a very recent review in 2021 [14] proposed that “the catch-all term AL should be deconstructed and subdivided into three different entities”: 1) levitation and finger writhing, likely related to involuntary activation; 2) hemiasomatognosia, as a result of parietal lobe alterations; and 3) complex finalized movements with or without intermanual conflict [14].
Objective
We present a patient with CBS and mild cognitive impairment (MCI) who showed the presence of α-synuclein aggregates in the cerebrospinal fluid (CSF) and in the olfactory mucosa (OM) samples, and a quantitative electroencephalography (QEEG) pattern of early-stage dementia with Lewy bodies (DLB).
Case presentation
An 82-year-old right-handed man, a retired teacher, was referred to the Treviso Cognitive Impairment Center (TCIC) in September 2018, complaining of increasing motor impairments that had arisen three years before. He reported difficulties in moving his left arm and foot, tremor in his left hand and gestures of the left upper limb that appeared to be independent of his will, characteristic of anarchic hand phenomenon [12]. He also reported needing visual confirmation to be aware of the movement made by his left arm, suggesting the presence of alien limb phenomenon. A fall to the ground, which occurred a month earlier, due to probable failure of the left lower limb, was also reported.
The patient, with 17 years of education, worked as a teacher of Italian Literature. He drank a glass of wine a day and did not smoke. He suffered from malaria at a young age and reported a transient ischemic attack in 2003. No family history of neurodegenerative or psychiatric diseases was reported.
Upon physical examination, the patient was alert and cooperative. No apparent visual field deficits were detected; the pupils were isocyclic, isochoric; ocular movements were normal. No asymmetries of the face were detectable. The palatal velum was in axis and the tongue midlined and protrusible. The patient presented resting tremor, mild extrapyramidal hypertonus, mild bradykinesia on the left side, and severe apraxia on the left upper limb. The patient reported that the tremor in his left upper limb was accentuated by emotional tension, consequent to the awareness of his motor limitations. His sense of smell was preserved.
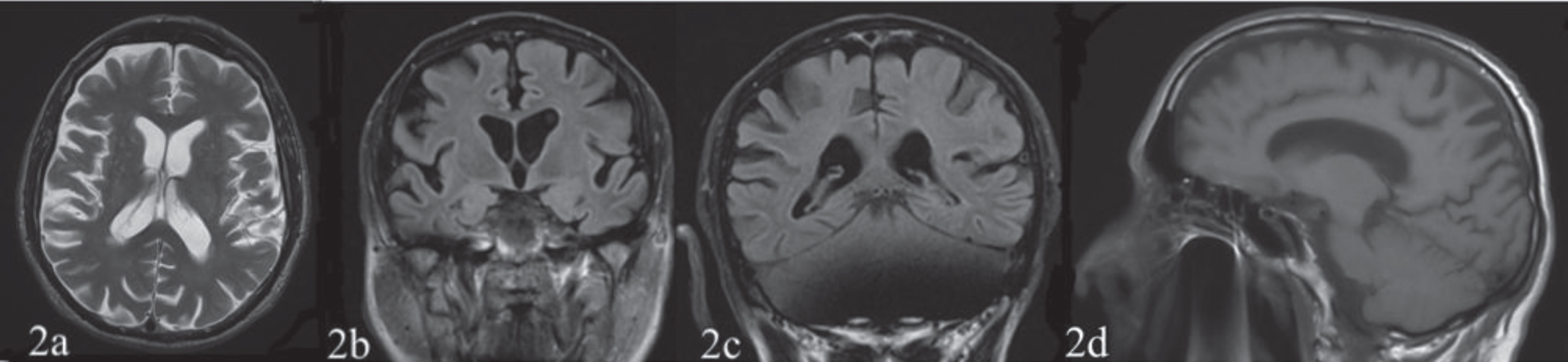
Osteo-tendon reflexes were prevalent on the left. Mild muscle strength deficit, 4/5 on the Medical Research Council Scale [15] and mild hypoesthesia were observed on the left side. The patient showed signs of apraxia on the left limbs. Eye movements were normal. Brain CT and MRI, performed in 2018 before the patient was referred to the TCIC, showed a posterior parietal (Figs. 1c, 1d, 2b, 2c) and temporal cortical atrophy (Fig. 1a) prevalent in the right hemisphere and a moderate atrophy of the precuneus (Figs. 1e, 2d) and frontal cortex (Figs. 1a, 1b, 2a).
Fig. 1
CT scan (February 17, 2018), with no contrast, showing a posterior parietal (1c, 1d) and temporal cortical atrophy (1a) prevalent on the right hemisphere and a moderate atrophy of the precuneus (1e) and frontal cortex (1a, 1b). The hippocampus and the mesial temporal area appeared fairly preserved.
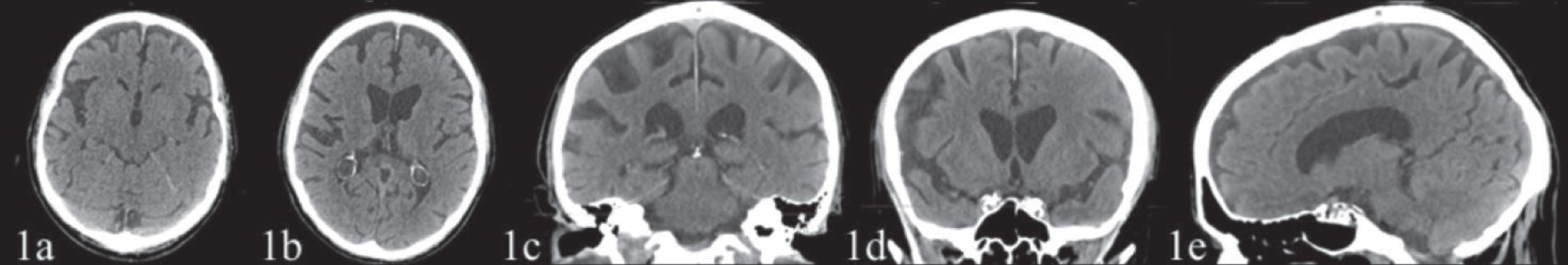
Fig. 2
The first (March 3, 2018) and the second (December 14, 2019, not showed) structural MRI T1 and T2 weighted sequences reported a posterior parietal (2b, 2c), temporal (not shown) and frontal cortex (2a) atrophy prevalent on the right hemisphere and an atrophy of the precuneus (2d).
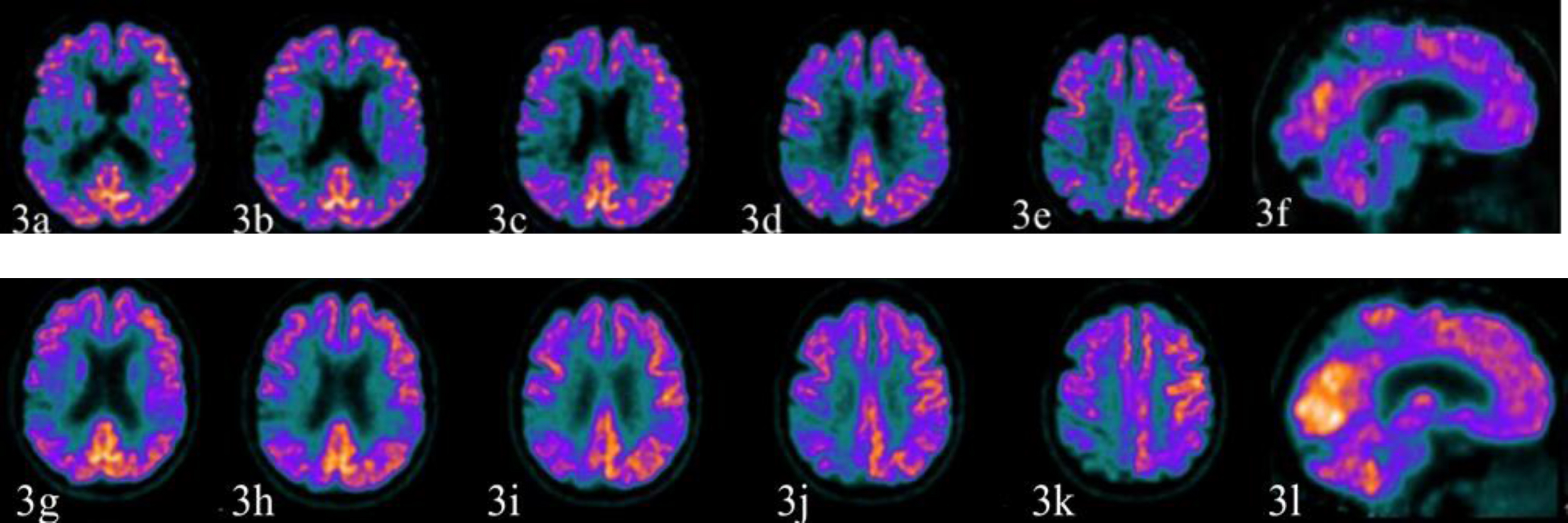
In November 2018, the patient underwent a 18F-FDG PET showing reduced glucose metabolism in the occipital, parietal, temporal, and lateral frontal cortices on the right side along with the involvement of the right supplementary motor area (Fig. 3a–3e), of the precuneus (Fig. 3f), and partially of the primary sensory-motor area.
Fig. 3
The first 18F-FDG PET study (November 13, 2018) is illustrated by the images above (3a-3e axial sections; 3f sagittal section) while the second study (December 6, 2019) produced the images below (3g–3k axial sections; 3l sagittal section). The first 18F-FDG PET study showed reduced glucose metabolism in the occipital, parietal, temporal, lateral frontal cortex on the right side along with the involvement of right supplementary motor area (3a–3e), of the precuneus (3e, 3f), and partially of the primary sensory-motor area. The hypometabolism of the precuneus on the right side suggested an initial sign of the posterior cingulate island on the right (3e). The right putamen was relatively less metabolically active (not shown). The left parietal and the temporal cortex were also moderately hypometabolic. The second study produced images substantially unchanged from the previous ones.
In December 2018 the patient performed a 123I-ioflupane SPECT (DaT-SPECT) which showed a mild, albeit non-pathological at DaTQUANT (see methods), thinning of the posterior portion of the right putamen (Fig. 4). A first neuropsychological evaluation was carried out on September 27, 2018 and showed mild deficits in visual-perceptive memory (Table 1).
Fig. 4
The 123I-ioflupane SPECT (DaT-SPECT), performed on December 20, 2018. It showed a mild thinning of the posterior portion of the right putamen that did not appear to be pathological in the semi-quantitative analysis (DaTQUANT). The second study (December 12, 2019, not shown) produced images and semi-quantitative analysis essentially unchanged.
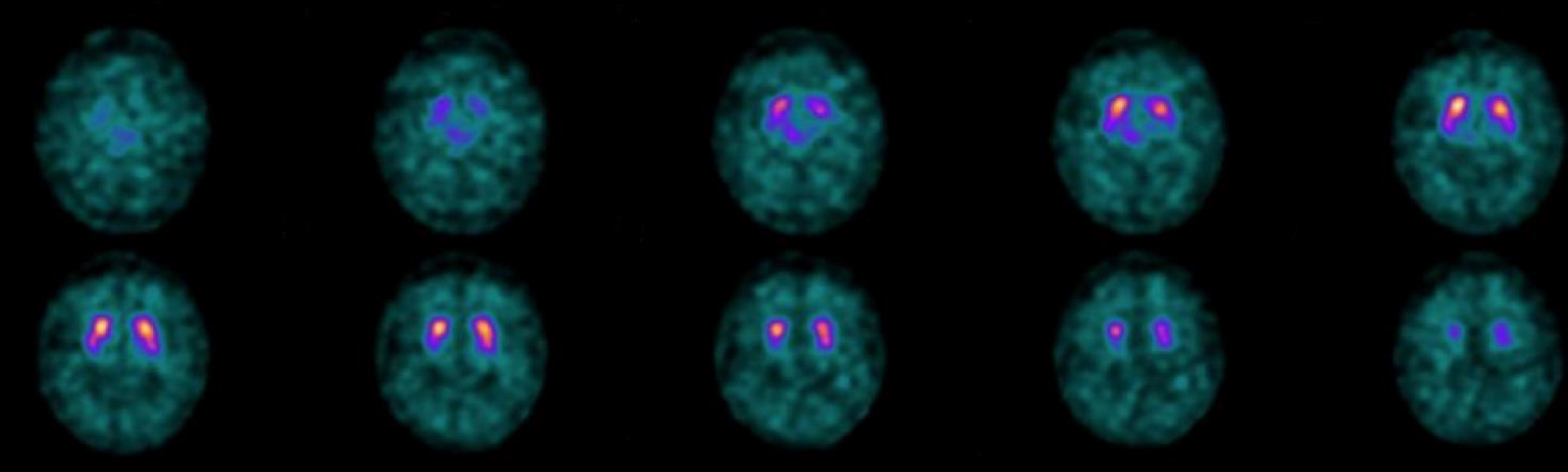
Table 1
Neuropsychological evaluations. The table shows the patient’s performance on tests of memory, attention, language, apraxias, visual perception, executive functions, agnosia, and the comprehensive neuropsychiatric profile, during a period of two years (raw scores, corrected scores, and cut-off values are reported)
| Neuropsychological test | First evaluation (27/09/18) | Third evaluation (16/05/19) | Fifth Evaluation (04/12/19) | Seventh Evaluation (06/10/20) | Cut-off scores | Cognitive Domain | ||||
| Raw score | Corrected score | Raw score | Corrected score | Raw score | Corrected score | Raw score | Corrected score | |||
| Mini-Mental State Examination [18] | 28 | 27.1 | 27 | 26.1 | 27 | 26.1 | 27 | 26.1 | ≥26 | General Cognitive Abilities |
| Clinical Dementia Rating [17] | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | General Cognitive Abilities | ||||
| RAVLT [21] | ||||||||||
| Immediate Recall | 25 | 30.6 | 28 | 33.6 | 23 | 28.6 | 17 | 22.6 | IR > 28.53 | Memory |
| Delayed Recall | 3 | 5.2 | 3 | 5.2 | 2 | 4.2 | 1 | 3.2 | DR>4.69 | Memory |
| Digit Span [19] | 6 | 5.75 | 8 | 7.75 | 7 | 6.75 | 6 | 5.75 | >3,75 | Memory |
| Visuo-Spatial Span [20] | 2 | 2.25 (Z = –2.40) | N.A. | 3 | 3.25 (Z = –1.47) | 2 | 2.25 (Z = –2.40) | –1.5 < Z < 1.5 | Memory | |
| Short-Story Memory Test [20] | 12.6 | 12.1 (Z = 0.51) | 2.2 | 1.7 (Z = –2.52) | 8.8 | 8.3 (Z = –0.60) | 5.6 | 5.1 (Z = –1.53) | –1.5 < Z < 1.5 | Memory |
| Attentive Matrices [20] | 51 | 47.5 (Z = 1.06) | 54 | 50.5 (Z = 1.31) | 51 | 47.5 (Z = 1.06) | N.A. | –1.5 < Z < 1.5 | Attention | |
| Phonemic Verbal Fluency Test [20] | 29 | 24.3 | 38 | 33.3 | 32 | 27.3 | 38 | 33.3 | ≥17.35 | Language |
| Semantic Verbal Fluency Test [20] | 13.5 | 11.25 | 14.25 | 12 | 13.75 | 11.5 | 14.25 | 12 | ≥7.25 | Language |
| Token Test [20] | 32.5 | 31.25 | 33 | 31.75 | 30 | 28.75 | N.E. | ≥26.5 | Language | |
| Drawing Copy Test [23] | 7 | 6.7 (Z = –0.75) | 6 | 5.7 (–1.19) | 5 | 4.7 (Z = 1.63) | N.E. | –1.5 < Z < 1.5 | Constructional Praxia | |
| Clock Drawing Test [23] | 9.5/10 | 6/10 | 9.5/10 | 0/10 | 6/10 | Executive Functions | ||||
| Cognitive Estimation Test [28] | 5/5 | 5/5 | 5/5 | 5/5 | 3/5 | Executive Functions | ||||
| ROCF [22] | ||||||||||
| Immediate Copy | 35 | 36.25 | N.A. | N.A. | N.A. | 28 | Memory | |||
| Delayed recall | 6 | 8.75 | N.A. | N.A. | N.A. | 6.2 | Constructional Praxia | |||
| Ideational Apraxia [24] | N.A. | N.A. | 20/20* | 20/20* | 18/20 | Apraxia | ||||
| Ideomotor Apraxia Test [25] | 20/20* | N.A. | 20/20* | Right 20/20; Left 14/20 ** | 16/20 | Apraxia | ||||
| Buccofacial apraxia [26] | 20/20 | N.A. | 20/20 | 20/20 | 17/20 | Apraxia | ||||
| X-O-N Warrington and Taylor [29] | N.A. | N.A. | N.A. | 21/33 | ≥29.03 | Apperceptive agnosia | ||||
| Ghent overlapping figures [27] | N.A | N.A | N.A | N.E. | Agnosia | |||||
| NPI F X G [30] | 10/144 | 0/144 | 18/144 | 22/144 | N.A. | Neuropsychiatric Profile | ||||
N.A., test not applied for that evaluation; N.E., not possible to execute; RAVLT, Rey-Auditory Verbal Learning Test; IR, Immediate Recall; DR, Delayed Recall; ROCF, Rey-Osterrieth Complex Figure Test; NPI F X G, Neuropsychiatry Inventory Frequency and Gravity score. Scores outside the normal range are shown in bold; *applied on dominant side as indicated by test protocol; **applied on both sides. The z-score is the raw score of the neuropsychological test minus the population mean, divided by the population standard deviation. The neuropsychological assessment showed memory deficits already in the first two evaluations and later constructional apraxia and executive functions deficit appeared. In the last evaluation, apperceptive agnosia and ideomotor apraxia (on the left side) also emerged.
In January 2019, the patient was clinically re-evaluated: pendolar movements of the left upper limb were reduced during walking. The left hand, after a few steps, assumed a dystonic posture with levitation. Rest tremor, plastic rigidity, and bradykinesia were prevalent on the left side.
The onset with memory deficits and atrophy and hypometabolism in right parieto-temporal cortical areas was diagnosed as MCI/prodromal Alzheimer’s disease (AD) presenting with CBS. Levodopa/Carbidopa 100/25 mg three times a day was initiated with minimal benefit on the rigidity. Participation in the walking and reading groups for people with MCI, organized by the TCIC, was proposed together with moderate but continuous physical activity, Mediterranean diet adherence, and socialization.
In August 2019 the patient was re-evaluated. He presented with a worsening of motor signs with instability in walking and loss of balance, resulting in frequent falls. He complained of difficulty in coordinating the two hands and signaled that his left upper limb was increasingly escaping his control. At physical examination, compared to the previous evaluations, there was an evident apraxia of the left hand during tasks that required synergistic coordination of both hands, e.g., buttoning a shirt or tying shoelaces.
In October 2019 the patient showed cautious and insecure walking with a tendency to lose balance. Standing was possible on a restricted basis but with a tendency to retropulsion. In addition to the ongoing therapy, the patient started a course of physiokinetic therapy. Further neuropsychological evaluations were carried out in 2019, respectively on February 6 (not shown in Table 1), May 16, August 1 (not shown in Table 1), and December 4, and showed overall mild memory deficit, constructive apraxia, and borderline executive functions (Table 1). The cognitive profile and the other clinical and instrumental elements still supported the diagnosis of MCI/prodromal AD presenting as CBS.
In November 2019, the patient was admitted to the emergency room for vasovagal syncope. Due to the physical deterioration found in October 2019, structural and metabolic imaging techniques were repeated. A brain MRI, performed on December 14, 2019 (not shown), a second 18F-FDG PET, and a further 123I-ioflupane SPECT (DaT-SPECT, not shown) were substantially unchanged from the previous ones.
The patient was subsequently evaluated in January and in October 2020. The patient reported a worsening of his motor performance, in particular in concomitance and after the period of social restrictions related to the SARS-CoV2 pandemic. By October 2020, the situation had objectively worsened further. The patient presented hypomimia. Verbal fluency was intact but with monotone speech. The patient reported tactile hypoesthesia of the left side of the face. Changes in posture were performed with extreme uncertainty and with the examiner’s help. The patient showed precarious control of the trunk when sitting, with a tendency to retropulsion. In a standing position his balance was facilitated by placing his feet further apart. The patient was also supported by the examiner; Romberg’s test was positive. We observed freezing of gait, possible only with unilateral support by the examiner and constant search for support with the contralateral limb. While walking, the feet had to keep a certain distance, otherwise the left leg tended to hinder the step of the right leg.
The patient showed severe apraxia of the left upper limb in transitive and intransitive gestures, symbolic and imitative. Graphesthesia and spatial discrimination of the tactile stimulus were not clearly interpretable in relation to the patient’s responses. Astereognosis was present on the left upper limb. The left upper limb could not be kept extended forward. Plastic hypertonia of upper limbs (left > right), and paratonia to lower limbs were present along with neck rigidity. The patient reported tactile hypoesthesia on the left side. Plantar reflex was in flexion on the right, no response was found on the left. The index-nose test was correctly performed on the right, but not on the left.
Two new neuropsychological evaluations were carried out on February 2 (not shown in Table 1) and on October 6, 2020 (Table 1). The results showed short, long-term, and visuospatial memory loss, executive function deficits, apraxia on the left side, and apperceptive agnosia.
At the end of 2020 we concluded with a diagnosis of MCI/prodromal AD presenting as CBS. At that time, the patient’s therapy included Levodopa/Carbidopa 100/25 mg 1 tablet three times a day, Simvastatin 20 mg 1 tablet a day, Perindopril 4 mg 1 tablet a day, and Ticlopidine 250 mg 1 tablet a day.
Suspecting a possible evolution of MCI towards a disease in the spectrum of tauopathies, the determination of CSF markers for AD, including amyloid-β, tau, phospho-tau, was carried out. The presence of AD related protein level abnormalities was excluded. The determination of α-synuclein in CSF and in samples of the nasal mucosa were subsequently performed and a QEEG assessment was completed as well.
Alpha-synuclein aggregates were found in both CSF and in OM samples. Quantitative spectral analysis of the EEG showed a typical pattern of early stage DLB with the presence of a dominant pre-alpha band in posterior derivations.
The finding of α-synuclein deposits in two different tissues was surprising because CBS is usually associated with tauopathies. In 2021, the patient’s condition worsened further. He stopped walking independently. His mood became depressed and an antidepressant therapy with Escitalopram, 10 mg 1 tablet daily, was started.
The general conditions of the patient and his concomitant poor compliance did not allow 123I-metaiodobenzylguanidine cardiac sympathetic imaging, which could have further clarified the DLB pathology. The patient’s general condition further deteriorated, with urinary incontinence, further reduced mobility and secondary difficulty in caring for him, until his death in February 2022.
METHODS
Clinical, neuropsychological, MRI, 18F-FDG PET, and DaT-SPECT imaging and biomarkers evaluations, including tau and amyloid-β levels in the CSF and RT-QuIC assay for α-synuclein both in the CSF and OM, along with a QEEG assessment, were conducted. The case presented was a participant in the TREDEM Study [16] and Registry which was approved by the Treviso Province Ethics Committee (protocol no. 42326 of April 10th 2015).
Neuropsychological evaluations
Neuropsychological evaluations were carried out seven times (of these, four illustrated in Table 1) from admission in September 27, 2018 to October 6, 2020 (the last one performed at patient’s home, due to the worsening of his mobility). Psychometric test results are included in Table 1 (raw scores, corrected scores, and cut-offs). The z-score was also calculated. It is the raw score of the neuropsychological test minus the population mean, divided by the population standard deviation. Such normative data (mean and standard deviation) are provided by the authors of the respective neuropsychological tests.
The evaluation included the following tests: Clinical Dementia Rating [17], Mini-Mental State Examination [18], Digit Span [19], Visuo-Spatial Span [20], Short Story Memory Test [20], Rey-Auditory Verbal Learning Test [21], Rey-Osterrieth Complex Figure test [22], Attentive Matrices [20], Semantic and Phonemic Verbal Fluencies [20], Token Test [20], Drawing Copy Test [23], Ideational Apraxia [24], Ideomotor Apraxia [25], Buccofacial Apraxia [26], Poppelreuter-Ghent’s overlapping figures test [27], Clock Drawing Test [23], Cognitive Estimation Test [28], X-O-N- Warrington Test [29], and the Neuropsychiatric Inventory [30].
Structural and nuclear medicine imaging
The CT scans were acquired with an EMOTION 6 Siemens volumetric scanner. Section orientation was parallel to the orbitomeatal plane. Sections on the same plane (time of 2 s, 120 kV, 130 mA, section thickness of 5 mm, no intersection gap) covered the remaining brain from the inferior aspect of the cerebellum to the vertex of the cranium.
The structural MRI scan was performed with 3-T Magnetom 135 mT of gradients with 64 channels (Siemens Magnetom Vida). T1, T2, and T2 FLAIR weighted sequences were extracted.
The patient underwent an 18F-FDG PET brain scan using a PET tomograph Discovery MI (General Electric Healthcare). The CT scan was used for attenuation and scatter correction with set voltage tuned to 120 kV. The scan was obtained over 10 min, starting 60 min after i.v. injection of 227 MBq, and the images of the two scans were visually assessed.
Moreover, to obtain the DaT-SPECT images, the patient was administered two capsules of 200 mg of potassium perchlorate orally, approximately 1 h before the injection of 123I-ioflupane. The patient was administered 170 MBq of 123I-ioflupane intravenously, and SPECT imaging was detected 3 h post-injection. Furthermore, a DaTQUANT analysis, an adjunct processing tool with advanced quantitative uptake methods and a designated normals database, for a more accurate assessment of a patient’s case, was also performed [31, 32].
An amyloid PET was not performed given the patient’s advanced age at first evaluation at our center. Indeed, the prevalence of amyloid PET positivity among healthy older adults increases exponentially with age and, in particular, is more than 50% in persons aged 80–90 years [33].
Quantitative electroencephalography
EEG and QEEG assessments were performed with nineteen Ag/AgCl disk scalp electrodes, placed according to the international 10–20 system, recorded EEG from Fp1, Fp2, Fz, F3, F4, F7, F8, Cz, C3, C4, Pz, P3, P4, T3, T4, T5, T6, O1, and O2. EEG activity was analyzed from single or multiple leads grouped to define the following scalp regions: anterior (Fz, Fp2, F7, Fp1, F3, F4, F8), central (Cz, C3, C4), posterior (Pz, P3, P4, O1, O2), temporal (T3, T4, T5, T6), peripheral (Fp1, Fp2, F8, T4, T6, O1, O2, T3, T5, Fz), and internal (F3, F4, Fz, C3, Cz, C4, P3, Pz, P4). Recordings were obtained with the patient resting comfortably, with eyes closed. Patient’s wakefulness was ascertained every about 2 min inviting him to open his eyes and checking block reactions. Electrodes to record electrocardiogram were also applied. EEG was acquired as a continuous signal for 20 min and visually inspected for current clinical interpretation or detection of artefacts and stored in order to be epoched in off-analysis setting as series of 2 s-long epochs. Two electrodes from posterior derivations (O1, O2) were considered for the analysis.
Ninety blocks of artefact-free 2-s-long epochs appearing consecutively were selected off-line by visual inspection. Fast Fourier transform was applied on each epoch allowing a frequency resolution of 0.5 Hz. The obtained spectra values were then processed in order to compute a mean Power Spectrum for each channel and divided automatically into 4 frequency bands: 3–4 Hz (delta), 4.5–5.5 Hz (theta), 6–7.5 Hz (pre-alpha), and 8–12 Hz (alpha) [34]. EEG traces were quantified by the following mathematical descriptors: DF, i.e., the frequency where the spectral power value was greatest, was evaluated for each epoch; DFV expressing the variability of DF across the 90 analyzed epochs; FP, i.e., percent of epochs where a dominant frequency band was observed (1–100%) in order to define the compressed spectral array pattern of the recording, according to Bonanni [34].
Cerebrospinal fluid analyses
CSF Aβ42, total Tau (tTau), and phosphorylated Tau (pTau181) levels were determined by using commercially available INNOTEST ELISA kit (Fujirebio Europe N.V., Ghent, Belgium), as previously described [35]. CSF Aβ42, tTau, and pTau181 levels were checked in duplicate. Pathological values orienting toward an AD pathology were assumed for total Tau >350 pg/mL and Aβ42/pTau181 ratio <7 [35]. Alpha-synuclein RT-QuIC assay was performed as previously described [36].
Olfactory brushing and α-synuclein RT-QuIC analysis
An otolaryngologist visualized the nasal cavity using a 0° endoscope. Four OM samples were collected through the middle turbinate using flocked swabs (COPAN Italia, Technologies) and OM samples were processed for RT-QuIC analysis. Alpha-synuclein RT-QuIC assay in OM and CSF samples was performed as previously reported in patients with Parkinson’s disease, DLB, and isolated REM sleep behavior disorder [37, 38].
RESULTS
Neuropsychological evaluation
Seven neuropsychological assessments were conducted between September 27, 2018 and October 6, 2020; for the sake of brevity, only the four most significant of these are illustrated in Table 1. The neuropsychological evaluations carried out were numerous because four of these were part of TCIC protocol for the prevention of cognitive deterioration for people with MCI [39, 40], in which the patient was included. The patient always came to the clinical interviews accompanied by his wife. The last evaluation took place at the patient’s house because of his severe disability at that time.
During the first evaluation, the patient showed an isolated deficit of the visuo-spatial memory domain. In the second evaluation (February 6, 2019; not shown) the patient performed poorly on the Drawing Copy Test and on immediate recall as measured by the Rey-Auditory Verbal Learning Test.
The third evaluation (May 16, 2019; Table 1) showed a deficit in memory (Short Story Test) and borderline executive functions (Clock Drawing Test). The fourth evaluation (August 1, 2019; not shown) did not show significant changes compared to the previous ones.
The fifth evaluation (December 4, 2019; Table 1) showed memory deficits (delayed recall, Rey-Auditory Verbal Learning Test, and Visuo-Spatial Span with borderline score) and constructional apraxia (Drawing Copy Test).
The sixth evaluation (February 2, 2020; not shown) did not show significant changes compared to the previous one. The last evaluation (October 6, 2020) showed memory deficits (Immediate and Delayed Recall Rey-Auditory Verbal Learning Test; Visuo-Spatial Span; Short-Story Memory Test), executive deficits (Clock Drawing Test) and severe apperceptive agnosia (X-O-N Warrington and Taylor test) and ideomotor apraxia on the left side.
Structural and nuclear medicine imaging
The CT scans (February 17, 2018) showed a posterior parietal cortical atrophy prevalent on the right hemisphere (Fig. 1c, 1d), atrophy of the precuneus area (Fig. 1e), of the temporal and of frontal cortex (Fig. 1a, 1b). The hippocampus and the mesial temporal area appeared fairly preserved.
The first (March 3, 2018) and the second (December 14, 2019, not showed) structural MRI T1 and T2 weighted sequences reported a posterior parietal (Fig. 2b, 2c), temporal (not shown) and frontal cortex (Fig. 2a) atrophy prevalent on the right hemisphere and an atrophy of the precuneus (Fig. 2)d.
The first 18F-FDG PET study (November 13, 2018) showed reduced glucose metabolism in the occipital, parietal, temporal, and lateral frontal cortex on the right side along with the involvement of right supplementary motor area (Fig. 3a–3e), of the precuneus (Fig. 3e, 3f), and partially of the primary sensory-motor area. The hypometabolism of the precuneus on the right suggested the sign of the posterior cingulate island on the right (Fig. 3e). The right putamen was relatively less metabolically active (not shown). The left parietal and the temporal cortex were also moderately hypometabolic. The second study (December 6, 2019) produced images substantially unchanged from the previous ones.
The 123I-ioflupane SPECT (DaT-SPECT), performed on December 20, 2018, showed a mild reduced uptake of the posterior portion of the right putamen that did not appear to be pathological in the semi-quantitative analysis (DaTQUANT). The second study (December 12, 2019, not shown) produced images and semi-quantitative analysis that were essentially unchanged.
Cerebrospinal fluid analysis
The CSF analysis revealed a Aβ42 level of 403 pg/ml, t-tau of 395 pg/ml, p-tau 181 of 59 pg/ml, and Aβ42 to p-tau 181 ratio of 6.8.
AD biomarker assays were not clearly pathological. RT-QuIC for α-synuclein in CSF was positive.
Olfactory mucosa brushing analysis
RT-QuIC for α-synuclein in olfactory mucosa samples was positive.
Quantitative electroencephalography
The spectral analysis showed a dominant frequency varying between 6 and 10 Hzs in the posterior derivations, a supportive biomarker of DLB diagnosis [41].
DISCUSSION
The case described shows a CBS in possible MCI-LB diagnosis. CBS is a syndrome frequently associated with tauopathies; however, it is very rarely reported in synucleinopathies.
Lewy body-related pathology is rarely the underlying substrate of CBS [42]. Two published reports have described autopsy-confirmed diffuse Lewy body disease (DLBD) manifesting as CBS [43–45].
Recently, Kasanuki reviewed 523 cases in which α-synuclein immunohistochemistry was used to quantify Lewy body-related pathology from a brain bank of neurodegenerative disorders [46]; he identified 11 cases of DLBD with antemortem CBS: four with concomitant AD, three with concomitant progressive supranuclear palsy, but also four with concomitant DLB, suggesting a frequency of 0.8%. Interestingly, all these 11 cases with antemortem CBS had 14-fold more Lewy bodies and 6-fold greater α-synuclein burden in the motor cortex than DLBD cases with clinical features of DLB.
The case presented shows a CBS that progressively worsened over a three-year period.
The phenomenon of the left anarchic hand, anterior sign [12, 13], shown by our patient, could be supported by the hypometabolism observed at 18F-FDG PET also in the right supplementary motor area. The fact that the patient required visual confirmation of the position of the left arm, suggesting the AL phenomenon, could be explained by the parietal lobe atrophy and hypometabolism.
The objective finding, which appeared in October 2020, that the left leg tended to hinder the step of the right leg, could be the expression of the worsening and extension of the pathology representing phenomena of interference and conflict with the movements of the healthy side of the body [13, 14].
The onset with memory deficits and parietal cerebral atrophy and hypometabolism in parieto- temporal cortical areas led us to hypothesize prodromal AD.
Although our patient was assigned a clinical diagnosis of probable CBS, usually an expression of tauopathies, unexpectedly, α-synuclein RT-QuIC assay detected α-synuclein aggregates both in CSF and in OM samples, indicating a probable α-synuclein pathology.
The QEEG confirmed the evidence emerged from the RT-QuIC showing an electrophysiological pattern of early stage DLB [34, 41].
In summary, the patient presented with resting tremor, mild extrapyramidal rigidity, mild bradykinesia, and postural instability, repeated falls, one vasovagal syncope, anxiety, and depression. DaT-SPECT showed only a mild reduced uptake of the posterior portion of the right putamen that did not appear to be pathological in the semi-quantitative analysis (DaTQUANT). The PET images showed a marked hypometabolism in the right parieto-occipital cortex and precuneus but a discrete sparing of the posterior cingulate cortex, suggesting a possible cingulate island sign. Furthermore, considering the recent research criteria for the diagnosis of prodromal DLB [47] the patient satisfied the criteria for possible MCI-LB. Indeed, he presented a core clinical feature (extrapiramidal signs) with different potential biomarkers, such as low occipital and right putamen uptake on the 18F-FDG PET, along with the cingulate island sign, preservation of the medial temporal lobe structures on structural imaging, and quantitative EEG showing slowing and dominant frequency variability [47].
Our patient also showed supportive clinical features such as postural instability, repeated falls, vasovagal syncope, anxiety, depression, and urinary incontinence [47].
In the recent research criteria for the diagnosis of prodromal DLB, RT-QuIC for the research on α-synuclein in different biofluids including saliva, plasma, serum, or urine is considered promising [47].
The detection of deposits of the α-synuclein in two different tissues, CSF and nasal mucosa, strongly reinforced the diagnosis of synucleinopathy in our patient.
RT-QuIC provided a better definition of underlying pathologies in neurodegenerative disorders with prognostic and potentially therapeutic implications. It also offers a re-appreciation of CBS as the expression of different underlying pathologies, including synucleinopathy.
The clinical case presented is certainly atypical, both because of certain phenotypic aspects and because of the biological markers and the QEEG detected. We argue that clinical diagnostic criteria are insufficient and there is an increasing need to include reliable and sensitive markers.
It was not possible to conduct an autopsy, nonetheless, it is the authors’ hope that the evidence presented in this work stimulates a debate on the issues addressed.
Conclusions
We discussed the case of a patient who showed a CBS clinical phenotype and α-synuclein deposits in CSF and OM samples together with one core clinical feature and potential biomarkers for the research diagnostic criteria of possible MCI-LB.
The clinical case presented is in addition to the very few examples of CBS with biological evidence of LB pathology already described in the literature.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Chahine LM , Rebeiz T , Rebeiz JJ , Grossman M , Gross RG ((2014) ) Corticobasal syndrome: Five new things. Neurol Clin Pract 4: , 304–312. |
[2] | Rebeiz JJ , Kolodny EH , Richardson EP Jr ((1968) ) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18: , 20–33. |
[3] | Rebeiz JJ , Kolodny EH , Richardson EP Jr ((1967) ) Corticodentatonigral degeneration with neuronal achromasia: A progressive disorder of late adult life. Trans Am Neurol Assoc 92: , 23–26. |
[4] | Gibb WR , Luthert PJ , Marsden CD ((1989) ) Corticobasal degeneration. Brain 112: (Pt 5), 1171–1192. |
[5] | Armstrong MJ , Litvan I , Lang AE , Bak TH , Bhatia KP , Borroni B , Boxer AL , Dickson DW , Grossman M , Hallett M , Josephs KA , Kertesz A , Lee SE , Miller BL , Reich SG , Riley DE , Tolosa E , Troster AI , Vidailhet M , Weiner WJ ((2013) ) Criteria for the diagnosis of corticobasal degeneration. Neurology 80: , 496–503. |
[6] | Parmera JB , Rodriguez RD , Studart Neto A , Nitrini R , Brucki SMD ((2016) ) Corticobasal syndrome: A diagnostic conundrum. Dement Neuropsychol 10: , 267–275. |
[7] | Biran I , Giovannetti T , Buxbaum L , Chatterjee A ((2006) ) The alien hand syndrome: What makes the alien hand alien? . Cogn Neuropsychol 23: , 563–582. |
[8] | Doody RS , Jankovic J ((1992) ) The alien hand and related signs. J Neurol Neurosurg Psychiatry 55: , 806–810. |
[9] | Fisher CM ((2000) ) Alien hand phenomena: A review with the addition of six personal cases. Can J Neurol Sci 27: , 192–203. |
[10] | Josephs KA , Rossor MN ((2004) ) The alien limb. Pract Neurol 4: , 44–45. |
[11] | Rowe JB , Wolpe N WolpeN(2014) Disorders of volition from neurological disease: Altered awareness of action in neurological disorders. In The sense of agency, Haggard P, Eitam B, eds. University Press, Oxford, Oxford. |
[12] | Marchetti C , Della Sala S ((1998) ) Disentangling the alien and anarchic hand. Cogn Neuropsychiatry 3: , 191–207. |
[13] | Scepkowski LA , Cronin-Golomb A ((2003) ) The alien hand: Cases, categorizations, and anatomical correlates. Behav Cogn Neurosci Rev 2: , 261–277. |
[14] | Di Pietro M , Russo M , Dono F , Carrarini C , Thomas A , Di Stefano V , Telese R , Bonanni L , Sensi SL , Onofrj M , Franciotti R ((2021) ) A critical review of alien limb-related phenomena and implications for functional magnetic resonance imaging studies. Front Neurol 12: , 661130. |
[15] | John J ((1984) ) Grading of muscle power: Comparison of MRC and analogue scales by physiotherapists. Medical Research Council. Int J Rehabil Res 7: , 173–181. |
[16] | Gallucci M , Mariotti E , Saraggi D , Stecca T , Oddo MG , Bergamelli C , Boldrini P , Mazzuco S , Ongaro F , Mecocci P , Di Paola F , Bendini M , Forloni GL , Albani D , Antuono P , Caberlotto L , Zanardo A , Siculi M , Gajo GB , Durante E , Buscato G ((2012) ) The Treviso Dementia (TREDEM) Study: A biomedical, neuroradiological, neuropsychological and social investigation of dementia in North-Eastern Italy. J Frailty Aging 1: , 24–31. |
[17] | Berg L ((1984) ) Clinical dementia rating. Br J Psychiatry 145: , 339. |
[18] | Measso G , Cavarzeran F , Zappalà G , Lebowitz BD , Crook TH , Pirozzolo FJ , Amaducci LA , Massari DFG ((1993) ) The mini mental stateexamination: Normative study of an Italian random sample. DevNeuropsychol 9: , 77–95. |
[19] | Orsini A , Grossi D , Capitani E , Laiacona M , Papagno C , Vallar G ((1987) ) Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8: , 539–548. |
[20] | Spinnler H , Tognoni G ((1987) ) Standardizzazione e Taratura italianadi Test Neuropsicologici. Ital J Neurol Sci 6: , 1–120. |
[21] | Rey A (1964) L’examen clinique en psychologie, Presses Universitaires de France, Paris. |
[22] | Caffarra P , Vezzadini G , Dieci F , Zonato F , Venneri A ((2002) ) Rey-Osterrieth complex figure: Normative values in an Italian population sample. }. Neurol Sci 22: , 443–447. |
[23] | Carlesimo GA , Caltagirone C , Gainotti G , Nocentini U ((1995) ) Batteriaper la valutazione del deterioramento mentale (Parte II):Standardizzazione e affidabilità diagnosticanell’identificazione dei pazienti affetti da sindrome demenziale.. Arch Psicol Neurol Psichiatr 471–488. |
[24] | De Renzi E , Lucchelli F ((1988) ) Ideational apraxia. Brain 111: (Pt 5), 1173–1185. |
[25] | De Renzi E , Motti F , Nichelli P ((1980) ) Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol 37: , 6–10. |
[26] | De Renzi E , Pieczuro A , Vignolo LA ((1966) ) Oral apraxia and aphasia. Cortex 2: , 50–73. |
[27] | De Renzi E , Scotti G , Spinnler H ((1969) ) Perceptual and associativedisorders of visual recognition. Relationship to the side of thecerebral lesion. Neurology 19: , 634–642. |
[28] | Mondini S , Mapelli D , Vestri A , Arcara G , Bisiacchi PS (2011) Esame Neuropsicologico Breve 2. Una batteria di test per lo screening neuropsicologico, Raffaello Cortina Editore. |
[29] | Warrington EK , James M JamesM(1991) The visual object and space perception battery, Thames Valley Test Company, Bury St. Edmunds. |
[30] | Cummings JL ((1997) ) The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 48: , S10–S16.. |
[31] | Neill M , Fisher JM , Brand C , Lei H , Sherman SJ , Chou YH , Kuo PH ((2021) ) Practical application of DaTQUANT with optimal threshold fordiagnostic accuracy of dopamine transporter SPECT.. Tomography 7: , 980–989. |
[32] | Brogley JE ((2019) ) DaTQUANT: The future of diagnosing Parkinsondisease. J Nucl Med Technol 47: , 21–26. |
[33] | Suppiah S , Didier MA , Vinjamuri S ((2019) ) The who, when, why, and how of pet amyloid imaging in management of Alzheimer’s disease-review of literature and interesting images. Diagnostics (Basel) 9: , 65. |
[34] | Bonanni L , Thomas A , Tiraboschi P , Perfetti B , Varanese S , Onofrj M ((2008) ) EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain 131: , 690–705. |
[35] | Fiorini M , Bongianni M , Benedetti MD , Monaco S , Zanusso G ((2018) ) Reappraisal of Abeta40 and Abeta42 peptides measurements incerebrospinal fluid of patients with Alzheimer’s disease. JAlzheimers Dis 66: , 219–227. |
[36] | Bongianni M , Ladogana A , Capaldi S , Klotz S , Baiardi S , Cagnin A , Perra D , Fiorini M , Poleggi A , Legname G , Cattaruzza T , Janes F , Tabaton M , Ghetti B , Monaco S , Kovacs GG , Parchi P , Pocchiari M , Zanusso G ((2019) ) alpha-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol 6: , 2120–2126. |
[37] | Stefani A , Iranzo A , Holzknecht E , Perra D , Bongianni M , Gaig C , Heim B , Serradell M , Sacchetto L , Garrido A , Capaldi S , Sánchez-Gómez A , Cecchini MP , Mariotto S , Ferrari S , Fiorini M , Schmutzhard J , Cocchiara P , Vilaseca I ((2021) ) Alpha-synucleinseeds in olfactory mucosa of patients with isolated REM sleepbehaviour disorder. Brain 144: , 1118–1126. |
[38] | Perra D , Bongianni M , Novi G , Janes F , Bessi V , Capaldi S , Sacchetto L , Tagliapietra M , Schenone G , Morbelli S , Fiorini M , Cattaruzza T , Mazzon G , Orru CD , Catalan M , Polverino P , Bernardini A , Pellitteri G , Valente M , Bertolotti C , Nacmias B , Maggiore G , Cavallaro T , Manganotti P , Gigli G , Monaco S , Nobili F , Zanusso G ((2021) ) Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun 3: , fcab045. |
[39] | Gallucci M , Mazzetto M , Spagnolo P , Arico M , Bergamelli C , Oddo MG , Gallo G , Astolfo P , Di Giorgi E , Vanacore N ((2016) ) Mild cognitive impairment, from theory to practical intervention: “Camminando e leggendo... ricordo” (Walking and reading... I remember), an action plan. The Treviso Dementia (TREDEM) Registry. Ann Ist Super Sanita 52: , 240–248. |
[40] | Gallucci M , Mazzarolo AP , Focella L , Piovesan C , Mazzetto M , Ramigni M , Marzetti E ((2020) ) “Camminando e leggendo... ricordo” (Walking and Reading... I Remember): Prevention of frailty through the promotion of physical activity and reading in people with mild cognitive impairment. Results from the TREDEM registry. J Alzheimers Dis 77: , 689–699. |
[41] | Bonanni L , Perfetti B , Bifolchetti S , Taylor JP , Franciotti R , Parnetti L , Thomas A , Onofrj M ((2015) ) Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiol Aging 36: , 434–445. |
[42] | Ling H , O’Sullivan SS , Holton JL , Revesz T , Massey LA , Williams DR , Paviour DC , Lees AJ ((2010) ) Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 133: , 2045–2057. |
[43] | Horoupian DS , Wasserstein PH ((1999) ) Alzheimer’s disease pathology in motor cortex in dementia with Lewy bodies clinically mimicking corticobasal degeneration. Acta Neuropathol 98: , 317–322. |
[44] | Haug A , Boyer P , Kluger B ((2013) ) Diffuse lewy body diseasepresenting as corticobasal syndrome and progressive supranuclearpalsy syndrome. Mov Disord 28: , 1153–1155. |
[45] | Ichinose K , Watanabe M , Mizutani S , Tanizawa T , Uchihara T , Fujigasaki H ((2021) ) An autopsy case of corticobasal syndrome with pure diffuse Lewy body disease. Neurocase 27: , 231–237. |
[46] | Kasanuki K , Josephs KA , Ferman TJ , Murray ME , Koga S , Konno T , Sakae N , Parks A , Uitti RJ , Van Gerpen JA , Graff-Radford NR , Wszolek ZK , Dickson DW ((2018) ) Diffuse Lewy body disease manifesting ascorticobasal syndrome: A rare form of Lewy body disease. Neurology 91: , e268–e279. |
[47] | McKeith IG , Ferman TJ , Thomas AJ , Blanc F , Boeve BF , Fujishiro H , Kantarci K , Muscio C , O’Brien JT , Postuma RB , Aarsland D , Ballard C , Bonanni L , Donaghy P , Emre M , Galvin JE , Galasko D , Goldman JG , Gomperts SN , Honig LS , Ikeda M , Leverenz JB , Lewis SJG , Marder KS , Masellis M , Salmon DP , Taylor JP , Tsuang DW , Walker Z , Tiraboschi P , prodromal DLBDSG ((2020) ) Researchcriteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 94: , 743–755. |




