Association Between Low- and High-Value Medication and Hospital Referrals by General Practitioners in Patients Living with Dementia
Abstract
Background:
Previous studies revealed that low-value medication (LvM), drugs that provide little or no benefit but have the potential to cause harm, are associated with hospitalizations in dementia. Recommended medications, referred to as high-value medication (HvM), can be used alternately. However, the effect of LvM and HvM on hospitalizations is uncertain.
Objective:
To determine the prevalence of LvM and HvM in hospitalized and non-hospitalized patients living with dementia (PwD) and the odds for hospital referrals in PwD receiving LvM or HvM.
Methods:
The analysis was based on 47,446 PwD who visited a general practitioner practice between 2017 and 2019. Different guidelines were used to elicit LvM and HvM, resulting in 185 LvM and HvM related recommendations. Of these, 117 recommendations (83 for LvM, 34 for HvM) were categorized into thirteen therapy classes. The association of hospital referrals issued by general practitioners and receiving LvM or HvM was assessed using multiple logistic regression models.
Results:
20.4% of PWD received LvM. Most frequently prescribed LvM were non-recommended sedatives and hypnotics, analgesics, and antidepressants. Recommended HvM were 3.4 (69.9%) more frequently prescribed than LvM. Most commonly prescribed HvM were recommended antihypertensives, antiplatelet agents, and antiarrhythmics. Both receiving LvM and receiving HvM were associated with higher odds for hospital referrals. When receiving LvM were compared to HvM, no significant differences could be found in hospital referrals.
Conclusion:
LvM is highly prevalent but did not cause more likely hospital referrals than HvM. Further research should focus on acute hospitalizations, not only on planned hospital referrals.
INTRODUCTION
Worldwide, the population is aging rapidly, which is associated with an increase in the prevalence of age-associated illnesses, such as dementia diseases [1]. More than 50 million people were living with dementia (PwD) worldwide in 2019. This number is expected to reach 152 million within the next 30 years, representing a considerable societal and economic burden [2, 3].
In times of scarce healthcare resources, these rapidly increasing healthcare expenditures are likewise massive global problems. These expenditures are not only caused by demographic changes, new treatment possibilities, and growing demand. Shrank et al. [4] revealed that 10% of the united healthcare spending was related to unnecessary or even harmful diagnostic procedures, treatments, and care, summarized as overuse, overtreatment, failures, or low-value care or medication (LvM). LvM can be defined as care unlikely to benefit the patient regarding the patient’s preferences, potential harms, costs, or available alternatives [4–7]. Inappropriate drug use, overdiagnosis, overtreatment, and overtesting are overlapping concepts that address medical overuse along the entire care pathway. Thus, these concepts are of particular relevance to LvM with a substantial overlap [8, 9]. However, LvM goes beyond this concept by additionally incorporating medications where the costs exceed their benefits.
Instead of prescribing LvM, different guidelines explicitly recommend equally effective and tolerable medications that can provide a benefit under consideration of all the mentioned aspects and besides potential risks they have, which can be defined as high-value medication (HvM) [10]. However, it is difficult to differentiate between inappropriate and appropriate health care service provision [9]. For this purpose, guidelines provide support by issuing recommendations for or against health services representing over- and underuse or by listing potentially inadequate medication [11–13].
PwD are a multimorbid population. Polypharmacy occurs very frequently. A study by Amann et al. [14] revealed that nearly every fourth elderly individual receives potentially inadequate drugs, predominantly female patients at higher ages [14]. A further study has shown that a large proportion of PwD is affected by additional drug-related problems [15]. The likelihood of receiving LvM increases with age, higher comorbidity, and higher deficits in their daily living [16, 17].
Reducing LvM could simultaneously lead to greater efficiency in the healthcare system and higher value for patient-centered outcomes [18]. A study by Platen et al. demonstrates that LvM is highly present in routine care. Also, PwD receiving LvM were significantly more hospitalized and had lower quality of life [19]. However, results concerning the impact of recommended HvM remained uncertain. Also, the analysis was based on small sample size, limiting the demonstrated association’s generalizability. Therefore, further research is needed to evaluate the association between LvM and cost-intensive hospitalizations in PwD. Also, studies that consider both LvM and HvM simultaneously to examine their respective associations with patient-reported data, such as hospitalizations, are currently rare. Moreover, it has not yet been conclusively determined which type of care is of a higher value. Therefore, the objective of this study is to demonstrate the prevalence of LvM and HvM in hospitalized PwD and to evaluate the associations between receiving LvM or HvM and hospital referrals given by general practitioners in primary care.
MATERIALS AND METHODS
Study sample and participant flow
This retrospective cross-sectional study was based on medical record data from the Disease Analyzer database (IQVIA). This database compiles drug prescriptions, diagnoses, and basic medical and demographic data obtained directly in anonymous format from computer systems used in the practices of general practitioners (GPs) [20]. Diagnoses, prescriptions, and the quality of reported data are monitored by IQVIA based on an array of criteria. In Germany, the sampling methods used to select physicians’ practices have been shown to be appropriate for obtaining a population-representative database of primary care [20].
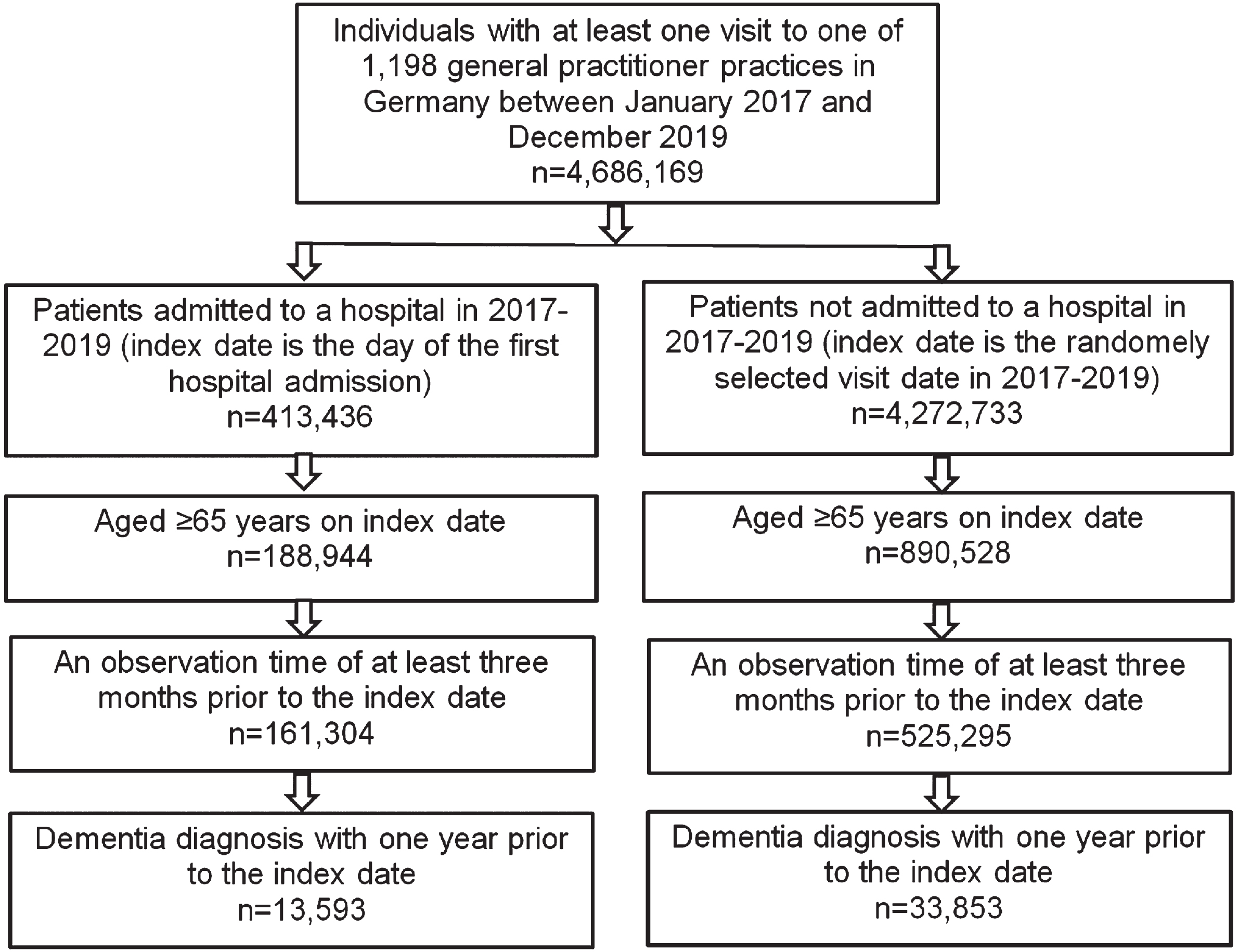
Overall, 4,686,169 patients visited at least one out of 1,198 GPs and internal specialists practices in Germany between January 2017 and December 2019. Totally, 413,436 (8.8%) of these patients were admitted to a hospital during the study period, 4,272,733 (91.2%) were not admitted. Also, 188,944 hospitalized patients and 890,528 non-hospitalized patients were at the age of 65 and older. For some patients (686,599, 14.7%), the observation time of at least three months before the index date was not available, resulting in a subsample of 686,599 patients. Finally, n = 13,593 hospitalized and n = 33,853 non-hospitalized patients had a formal dementia diagnosis. The data flow chart is shown in Fig. 1.
Fig. 1
Selection of study patients.
Study outcomes
The main outcomes of this study were the prescription prevalence of LvM and HvM and dementia patients that were admitted or not admitted to hospitals by GPs. Primarily, we will demonstrate the prevalence of different LvM and HvM therapy classes of patients admitted compared to those not admitted to hospitals by GPs. Hospital admissions were non-urgent, elective admissions. The data given by GP practices did not capture acute hospital admissions.
To indicate LvM and HvM treatments, we used the German “S3-guideline: Dementia” published by the German Association for Psychiatry, Psychotherapy and Psychosomatics and the German Society for Neuroscience, selecting treatments, procedures, and drugs that are effective, helpful, and highly recommended in their use (representing HvM) or should be omitted or avoided (representing LvM) [12]. Additionally, defined positive and negative recommendations of the international “Choosing Wisely” campaign were used to identify further LvM and HvM treatments [11]. Finally, we used the PRISCUS list, comprising a list of 83 substances of 18 drug classes that are potentially inadequate for older people. This list includes recommendations (LvM) and alternatives (HvM), representing a decision-making aid [13].
Two independent reviewers (MP and BM) reviewed all drug-related recommendations. According to previous studies, the selection was made after a discussion in terms of relevance, targeted audience, differentiation possibilities, and existence in the data set used for this analysis [21]. Since they are different sources with similar goals, there was some overlap [22]. A total of 68 out of 185 recommendations of the three independent sources had to be excluded because they did not meet the mentioned criteria. Of the remaining 117 recommendations, 83 recommendations could be assigned to LvM and 34 to HvM. The individual substances were grouped according to their drug classes following the PRISCUS list. In conclusion, 13 measurable LvM and 13 measurable HvM treatments provided the basis for this analysis. We used ATC codes of LvM and HvM for identification in the data set. Used LvM and HvM therapy classes are shown in Table 1.
Table 1
Low-value medication and high-value medication classification
| Low-value Care –Drug class (included substances) | High-value medication –Drug class (included substances) |
| Low-value antiphlogistics/analgesics (Dexketoprofen, etoricoxib, indometacin, meloxicam, naproxen, diclofenac, rofecoxib, acemetacin, phenylbutazone, piroxicam, pethidine) | High-value antiphlogistics/ analgesics (Paracetamol, tramadol, codeine, ibuprofen) |
| Low-value antidementive drugs (Naftidrofuryl, piracetam, dihydroergotoxine, nicergoline, nimodipine, pentoxifylline) | High-value antidementia drugs (Donepezil, galantamine, rivastigmine, memantine) |
| Low-value antipsychotics (Levomepromazine, olanzapine, haloperidol, perphenazine, clozapine, thioridazine, fluphenazine) | High-value antipsychotics (Risperidone, melperone, pipamperone) |
| Low-value sedatives/ hypnotics (Chloral hydrate, chlordiazepoxide, clobazam, diazepam, zopiclon, diphenhydramine, doxylamine, medazepam, nitrazepam, zolpidem, flurazepam, clorazepate, bromazepam, prazepam, flunitrazepam, alprazolam, temazepam, triazolam, lorazepam, oxazepam, lormetazepam, brotizolam, zaleplon, doxylamine) | High-value sedatives/ hypnotics (Trazodone, mianserin, opipramol) |
| Low-value antidepressants (Amitriptyline, amitriptylinoxide, doxepin, trimipramine, imipramine, clomipramine, maprotiline, fluoxetine, tranylcypromine) | High-value antidepressants (Citalopram, escitalopram, sertraline, mirtazapine, opipramol) |
| Low-value antihypertensives (Clonidine, doxazosin, methyldopa,, prazosin, terazosin, reserpine, nifedipin, nifedipine) | High-value antihypertensives (ACE inhibitor, diuretics) |
| Low-value laxative (Liquid paraffin) | High-value laxative (Macrogol, lactulose) |
| Low-value antiarrhythmics (Acetyldigoxin, flecainide, sotalol, quinidine, digoxin, metildigoxin) | High-value antiarrhythmics (Amiodarone, verapamil, diltiazem, bisoprolol, carvedilol, propafenone) |
| Low-value antiemetics (Dimenhydrinate) | High-value antiemetics (Domperidone, metoclopramide) |
| Low-value muscle relaxants (Baclofen, tetrazepam) | High-value muscle relaxants (Tolperisone, tizanidine) |
| Low-value anticholinergics (Hydroxyzine, clemastine, dimetindene, chlorphenamine, triprolidine, oxybutynin, tolterodine, solifenacin) | High-value anticholinergics (Cetirizine, desloratadine, loratadine, mizolastine, azelastine, ebastine, trospium) |
| Low-value antibiotics (Nitrofurantoin) | High-value antibiotics (Cephalosporin, trimethoprim/sulfamethoxazole, trimethoprim) |
| Low-value antiplatelet drugs (Ticlopidine, prasugrel) | Low-value antiplatelet drugs (Acetylsalicylic acid, clopidogrel) |
Also, demographic data (age, sex) the following ICD-10 diagnoses (International Statistical Classification of Diseases and Related Health Problems) were used to adjust for coexisting diseases: diabetes mellitus (E10-14), stroke including transient ischemic attack (I63, I64, G45), epilepsy (G40), Parkinson’s disease (G20, G21), depression (F32, F33), cancer (C00–C97), chronic bronchitis and COPD (J42–J44), as well as myocardial infarction (I21, I22) and coronary heart disease (I24, I25).
Statistical analysis
The study participants’ demographic and clinical characteristics and the prevalence of LvM and HvM treatments over both groups (admitted to hospital versus not admitted) were presented using descriptive statistics. Differences between admitted and non-admitted hospitals patients were assessed using Wilcoxon-tests, and chi-squared tests. To assess the associations between hospital admissions and the presence of LvM and HvM, multivariable linear regression models were fitted. The first model Models tested the association with hospital admission between those patients that ever used LvM (HvM) versus those that never used LvM (HvM) within three months before the hospital admission. The second model analyzed the association with hospital admission between patients who used LvM versus those who used HvM three months before the hospital admissions. Models were adjusted for age, sex, Charlson comorbidity score, and coexisting comorbidities. Results were expressed by odds ration and 95% confidence intervals. All statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, US).
RESULTS
Demographic and clinical characteristics
The study participants were on average 83 years old and primarily female (64%). According to the Charlson Comorbidity Index, patients had very high comorbidity (mean 3.8). Hypertension (60.7%), diabetes mellitus (27.0%), osteoarthritis (24.7%), and depression (24.1%) were the most common coexisting diseases. PwD who were admitted to a hospital were significantly more likely male (39.6% versus 34.4%), younger (82.9 versus 83.6), and had a higher comorbidity index (4.2 versus 3.6) than those not admitted to a hospital during the observation period. The sample characteristics are presented in Table 2.
Table 2
Baseline characteristics of study patients
| Variable N | Overall (n = 47,446) | Patients with referral to a hospital(n = 13,593) | Patients without referral to a hospital(n = 33,853) | p |
| Women | 64.1 | 60,4 | 65,6 | <0.001 |
| Mean age in years (standard deviation) | 83,4 (6,8) | 82,9 (6,7) | 83,6 (6,9) | <0.001 |
| Age 65–70 y | 4.3 | 4,6 | 4,2 | |
| Age 71–75 y | 8.1 | 8,5 | 8,0 | |
| Age 75–80 y | 20.3 | 22,1 | 19,6 | <0.001 |
| Age 81–85 y | 27.2 | 28,1 | 26,8 | |
| Age >85 y | 40.1 | 36,7 | 41,5 | |
| Charlson Comorbidity Index (mean, SD) | 3.8 | 4,2 (2,5) | 3,6 (2,3) | <0.001 |
| Diabetes mellitus | 27.0 | 29,9 | 25,9 | <0.001 |
| Hypertension | 60.7 | 65,2 | 58,9 | <0.001 |
| Lipid metabolism disorders | 29.7 | 33,6 | 28,2 | <0.001 |
| Heart failure | 22.3 | 26,1 | 20,8 | <0.001 |
| Chronic coronary heart disease | 20.9 | 24,6 | 19,5 | <0.001 |
| History of stroke/TIA | 23.4 | 25,5 | 22,6 | <0.001 |
| History of myocardial infarction | 4.7 | 6,1 | 4,2 | <0.001 |
| Depression | 24.1 | 26,9 | 23,0 | <0.001 |
| Cancer | 12.5 | 15,4 | 11,4 | <0.001 |
| Osteoarthritis | 24.7 | 28,5 | 23,2 | <0.001 |
Data are absolute numbers and percentages unless otherwise specified.
Prevalence of low- and high-value medication
20.4% of patients received LvM. Considering the prescribed LvM, non-recommended Sedatives and Hypnotics (8.8%), Analgesics and Anti-inflammatories (3.8%), and Antidepressants (2.2%) were most commonly prescribed LvM in patients living with dementia. In contrast, and following the evidence-based guidelines, recommended HvM were significantly more often prescribed (69.9%) than LvM. The most commonly HvM taken were Antihypertensive (43.5%), Antiplatelet agents (20.1%), and Antiarrhythmics (17.0%). Overall, HvM were eight times (range: 1.5 times to 32 times) more frequently prescribed than LvM, except muscle relaxants that were more frequently prescribed as LvM than as HvM (0.3% versus 0.2%). Also, PwD that were admitted to a hospital take more frequently LvM, but also more frequently HvM. The prevalence of LvM and HvM in patients living with dementia were shown in Table 3.
Table 3
Association between defined therapies and referral to hospital in patients living with dementia analyzed in general practices in the Germany –Logistic regression model: ever use LvM/ HvM versus never use LvM/ HvM within 3 months prior to index date
| LvM | HvM | |||||||
| Therapy class | % of patients with at least one prescription among patients with referral to hospital | % of patients with at least one prescription among patients without referral to hospital | Odds ratio (95% confidence interval) | p | % of patients with at least one prescription among patients with referral to hospital | % of patients with at least one prescription among patients without referral to hospital | Odds ratio (95% confidence interval) | p |
| Antidementida drugs | 0.7 | 0.6 | 1.02 (0.80–1.31) | 0.856 | 9,0 | 7,5 | 1.18 (1.10–1.27) | <0.001 |
| Antipsychotics | 1.5 | 1.1 | 1.16 (0.97–1.38) | 0.105 | 16,0 | 12,6 | 1.08 (1.00–1.18) | 0.064 |
| Antiphlogistics/analgesics | 5.2 | 3.3 | 1.36 (1.23–1.50) | <0.001 | 11,3 | 7,3 | 1.42 (1.33–1.52) | <0.001 |
| Antiarrhythmics | 1.3 | 0.8 | 1.39 (1.14–1.68) | <0.001 | 21,1 | 15,4 | 1.25 (1.17–1.30) | <0.001 |
| Antibiotics | 0.8 | 0.4 | 1.53 (1.18–1.97) | 0.001 | 1,9 | 1,0 | 1.63 (1.38–1.92) | <0.001 |
| Anticholinergics | 1.9 | 1.3 | 1.28 (1.09–1.50) | 0.003 | 6,7 | 5,2 | 1.02 (0.93–1.12) | 0.617 |
| Antiplatelet drugs | 0.1 | 0.0 | 2.07 (0.68–6.31) | 0.199 | 23,5 | 18,7 | 1.10 (1.04–1.16) | <0.001 |
| Antidepressant | 2.9 | 1.9 | 1.30 (1.14–1.49) | <0.001 | 10,5 | 7,8 | 1.10 (1.01–1.19) | 0.022 |
| Antiemetics | 2.1 | 1.3 | 1.37 (1.17–1.60) | <0.001 | 3,6 | 2,0 | 1.55 (1.37–1.75) | <0.001 |
| Antihypertensives | 1.8 | 1.2 | 1.32 (1.12–1.56) | <0.001 | 52,3 | 40,0 | 1.42 (1.36–1.49) | <0.001 |
| Laxative | 1.0 | 0.8 | 1.21 (0.98.1.50) | 0.082 | 12,2 | 9,9 | 1.08 (1.01–1.15) | 0.023 |
| Muscle relaxants | 0.4 | 0.3 | 1.02 (0.71–1.45) | 0.932 | 0,2 | 0,2 | 0.76 (0.48–1.19) | 0.232 |
| Sedatives, hypnotics | 11.4 | 7.8 | 1.27 (1.19–1.36) | <0.001 | 17,2 | 12,7 | 1.18 (1.08–1.28) | <0.001 |
LvM, Low-value medication; HvM, High-value medicaition.
Associations between low- and high-value medication and hospital admissions
Receiving at least one LvM compared to never receiving LvM was significantly associated with higher odds of hospital admissions for all LvM therapy classes, except for low-value Anti-dementia drugs. Highest odds were seen for low-value Antibiotics (OR = 1.53; 95% CI 1.18–1.97), Antiarrhythmics (OR = 1.39; 95% CI 1.14–1.68), Antiemetics (OR = 1.37; 95% CI 1.17–1.60), and Analgetic (OR = 1.36; 95% CI 1.23–1.50).
However, significantly higher odds for hospital admissions were also seen for nearly all HvM therapy classes, except Neuroleptics. The highest odds were seen for Antibiotics (OR = 1.63; 95% CI 1.387–1.92), Antiemetics (OR = 1.42; 95% CI 1.33–1.52), Antihypertensives (OR = 1.42; 95% CI 1.36–1.49), and Analgesics (OR = 1.52; 95% CI 1.34–1.73). Even though odds for hospital admissions were in most therapy classes slightly higher in patients receiving LvM than in patients receiving HvM, the results suggest that both receiving LvM and HvM were significantly associated with a higher likelihood for a hospital admission issued by GPs. This was confirmed by the logistic regressions models that compared those patients that received LvM with those receiving HvM and the likelihood for hospital admissions issued by GPs, where no significant association could be found. The odds ratios of all therapy classes in both groups are shown in Tables 3 and 4.
Table 4
Association between defined LvM therapies compared to HvM therapies and referral to hospital in patients analyzed in general practices in the Germany (Model II: LvM versus HvM within 3 months prior to index date)
| Therapy class | Dementia patients | |
| Odds ratio (95% confidence interval) for LvM versus HvM(reference) | p | |
| Antidementida drugs | 0.84 (0.64–1.10) | 0.216 |
| Antipsychotics | 1.05 (0.84–1.31) | 0.668 |
| Antiphlogistics/analgesics | 0.93 (0.82–1.05) | 0.256 |
| Antiarrhythmics | 1.01 (0.80–1.29) | 0.919 |
| Antibiotics | 1.00 (0.73–1.39) | 0.978 |
| Anticholinergics | 1.07 (0.89–1.29) | 0.481 |
| Antiplatelet drugs | 1.67 (0.49–5.92) | 0.425 |
| Antidepressant | 1.13 (0.98–1.31) | 0.104 |
| Antiemetics | 0.87 (0.72–1.07) | 0.186 |
| Antihypertensives | 1.14 (0.87–1.50) | 0.339 |
| Laxative | 1.13 (0.89–1.45) | 0.312 |
| Muscle relaxants | 1.23 (0.67.2.28) | 0.505 |
| Sedatives, hypnotics | 1.03 (0.94–1.13) | 0.542 |
LvM, Low-value medication; HvM, High-value medication.
DISCUSSION
This study aimed to examine the associations between receiving LvM and HvM and patients’ hospital admissions issued by GPs. 20.4% of primary care patients living with dementia received LvM treatments, indicating that LvM was highly present in community-dwelling PwD. Contrary to this, in 69.9% of patients, recommended HvM were prescribed, demonstrating that HvM were x-times more frequently taken than LvM. Hospital admissions were more like issued in younger, male patients with a very high comorbidity. LvM was mainly caused by sedatives and hypnotics, analgesics, antidepressants, and anticholinergics. HvM was particularly prescribed in antihypertensives, antiplatelet agents, and antiarrhythmics. Both taking LvM and receiving HvM were associated with higher odds for hospital admissions issued by GPs compared to those patients who never received LvM or HvM, respectively. However, comparing those receiving LvM with those receiving HvM, no significant differences could be seen in hospital admissions, underlining the missing association between LvM and hospital admissions issued by GP in primary dementia care.
Several studies have already focused on the prescription of LvM in the elderly, including PwD. The risk for receiving LvM is age-related and associated with a higher degree of comorbidity. Additionally, potentially inadequate drugs, in particular, are more frequently prescribed to women [14, 16, 17]. Results of previous studies are partly in line with the presented results of this study, underlining that PwD are a high-risk group for receiving LvM, especially female patients that are more often affected by dementia and other coexisting diseases due to the higher life expectancy [23].
This study revealed that sedative or hypnotics, analgesics, and antidepressants were the most commonly prescribed LvM. Inappropriate sedatives and hypnotics are mainly benzodiazepines that are associated with higher risks of falls and fractures that cause hospitalizations in the elderly [11]. Further studies revealed that the prescription and intake of antidepressants is also associated with an increased risk of hospitalization [24, 25]. Thus, our findings are in line with these studies, demonstrating a prevalence of these LvM in patients admitted to hospitals by GPs. Also, receiving LvM was significantly associated with a higher probability of hospitalization for nearly all individual therapy classes.
Considering HvM, recent studies have shown that the probability of receiving care according to the guidelines for PwD depends on a patient’s age, severity, and comorbidity [26, 27]. Our results reveal that recommended HvM was 3.4-times more frequently prescribed than LvM (69.9% versus 20.4%), especially antihypertensives, antiplatelet agents, and antiarrhythmics. However, the association between receiving HvM and patients’ hospital admissions was comparable to the higher odds of hospital admission in patients receiving LvM. Our results reveal that receiving HvM was also significantly associated with an increased risk of hospital admissions compared to patients who never received recommended HvM. Comparing those patients who receive LvM with those who receive HvM, there was evidence of the superiority or inferiority of HvM or LvM, respectively.
Our findings emphasize that HvM is not the simple opposite of LvM. Platen et al. also highlighted this issue [19], demonstrating on a data basis of patients living with dementia in Germany primary care that, in contrast to LvC, recommended HvM not guarantee a positive patient-reported outcome. Their study revealed that guideline-based prescription of memantine significantly increases the health-related quality of life. In contrast, the prescription of recommended antidepressant drugs was associated with lower health-related quality of life [19]. Further studies by Banerjee et al. [28, 29] confirmed the missing superiority of the prescription of recommended antidepressant drugs over a placebo. Thus, evidence about the superiority of HvM over LvM is still uncertain or ambiguous.
It seems unsurprising that there were no differences between LvM and HvM. Even though HvM has a lower potential for harm as it brings benefits to the patients, such medications still have potential side effects and risks that could also cause hospitalizations. Unfortunately, this analysis solely focused on planned hospitalization and did not consider emergencies, which could change the presented results. However, it seems that outcomes of HvM and LvM still tend to bring adverse consequences to the patients, as shown in planned hospitalization. Therefore, the adoption of HvM needs to consider the clinical context and the organization of health care provision should be taken into account. This would be more beneficial than focusing on the quality of single treatments only. Thus, dementia care seems to require a more comprehensive disease management approach [30].
However, it has to be noted that this analysis use planned hospital admissions by GPs as an outcome of interest. LvM are associated with higher risks of falls and fractures that cause acute instead of hospitalizations instead of planned hospital admissions issued by GPs [11]. Also, hospital admissions by other specialist’s practices were not included in this analysis, which further limits the generalizability of the presented results. Thus, no simple conclusion can be drawn from this analysis that demonstrated no evidence for the superiority of recommended HvM over LvM in consideration of hospital admissions. Instead, it seems that the patients’ underlying diseases determine the outcomes rather than the non-recommended or recommended medication. In this case, a higher hospital admission rate. Therefore, further studies are urgently needed to clarify why certain LvM were prescribed and how these medications affect patients’ outcomes more detailed and comprehensively in comparison to recommended HvM by using planned and acute hospitalization data. Cross-sectional data alone cannot establish cause and effect. Hospitalized PwD may be treated with LvM or HvM after discharge. Thus, hospital admissions cannot be considered as a consequence of receiving LvM or HvM. Therefore, further research should evaluate the differences in the benefits and hamrs of LvM and HvM including the patient and the healthcare provider perspective. Furthermore, the associations between LvM and HvM and patient-reported outcomes should be evaluated in in a longitudinal approach. There is also a need to identify relevant subgroups of PwD that could benefit most from cancelling LvM or for those HvM would be superior over LvM.
Limitations
There are some limitations in the accuracy of the data. Data documentation by GP may be less accurate than usual due to the organizational challenges faced by physician practices. Also, the database does not allow for establishing a patient-related connection between different specialists. Therefore, hospital admissions and drug prescriptions other specialties could be missing. However, these prescriptions would have been essential for more precise and complete identification of LvM and HvM across specialists’ discipline. Also, the used outcomes of hospital admissions have several limitations because LvM are mainly associated with a higher risk of falls and fractures that could cause acute hospitalization rather than planned hospital admission issued by GPs. Therefore, the limitations of this study limit the generalizability of the conclusions drawn from this analysis.
Living in a nursing home or community-dwelling could affect the association between LvM and hospitalization differently. Unfortunately, there was no information about the patient’s living situation. Thus, this important variable was not considered in our multivariate models, which limits the generalizability of the presented results.
In addition, the results may be limited because some clinical contextual factors, such as indications, were missing from the secondary data for classifying the prescription as low- or high-value care. Therefore, prescriptions may have been recorded as low-value medication even though the treatment provided was appropriate, and vice versa. Further research with patient-level primary data is needed to incorporate more clinical contextual factors and minimize this problem already described by Schwartz et al. [9]. Also, the generalizability is limited because the classification of treatments as low-value depends on countries and their health systems. Campaigns such as Choosing Wiseley or an international network of healthcare stakeholders could help introduce cross-national formulation and further common standards to make outcomes generalizable and comparable across healthcare systems.
Finally, the LvM-related findings are limited to drug-associated treatments and are non-applicable to non-drug treatments, like surgeries or diagnostic tests. As a result, the prevalence of LvM and HvM is somewhat underestimated.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Prince M , Guerchet M , Prina M (2015) The Epidemiology and Impact of Dementia - Current State and Future Trends. WHO Thematic Briefing. World Health Organization (WHO). http://www.who.int/mental_health/neurology/dementia/thematic_briefs_dementia/en/. |
[2] | Alzheimer’s Disease International (2019) World Alzheimer Report 2019. Attitudes to Dementia. Alzheimer’s Disease International, London. |
[3] | Wimo A , Guerchet M , Ali GC , Wu YT , Prina AM , Winblad B , Jonsson L , Liu Z , Prince M ((2017) ) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13: , 1–7. |
[4] | Shrank WH , Rogstad TL , Parekh N ((2019) ) Waste in the US health care system: Estimated costs and potential for savings. JAMA 322: , 1501–1509. |
[5] | Berwick DM , Hackbarth AD ((2012) ) Eliminating waste in US health care. JAMA 307: , 1513–1516. |
[6] | Elshaug AG , Rosenthal MB , Lavis JN , Brownlee S , Schmidt H , Nagpal S , Littlejohns P , Srivastava D , Tunis S , Saini V ((2017) ) Levers for addressing medical underuse and overuse: Achieving high-value health care. Lancet 390: , 191–202. |
[7] | Verkerk EW , Tanke MAC , Kool RB , van Dulmen SA , Westert GP ((2018) ) Limit, lean or listen? A typology of low-value care that gives direction in de-implementation. Int J Qual Health Care 30: , 736–739. |
[8] | Johnell K ((2015) ) Inappropriate drug use in people with cognitive impairment and dementia: A systematic review. Curr Clin Pharmacol 10: , 178–184. |
[9] | Schwartz AL , Landon BE , Elshaug AG , Chernew ME , McWilliams JM ((2014) ) Measuring low-value care in Medicare. JAMA Intern Med 174: , 1067–1076. |
[10] | Owens DK , Qaseem A , Chou R , Shekelle P ((2011) ) High-value, cost-conscious health care: Concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med 154: , 174–180. |
[11] | Deutsche Gesellschaft für Innere Medizin e.V. ((2019) ) Sammelband Initiative “Klug Entscheiden". Deutsches Ärzteblatt 115: . |
[12] | Deutsche Gesellschaft für Psychiatrie Psychotherapie und Nervenheilkunde und Deutsche Gesellschaft für Neurologie((2017) ), S3-Leitlinie Demenzen Springer-Verlag, Berlin. |
[13] | Holt S , Schmiedl S , Thürmann PA ((2010) ) Potenziell inadäquate Medikation für ältere Menschen. Dtsch Arztebl Int 107: , 543–551. |
[14] | Amann U , Schmedt N , Garbe E ((2012) ) Ärztliche Verordnungen von potenziell inadäquater Medikation bei Älteren. Dtsch Arztebl Int 109: , 69–75. |
[15] | Wohlgemuth A , Michalowsky B , Wucherer D , Eichler T , Thyrian JR , Zwingmann I , Rädke A , Hoffmann W ((2020) ) Drug-related problems increase healthcare costs for people living with dementia. J Alzheimers Dis 73: , 791–799. |
[16] | Brett J , Zoega H , Buckley NA , Daniels BJ , Elshaug AG , Pearson SA ((2018) ) Choosing wisely? Quantifying the extent of three low value psychotropic prescribing practices in Australia. BMC Health Serv Res 18: , 1009. |
[17] | Nili M , Shen C , Sambamoorthi U ((2020) ) Low-value care: Antipsychotic medication use among community-dwelling Medicare beneficiaries with Alzheimer’s disease and related dementias and without severe mental illness. Aging Ment Health 24: , 504–510. |
[18] | Badgery-Parker T , Pearson SA , Chalmers K , Brett J , Scott IA , Dunn S , Onley N , Elshaug AG ((2019) ) Low-value care in Australian public hospitals: Prevalence and trends over time. BMJ Qual Saf 28: , 205–214. |
[19] | Platen M , Fleßa S , Rädke A , Wucherer D , Thyrian JR , Mohr W , Scharf A , Mühlichen F , Hoffmann W , Michalowsky B ((2021) ) Prevalence of low-value care and its associations with patient-centered outcomes in dementia. J Alzheimers Dis 83: , 1775–1787. |
[20] | Rathmann W , Bongaerts B , Carius HJ , Kruppert S , Kostev K ((2018) ) Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther 56: , 459–466. |
[21] | Chalmers K , Badgery-Parker T , Pearson SA , Brett J , Scott IA , Elshaug AG ((2018) ) Developing indicators for measuring low-value care: Mapping Choosing Wisely recommendations to hospital data. BMC Res Notes 11: , 163. |
[22] | Brett J , Elshaug AG , Bhatia RS , Chalmers K , Badgery-Parker T , Pearson SA ((2017) ) A methodological protocol for selecting and quantifying low-value prescribing practices in routinely collected data: An Australian case study. Implement Sci 12: , 58. |
[23] | Bickel H (2018) Die Häufigkeit von Demenzerkrankungen. Informationsblätter der Deutschen Alzheimer Gesellschaft, 8. |
[24] | Kalisch Ellett LM , Pratt NL , Ramsay EN , Barratt JD , Roughead EE ((2014) ) Multiple anticholinergic medication use and risk of hospital admission for confusion or dementia. J Am Geriatr Soc 62: , 1916–1922. |
[25] | Uusvaara J , Pitkala KH , Kautiainen H , Tilvis RS , Strandberg TE ((2011) ) Association of anticholinergic drugs with hospitalization and mortality among older cardiovascular patients. Drugs Aging 28: , 131–138. |
[26] | Michalowsky B , Flessa S , Eichler T , Hertel J , Dreier A , Zwingmann I , Wucherer D , Rau H , Thyrian JR , Hoffmann W ((2018) ) Healthcare utilization and costs in primary care patients with dementia: Baseline results of the DelpHi-trial. Eur J Health Econ 19: , 87–102. |
[27] | Wübbeler M , Thyrian JR , Michalowsky B , Hertel J , Laporte Uribe F , Wolf-Ostermann K , Schäfer-Walkmann S , Hoffmann W ((2015) ) Nonpharmacological therapies and provision of aids in outpatient dementia networks in Germany: Utilization rates and associated factors. J Multidiscip Healthc 8: , 229–236. |
[28] | Banerjee S , Hellier J , Dewey M , Romeo R , Ballard C , Baldwin R , Bentham P , Fox C , Holmes C , Katona C , Knapp M , Lawton C , Lindesay J , Livingston G , McCrae N , Moniz-Cook E , Murray J , Nurock S , Orrell M , O’Brien J , Poppe M , Thomas A , Walwyn R , Wilson K , Burns A ((2011) ) Sertraline or mirtazapine for depression in dementia (HTA-SADD): A randomised, multicentre, double-blind, placebo-controlled trial. Lancet 378: , 403–411. |
[29] | Banerjee S , High J , Stirling S , Shepstone L , Swart AM , Telling T , Henderson C , Ballard C , Bentham P , Burns A , Farina N , Fox C , Francis P , Howard R , Knapp M , Leroi I , Livingston G , Nilforooshan R , Nurock S , O’Brien J , Price A , Thomas AJ , Tabet N ((2021) ) Study of mirtazapine for agitated behaviours in dementia (SYMBAD): A randomised, double-blind, placebo-controlled trial. Lancet 398: , 1487–1497. |
[30] | Borson S , Frank L , Bayley PJ , Boustani M , Dean M , Lin P-J , McCarten JR , Morris JC , Salmon DP , Schmitt FA , Stefanacci RG , Mendiondo MS , Peschin S , Hall EJ , Fillit H , Ashford JW ((2013) ) Improving dementia care: The role of screening and detection of cognitive impairment. Alzheimers Dement 9: , 151–159. |




