Association of Insulin Receptor Substrate-1 Gene Polymorphism (rs1801278) with Alzheimer’s Disease
Abstract
Background:
Alzheimer’s disease (AD) is the most common form of dementia. AD is also the leading cause of morbidity and mortality due to dementia worldwide. It has been shown that AD is associated with type 2 diabetes mellitus (T2DM) and brain insulin resistance. Rs1801278 is a polymorphism in insulin receptor substrate-1 (IRS-1) gene which changes the amino acid Arg972. This polymorphism has been found to be associated with susceptibility to AD in some populations.
Objective:
In the present study, our aim was to investigate the association of Arg972 IRS-1 (rs1801278) gene polymorphism and late-onset Alzheimer’s disease (LOAD) in an Iranian population.
Methods:
In this case-control study, 150 patients with LOAD and 150 unrelated healthy controls were recruited. Polymerase chain reaction (PCR) was performed to amplify a DNA segment of 263 base-pair (bp) length containing the single nucleotide polymorphism (SNP). The PCR product was then incubated with MvaI restriction enzyme to undergo enzymatic cleavage. Electrophoresis was thereafter carried out using agarose gel and DNA safe stain. The gel was ultimately visualized under a UV trans-illuminator. Allelic and genotypic frequencies were then compared.
Results:
A allele (mutant) of the gene was significantly associated with the risk of AD after adjustment for sex and age (p = 0.04, adjusted OR:1.77, 95% CI:1.00–3.11). Only AA genotype (mutant homozygote) was significantly associated with the risk of AD after adjustment for sex and age (p = 0.01, adjusted OR:2.39, 95% CI:1.22–4.66).
Conclusion:
SNP rs1801278 is significantly associated with the risk of developing AD in the studied Iranian population.
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia and also the leading cause of morbidity and mortality due to dementia worldwide [1]. AD is characterized by neuronal cell death [2] which arises from extracellular amyloid-β (Aβ) plaques and intracellular tau neurofibrillary tangles (NFTs) [3]. The World Alzheimer Report indicated about 47 million AD or AD-related dementia throughout the world in 2015 [4]. It is anticipated that, as the world’s population is aging, the number of patients with dementia, will grow to 130 million worldwide in 2050 [1, 4]. AD is classified into two forms of familial early-onset AD and sporadic late-onset AD (LOAD), constituting 3% and 97% of the AD cases, respectively [5]. The progression of LOAD occurs normally after 65 years of age [6].
It is believed that AD is associated with some gene mutations. Mutations of Aβ precursor protein and presenilin 1 and 2 in familial AD or LOAD and mutations of ɛ4 allele of APOE (APOE ɛ4) gene in sporadic cases of AD have been reported [7, 8]. AD is also associated with polymorphisms of TREM2 [9], MS4A6A [10], and CD33 genes [11].
Epidemiological and experimental studies suggest that insulin resistance is a key risk factor for type 2 diabetes mellitus (T2DM), AD, and other AD-related dementias [12]. An epidemiological study showed that T2DM is associated with an increased risk of AD [3]. The incidence of dementia increases by 50–100% relative to people without diabetes [13]. In Iran, prevalence of impaired fasting glucose and total DM were 14.60% (95% CI: 12.41–16.78) and 11.37% (95% CI: 9.86–12.89) among individuals with 25–70 years of age, respectively, in 2011 [14]. The number of DM patients is estimated to rise to 9.24 million cases by 2030 in Iran [15]. In a cohort study carried out in Guilan province of Iran (2014–2017), prevalence of DM was 24.1% [16].
Recently, it has been shown that the central nervous system (CNS) insulin resistance can be present apart from peripheral insulin resistance of T2DM in individuals with LOAD [17–20].
Neuronal growth, development, and neurogenesis have important roles in insulin function in the CNS [18, 19]. The role of insulin in neuronal cells is mediated by two main signaling pathways, that is to say, insulin –insulin receptor substrate (IRS) –protein kinase B (AKT) pathway and mitogen-activated protein kinase pathway [20]. Insulin –IRS1 –PI3K (phosphoinositide 3-kinase) –AKT –TBC1D4 signaling induces cognition and emotional function in hippocampus. In addition, insulin –IRS1 –PI3K –AKT –GSK3β (glycogen synthase kinase 3) has a role in neuroplasticity and neurogenesis [20].
In animal models, brain insulin level alterations can influence neuronal glucose uptake and metabolism due to glucose transporter type 4 (GLUT4) translocation arising from insulin –IRS1 –AKT signaling pathway in the basal forebrain, hippocampus, amygdala, and to a lesser degree, in the cerebral cortex and cerebellum [21–24]. Under the condition of high-energy demand during spontaneous alternation tests of spatial working memory, this mechanism can promote cognitive activity in the hippocampus in rats [21–23].
Brain insulin resistance can result from insulin-like growth factor-1 (IGF-1) resistance and IRS-1, 2 malfunctions induced by Aβ accumulation [25]. There is a two-sided relationship between brain insulin resistance and Aβ plaque deposition in the brain [26].
Arg972 IRS-1 gene polymorphism (rs1801278) results in a Gly/Arg substitution at codon 972 (Arg972) that impairs IRS-1 ability to activate the downstream PI3K signaling pathway. Therefore, it can lead to insulin signaling malfunction [6]. Lack of insulin signaling is proposed to upregulate amyloidogenic and Tau NFT-genic pathways. There is a higher prevalence of this polymorphism in T2DM [27] and AD patients [28].
Albegali et al. [29] and Alharbi et al. [30] have found significant association of rs1801278 variant with T2DM (p < 0.001 and p = 0.04, respectively). Li et al. [31] in a meta-analysis, found significant association between rs1801278 and T2DM risk in recessive model (AA versus GA + GG, p = 0.043) and codominant model (AA versus GG, p = 0.007).
In contrast, some studies have shown no association of IRS-1 gene polymorphisms with AD in Caucasian populations [27, 32–35], but a Chinese study evaluating the association of rs1801278 polymorphism with AD in a Han Chinese population revealed a significant association of the polymorphism and AD [28].
An explanation for this discrepancy could be the possible effects of ethnic populations on the mentioned association [28].
As little is known about the association of Arg972 IRS-1 (rs1801278) with AD in the Iranian ethnic population, we set out the present study to test the hypothesis of the presence of the association between the polymorphism and AD in an Iranian population in the north of Iran.
MATERIALS AND METHODS
Subjects
Given the results of previous published research [28] and considering type 1 error (α) = 0.05, type 2 error (β) = 0.1 (power of study = 90%), and a case-control ratio of one-to-one, a minimum sample size of 148 participants in each group was calculated and considered as a prerequirement for this study. Therefore, 150 patients with LOAD and 150 unrelated healthy subjects were enrolled in Guilan province in the north of Iran. Inclusion criteria included patients with probable LOAD diagnosed according to the National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic criteria of AD [36]. Exclusion criteria for the patients were the history of head trauma, stroke, motor neuron disease, congenital dementia, neurological infections, neuropsychologic systemic lupus erythematosus, sarcoidosis, multiple sclerosis, other neurodegenerative diseases, and familial history of AD in first-class relatives. Informed consent was obtained from all the participants. This study was approved by the ethics committee of Guilan University of Medical Sciences.
Genotyping
Five milliliters of peripheral blood sample were collected from the participants and stored in Tris-EDTA 5% buffer at –30°C. DNA was extracted by a salting-out method from white blood cells. Forward and reverse primers were synthesized and used for PCR amplification. Restriction enzyme MvaI was used for Restriction Fragment Length Polymorphism (RFLP) as previously described [37].
PCR amplification was performed for all samples using respective forward and reverse primers. In the next step, PCR products were incubated and digested by MvaI. PCR-RFLP characteristics are shown in Table 1.
Table 1
Enzymatic digestion reaction characteristics
| Forward primer | 5′- CTT CTG TCA GGT GTC CAT CC-3′ |
| Reverse primer | 5′- TGG CGA GGT GTC CAC GTA GC-3′ |
| Restriction enzyme | MvaI |
| Recognition | 5′…C C↓W G G…3′ |
| sequence | 3′…G G W↑ C C…5′ |
| Restriction | GG genotype: 263 bp |
| fragments | GA genotype: 263, 180, and 83 bp |
| AA genotype: 180 and 83 bp |
Electrophoresis was carried out using 3% agarose gel and visualized by DNA-safe stain using a UV trans-illuminator (Figs. 1 and 2). A few samples were sequenced to illustrate wild-type and variant allele sequences.
Fig. 1
Electrophoresis of undigested PCR products. M, marker ladder; NTC, no template control ladder.
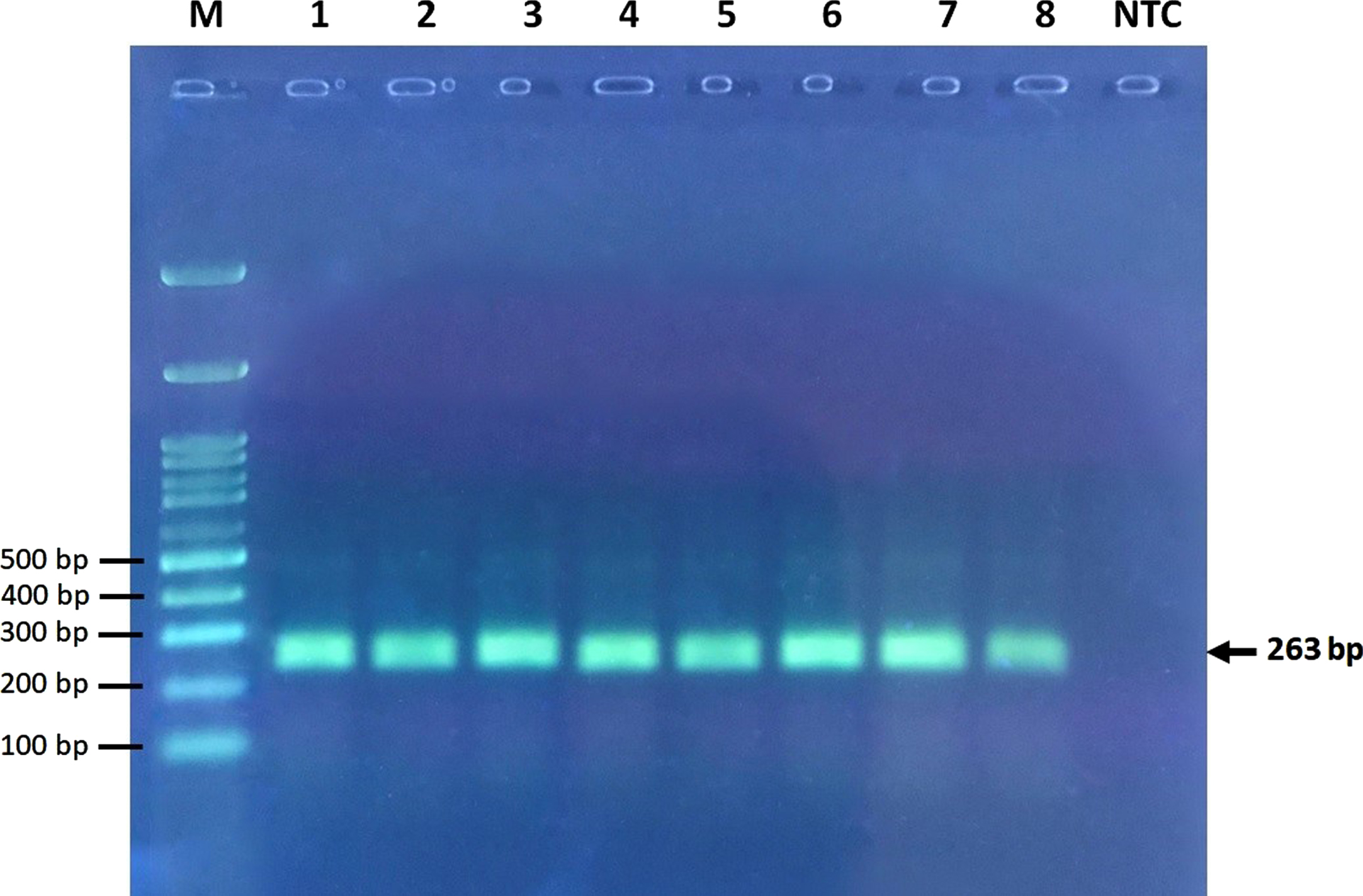
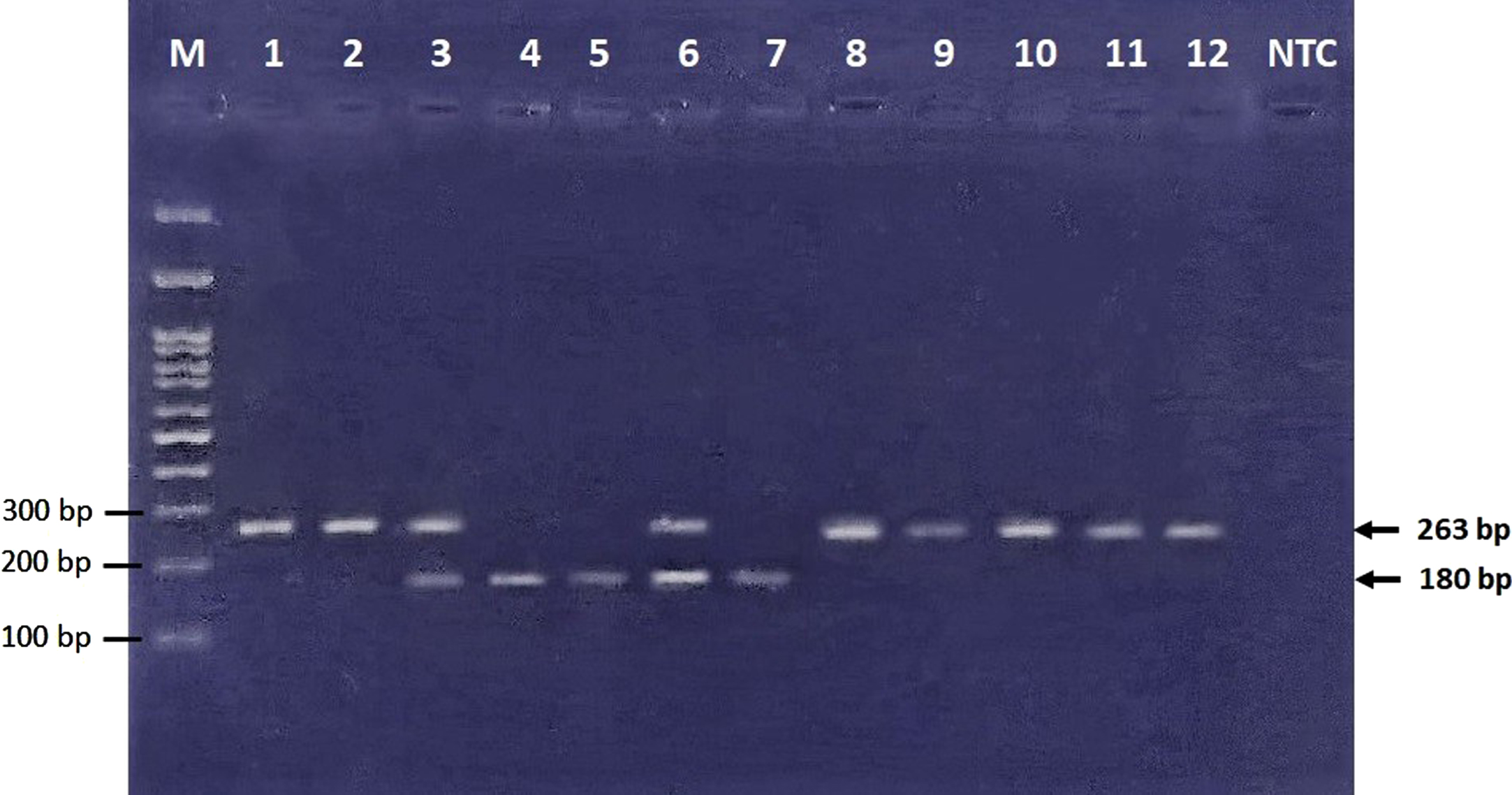
Fig. 2
Electrophoresis of restriction products. 83 bp band cannot be visualized in this figure due to usage of 3% agarose gel, which can show DNA fragment size of larger than 100 bp. GG (wild type homozygote genotype) ⟶ Bands No. 1, 2, 8–12 (263 bp). GA (heterozygote genotype) ⟶ Bands No. 3, 6 (263, 180, and 83 bp). AA (mutant homozygote genotype) ⟶ Bands No. 4, 5, 7 (180 and 83 bp).
Statistical analysis
Hardy-Weinberg equilibrium analysis was performed with chi-square goodness-of-fit test. Categorical variables were expressed as n (%) and analyzed with chi-square test or Fisher’s exact test where appropriate. Continuous variables were expressed as mean±standard deviation (SD) and analyzed with independent samples T-test. Comparison of genotype and allele frequencies between patients and controls were made using the logistic regression analysis to assess odds ratio (OR) and 95% confidence interval (CI). Statistical analyses were performed using SPSS (version 26). α= 0.05 was used as statistical level of significance.
RESULTS
Data from 300 subjects in two groups of cases and controls were collected and analyzed. The mean±SD of age in the case and control groups was 83.01±7.24 and 76.94±9.54, respectively (p < 0.001). Thirty-eight (25.3%) and sixty-two (41.3%) were male in the case and control groups, respectively (p = 0.002).
The genotype frequencies of the IRS-1 gene rs1801278 polymorphism were not in Hardy-Wein-berg equilibrium in both groups.
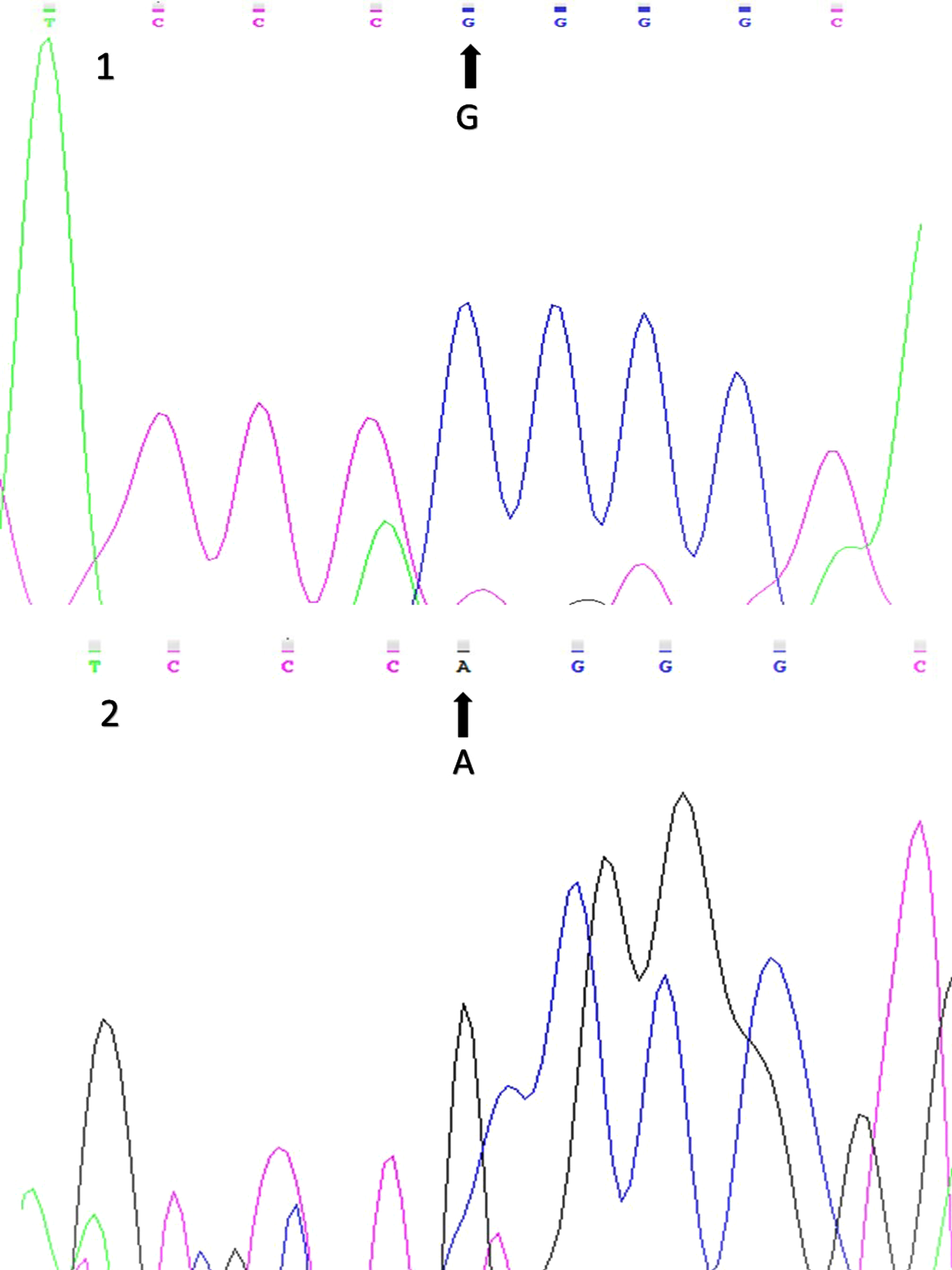
Figure 3 displays sequencing results of the wild-type and variant alleles of the Arg972 IRS-1 gene polymorphism (rs1801278).
Fig. 3
Sequencing results for the Arg972 IRS-1 (rs1801278) gene polymorphism (1: wild-type allele; 2: variant allele).
The prevalence of A (mutant) allele in case and control groups were 31.67% and 19.67%, respectively. By using logistic regression analysis and considering age, sex, and allelic frequencies as cov-ariates, A allele was significantly associated with the risk of AD after adjustment for sex and age (p = 0.04, adjusted OR: 1.77, 95% CI: 1.00–3.11) (Table 2).
Table 2
Comparison of genotypic and allelic frequencies of Arg972 IRS-1 gene polymorphism between AD cases (N = 150) and healthy controls (N = 150)
| Genotype & Allele | Cases N (%) | Controls N (%) | Adjusted OR (95% CI) | p |
| GG | 94 (% 62.67) | 108 (% 72) | 1.00 a | – |
| (Reference) | ||||
| GA | 17 (% 11.33) | 25 (% 16.67) | 0.71 a | 0.37 |
| (0.34–1.48) | ||||
| AA | 39 (% 26) | 17 (% 11.33) | 2.39 a | 0.01 |
| (1.22–4.66) | ||||
| G | 205 (% 68.3) | 241 (% 80.3) | 1.00 b | – |
| (Reference) | ||||
| A | 95 (% 31.67) | 59 (% 19.67) | 1.77 b | 0.04 |
| (1.00–3.11) |
aAdjusted OR is based on the logistic regression with covariates to be age, sex, and genotypic frequency. bAdjusted OR is based on the logistic regression with covariates to be age, sex, and allelic frequency.
The prevalence of AA (mutant homozygote IRS-1 polymorphism) genotype in case and control groups were 26% and 11.33%, respectively (Table 2). The prevalence of GA (heterozygote IRS-1 polymorphism) genotype in the case and the control groups were 11.33% and 16.67%, respectively. By using logistic regression analysis and considering age, sex, and genotypic frequencies as covariates, only AA genotype was significantly associated with the risk of AD after adjustment for sex and age (p = 0.01, adjusted OR: 2.39, 95% CI: 1.22–4.66). GA genotype was not associated with the risk of AD after adjustment for sex and age (p = 0.37, adjusted OR: 0.71, 95% CI: 0.34–1.48).
DISCUSSION
In this case-control study, we enrolled 150 LOAD patients and 150 unrelated controls for evaluation of the association of Arg972 IRS-1 gene polymorphism with the risk of AD in an Iranian population. A allele was significantly associated with the risk of AD, compared to G allele after adjustment for sex and age. Furthermore, AA genotype was significantly associated with the risk of AD, in contrast to GG and GA genotypes after adjustment for sex and age.
To the best of our knowledge, only one study evaluated the association of Arg972 IRS-1 (rs1801278) gene polymorphism with the risk of AD [28]. Wang et al. [28] in a case-control study in 2014, evaluated 1113 AD patients and 1113 healthy controls which were matched for sex, age, body mass index, residence area, and education level. The authors showed that 5.7% of the patients and 2% of the controls had AA genotype (mutant homozygote), respectively and 42.3% of the patients and 20.1% of the controls had GA genotype (heterozygote Arg972 IRS-1), respectively. Moreover, the authors found that both genotypes were significantly associated with an increased risk of AD after adjustment for comorbidities including T2DM, coronary artery disease, and hypertension (p < 0.001) [28]. Also, 26.8% of the patients and 12% of the controls carried the mutant A allele, respectively and the A allele was significantly associated with an increased risk of AD after adjustment for the above-mentioned comorbidities (p < 0.001). On the other hand, in each age sub-group of the cases, the prevalence of AA genotype, unlike GG genotype, in patients with a Mini-Mental State Examination (MMSE) score≤14 was high, compared with that in patients with MMSE score≥15–26 (p < 0.001). Furthermore, it was demonstrated that patients with GG genotype had higher MMSE score in each age group, compared with GA and AA genotypes (p < 0.05). It was concluded that Arg972 IRS-1 (rs1801278) gene polymorphism is significantly associated with AD in Han Chinese population. Moreover, the authors stated that this polymorphism may also have a prognostic value for AD [28].
The findings of the study of Wang et al. [28], were in agreement with those of our study demonstrating a significant association of A allele and AA genotype with increased risk of AD. Unlike the findings of Wang et al. [28], we did not find a higher prevalence of GA genotype in the patients even after adjustment for sex and age.
Hamilton et al. [33] investigated the role of insulin signaling-related candidate gene polymorphisms in 954 LOAD patients higher than 60 years of age and 1,106 controls in a Caucasian population in England. The authors showed that there was no significant association of IRS-1 polymorphism (rs1801123) with AD.
Giedraitis et al. [32] used available data from two genome-wide association studies [34, 35]. They showed that IRS-1 polymorphism (rs10187726) had a significant association with LOAD in one of the mentioned studies [34].
In addition, the literature suggests no association of other IRS-1 gene variants (rs2234931, rs2943634) with AD in Caucasian populations [28, 32, 33, 35, 38, 39].
The above-mentioned studies suggest possible ethnic influences on the association between IRS-1 gene variants and the risk of AD.
There are caveats with respect to the present study. First, data concerning comorbidities and clinical characteristics such as T2DM, plasma glucose, heart disease, hypertension, weight, height, body mass index, and waist/hip ratio of the participants were not collected and therefore we were not able to adjust the results accordingly. For example, an association of rs1801278 with the risk of T2DM has been reported [29–31]. Therefore, future studies with adjustment of these parameters would be helpful. Second, the genotype frequencies of the gene variant were not in Hardy-Weinberg equilibrium. However, inasmuch as 10% of all genotype–phenotype association studies show deviation from Hardy-Weinberg equilibrium [40], the results of our trial cannot be considered abnormal. Nevertheless, we suggest future investigations with larger sample sizes.
To sum up, the findings from the present study indicate a significant association of mutant A allele and mutant homozygote AA genotype with the risk of AD after adjustment for sex and age. Further investigation is needed to assess the effects of rs1801278 polymorphism on the severity of cognitive impairment to assess prognostic values.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
Guilan University of Medical Sciences provided financial support for this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
[1] | Olayinka O , Olayinka O , Alemu B , Akpinar-Elci M , Grossberg G ((2019) ) Toxic environmental risk factors for Alzheimer’s disease: A systematic review. Aging Med Healthcare 10: , 4–17. |
[2] | Heidari F , Ansstas G , Ajamian F (2021) CD33 mRNA has elevated expression levels in the leukocytes of peripheral blood in patients with late-onset Alzheimer’s disease. Gerontology. doi: 10.1159/000518820. |
[3] | Andalib S , Ghayeghran A , Moadabi Y , Asadi K , Mohammadpour M , Ghorbani-Shirkouhi S ((2019) ) Association of diabetes mellitus type 2 and Alzheimer’s disease. Caspian J Health Res 4: , 86–89. |
[4] | Prince MJ , Wimo A , Guerchet MM , Ali GC , Wu Y-T , Prina M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London. |
[5] | Ettcheto M , Cano A , Busquets O , Manzine PR , Sánchez-López E , Castro-Torres RD , Beas-Zarate C , Verdaguer E , García ML , Olloquequi J ((2019) ) A metabolic perspective of late onset Alzheimer’s disease. Pharmacol Res 145: , 104255. |
[6] | Florez JC , Sjögren M , Burtt N , Orho-Melander M , Schayer S , Sun M , Almgren P , Lindblad U , Tuomi T , Gaudet D ((2004) ) Association testing in 9,000 people fails to confirm the association of the insulin receptor substrate-1G972R polymorphism with type 2 diabetes. Diabetes 53: , 3313–3318. |
[7] | Huang X , Liu H , Li X , Guan L , Li J , Tellier LCAM , Yang H , Wang J , Zhang J ((2018) ) Revealing Alzheimer’s disease genes spectrum in the whole-genome by machine learning. BMC Neurol 18: , 5. |
[8] | Moreno DJ , Ruiz S , Ríos Á , Lopera F , Ostos H , Via M , Bedoya G ((2017) ) Association of GWAS top genes withlate-onset Alzheimer’s disease in Colombian population. Am J Alzheimers Dis Other Demen 32: , 27–35. |
[9] | Jonsson T , Stefansson H , Steinberg S , Jonsdottir I , Jonsson PV , Snaedal J , Bjornsson S , Huttenlocher J , Levey AI , Lah JJ ((2013) ) Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368: , 107–116. |
[10] | Antúnez C , Boada M , Gonzáalez-Pérez A , Gayán J , Ramírez-Lorca R , Marín J , Hernández I , Moreno-Rey C , Morón FJ , López-Arrieta J ((2011) ) Themembrane-spanning 4-domains, subfamily A (MS4A) gene cluster contains a common variant associated with Alzheimer’s disease. Genome Med 3: , 1–8. |
[11] | Mehdizadeh E , Khalaj-Kondori M , Shaghaghi-Tarakdari Z , Sadigh-Eteghad S , Talebi M , Andalib S ((2019) ) Association of MS4A6A, CD33, and TREM2 gene polymorphisms with the late-onset Alzheimer’s disease. Bioimpacts 9: , 219. |
[12] | Kuusisto J , Koivisto K , Mykkänen L , Helkala E-L , Vanhanen M , Hänninen T , Kervinen K , Kesäniemi YA , Riekkinen PJ , Laakso M ((1997) ) Association between features of the insulin resistance syndrome and Alzheimer’sdisease independently of apolipoprotein E4 phenotype: Cross sectional population based study.. BMJ 315: , 1045–1049. |
[13] | Biessels GJ , Staekenborg S , Brunner E , Brayne C , Scheltens P ((2006) ) Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol 5: , 64–74. |
[14] | Esteghamati A , Etemad K , Koohpayehzadeh J , Abbasi M , Meysamie A , Noshad S , Asgari F , Mousavizadeh M , Rafei A , Khajeh E ((2014) ) Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract 103: , 319–327. |
[15] | Javanbakht M , Mashayekhi A , Baradaran HR , Haghdoost A , Afshin A ((2015) ) Projection of diabetes population size and associated economic burden through 2030 in Iran: Evidence from micro-simulation Markov model and Bayesian meta-analysis. PloS One 10: , e0132505. |
[16] | Hasandokht T , Joukar F , Marroufizadeh S , Sibevey S , Naghipour M , Mansour-Ghanaei F (2020) Detection of high risk people for diabetes by American Diabetes Association Risk Score in PERSIAN Guilan Cohort Study. Research Square, https://doi.org/10.21203/rs.3.rs-111051/v1. |
[17] | Ferrario CR , Reagan LP ((2018) ) Insulin-mediated synaptic plasticity in the CNS: Anatomical, functional and temporal contexts. Neuropharmacology 136: , 182–191. |
[18] | Chiu S-L , Cline HT ((2010) ) Insulin receptor signaling in the development of neuronal structure and function. Neural Dev 5: , 7. |
[19] | Park C ((2001) ) Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev 25: , 311–323. |
[20] | Arnold SE , Arvanitakis Z , Macauley-Rambach SL , Koenig AM , Wang H-Y , Ahima RS , Craft S , Gandy S , Buettner C , Stoeckel LE ((2018) ) Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat Rev Neurol 14: , 168. |
[21] | McNay EC , Fries TM , Gold PE ((2000) ) Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A 97: , 2881–2885. |
[22] | McNay EC , Gold PE ((2002) ) Food for thought: Fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav Cogn Neurosci Rev 1: , 264–280. |
[23] | McNay EC , Cotero VE ((2010) ) Mini-review: Impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav 100: , 234–238. |
[24] | Apelt J , Mehlhorn G , Schliebs R ((1999) ) Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res 57: , 693–705. |
[25] | Mayeda ER ((2019) ) Invited commentary: Examining sex/gender differences in risk of Alzheimer disease and related dementias—challenges and future directions. Am J Epidemiol 188: , 1224–1227. |
[26] | Blázquez E , Velázquez E , Hurtado-Carneiro V , Ruiz-Albusac JM ((2014) ) Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol 5: , 161. |
[27] | Sesti G , Federici M , Hribal ML , Lauro D , Sbraccia P , Lauro R ((2001) ) Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J 15: , 2099–2111. |
[28] | Wang W , Yang L , Tan L , Wu X , Jiang B , Shen X ((2014) ) Arg972 insulin receptor substrate-1 polymorphism and risk and severity of Alzheimer’s disease. J Clin Neurosci 21: , 1233–1237. |
[29] | Albegali AA , Shahzad M , Mahmood S , Ullah MI ((2019) ) Genetic association of insulin receptor substrate-1 (IRS-1, rs1801278) gene with insulin resistant of type 2 diabetes mellitus in a Pakistani population. Mol Biol Rep 46: , 6065–6070. |
[30] | Alharbi KK , Khan IA , Munshi A , Alharbi FK , Al-Sheikh Y , Alnbaheen MS ((2014) ) Association of the genetic variants of insulin receptor substrate 1 (IRS-1) with type 2 diabetes mellitus in a Saudi population. Endocrine 47: , 472–477. |
[31] | Li Q , Qiao Y , Wang C , Zhang G , Zhang X , Xu L ((2016) ) Associations between two single-nucleotide polymorphisms (rs1801278 and rs2943641) of insulin receptor substrate 1 gene and Type 2 diabetes susceptibility: A meta-analysis. Endocrine 51: , 52–62. |
[32] | Giedraitis V , Kilander L , Degerman-Gunnarsson M , Sundelöf J , Axelsson T , Syvänen A-C , Lannfelt L , Glaser A ((2009) ) Genetic analysis of Alzheimer’s disease in the Uppsala Longitudinal Study of Adult Men. Dement Geriatr Cogn Disord 27: , 59–68. |
[33] | Hamilton G , Proitsi P , Jehu L , Morgan A , Williams J , O’Donovan MC , Owen MJ , Powell JF , Lovestone S ((2007) ) Candidate gene association study of insulin signaling genes and Alzheimer’s disease: Evidence for SOS2, PCK1, and PPARγ as susceptibility loci. Am J Med Genet B Neuropsychiatr Genet 144: , 508–516. |
[34] | Coon KD , Myers AJ , Craig DW , Webster JA , Pearson JV , Lince DH , Zismann VL , Beach TG , Leung D , Bryden L ((2007) ) A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry 68: , 613–618. |
[35] | Li H , Wetten S , Li L , Jean PLS , Upmanyu R , Surh L , Hosford D , Barnes MR , Briley JD , Borrie M ((2008) ) Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol 65: , 45–53. |
[36] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr, Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from theNational Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelinesfor Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[37] | Federici M , Petrone A , Porzio O , Bizzarri C , Lauro D , D’Alfonso R , Patera I , Cappa M , Nisticò L , Baroni M ((2003) ) The Gly972⟶ Arg IRS-1 variant is associated with type 1 diabetes in continental Italy. Diabetes 52: , 887–890. |
[38] | Reiman EM , Webster JA , Myers AJ , Hardy J , Dunckley T , Zismann VL , Joshipura KD , Pearson JV , Hu-Lince D , Huentelman MJ ((2007) ) GAB2 alleles modify Alzheimer’s risk in APOE ɛ4 carriers. Neuron 54: , 713–720. |
[39] | Figgins JA , Minster RL , Demirci FY , DeKosky ST , Kamboh MI ((2009) ) Association studies of 22 candidate SNPs with late-onset Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet 150: , 520–526. |
[40] | Trikalinos TA , Salanti G , Khoury MJ , Ioannidis JP ((2006) ) Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol 163: , 300–309. |




