MoCA 7.1: Multicenter Validation of the First Italian Version of Montreal Cognitive Assessment
Abstract
Background:
The early detection of neurocognitive disorders, especially when mild, is a key issue of health care systems including the Italian Dementia National Plan. The Mini-Mental State Examination (MMSE), i.e., the reference screening tool for dementia in Italian Memory Clinics, has low sensitivity in detecting mild cognitive impairment (MCI) or mild dementia.
Objective:
Availability of a 10-minute screening test sensitive to MCI and mild dementia, such as the Montreal Cognitive Assessment (MoCA), is relevant in the field. This study presents initial validity and reliability data for the Italian version of MoCA 7.1 that is being collected as part of a large ongoing longitudinal study to evaluate the rate of incident MCI and dementia in older adults.
Methods:
MoCA 7.1 and MMSE were administered to cognitive impaired patients (n = 469; 214 with MCI, 255 with dementia; mean age: 75.5; 52% females,) and healthy older adults (n = 123, mean age: 69.7, 64 % females).
Results:
Test-retest (0.945, p < 0.001) and inter-rater (0.999, p < 0.001) reliability of MoCA 7.1, assessed on randomly selected participants with normal cognition, MCI, dementia, were significant. MoCA 7.1 showed adequate sensitivity (95.3%) and specificity (84.5%) in detecting MCI compared to MMSE (sensitivity: 53.8%; specificity: 87.5%). The Area Under the Curve of MoCA 7.1 was significantly greater than that of MMSE (0.963 versus 0.742). MoCA 7.1 showed similar results in detecting both MCI and dementia.
Conclusion:
MoCA 7.1 is a reliable and useful tool that can aid in the diagnosis of MCI and dementia in the Italian population.
INTRODUCTION
The early diagnosis of neurocognitive disorders provides relevant benefits to patients and families [1]; it represents a cornerstone of the policies for dementia across health care systems [2]. Mild cognitive impairment (MCI) is the intermediate stage between the normal and usual cognitive performance and dementia. In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [3], MCI is defined as “Mild Neurocognitive Disorder” (Mild NCD) whereas dementias have been grouped in “Major NCDs” [4].
The novelty of these diagnostic criteria depends on both the identification of six cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor, social cognition) and on the assessment of the related functions. On this basis, the diagnosis of Mild NCD stems from “...evidence of modest cognitive decline from a previous level of performance in one or more cognitive domains (complex attention, executive function, . . .) . . . that do not interfere with capacity for independence in everyday complex activities of daily living but greater effort, compensatory strategies, or accommodation may be required” [3, 4].
The Major NCDs differ from Mild NCD because the cognitive decline interferes with independence in the complex activities of daily living [3–5]. This framework overlaps the staging of dementia based on the Clinical Dementia Rating scale (CDR), a standard staging tool that evaluates six cognitive and functional domains with a five-point scale [6]: Mild NCD is aligned to a score of 0.5, where normal cognition of 0 and Major NCD of >1.
The risk factors for developing Mild NCD are aging, belonging to the male gender, and low education [7]. Mild NCD may progress to dementia but, importantly, it may not [8]. Cognitive screening represents the initial step in a process of further assessment for NCD and can help identify potential patients.
The Mini-Mental State Examination (MMSE) is a widespread cross-cultural tool for the initial screening of dementia [9]. The MMSE, however, has limitations: healthy elderly population with normal scores could show only a slight cognitive impairment particularly in attention and executive functioning [10] thus yielding a low sensitivity towards Mild NCD, especially among individuals with high cognitive reserve and/or education [11].
The Montreal Cognitive Assessment (MoCA) was developed as the first “brief screening tool” to detect Mild NCD in cognitively impaired participants who would score in the normal range on the MMSE [12]. MoCA has improved the management of the increasing volume of referrals to memory clinics for cognitive concerns [13]: it evaluates the six main cognitive domains and has a good convergent validity with more intensive neuropsychological batteries [14–16]. In addition, MoCA presents meaningful correlations with findings from structural brain imaging [17].
Results of MoCA have demonstrated to be age and gender independent [18, 19] and only minimally influenced by education levels [20, 21] partly due to its correction factors. Thus, MoCA does not need to be corrected for education; such adjustment usually has a detrimental effect on sensitivity and a slight increase in specificity, considering that educational levels do not always correlate with premorbid intellectual function [22]. Furthermore, MoCA correlates with cognitive reserve [23].
Over the years, MoCA has been adapted to meet several of the issues of cognitive assessment (https://www.mocatest.org): illiteracy (MoCA-B), sensory impairments (MoCA Blind/Hearing impairment), and remote assessment (T-MoCA) for community-residing people or unavailability of in-clinic assessments, as happened during the COVID-19 pandemic.
Scientific societies strongly recommend the timely diagnosis of Major NCD [1, 24] especially at the prodromal stage of Mild NCD [25–29].
A diagnosis of Mild NCD can be both stigmatizing and anxiogenic, and we acknowledge that many clinicians may not wish to screen for Mild NCD, given it has a variable natural history and that treatment is largely supportive. Nonetheless, diagnosis of Mild NCD must be made carefully; patients must be counselled that it has a variable natural history and prognosis and that they will not necessarily develop Major NCD. Therefore, both in the clinical setting and health policy it is pivotal to increase knowledge on prognostic perspectives.
Aims
We designed a longitudinal study aimed at evaluating the rates of Mild NCD developed by people with normal cognition and of Major NCD from those with Mild NCD.
The study started in 2016, and was scheduled to end in 2020, but the COVID-19 pandemic has delayed the follow-up to autumn 2021 and the subsequent completion of the study to end of 2022. The first step of the longitudinal study has been to compare the psychometric properties of two neuropsychological tools: the first Italian version of MoCA (MoCA 7.1, 2006) [30] and MMSE.
The purpose of this study is to investigate: a) test-retest and interrater reliability of the Italian version of the MoCA, originally developed in 2006, with this longitudinal cohort; b) the effects of age, gender, education and normative data in diagnostic accuracy; c) the optimal balance of sensitivity/specificity of MoCA 7.1 and MMSE for detecting MCI and dementia.
This design expands upon the initial validation work of MoCA 7.1 aiming to add incremental knowledge about the utility of the measure in the longitudinal cohort of normal older adults versus at-risk for cognitive impairment.
METHODS
Rationale of the implementation of MoCA 7.1
The validation process followed the criteria of DSM-5 to diagnose NCDs as acquired conditions [31] that are age-related but not age-dependent [32] where the individual, directly or through an informant, reports cognitive concerns regardless of age, gender, and education [33–35].
As MoCA reflects the cognitive reserve [23], it allows assessment along the two trajectories of cognition in aging: 1) normality: cognitive idemescence [36] or absence of anatomical-structural pathological modifications of the brain [37–40] with preservation of the functional network reorganization in the adults [41, 42], therefore maintaining the usual level of cognition [43] and related complex/advanced activity of daily living [44–46]; 2) cognitive impairment: the NCDs usually appear after the fifth decade and increase with aging [47–51].
Participants
We recruited: 1) participants with memory complaints perceived directly by themselves and/or by an informant (Patient Group, PG); 2) community-dwelling elderly without known memory and functional complaints (CDR Global and Sum of Boxes scores equal to 0) and without sensory impairments (normal elderly controls, NECs). Both groups were recruited from three Center for Cognitive Disorder and Dementia (CCDD) located in northern Italy: one in a rural town (Cento) and two in a middle-sized industrial city (Modena).
Oral and written informed consent were obtained according to protocols approved by the local institutional review board.
Assessments and inclusion criteria
1) Participants with memory complaints were evaluated with a comprehensive assessment of clinical, cognitive, affective, and functional status.
Clinical assessment: medical history, physical comorbidities assessed with the Cumulative Illness Rating Scale (CIRS) [52], physical and neurologic examination, medications and laboratory testing covering: Complete Blood Count, Thyroid-Stimulating Hormone, B-12, folic acid, serum calcium, liver and kidney function, electrolytes.
Cognitive assessment: MMSE [9, 53], standard neuropsychological tests in participants referring cognitive impairments but performing the MMSE in the normal score range [54], MoCA 7.1 [12, 30]. At baseline, the MMSE and MoCA 7.1 were administered on the same day or within 1 month for all participants.
For the MMSE, a cut-off score lower than 27 is the threshold for the diagnosis of Major NCD [53], as approved by the Italian Medicines Agency (AIFA). This allows the prescription of cholinesterase inhibitors or memantine, free of charge, and only through specialists working within public CCDDs.
In the following analysis we considered the MMSE both as a raw (MMSE-RS) or as a normative score adjusted for age and education (MMSE-NS) [53].
The suggested cut-off score for MoCA 7.1 is 26 (score ≤25 to indicate impairment). The original cut-off score is determined with two types of correction for the effect of education. The first is general, relative to the serial-7 subtraction where one error is allowed thus, 4 to 5 correct answers correspond to a maximum score of 3 points instead of a maximum of 5 as in the MMSE; the second is variable where one point is added to the score of participants with 12 years of education or less if the total MoCA score is lower than 30 [12].
Other assessments included: Geriatric Depression Scale 15-item (GDS) [55]; Activities of Daily Living (ADL) [56] and Instrumental Activities of Daily Living (IADL) [57]; Clinical Dementia Rating scale (CDR) [6, 58] indexed by the Global score (CDR-G), and CDR Sum of Boxes score (CDR-SB).
The ADL, IADL and CDR interviews were conducted with each examinee and their informant partners as the informant-based functional measures improve overall diagnostic accuracy of the psychometric tests [59].
Diagnosis of Mild or Major NCD. Diagnoses were made for patients with cognitive complaints who fulfilled DSM-5 criteria [3, 4] and reported a CDR-G ≤ 0.5. All diagnoses were made at the end of the assessment and were reviewed by an expert geriatrician or neurologist blind to the MoCA 7.1 score: the clinic-based out-patients with Mild or Major NCD were admitted to the PG.
2) Normal elderly controls were healthy elderly volunteers randomly recruited from the community. Inclusion criteria: complete independence, absence of memory-related and functional complaints confirmed by an informant partner, well compensated sensory impairments. All participants underwent assessment with the MMSE, MoCA 7.1, IADL, and CDR. Only participants with intact IADL and CDR- Global and -Sum of Boxes scores [60] were included in the NECs. This procedure is highly successful to avoid including older adults with early-stage unrecognized cognitive impairment (CDR-G: 0.5) [61, 62] who display a performance within normative values [60, 63]. Underdetection of early or mild cognitive impairment (CDR-G: 0.5–1) among independent elderly may be frequent due to lack of insight [64].
Test-retest and inter-rater reliability
Measures were retested 30–60 days apart: the time interval was chosen to minimize practice effects [65]. Test-retest and inter-rater reliability were assessed respectively on randomly selected sub-samples of 86 and 77 participants stratified by CDR-G scores of 0, 0.5 and 1.
Statistical analysis
First, we compared PG and NECs. Secondly, we assessed the test-retest and inter-rater reliability of MoCA 7.1 computing the intra-class correlation coefficient (ICC) between the first and second administration and the two raters respectively. Moreover, we assessed the diagnostic accuracy of MoCA 7.1 towards healthy cognitive status, Mild and Major NCDs by calculating their Area Under the Curve of the Receiver Operating Characteristic curve (AUC-ROC). The Youden index was used to estimate the optimal cut-off score to discriminate the presence of NCDs. We report the predictive accuracy as the probability of correct classification of participants with or without cognitive impairment. Furthermore, we applied an index of best fitting in the data analysis to verify if the sensitivity/specificity ratio could be significantly modified. Finally, we used logistic regression with stepwise variable selection to identify domains that most contributed to differentiate NECs from NCDs.
Statistical analyses were performed using SPSS version 25.0 (SPSS, Inc., Chicago, IL).
RESULTS
Participants
The NECs group included 123 participants and their informant partners were: spouse (51%), children (31%), other relatives (18%).
The PG included 469 participants: 214 had Mild NCD and 255 Major NCD. The informant partners of the PG were the informal caregivers as follows: spouse (Mild NCD 42%, Major NCD 33%), children (Mild NCD 44%, Major NCD 56%), other relatives (Mild NCD 14%, Major NCD 11%).
Table 1 provides an overview of the characteristics of participants. The PG was significantly older and less educated than NECs. Among NECs, the education effect was assessed splitting the school years in four subgroups: 1) 3–6 years; 2) 7–11 years; 3) 12-13 years; 4) 14–20 years (Table 2). The four subgroups did not show significant differences in mean age, MMSE-RS, and MoCA 7.1 scores. Mean MMSE-RS was significantly higher than MMSE-NS where, in particular, the intermediate education level (subgroup 3) showed a mean score significantly lower than lower education levels (subgroup 1 and 2). Gender was not associated to differences in cognitive performance.
Table 1
Characteristics of the study participants
| Age | Education | ||
| Characteristics | Average±Standard Deviation | Female n (%) | |
| Controls (n = 123) | 69.75±9.02† | 11.62±4.07‡ | 79 (64.2) |
| Mild NCD (n = 214) | 74.35±8.32† | 8.74±4.19‡ | 110 (51.4) |
| Major NCD (n = 255) | 76.75±6.86† | 7.60±3.86‡ | 135 (52.9) |
| Total (n = 592) | 74.42±8.30 | 8.85±4.29 | 324 (54.7) |
NCD, Neurocognitive Disorder; †Analysis of Variance: F = 32.947, p < 0.05; Bonferroni Post Hoc: p < 0.05; ‡Analysis of Variance: F = 38.758, p < 0.05; Bonferroni Post Hoc: p < 0.05.
Table 2
Demographic and cognitive performance by education in the normal elderly group
| Education (y) | Participants (Female %) | Mean | Standard dev. | 95% confidence interval | ANOVA | ||||
| Lower Limit | Upper Limit | F | Significance | ||||||
| Age | 1) 3–6 | 18 (77) | 73.78 | 8.17 | 69.71 | 77.84 | |||
| 2) 7–11 | 30 (53) | 67.43 | 9.09 | 64.04 | 70.83 | ||||
| 3) 12–13 | 52 (70) | 69.79 | 8.20 | 67.50 | 72.07 | ||||
| 4) 14–20 | 23 (54) | 69.52 | 10.65 | 64.92 | 74.13 | ||||
| Total | 123 (64) | 69.68 | 9.01 | 68.14 | 71.36 | 1.904 | 0.133 | ||
| MoCA 7.1 | 1) 3–6 | 18 (77) | 26.28 | 1.84 | 25.36 | 27.19 | |||
| 2) 7–11 | 30 (53) | 27.23 | 1.69 | 26.60 | 27.86 | ||||
| 3) 12–13 | 52 (70) | 26.94 | 1.38 | 26.56 | 27.33 | ||||
| 4) 14–20 | 23 (54) | 27.61 | 1.62 | 26.91 | 28.31 | ||||
| Total | 123 (64) | 27.04 | 1.61 | 26.75 | 27.33 | 2.621 | 0.064 | ||
| MMSE-RS | 1) 3–6 | 18 (77) | 29.06 | 0.80 | 28.66 | 29.45 | |||
| 2) 7–11 | 30 (53) | 29.27 | 1.04 | 28.88 | 29.66 | ||||
| 3) 12–13 | 52 (70) | 29.02 | 1.01 | 28.74 | 29.30 | ||||
| 4) 14–20 | 23 (54) | 29.17 | 0.93 | 28.77 | 29.58 | ||||
| Total | 123 (64) | 29.12¶ | 0.97 | 28.94 | 29.29 | 0.452 | 0.717 | ||
| MMSE-NS | 1) 3–6 | 18 (77) | 28.96§ | 0.86 | 28.53 | 29.39 | |||
| 2) 7–11 | 30 (53) | 28.51§ | 1.14 | 28.08 | 28.93 | ||||
| 3) 12–13 | 52 (70) | 27.25§ | 1.44 | 26.85 | 27.65 | ||||
| 4) 14–20 | 23 (54) | 27.96 | 1.49 | 27.32 | 28.61 | ||||
| Total | 123 (64) | 27.94¶ | 1.45 | 27.68 | 28.20 | 10.158 | 0.000§ | ||
MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; RS, raw score; NS, normative score; §ANOVA, Analysis of Variance: Bonferroni Post Hoc: p < 0.05; ¶T-test: p < 0.05
Test-retest and inter-rater reliability
The mean difference in the total score of MoCA 7.1 between the first and second administration was 1.04±1.33 points: test-retest and inter-rater reliability showed a statistically significant ICC (0.945, p < 0.001 and 0.999, p < 0.001).
Discriminant power of the MoCA 7.1. and MMSE
Average MMSE-RS, MMSE-NS, and MoCA 7.1 scores of normal, Mild and Major NCD participants differed significantly from each other (respectively F 168,07, p < 0.001; F 113,74, p < 0.001, F 383,731, p < 0.001) (Table 3).
Table 3
MMSE and MoCA 7.1 mean scores±standard deviations for normal controls and participants with Mild and Major Neurocognitive Disorder
| N | Mean | Standard | 95% Confidence Interval | ANOVA | ||||
| deviation | Lower Limit | Upper Limit | F | Sign. | ||||
| MOCA 7.1 | Normal | 123 | 27.04 | 1.62 | 26.75 | 27.33 | ||
| Mild NCD | 214 | 21.60 | 2.94 | 21.20 | 22.00 | |||
| Major NCD | 255 | 18.27 | 3.25 | 17.87 | 18.67 | 383.731 | 0.000§ | |
| Total | 592 | 21.27 | 4.35 | 20.92 | 21.63 | |||
| MMSE-RS | Normal | 123 | 29.12 | 0.98 | 28.95 | 29.30 | ||
| Mild NCD | 214 | 27.26 | 1.97 | 27.00 | 27.53 | |||
| Major NCD | 255 | 24.81 | 2.80 | 24.46 | 25.15 | 168.070 | 0.000§ | |
| Total | 592 | 26.58 | 2.80 | 26.35 | 26.81 | |||
| MMSE-NS | Normal | 123 | 27.94 | 1.45 | 27.68 | 28.20 | ||
| Mild NCD | 214 | 26.53 | 2.02 | 26.26 | 26.81 | |||
| Major NCD | 255 | 24.36 | 2.71 | 24.03 | 24.70 | 113.740 | 0.000§ | |
| Total | 592 | 25.58 | 2.66 | 25.66 | 26.09 | |||
MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; RS, raw score; NS, normative score; NCD, Neurocognitive Disorder; §ANOVA, Analysis of Variance: Bonferroni Post Hoc: p < 0.05.
Differences were more pronounced with MoCA 7.1 than MMSE: in particular the mean score of the Mild NCDs falls within the normal range on the MMSE and in the abnormal range on MoCA 7.1.
The magnitude of differences in mean MoCA 7.1 and MMSE scores displayed an overlap with the values of the conversion table for MoCA and MMSE [59], confirming to be more relevant, i.e., greater than 1 standard deviation in Mild (MoCA 5.66–4.93 points < MMSE-RS/NS) and Major NCD (MoCA 6.54–6.09 points < MMSE-RS/NS) groups.
3.4Discriminant validity of MoCA 7.1 and MMSE
The predictive accuracy of MoCA 7.1 was higher than that of the MMSE, with excellent sensitivity in identifying Mild NCDs (95.3%) (Table 4). The sensitivity of MMSE resulted low in detecting Mild NCDs both for raw (28.5%) and normative scores (53.8%). Specificity was the percentage of NECs that scored at or above the cut-off of 26 for MoCA 7.1. and 27 for MMSE-RS and MMSE-NS.
Table 4
The criterion values and the coordinates of the ROC curves examined in normal and Mild Neurocognitive Disorders for MoCA 7.1 and MMSE both as raw and normative scores
| Normal versus Mild NCDs | Standard cut-off | Sensitivity | Specificity | Best Fitting cut-off | Sensitivity | Specificity |
| MoCA 7.1 | ≤25 | 95.33 | 84.55 | ≤25 | 95.33 | 84.55 |
| MMSE-RS | ≤26 | 28.5 | 99.19 | ≤28 | 73.83 | 78.05 |
| MMSE-NS | ≤26.9 | 53.87 | 85.71 | ≤27.3 | 65.38 | 71.43 |
ROC, Receiver Operating Characteristic curve; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; RS, raw score; NS, normative score; NCD, Neurocognitive Disorder.
MoCA 7.1 showed a good specificity (84.5%), confirming the standard cut-off as the best fitting one. The MMSE-RS showed a high specificity, identifying 99.1% of the NECs while the MMSE-NS lowers to 85.7%. The MMSE yielded a better trade-off between sensitivity and specificity for Mild NCDs with different cut-off scores: ≤28 for raw scores and ≤27.3 for normative scores.
Nonetheless, the diagnostic accuracy of MoCA 7.1 for overall cognitive impairment was higher than MMSE (Fig. 1). MoCA 7.1 showed a good performance in the detection of Mild NCD with an AUC (0.963, sig. 0.00001) higher than MMSE (MMSE-RS: 0.803, sig. 0.00001, MMSE-NS: 0.742, sig. 0.0001).
Fig. 1
Normal versus Mild NCD. ROC curve, MMSE raw and normative scores and MOCA 7.1.
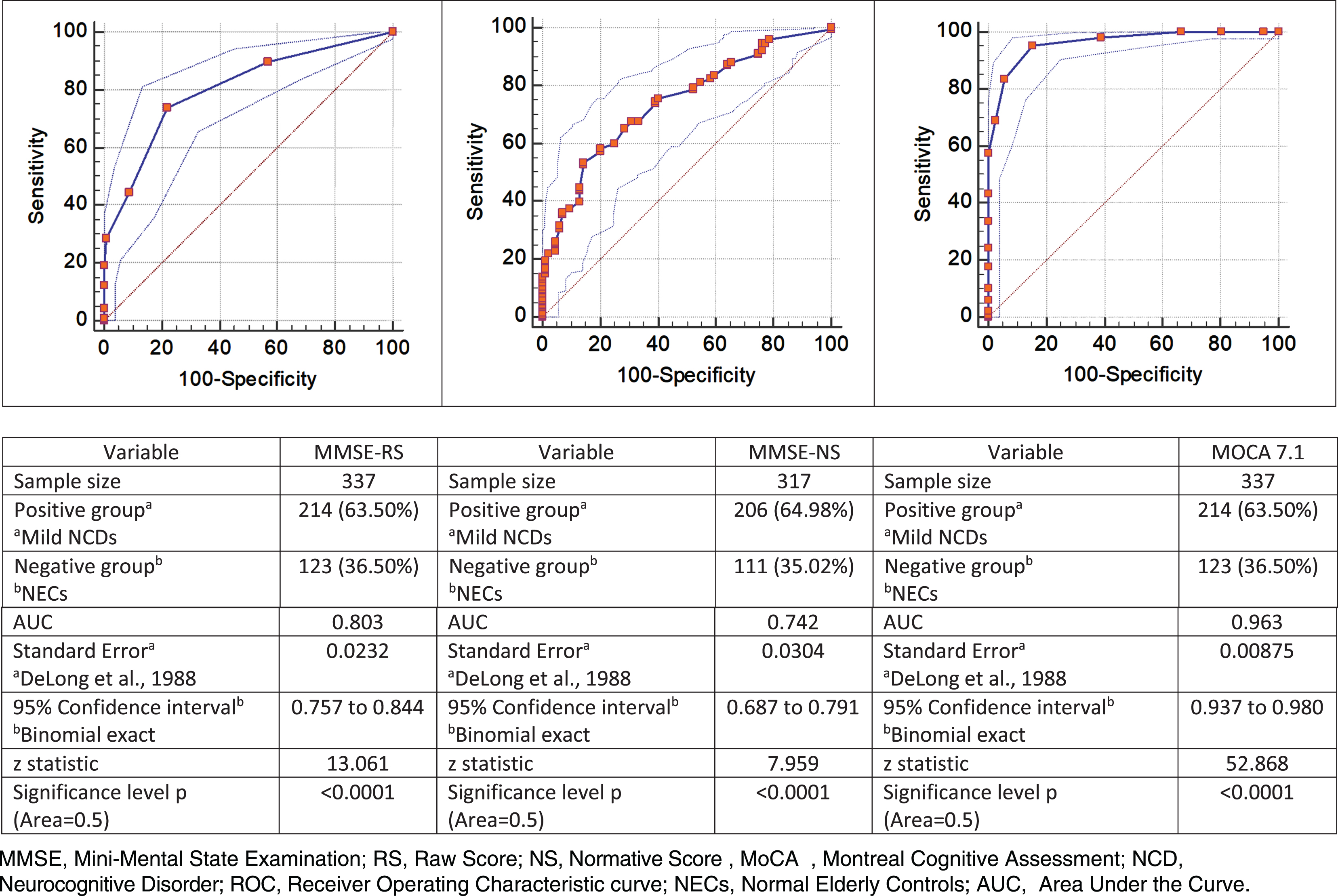
The diagnostic accuracy of MoCA 7.1 was examined to identify the items with greater sensitivity towards Mild NCD. The factorial analysis showed that 5 items of MoCA 7.1 distinguished significantly NECs from Mild NCDs: visuospatial/executive, digit span, serial 7’s subtraction, abstraction, free delayed recall (Table 5).
Table 5
Stepwise logistic regression analyses of MoCA 7.1 items in the normal elderly and Mild Neurocognitive Disorder groups
| Coefficients | ||||||
| MoCA 7.1 Items | Non-standardized coefficients | Standardized coefficients | t | Sign. | ||
| B | Standard Error | Beta | Standard Error | |||
| 2.793 | 0.216 | 12.930 | 0.000 | |||
| Visuospatial/Executive | –0.080 | 0.018 | –0.233 | 0.053 | –4.401 | 0.000* |
| Naming | –0.073 | 0.047 | –0.068 | 0.043 | –1.570 | 0.118 |
| Digit span (5 forward, 3 backward) | –0.080 | 0.031 | –0.109 | 0.043 | –2.562 | 0.011* |
| Letter A tapping | –0.023 | 0.059 | –0.016 | 0.043 | –0.382 | 0.703 |
| Serial 7’s subtraction | –0.065 | 0.033 | –0.084 | 0.043 | –1.968 | 0.048* |
| Repetition (2 longer sentences) | –0.013 | 0.034 | –0.016 | 0.044 | –0.373 | 0.709 |
| Verbal fluency for letter F | –0.058 | 0.040 | –0.063 | 0.044 | –1.442 | 0.151 |
| Abstraction | –0.098 | 0.029 | –0.155 | 0.045 | –3.444 | 0.001* |
| Free delayed recall | –0.132 | 0.013 | –0.464 | 0.047 | –9.869 | 0.000* |
| Orientation to time and place | 0.013 | 0.027 | 0.022 | 0.044 | 0.502 | 0.616 |
*p < 0.05.
DISCUSSION
This study examined the psychometric properties of the Italian version of MoCA 7.1 in older adults.
MoCA 7.1 showed optimal test-retest and inter-rater reliability which may depend on its simple structure and clarity of the instructions. As the original version [12], MoCA 7.1 yielded higher diagnostic accuracy than the MMSE, differentiating normal cognitive performance from early impairment (Table 4, Fig. 1) [13, 59, 66].
The diagnostic accuracy of MoCA is explained by its robust content and construct validity. First, the psychometric structure of MoCA contains five of ten items that are essential for evaluating the early transition from healthy cognition to NCDs (Table 5) [38, 67]. These items account for 50% of the total score variance and evaluate five of the six main cognitive domains associated with the anatomical-structural changes of the brain underlying NCDs [47, 68, 69]. Secondly, the length of the interval time for the delayed recall of MoCA matches the recommended standard of 3–5 minutes, which is relevant to ensure the similar ability of the long-term delayed recall (20-minute delay time) to discriminate Mild NCD from cognitively normal participants [70]. In contrast, the MMSE contains only three items relevant for early detection of NCDs and has a delay time (2 minutes) for short-term delayed recall with a number of recalled items (3 versus 5) below the thresholds of the standardized neuropsychological tests. [14, 71].
MoCA 7.1 scores among NECs did not display relevant effects due to age and education, thus confirming the chance to preserve the usual level of cognition of the adulthood across lifespan (Table 2) [41, 68].
The comparison of the MMSE-RS with MMSE-NS confirmed that adjustment for age and education is questionable and may mislead the clinical diagnosis (Table 2) [22, 72].
Administering MoCA 7.1 without adjustment for age and education unlike other studies did [73–77], yielded the optimal balance among sensitivity and specificity towards Mild NCDs (Table 4). In contrast, the MMSE-NS confirmed the well-known low or very low sensitivity towards Mild NCDs.
These findings are consistent with some prior studies. One twin study suggested that most of the association between MMSE scores and education reflects genetically-mediated differences in cognitive capacity rather than educational biases [78]. The second examined the Differential Item Functioning and found educational effects on serial subtractions, spelling backwards, writing a sentence; however, such effects explained less than 2% of differences participants with high and low educational attainment [79].
Our findings are aligned also with other studies [18, 19, 21, 22] and confirm the application of the original cut-off score of MoCA for detecting mild NCD in the Italian population while other Italian contributions have proposed five different lower cut-off scores ranging from 15,5 [74, 77], 17 through 19 [75], 23,28 [73] to 24,17 [76].
Among the aims of these studies there was not the validation of MoCA 7.1 to detect Mild NCD [3, 4, 12]: three were aimed to determine normative data [73, 74, 76], one to detect “probable cognitive impairment” and “probable AD” [75] and one “cognitive impairment” [77].
The diagnostic accuracy of MoCA 7.1 is important for clinical practice allowing a limited threshold of excluding false negatives counterbalanced by an acceptable risk of monitoring patients referring memory impairment but not definitively classifiable as normal or impaired cognition [28]. When administering only MMSE, the clinician should expect that some cases of Mild NCD could be labeled as “normal”, failing to schedule follow-up.
MoCA 7.1 has shown a convergent validity with the Italian version of the General Practitioner assessment of Cognition (GPCog) [80, 81], a brief cognitive screening test used by the Italian general practitioners (https://www.demenzemedicinagenerale.net).
While the comparison of the diagnostic accuracy confirmed that MoCA 7.1 overcomes the well-known ceiling effect of MMSE in detecting Mild and, not rarely, Major NCDs [82, 83], conversely, MoCA 7.1 showed a greater floor effect than MMSE [59], making the last one preferable to follow-up the advanced stages of Major NCD (CDR-G ≥3).
The diagnostic accuracy of MoCA provides a solid prognostic basis to the cognitive health as reported recently [68] Table 1 or, conversely, to detect the subtle abnormalities in prodromal neurocognitive disorders [84].
These characteristics of MoCA are relevant to update the algorithm of the basic approach to the assessment of cognitive functions in Mild NCD [85].
Limitations
This study has some limitations. First, the performance of MoCA 7.1 was analyzed only for the Mild and Major NCD, without considering its accuracy towards subtypes [86, 87]. Second, the NECs group had a limited age range (69.75±9.02) with a reduced proportion of examinees younger than 60 and older than 80 [88]. This limits the strength of our data when confirming the absence of the generalized effect of age and education applied to the entire population while a part preserves the usual level of cognition of adulthood along with aging. Nevertheless, this limit may be counterbalanced by the inclusion criteria of the NECs (intact IADL and CDR-G: 0). Finally, we did not assess the role of premorbid intellectual function [23].
MoCA 7.1 can be considered an ideal basic minimum data set of neuropsychological assessment for the detection mainly of Mild NCD, as the original.
The original cut-off ensures an optimal balance between using it as either a screening or a diagnostic tool. Nevertheless, other cut-offs may be used, depending on whether the test is being used as a screening (high sensitivity required) or as a diagnostic tool (high specificity required).
The optimal diagnostic accuracy of MoCA 7.1 allows clinicians and neuropsychologists to consistently reduce the need to administrate standardized sets of complex and time-consuming neuropsychological tests, reserving them preferably to the patients clinically positive but with normal MoCA 7.1 and MMSE.
Consequently, the professionals working in the CCDD can save time for the implementation of non-pharmacologic interventions for person with Mild or Major NCDs such as cognitive training, counselling, psychotherapy, psychosocial interventions.
The most relevant result of this study consists in the timely detection of cognitive impairment to allow professionals and caregivers to administrate timely preventive, pharmacologic and non-pharmacologic interventions with better outcomes with respect to more advanced stages (CDR-G score ≥2) [1].
In clinical practice, the routinely administration of both MMSE and MoCA 7.1 is recommended to avoid false negatives in the normal score range of MMSE especially in people with elevated premorbid intelligence.
The elderly achieving normal scores of MoCA and CDR-G and SB = 0 may be reasonably confident to maintain a healthy cognition longer than reporting normal scores of the MMSE.
ACKNOWLEDGMENTS
In memory of Prof. Rita Levi Montalcini who in person showed to AP the significance of normal cognition in near centenarian.
Cavicchi Eva born on February 22, 1919, elementary school, housewife, widow, only one of 3 children living, institutionalized when 98 years old, still solving daily challenging crossword puzzles at the age of 100 and performing MMSE 28/30 and MoCA 22/30 even if with relevant eye and hearing impairments.
Dr. Davide Zaccherini for his collaboration in administering firstly MoCA 7.1 in the Center for Cognitive Disorder and Dementia of Cento (Health County of Ferrara, Ovest District).
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
Alessandro Pirani, Andrea Fabbo, Marco Bruno Rocchi, Cristina Tulipani, Matteo Galassi, and Mirco Neri have no conflict of interest to report.
Ziad Nasreddine is the copyright owner of the MoCA test and received grants from Eli Lilly, Roche, Biogen for clinical trials, royalties for the use of the MoCA test in clinical trials, consulting fees from Biogen, Eli Lilly and Roche, payments from Biogen and Roche for data safety monitoring board or advisory board.
Francesca Neviani received honoraria from Medicalnet and Aristea. The payments were made to her.
Marco Bertolotti received support from Mylan Drug Company for attending the meeting of the European Atherosclerosis Society (Maastricht, Netherlands, 2019) and serves as member of the national board of the Italian Society for Cardiovascular Prevention and the regional board of the Italian Society of Gerontology and Geriatrics; he served as a former member of the regional board of the Italian Society for the Study of Atherosclerosis.
Martino Belvederi Murri received an honorarium for a lecture on depression by an educational agency (Lopez Eventi e Congressi) for which he had to declare absence of conflict of interests. The payment was made to him.
REFERENCES
[1] | Prince M , Bryce R , Ferri C (2011) World Alzheimer Report 2011: The benefits of early diagnosis and intervention. Alzheimer–s Disease International. London, UK. |
[2] | Di Fiandra T , Canevelli M , Di Pucchio A , Vanacore N & Italian Dementia National Plan Working Group ((2015) ) The Italian Dementia National Plan. Commentary.à. Ann Ist Super Sanit 51: , 261–264. |
[3] | American Psychiatric Association (APA) (2013) Diagnostic and Statistical Manual of Mental Disorders. DSM-5, 5th edn. Washington, DC. |
[4] | Sachdev PS , Blacker D , Blazer DG , Ganguli M , Jeste DV , Paulsen JS , Petersen RC ((2014) ) Classifying neurocognitive disorders: The DSM-5 approach. Nat Rev Neurol 10: , 634–642. |
[5] | Marshall GA , Amariglio RE , Sperling RA , Rentz DM ((2012) ) Activities of daily living: Where do they fit in the diagnosis of Alzheimer’s disease? . Neurodegener Dis Manag 2: , 483–491. |
[6] | Morris JC ((1993) ) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43: , 2412–2414. |
[7] | Petersen RC , Lopez O , Armstrong MJ , Getchius TSD , Ganguli M , Gloss D , Gronseth GS , Marson D , Pringsheim T , Day GS , Sager M , Stevens J , Rae-Grant A ((2018) ) Practice guideline update summary: Mild cognitive impairment: Report of the Guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology 90: , 126–135. |
[8] | Sachs-Ericsson N , Blazer DG ((2015) ) The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Mental Health 19: , 2–12. |
[9] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[10] | Votruba KL , Persad C , Giordani B ((2016) ) Cognitive deficits in healthy elderly population with “normal” scores on the Mini-Mental State Examination. J Geriatr Psychiatry Neurol 29: , 126–132. |
[11] | Strauss E , Sherman EMS , Spreen O ((2006) ) A compendium of neuropsychological test, 3rd Edition. Oxford University Press, pp. 169–189. |
[12] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , WhiteheadV , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal CognitiveAssessment, MoCA: A brief screening tool for Mild CognitiveImpairment. J Am Geriatr Soc 53: , 695–699. |
[13] | Larner AJ ((2012) ) Screening utility of the Montreal Cognitive Assessment (MoCA): In place of–or as well as–the MMSE? . Int Psychogeriatr 24: , 391–396. |
[14] | Lam B , Middleton LE , Masellis M , Stuss DT , Harry RD , Kiss A , Black SE ((2013) ) Criterion and convergent validity of the Montreal Cognitive Assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc 61: , 2181–2185. |
[15] | Martinelli JE , Cecato JF , Bartholomeu D , Montiel JM ((2014) ) Comparison of the diagnostic accuracy of neuropsychological tests in differentiating Alzheimer’s disease from Mild Cognitive Impairment: Can the Montreal Cognitive Assessment be better than the Cambridge Cognitive Examination? . Dement Geriatr Cogn Dis Extra 4: , 113–121. |
[16] | Vogel SJ , Banks SJ , Cummings JL , Miller JB , ((2015) ) Concordance of the Montreal Cognitive Assessment with standard neuropsychological measures.. Alzheimers Dement (Amst) 1: , 289–294. |
[17] | Del Brutto OH , Mera RM , Zambrano M , Soriano F , Lama J ((2015) ) Global cortical atrophy (GCA) associates with worse performance in the Montreal Cognitive Assessment (MoCA). A population-based study in community-dwelling elders living in rural Ecuador.. Arch Gerontol Geriatr 60: , 206–209. |
[18] | Bernstein IH , Lacritz L , Barlow CE , Weiner MF , Defina LF ((2011) ) Psychometric evaluation of the Montreal Cognitive Assessment (MoCA) in three diverse samples. Clin Neuropsychol 25: , 119–126. |
[19] | Hu JB , Zhou WH , Hu SH , Huang ML , Wei N , Qi HL , Huang JW , Xu Y ((2013) ) Cross-cultural difference and validation of the Chinese version of Montreal Cognitive Assessment in older adults residing in Eastern China: Preliminary findings. Arch Gerontol Geriatr 56: , 38–43. |
[20] | Julayanont P , Nasreddine ZS ((2017) ) Montreal Cognitive Assessment (MoCA): Concept and Clinical Review. In Cognitive Screening Instruments, Larner AJ, ed. Springer, Cham, pp. 139–195. |
[21] | Pugh EA , Kemp EC , van Dyck CH , Mecca AP , Sharp ES , Alzheimer’s Disease Neuroimaging Initiative ((2018) ) Effects of normative adjustments to the Montreal Cognitive Assessment. Am J Geriatr Psychiatry 26: , 1258–1267. |
[22] | Gagnon G , Hansen KT , Woolmore-Goodwin S , Gutmanis I , Wells J , Borrie M , Fogarty J ((2013) ) Correcting the MoCA for education: Effect on sensitivity. Can J Neurol Sci 40: , 678–683. |
[23] | Kang JM , Cho YS , Park S , Lee BH , Sohn BK , Choi CH , Choi JS , Jeong HY , Cho SJ , Lee JH , Lee JY ((2018) ) Montreal Cognitive Assessment reflects cognitive reserve. BMC Geriatr 18: , 261. |
[24] | Morley JE , Morris JC , Berg-Weger M , Borson S , Carpenter BD , Del Campo N , Dubois B , Fargo K , Fitten LJ , Flaherty JH , Ganguli M , Grossberg GT , Malmstrom TK , Petersen RD , Rodriguez C , Saykin AJ , Scheltens P , Tangalos EG , Verghese J , Wilcock G , Winblad B , Woo J , Vellas B ((2015) ) Brain health: The importance of recognizing cognitive impairment: An IAGG consensus conference. J Am Med Dir Assoc 16: , 731–739. |
[25] | Petersen RC , Smith GE , Waring SC , Ivnik RJ , Tangalos EG , Kokmen E ((1999) ) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56: , 303–308. |
[26] | Petersen RC ((2016) ) Mild Cognitive Impairment. Continuum (Minneap Minn) 22: , 404–418. |
[27] | Winblad B , Palmer K , Kivipelto M , Jelic V , Fratiglioni L , Wahlund LO , Nordberg A , Bäckman L , Albert M , Almkvist O , Arai H , Basun H , Blennow K , de Leon M , DeCarli C , Erkinjuntti T , Giacobini E , Graff C , Hardy J , Jack C , Jorm A , Ritchie K , van Duijn C , Visser P , Petersen RC ((2004) ) Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256: , 240–246. |
[28] | Reisberg B , Prichep L , Mosconi L , John ER , Glodzik-Sobanska L , Boksay I , Monteiro I , Torossian C , Vedvyas A , Ashraf N , Jamil IA , de Leon MJ ((2008) ) The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement 4: (1 Suppl 1), S98–S108. |
[29] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[30] | Pirani A , Nasreddine ZS , Tulipani C , Zaccherini D , Martini E , Neri M (2007) Montreal Cognitive Assessment (MoCA): Uno strumento rapido per lo screening del Mild Cognitive Impairment. Dati preliminari della versione Italiana. Atti IV Congresso Regionale Associazione Italiana Psicogeriatria, Bologna, 15 Dicembre 2007. |
[31] | Boyle PA , Yang J , Yu L , Leurgans SE , Capuano AW , Schneider JA , Wilson RS , Bennett DA ((2017) ) Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain 140: , 804–812. |
[32] | Tucker-Drob EM ((2019) ) Cognitive aging and dementia: A life-span perspective. Ann Rev Dev Psychol 1: , 177–196. |
[33] | Wilson RS , Boyle PA , Yu L , Barnes LL , Schneider JA , Bennett DA ((2013) ) Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81: , 314–321. |
[34] | Wilson RS , Yu L , Lamar M , Schneider JA , Boyle PA , Bennett DA ((2019) ) Education and cognitive reserve in old age. Neurology 92: , e1041–e1050. |
[35] | Lu K , Nicholas JM , Collins JD , James SN , Parker TD , Lane CA , Keshavan A , Keuss SE , Buchanan SM , Murray-Smith H , Cash DM , Sudre CH , Malone IB , Coath W , Wong A , Henley SMD , Crutch SJ , Fox NC , Richards M , Schott JM ((2019) ) Cognition at age 70: Life course predictors and associations with brain pathologies. Neurology 3: , e2144–e2156. |
[36] | Sachdev PS ((2016) ) Idemescence: Ageing without decline. Curr Opin Psychiatry 29: , 155–158. |
[37] | Beker N , Ganz A , Hulsman M , Klausch T , Schmand BA , Scheltens P , Sikkes SAM , Holstege H ((2021) ) Association of cognitive functiontrajectories in centenarians with postmortem neuropathology,physical health, and other risk factors for cognitive decline. JAMA Netw Open 4: , e2031654. |
[38] | Johnson DK , Storandt M , Morris JC , Galvin JE ((2009) ) Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol 66: , 1254–1259. |
[39] | Bakkour A , Morris JC , Wolk DA , Dickerson BC ((2013) ) The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: Specificity and differential relationships with cognition. Neuroimage 76: , 332–344. |
[40] | Hoenig MC , Willscheid N , Bischof GN , van Eimeren T , Drzezga A , Initiative AND ((2020) ) Assessment of tau tangles and amyloid-β plaques among super agers using PET imaging. JAMA Netw Open 3: , e2028337. |
[41] | Stumme J , Jockwitz C , Hoffstaedter F , Amunts K , Caspers S ((2020) ) Functional network reorganization in older adults: Graph-theoretical analyses of age, cognition and sex. Neuroimage 214: , 116756. |
[42] | Jiang J , Liu T , Crawford JD , Kochan NA , Brodaty H , Sachdev PS , Wen W ((2020) ) Stronger bilateral functional connectivity of the frontoparietal control network in near-centenarians and centenarians without dementia. Neuroimage 15: , 215:116855. |
[43] | Miller IN , Himali JJ , Beiser AS , Murabito JM , Seshadri S , Wolf PA , Au R ((2015) ) Normative data for the cognitively intact oldest-old: The Framingham Heart Study. Exp Aging Res 41: , 386–409. |
[44] | Reuben DB , Solomon DH ((1989) ) Assessment in geriatrics. Of caveats and names. J Am Geriatr Soc 37: , 570–572. |
[45] | Reuben DB , Laliberte L , Hiris J , Mor V ((1990) ) A hierarchical exercise scale to measure function at the Advanced Activities of Daily Living (AADL) level. J Am Geriatr Soc 38: , 855–861. |
[46] | Jutten RJ , Peeters CFW , Leijdesdorff SMJ , Visser PJ , Maier AB , Terwee CB , Scheltens P , Sikkes SAM ((2017) ) Detecting functional decline from normal aging to dementia: Development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement (Amst) 8: , 26–35. |
[47] | Wilson RS , Leurgans SE , Boyle PA , Schneider JA , Bennett DA ((2010) ) Neurodegenerative basis of age-related cognitive decline. Neurology 75: , 1070–1078. |
[48] | Lucca U , Garrì M , Recchia A , Logroscino G , Tiraboschi P , Franceschi M , Bertinotti C , Biotti A , Gargantini E , Maragna M , Nobili A , Pasina L , Franchi C , Riva E , Tettamanti M ((2011) ) Apopulation-based study of dementia in the oldest old: The Monzino80-plus study. BMC Neurol 11: , 54. |
[49] | Samu D , Campbell KL , Tsvetanov KA , Shafto MA ,; Cam-CAN consortium Tyler LK ((2017) ) Preserved cognitive functions with age are determined by domain-dependent shifts in network responsivity. Nat Commun 8: , 14743. |
[50] | Ding X , Charnigo RJ , Schmitt FA , Kryscio RJ , Abner EL , Alzheimer’s Disease Neuroimaging Initiative ((2019) ) Evaluating trajectories of episodic memory in normal cognition and mild cognitive impairment: Results from ADNI.. PLoS One 14: , e0212435. |
[51] | Corrada MM , Brookmeyer R , Paganini-Hill A , Berlau D , Kawas CH ((2010) ) Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann Neurol 67: , 114–121. |
[52] | Parmelee PA , Thuras PD , Katz IR , Lawton MP ((1995) ) Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc 43: , 130–137. |
[53] | Magni E , Binetti G , Bianchetti A , Rozzini R , Trabucchi M ((1996) ) Mini-mental state examination: A normative study in Italian elderly population. Eur J Neurol 3: , 1–5. |
[54] | Di Pucchio A , Vanacore N , Marzolini F , Lacorte E , Di Fiandra T ; I-DemObs Group, Gasparini M ((2018) ) Use of neuropsychological tests for the diagnosis of dementia: A survey of Italian memory clinics. BMJ Open 8: , e017847. |
[55] | Sheikh JI , Yesavage JA ((1986) ) Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. In Clinical Gerontology: A Guide to Assessment and Intervention, Brink TL, ed. The Haworth Press Inc., New York, pp. 165–173. |
[56] | Katz S , Down TD , Cash HR , Grotz RC ((1970) ) Progress in the development of the index of ADL. Gerontologist 10: , 20–30. |
[57] | Lawton MP , Brody EM ((1969) ) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: , 179–186. |
[58] | Lucca U , Tettamanti M , Quadri P ((2008) ) The Italian version of Consortium to Establish a Registry of Alzheimer’s Disease (CERAD). Alzheimers Dement 4: , 310. |
[59] | Roalf DR , Moberg PJ , Xie SX , Wolk DA , Moelter ST , Arnold SE ((2013) ) Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement 9: , 529–537. |
[60] | Storandt M , Morris JC ((2010) ) Ascertainment bias in the clinical diagnosis of Alzheimer disease. Arch Neurol 67: , 1364–1369. |
[61] | Morris JC , Storandt M , McKeel DW Jr , Rubin EH , Price JL , Grant EA , Berg L ((1996) ) Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46: , 707–719. |
[62] | Berg L , McKeel DW Jr , Miller JP , Storandt M , Rubin EH , Morris JC , Baty J , Coats M , Norton J , Goate AM , Price JL , Gearing M , Mirra SS , Saunders AM ((1998) ) Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 55: , 326–335. |
[63] | Petersen RC , Thomas RG , Grundman M , Bennett D , Doody R , Ferris S , Galasko D , Jin S , Kaye J , Levey A , Pfeiffer E , Sano M , van Dyck CH , Thal LJ ; Alzheimer’s Disease Cooperative Study Group ((2005) ) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352: , 2379–2388. |
[64] | Bastin C , Giacomelli F , Miévis F , Lemaire C , Guillaume B , Salmon E ((2021) ) Anosognosia in Mild Cognitive impairment: Lack of awarenessof memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry 12: , 631518. |
[65] | Elman JA , Jak AJ , Panizzon MS , Tu XM , Chen T , Reynolds CA , Gustavson DE , Franz CE , Hatton SN , Jacobson KC , Toomey R , McKenzie R , Xian H , Lyons MJ , Kremen WS ((2018) ) Underdiagnosis of mild cognitive impairment: A consequence of ignoring practice effects. Alzheimers Dement (Amst) 10: , 372–381. |
[66] | Pinto TCC , Machado L , Bulgacov TM , Rodrigues-Júnior AL , Costa MLG , Ximenes RCC , Sougey EB ((2019) ) Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s disease (AD) in the elderly? Int Psychogeriatr 31: , 491–504. |
[67] | Goldstein FC , Milloy A , Loring DW ; for the Alzheimer’s Disease Neuroimaging Initiative ((2020) ) Incremental validity of Montreal Cognitive Assessment index scores in Mild Cognitive Impairment and Alzheimer disease. Dement Geriatr Cogn Disord 45: , 49–55. |
[68] | Chen Q , Baran TM , Rooks B , O’Banion MK , Mapstone M , Zhang Z , Lin F ; Alzheimer’s Disease Neuroimaging Initiative ((2020) ) Cognitively supernormal older adults maintain a unique structural connectome that is resistant to Alzheimer’s pathology. Neuroimage Clin 28: , 102413. |
[69] | Betthauser TJ , Koscik RL , Jonaitis EM , Allison SL , Cody KA , Erickson CM , Rowley HA , Stone CK , Mueller KD , Clark LR , Carlsson CM , Chin NA , Asthana S , Christian BT , Johnson SC ((2020) ) Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 143: , 320–335. |
[70] | Zhao Q , Lv Y , Zhou Y , Hong Z , Guo Q ((2012) ) Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One 7: , e51157. |
[71] | Reuter-Lorenz PA , Park DC ((2010) ) Human neuroscience and the aging mind: A new look at old problems. J Gerontol B Psychol Sci Soc Sci 65: , 405–415. |
[72] | Kraemer HC , Moritz DJ , Yesavage J ((1998) ) Adjusting Mini-Mental State Examination scores for age and educational level to screen for dementia: Correcting bias or reducing validity? . Int Psychogeriatr 10: , 43–51. |
[73] | Conti S , Bonazzi S , Laiacona M , Masina M , Coralli MV ((2015) ) Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol Sci 36: , 209–214. |
[74] | Santangelo G , Siciliano M , Pedone R , Vitale C , Falco F , Bisogno R , Siano P , Barone P , Grossi D , Santangelo F , Trojano L ((2015) ) Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci 36: , 585–591. |
[75] | Bosco A , Spano G , Caffò AO , Lopez A , Grattagliano I , Saracino G , Pinto K , Hoogeveen F , Lancioni GE ((2017) ) Italians do it worse. Montreal Cognitive Assessment (MoCA) optimal cut-off scores for people with probable Alzheimer’s disease and with probable cognitive impairment. Aging Clin Exp Res 29: , 1113–1120. |
[76] | Aiello EN , Gramegna C , Esposito A , Gazzaniga V , Zago S , Difonzo T , Maddaluno O , Appollonio I , Bolognini N ((2021) ) The Montreal Cognitive Assessment (MoCA): Updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res 34: , 375–382. |
[77] | Pirrotta F , Timpano F , Bonanno L , Nunnari D , Marino S , Bramanti P , Lanzafame P ((2015) ) Italian validation of Montreal Cognitive Assessment. Eur J Psychol Assess 31: , 131–137. |
[78] | Pedersen NL , Reynolds CA , Gatz M ((1996) ) Sources of covariation among Mini-Mental State Examination scores, education, and cognitive abilities. J Gerontol B Psychol Sci Soc Sci 51: , 55–63. |
[79] | Jones RN , Gallo JJ ((2001) ) Education bias in the Mini-Mental State Examination. Int Psychogeriatr 13: , 299–310. |
[80] | Pirani A , Zaccherini D , Tulipani C , Fabbo A , Neviani F , Neri M ((2015) ) Comparison of MMSE, MoCA and GPCog in early diagnosis of dementia. Int Psychogeriatr 27: (S1), S106–S107. |
[81] | Pirani A , Brodaty H , Martini E , Zaccherini D , Neviani F , Neri M ((2010) ) The validation of the Italian version of the GPCOG (GPCOG-It): A contribution to cross-national implementation of a screening test for dementia in general practice. Int Psychogeriatr 22: , 82–90. |
[82] | Bergeron D , Flynn K , Verret L , Poulin S , Bouchard RW , Bocti C , Fülöp T , Lacombe G , Gauthier S , Nasreddine Z , Laforce RJ ((2017) ) Multicenter validation of an MMSE-MoCA conversion table. J Am Geriatr Soc 65: , 1067–1072. |
[83] | Franco-Marina F , García-González JJ , Wagner-Echeagaray F , Gallo J , Ugalde O , Sánchez-García S , Espinel-Bermúdez C , Juárez-Cedillo T , Rodríguez MA , García-Peña C ((2010) ) The Mini-mental State Examination revisited: Ceiling and flooreffects after score adjustment for educational level in an agingMexican population. Int Psychogeriatr 22: , 72–81. |
[84] | Pini L , Geroldi C , Galluzzi S , Baruzzi R , Bertocchi M , Chitò E , Orini S , Romano M , Cotelli M , Rosini S , Magnaldi S , Morassi M , Cobelli M , Bonvicini C , Archetti S , Zanetti O , Frisoni GB , Pievani M ((2020) ) Age at onset reveals different functional connectivity abnormalities in prodromal Alzheimer’s disease. Brain Imaging Behav 14: , 2594–2605. |
[85] | Boccardi M , Nicolosi V , Festari C , Bianchetti A , Cappa S , Chiasserini D , Falini A , Guerra UP , Nobili F , Padovani A , Sancesario G , Morbelli S , Parnetti L , Tiraboschi P , Muscio C , Perani D , Pizzini FB , Beltramello A , Salvini Porro G , Ciaccio M , Schillaci O , Trabucchi M , Tagliavini F , Frisoni GB ((2020) ) Italian consensus recommendations for a biomarker-based aetiological diagnosis in mild cognitive impairment patients. Eur J Neurol 27: , 475–483. |
[86] | Pendlebury ST , Mariz J , Bull L , Mehta Z , Rothwell PM ((2012) ) MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke 43: , 464–469. |
[87] | Li X , Jia S , Zhou Z , Jin Y , Zhang X , Hou C , Zheng W , Rong P , Jiao J ((2018) ) The role of the Montreal Cognitive Assessment (MoCA) and its memory tasks for detecting mild cognitive impairment. Neurol Sci 39: , 1029–1034. |
[88] | Bruijnen CJWH , Dijkstra BAG , Walvoort SJW , Budy MJJ , Beurmanjer H , De Jong CAJ , Kessels RPC ((2020) ) Psychometric properties of the Montreal Cognitive Assessment (MoCA) in healthy participants aged 18-70. Int J Psychiatry Clin Pract 24: , 293–300. |



