CHROME Criteria and Quality of Life: A Pilot Study from Maria Wolff-Albertia
Abstract
Background:
Over- and potentially inappropriate prescribing of psychotropic medications is a major public health concern among people with dementia.
Objective:
Describe the CHemical Restraints avOidance MEthodology (CHROME) criteria and evaluate its effects on psychotropic prescribing and quality of life (QoL).
Methods:
Observational, prospective, two-wave study conducted in two nursing homes. A multicomponent program to eliminate chemical restraints and attain quality prescription of psychotropic medications was implemented. CHROME’s diagnostic criteria comprise constellations of behavioral and psychological symptoms of dementia under six primary syndromic diagnoses. Since pharmacologic treatment is aimed at only one syndrome, polypharmacy is avoided. Psychotropic prescription, QoL, neuropsychiatric symptoms (NPS), and other clinical measurements were collected before and one year after the intervention. Results are presented for all residents (n = 171) and for completer subjects (n = 115).
Results:
Mean age (SD) of the residents was 87.8 (5.7), 78.9% were women, and 68.5% suffered advanced dementia. Psychotropic prescriptions decreased from 1.9 (1.1) to 0.9 (1.0) (p < 0.0005). Substantive reduction in prescribing frequency was observed for antidepressants (76.9% pre-intervention, 33.8% post-intervention) and for atypical neuroleptics (38.8% pre-intervention, 15.1% post-intervention). There was improvement in patient’s response to surroundings (p < 0.0005) and total NPS (p < 0.01), but small worsening occurred in social interaction (p < 0.02, completer subjects). Safety measurements remained stable.
Conclusion:
CHROME criteria appear to optimize psychotropic prescriptions, avoid chemical restraints, and allow external verification of quality prescriptions. Extensive use seems feasible, related to substantial reduction of prescriptions, and of benefit for people with dementia as de-prescriptions are not associated to increased NPS or QoL loss.
INTRODUCTION
People with dementia are often prescribed psychotropic medications, despite limited efficacy and serious safety concerns, especially for neuroleptics and benzodiazepines [1–6]. High frequency of neuroleptic prescribing has been particularly reported in nursing homes [4, 7], together with potentially inappropriate use [7, 8].
Over-prescription of psychotropic drugs is a multifaceted phenomenon, not yet well understood. On many occasions, it may arise from a false belief that medications may solve behavioral or psychological problems more conveniently than with non-pharmacological means. Previous studies examined the association between neuropsychiatric symptoms (NPS) and psychotropic prescribing, but the strength of the association was limited [9], suggesting inappropriate or off-label use [10].
Several medical, societal, and economic factors may be influencing overprescribing of psychotropic medications in people with dementia living in nursing homes. Some of these factors are diagnostic errors [10, 11], treatment errors [12], and lack of constrained treatment periods or periodic treatment evaluation [13]. In addition, psychotropics may also be utilized for other indications (e.g., antidepressants and antiepileptics may be used for pain control).
Evidence of significant risks arising from short- and, particularly, long-term use of neuroleptics and other psychotropic medications led to an emphasis on deprescribing rather than pharmacological optimization [14, 15]. Several factors have been reported to influence deprescribing, including not only scientific evidence, but also social factors such as physician receptivity, residents’ goals of care, limited training, time constraints, and lack of interdisciplinary collaboration, among others [16–18].
In contrast to pure deprescribing, medication optimization is aimed at ensuring the safest and most effective use of medications [19]. Optimization, which implicitly includes deprescribing, thereby adds to quality prescribing. In this regard, a range of approaches have been proposed, from medical approaches based on efficacy and safety balance [20–22] to global approaches that include education and counseling for physicians, nurses, and nurse aids [23]. Global approaches usually implement environmental adaptation from a person-centered perspective, sometimes combined with non-pharmacological therapies [24], keeping pharmacological treatment for the more resistant or severe cases.
The CHemical Restraints avOidance MEthodology (CHROME) criteria were designed as a comprehensive and explicit methodology to guide, evaluate, and certify quality prescribing of psychotropic medications for people with dementia. By including an ad hoc definition of chemical restraint, the CHROME approach also pursues the elimination of over-prescribing and chemical restraints (Supplementary Material 1).
While interventions aiming at prescribing reduction, as well as CHROME implementation, were proven safe [2, 11], very few studies analyzed the effects of those interventions on NPS or quality of life (QoL) [25, 26]. This paucity in research is striking, as psychotropics are primarily prescribed with the goal of symptomatic improvement of both areas.
In this paper, we will describe the rationale and development of the CHROME criteria, along with the results of their implementation in a pilot study where frequency of prescription, NPS, and QoL were measured.
MATERIALS AND METHODS
CHROME development and description
As inappropriate prescribing and overprescribing appear because of so many contributing factors, CHROME, first devised in 2016 [27] and validated in 2019 [11] has multiple components, which can be structured in four functional groups: 1) Quality prescribing, 2) Patient rights and legal compliance, 3) Pharmacy, and 4) External audit and certification of CHROME compliance. Specifically, CHROME criteria substitute the traditional symptom-based prescription approach with explicit neuropsychiatric syndrome diagnostic criteria and their subsequent prescription recommendations (Table 1).
Table 1
Neuropsychiatric syndromes and indicated medications
| Core symptoms1 | Duration | Indicated medications | |
| Depression | Sadness, anhedonia, lack of hope | Most of the time for the last two weeks | - SSRI, SNRI, other antidepressants (mirtazapine, vortioxetine, bupropion) |
| Anxiety | Excessive/unjustified fear, feeling of loss of control, somatic complaints, repetitive thoughts or behaviors | Most of the time for the last two weeks | - SSRI, SNRI, other antidepressants (mirtazapine, trazodone) |
| - Short/middle half-life BZD, gabapentin, pregabalin2 | |||
| - Atypical antipsychotics3 | |||
| Psychotic syndrome | False beliefs or stories (ideas of theft, abandonment, prejudice, infidelity, etc.) or false perceptions (visual, auditory, etc.) | Most days for the last seven days | - Atypical antipsychotics |
| Impulsive syndrome | Lack of foresight or social tact | Most of the time for the last two weeks | - Serotoninergic medications (sertraline, citalopram, escitalopram, trazodone) |
| - Antiepileptic drugs (valproate, gabapentin, pregabalin, carbamazepine, oxcarbamazepine, zonisamide), atypical antipsychotics2 | |||
| Maniform syndrome | Elevated mood, overestimation of own capabilities, feeling abnormally energetic, hyperactive, decreased need for rest | Most of the time for the last week | - Antiepileptic drugs (valproate, carbamazepine, oxcarbamazepine, topiramate), atypical antipsychotics (e.g., quetiapine) |
| - Lithium2 | |||
| Sleep disturbance | Loss of the physiological sleep-wake cycle (hypersomnia, insomnia, cycle inversion, fragmented sleep, etc.) | Most days for the last two weeks | - Short half-life benzodiazepines (lorazepam, lormetazepam), benzodiazepine analogs (zolpidem, zopiclone), other medications (clomethiazole, trazodone, mirtazapine, gabapentin, pregabalin, melatonin), natural products (valeriana, passiflora) |
| - Atypical antipsychotics (quetiapine, olanzapine)2 |
1To qualify for diagnosis, symptoms should produce significant distress, loss of functioning, or risk; in addition, symptoms should not be a mere consequence of cognitive deterioration, medical process, unmet basic needs, inadequate environment, or other neuropsychiatric symptom; 2second choice; 3last choice. BZD, benzodiazepines; SNRI, Serotonin and norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
CHROME syndromic approach is consistent with the standards of diagnosis in Psychiatry, which relies on symptom constellations and expert consensus, rather than biomarkers [28, 29]. Since the focus was on pharmacological treatment, six relevant syndromes were defined based on specific treatment response observed in non-demented patients. To define these CHROME syndromes, we utilized previously published clinical pictures [30–32], although the diagnostic process was streamlined to allow feasibility in usual clinical practice. In line with the procedures of the Diagnostic and Statistical Manual of Mental Disorders [28], diagnosis was based on core symptoms, temporal pattern, exclusion criteria, and clinician judgment. As specific contributions, the spectrum of agitation/aggression was narrowed down and the maniform (that is, manic-like) syndrome was separately defined, according to distinct semiology and treatment response [33, 34]. A complete rationale of each CHROME syndrome was published elsewhere [35].
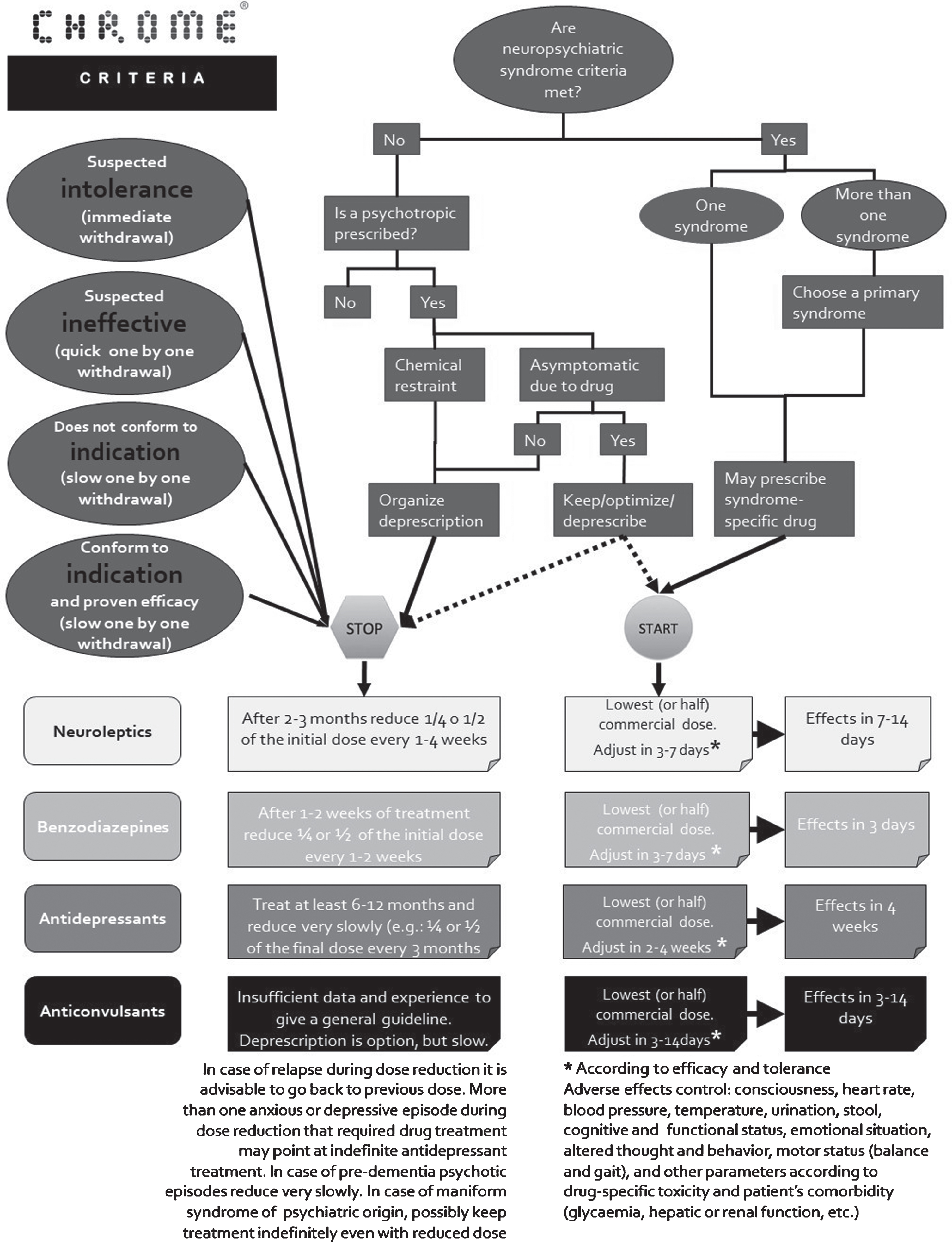
CHROME methodology combines deprescribing and syndrome-specific prescribing (Fig. 1). In case of patients receiving several psychotropic medications, deprescribing should invariably come first. Once indicated treatment is started, response and safety must be systematically monitored. Treatment benefits should be noticeable within 1–3 days of starting or increasing dose in the case of benzodiazepines, while up to one month of treatment may be necessary to observe positive effects of antidepressants. Vital signs, arousal, cognition, functional status, affective and behavioral symptoms, as well as patient-specific medical conditions, should be closely monitored, particularly during the first weeks of treatment or dose increase. A reliable informant having daily contact with the patient is useful and, in most cases, necessary to evaluate treatment response. Reduction of frequency or severity of symptoms qualifies as a positive response. Treatment is considered successful if improvement in symptoms outweighs unwanted adverse effects, provided a reasonable dose has been reached; failure if the opposite is the case. Even in case of success, treatment reduction or withdrawal should be periodically considered (for more details on CHROME-based prescription and chemical restraint elimination see Supplementary Material 1).
Fig. 1
Algorithm for psychotropic medication withdrawal, initiation, and effect control, according to CHROME criteria.
Study design
This was an observational, prospective, two-wave study conducted in two nursing homes from Albertia under usual care and practice conditions. Albertia Servicios Sociosanitarios is a chain of 14 nursing homes spread throughout Spain. In Spring 2018, its CEO and Medical Director (LP) decided to implement the CHROME chainwide. The present study reports the findings of two homes (Albertia “Valle de la Oliva” and Albertia “Las Palmeras”) located in the outskirts of the greater metropolitan area of Madrid. These two homes were audited and certified by an external auditor of the Spanish Alzheimer’s Society (CEAFA) as compliant with all CHROME criteria. Data were collected at two points in time (study waves), i.e., July 2018 and July 2019. Written informed consent for study participation was obtained from capable patients, according to the medical doctors criterium; otherwise, consent was provided by the patients’ legal representatives. Ethics committee consultation was not deemed necessary because CHROME implementation follows the standards of medical treatment and care.
As CHROME criteria are specific for cognitive deterioration, participants were all residents with a score≥3 in the Global Deterioration Scale (GDS) [36]. The first study wave (July 2018) reports the situation of the nursing homes right after only medical doctors received CHROME training. The two facilities employed three full-time medical doctors (LP, SR, and LG), LP being at that time also home administrator and medical director of the entire chain. The study director (JO) and the principal investigator (RM) ensured that LP had no influence on diagnoses or prescriptions of the other physicians. The second wave shows the data right after both homes passed the external audit (July 2019), performed by a senior psychiatrist.
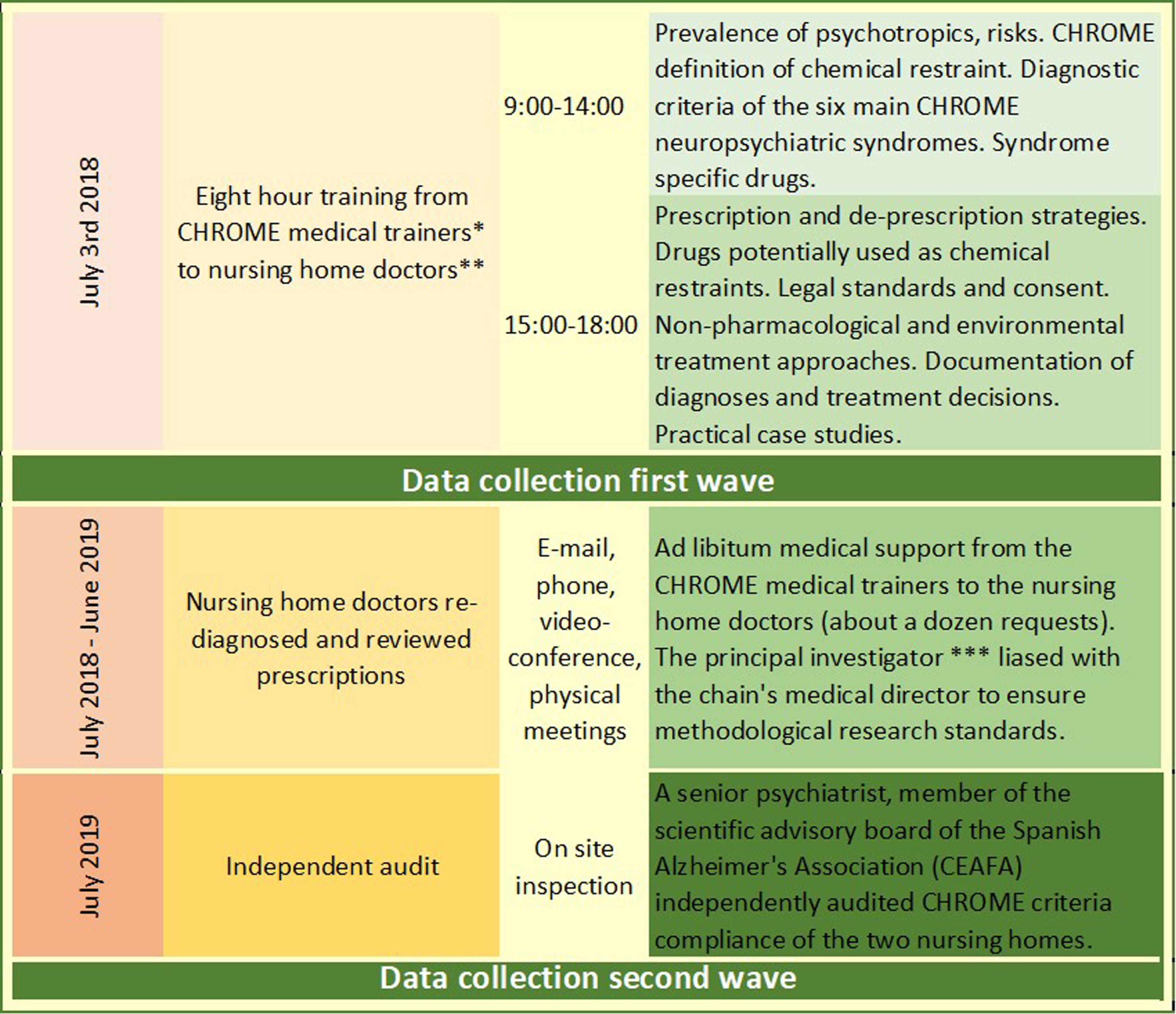
Training of eight hours was provided to the medical doctors by the Maria Wolff medical trainers (JO, JLA), including CHROME neuropsychiatric syndrome diagnosis, characteristics of drugs applicable to each syndrome, and deprescribing strategies, among other contents (Fig. 2 and Supplementary Material 2). Medical doctors could contact their trainers over the entire 12-month intervention period, on an as-needed basis.
Fig. 2
Study design, time-schedule, and intervention contents. *CHROME medical trainers: JO and JLA; **nursing home doctors: LP, SR, and LG; ***principal investigator: RM; medical director: LP; study director: JO.
To differentiate symptom-based pre-intervention diagnoses from post-intervention CHROME syndromic diagnoses, we called the former “diagnostic impressions” and the latter “CHROME diagnoses”. Medical doctors were asked to document their diagnostic impressions on a spreadsheet right after the initial training (July 2018). Over the ensuing months doctors got fully familiarized with CHROME syndrome-based diagnoses, which were documented as post-intervention CHROME diagnoses. The frequencies of those diagnostic impressions were recorded, together with CHROME diagnoses and all other scales reported below.
After first-wave data were recorded, doctors reviewed the diagnoses of patients on a one-by-one basis. Special emphasis was made to review usual overdiagnosis. Overdiagnosis ensues out of a) confusing symptoms with syndromes, b) inherited diagnoses or treatments of physicians no longer in charge of the residents, c) exaggerated symptom reports/perceptions, or d) relatives or co-workers pressuring for sedation out of convenience or ill understood humanity. Some of these prescribing practices are clear chemical restraints [11].
In case of two or more previously diagnosed concurrent syndromes, or clinical overlap between syndromes, physicians tried to identify a single underlying primary syndrome. This led to many residents, previously diagnosed with two or even three diagnoses and their corresponding drugs, to be diagnosed usually with only one or even no neuropsychiatric syndrome. Besides facilitating significant deprescribing, this procedure allowed “certain” or “possible” chemical restraints to be identified and eliminated (see Supplementary Table 1 for chemical restraint criteria). Drugs were then accordingly deprescribed. Once all residents’ diagnoses were reassessed and prescriptions reviewed according to CHROME guidelines and standards, the audit was performed in both homes. Post-audit data are shown in the July 2019 wave.
Psychotropic medications were divided according to the following categories: antidepressants, atypical neuroleptics, typical neuroleptics, short/medium half-life benzodiazepines (BZD), long half-life benzodiazepines, other hypnotics/sedatives, antiepileptic medications, cholinesterase inhibitors (CEI), and memantine. Psychotropic prescription was coded as present or not, regardless of its intentional use (pain, epilepsy, etc.), either continuous or PRN prescription, in the previous week to the study wave.
Quality of life was measured using the revised version of the Alzheimer’s Disease-Related Quality of Life scale (ADRQL) [37]. This instrument, which is answered by a close caregiver, measures QoL in five domains using a 0–100 scale. The use of a proxy, as well as the modest correlations of ADRQL scores with cognitive and functional disability, renders this instrument particularly well-suited for the study of QoL in people with advanced dementia.
In addition, the following secondary outcome measures were obtained:
- Performance of activities of daily living (ADL), according to the Functional Assessment Staging (FAST) [38]. The FAST evaluates functional dependence when secondary to cognitive deterioration and classifies patients according to seven principal levels of dependence. Since sub-scores are provided for some of those levels, a final score from 1 (no subjective or objective difficulty, FAST 1) to 16 (loss of ability to hold up head independently, FAST 7f) was obtained.
- Basic ADL, according to the Barthel Index [39]. This informant-based scale evaluates performance of 10 basic ADL with global score from 0 (total dependence) to 100 (independence).
- Cognitive performance, measured with the Mini-Examen Cognoscitivo (MEC) [40]. The MEC is a Spanish adaptation of Folstein’s Mini-Mental State, with possible scores between 0 (worst cognitive performance) and 35 (best cognitive performance).
- Dementia severity, according to the GDS [36]. Based on caregiver interview and patient examination, this scale grades dementia severity from 1 (no dementia symptoms) to 7 (severe dementia).
- Neuropsychiatric symptoms, according to the abridged version of the Neuropsychiatric Inventory (NPI-Q) [41]. Through interview with an informant, the NPI-Q assesses neuropsychiatric symptoms, yielding scores from 0 (no symptoms) to 3 (severe symptoms) for 12 symptom domains (total score ranges from 0 to 36).
- Any type of physical restraint during the last week, at the date of the study wave (excluding side rails).
- Falls accumulated in the six months before the date of the study wave. Four types of falls were separately recorded: non-injurious, with hip fracture, with other fracture, and with any other complication (e.g., head trauma, cutaneous wound, sprain).
- Referrals to the emergency room six months before the date of the study waves.
The ADRQL and all the secondary outcome measures were administered by qualified home staff, unaware of the study design (these employees performed those and other evaluations in their usual practice). The nursing home doctors provided neuropsychiatric diagnoses, according to the CHROME criteria [11].
Data regarding demographics and prescriptions were extracted from the IT Home Management Database/Software (ResiPlus® - ADD Informática, Torrent, Valencia). Although the evaluators cannot be considered blind, the external audit included more than 20% of cases being randomly selected and verified. No major discrepancies were found between the auditor and the three physicians: neither in diagnoses, nor in treatment adequacy. RM and LP reviewed data base consistency and accuracy at several checkup points.
Demographic and clinical variables were presented using indexes of frequency, central tendency, and dispersion. Confidence intervals were obtained for the categorical variables in the total sample and Wilcoxon test was utilized to analyze the evolution of the subjects that were evaluated at both study waves (completer subjects). Increase in confidence of neuropsychiatric diagnosis after full CHROME training was analyzed using the certainty increase ratio (CIR), defined as the odds ratio of certain versus possible diagnosis at the second wave measurement, in comparison with first-wave diagnostic impression.
The primary analysis was pre-post comparison of frequencies of psychotropic prescribing and QoL measurements. Assuming type I error of 0.05 and type II error of 0.80 and using the data obtained in the first wave, the study was powered to detect absolute reductions of 14.9%, 15.0%, and 10.7% in, respectively, antidepressant, atypical neuroleptic, and antiepileptic medications (total sample), as well as to detect a change of 3.3 points (effect size of 0.19) in the ADRQL total score (completer subjects). Since high interdependence between the different measures of effect was expected, multiple comparisons were controlled using the method of false discovery rate (FDR). As we conducted 28 effect comparisons, the level for statistical significance was set at p < 0.026 [42]. The statistical analyses were performed using the Statistical Package for Social Sciences version 15.0 software (SPSS, Chicago, IL).
RESULTS
A total of 171 residents were included in the study. They were predominantly women (78.9%) with a mean age (SD) of 87.8 (5.7). Mean (SD) number of medications was 7.8 (3.7) at study inclusion and 32.2% of the residents had some type of physical restraint. Severity of dementia was as follows: 15.2% mild cognitive impairment, 16.4% mild dementia, 21.1% moderate, 30.4% moderately severe, and 17.0% severe dementia.
The total sample consisted of 147 residents present in the first wave and 139 residents present a year later in the second wave (differences in numbers due to death [n = 22], leaving the home [n = 10], and new residents [n = 24]). Hence, the sub-sample of residents present at both waves was 115 (completer subjects). Demographic characteristics and study variables of the total sample and the completer subjects are presented in Table 2. The mean (SD) number of psychotropic prescriptions was reduced from 1.9 (1.1) to 0.9 (1.0), which represents an absolute reduction of one medication per patient and a relative reduction of 52.6%. The drugs most reduced in absolute terms were antidepressants (76.9% pre-intervention, 33.8% post-intervention) and atypical neuroleptics (38.8% pre-intervention, 15.1% post-intervention), but remarkable relative reductions of 87.0% and 65.0% were also achieved for, respectively, short/medium half-life and long half-life benzodiazepines (total sample).
Table 2
Demographic and clinical variables at both study waves
| Total sample | Completer residents | ||||
| July, 2018 | July, 2019 | July, 2018 | July, 2019 | p5 | |
| (pre-CHROME) | (post-CHROME) | (post-CHROME) | (post-CHROME) | ||
| (n = 147) | (n = 139) | (n = 115) | (n = 115) | ||
| Age | 87.8 (6.0) | 88.1 (5.6) | 88.1 (5.9) | 89.1 (5.9) | NA |
| Sex (% female) | 81.0 (74.6–87.3) | 80.6 (74.0–87.2) | 83.5 (76.7–90.3) | 83.5 (76.7–90.3) | NA |
| ADL performance (FAST) | 7.7 (3.6) | 8.6 (3.8) | 7.7 (3.7) | 8.7 (3.7) | 0.000 |
| Basic ADL (BI) | 34.7 (29.1) | 33.7 (29.6) | 36.8 (29.9) | 32.2 (28.7) | 0.003 |
| Cognition (MEC) | 12.7 (9.7) | 11.6 (9.3) | 13.0 (9.7) | 10.8 (9.2) | 0.000 |
| Dementia severity (GDS) | 5.1 (1.5) | 5.4 (1.4) | 5.1 (1.4) | 5.5 (1.4) | 0.000 |
| Neuropsychiatric symptoms (NPI-Q) | 2.5 (2.9) | 2.1 (2.6) | 2.5 (3.1) | 2.1 (2.5) | 0.008 |
| Total medications (n) | 7.8 (3.7) | 6.6 (3.5) | 7.7 (3.5) | 6.5 (3.4) | 0.000 |
| Psychotropic medications (n)1 | 1.9 (1.1) | 0.9 (1.0) | 1.9 (1.1) | 0.8 (1.0) | 0.000 |
| Antidepressants (%) | 76.9 (70.1–83.7) | 33.8 (25.9–41.7) | 79.1 (71.7–86.6) | 32.2 (23.6–40.7) | 0.000 |
| Atypical neuroleptics (%) | 38.8 (30.9–46.7) | 15.1 (9.2–21.1) | 40.0 (31.0–49.0) | 16.5 (9.7–23.3) | 0.000 |
| Typical neuroleptics (%) | 0.0 | 0.7 (0.0–2.1) | 0.0 | 0.9 (0.0–2.6) | 0.319 |
| Short/medium half-life BZD (%) | 5.4 (1.8–9.1) | 0.7 (0.0–2.1) | 4.3 (0.6–8.1) | 0.9 (0.0–2.6) | 0.045 |
| Long half-life BZD (%) | 2.0 (0.0–4.3) | 0.7 (0.0–2.1) | 1.7 (0.0–4.1) | 0.0 | 0.158 |
| Other hypnotics/sedatives (%) | 8.8 (4.3–13.4) | 11.5 (6.2–16.8) | 7.0 (2.3–11.6) | 9.6 (4.2–14.9) | 0.259 |
| Antiepileptic medications (%) | 17.7 (11.5–23.9) | 13.7 (8.0–19.4) | 17.4 (10.5–24.3) | 13.9 (7.6–20.2) | 0.207 |
| Dementia medications (%)2 | 21.1 (14.8–30.0) | 17.2 (11.2–23.3) | 22.6 (15.4–29.8) | 19.1 (12.2–26.0) | 0.341 |
| Physical restraint (%)3 | 34.5 (26.7–42.2) | 27.3 (19.9–34.7) | 31.9 (23.3–40.4) | 28.7 (20.4–37.0) | 0.408 |
| Double bed rail (%) | 52.4 (44.3–60.5) | 49.6 (41.3–58.0) | 49.6 (40.3–58.8) | 48.7 (39.6–57.8) | 0.783 |
| Non-injurious fall (%)4 | 50.3 (42.3–58.7) | 47.8 (39.2–55.8) | 47.8 (38.7–57.0) | 53.9 (44.8–63.0) | 0.286 |
| Fall with hip fracture (%)4 | 1.4 (0.0–3.2) | 4.3 (0.9–7.7) | 1.7 (0.0–4.1) | 3.5 (0.1–6.8) | 0.414 |
| Fall with other fracture (%)4 | 2.0 (0.0–4.3) | 2.2 (0.0–4.6) | 2.6 (0.0–5.5) | 2.6 (0.0–5.5) | 1.000 |
| Fall with other complication (%)4 | 2.0 (0.0–4.3) | 1.4 (0.0–3.4) | 1.7 (0.0) | 1.7 (4.1) | 1.000 |
| Emergency room referral (%)4 | 45.6 (37.5–53.6) | 32.4 (24.6–40.2) | 41.7 (32.7–50.8) | 32.2 (23.6–40.7) | 0.093 |
| Quality of life (ADRQL) | |||||
| Social interaction | 71.8 (26.9) | 70.9 (27.8) | 73.3 (26.3) | 69.2 (28.4) | 0.012 |
| Awareness of self | 49.1 (26.6) | 50.2 (28.4) | 49.1 (26.2) | 48.5 (29.1) | 0.541 |
| Feelings and mood | 74.0 (25.2) | 79.9 (22.7) | 73.6 (25.3) | 78.5 (23.1) | 0.037 |
| Enjoyment of activities | 52.2 (34.5) | 49.8 (38.5) | 50.8 (35.0) | 47.7 (38.8) | 0.247 |
| Response to surroundings | 70.9 (31.3) | 84.1 (26.8) | 72.1 (30.2) | 82.3 (27.9) | 0.000 |
| Total score | 66.0 (18.7) | 68.7 (20.4) | 66.3 (18.4) | 67.1 (20.8) | 0.541 |
Figures represent mean value (SD) or percentage (95% confidence interval). 1CEI and memantine were not included; 2CEI and/or memantine; 3bed rails were not included; 4residents with at least one event; 5Wilcoxon test for the completer group. ADL, activities of daily living; BI, Barthel Index; ADRQL, Alzheimer’s Disease-Related Quality of Life (0 worst, 100 best score); BI, Barthel Index (0 worst, 100 best score); BZD, benzodiazepines; CEI, cholinesterase inhibitors; CHROME, Chemical Restraints Avoidance Methodology; FAST, Functional Assessment Staging (1 best, 7 worst score); GDS, Global Deterioration Scale (1 best, 7 worst score); MEC, Mini-Examen Cognoscitivo (0 worst, 35 best score); NA, not applicable; NPI, Neuropsychiatric Inventory, abridged version (0 best, 36 worst score).
Response to surroundings displayed marked improvement from the first to the second measurement (pre-intervention mean value [SD] 72.1 [30.2], post-intervention mean value [SD] 82.3 [27.9], p < 0.0005), but social interaction deteriorated slightly (pre-intervention 73.3 [26.3], post-intervention 69.2 [28.4], p = 0.012). A trend of improvement in feelings and mood was observed (p = 0.037), while the QoL total score was not significantly changed (p = 0.541). There was mild improvement in NPS (pre-intervention 2.5 [3.1], post-intervention 2.1 [2.5], p = 0.008) and a trend of less emergency room referrals was also reported (p = 0.093). No significant differences or trends were observed in the occurrence of falls or restraints (Table 2).
A high frequency of diagnostic impressions was given in the first wave, particularly for sleep disturbance (61.2%), anxiety (60.5%), depression (57.8%), and psychotic syndrome (38.1%), which were significantly reduced after reviewing patients according to CHROME’s diagnostic criteria of neuropsychiatric syndromes (the respective frequencies being 33.1%, 37.4%, 30.9%, and 20.1%, total sample). The highest absolute reduction (28.1%) was observed for sleep disturbance, while maximal relative reduction (47.2%) was noted for impulsive and psychotic syndromes. The use of CHROME criteria only increased the diagnostic certainty for the impulsive syndrome (Table 3).
Table 3
Description of neuropsychiatric impressions and CHROME diagnoses
| Total sample | Completer residents | ||||
| July, 2018 (n = 147) | July, 2019 (n = 139) | July, 2018 (n = 115) | July, 2019 (n = 115) | p1 | |
| Diagnostic impression | CHROME diagnosis | Diagnostic impression | CHROME diagnosis | ||
| Depression | |||||
| Possible | 22.4 | 7.9 | 22.6 | 8.7 | |
| Certain | 35.4 | 23.0 | 35.7 | 19.1 | |
| Total | 57.8 (49.8–65.8) | 30.9 (23.3–38.6) | 58.3 (49.2–67.3) | 27.8 (19.6–36.0) | 0.000 |
| CIR | 1.84 (0.70–4.84) | 1.39 (0.53–3.63) | |||
| Anxiety | |||||
| Possible | 16.3 | 12.2 | 17.4 | 12.2 | |
| Certain | 44.2 | 25.2 | 44.3 | 22.6 | |
| Total | 60.5 (52.6–68.4) | 37.4 (29.4–45.5) | 61.7 (52.9–70.6) | 34.8 (26.1–43.5) | 0.000 |
| CIR | 0.76 (0.31–1.85) | 0.73 (0.30–1.77) | |||
| Psychotic syndrome | |||||
| Possible | 12.2 | 9.4 | 11.3 | 10.4 | |
| Certain | 25.9 | 10.8 | 27.0 | 12.2 | |
| Total | 38.1 (30.2–45.9) | 20.1 (13.5–26.8) | 38.3 (29.4–47.1) | 22.6 (15.0–30.3) | 0.000 |
| CIR | 0.54 (0.18–1.64) | 0.49 (0.17–1.45) | |||
| Impulsive syndrome | |||||
| Possible | 15.6 | 1.4 | 13.9 | 1.7 | |
| Certain | 6.1 | 10.1 | 5.2 | 10.4 | |
| Total | 21.8 (15.1–28.4) | 11.5 (6.2–16.8) | 19.1 (11.9–26.3) | 12.2 (6.2–18.2) | 0.033 |
| CIR | 18.45 (2.50–136.34) | 16.35 (2.42–110.32) | |||
| Maniform syndrome | |||||
| Possible | 0.7 | 0.7 | 0.9 | 0.0 | |
| Certain | 0.0 | 0.7 | 0.0 | 0.9 | |
| Total | 0.7 (0.0–2.0) | 1.4 (0.0–3.4) | 0.9 (0.0–2.6) | 0.9 (0.0–2.6) | 1.000 |
| CIR | NA | NA | |||
| Sleep disturbance | |||||
| Possible | 17.0 | 11.5 | 17.4 | 12.2 | |
| Certain | 44.2 | 21.6 | 41.7 | 18.3 | |
| Total | 61.2 (53.3–69.1) | 33.1 (25.3–40.9) | 59.1 (50.1–68.1) | 30.4 (22.0–38.8) | 0.000 |
| CIR | 0.72 (0.29–1.79) | 0.63 (0.25–1.56) | |||
Figures represent frequency (95% confidence interval), except for the CIR, which is expressed as odds ratio. CIR, certainty increase ratio (odds ratio of certain versus possible diagnosis); NA, not applicable; 1Wilcoxon test for the completer group.
Positive subjective experiences were spontaneously communicated by the medical doctors during the intervention period. Patients were seemingly more aware, and the home’s atmosphere had reportedly changed for the better. Moreover, nurses and nurse aides manifested to prefer working with more responsive residents.
DISCUSSION
The CHROME criteria were applied on a sample of very aged, fragile subjects, with a high frequency of psychotropic prescription, particularly for antidepressants and neuroleptics (Table 2). Sleep disturbance, anxiety, and depression were highly identified based on physicians’ first diagnostic impressions. However, the frequency of those labels dropped drastically after being reviewed by the stringently defined neuropsychiatric syndromes of the CHROME criteria and, consequently, psychotropic prescriptions were reduced (Tables 2 and 3).
Syndromic diagnoses and prescriptions were similar among both the completer and the total study samples, indicating successful application of the CHROME criteria, even in patients admitted to the nursing home during the study period whose medical history was often patchy, or unavailable.
Despite achieving important reduction of psychotropic prescribing, no drug category was completely deprescribed. These results are consistent with CHROME’s focus on treatment optimization, rather than exclusively deprescribing, thus allowing a subset of residents on medication, including neuroleptics, the most dangerous group. According to the present and previous studies, neuroleptic treatment may be indicated in 10–20% of institutionalized people with dementia [11, 27, 43, 44].
No significant changes were detected in total QoL after implementing CHROME criteria. One domain, however, improved (response to surroundings), while other (social interaction) slightly deteriorated (Table 2). Previous studies of natural evolution of QoL in institutionalized people with dementia yielded small increase, small decrease, or stabilization in mean QoL scores, but high interindividual variability was reported. The only study describing one-year evolution in the ADRQL reported significant improvement in feelings and mood along with trend of improvement in response to surroundings, which is quite consistent with our results, but deterioration in social interaction was not observed [45]. Worsening of social interaction in our residents could be caused by increased apathy, as severity of dementia advanced [46], while improvement in response to surroundings could result out of increased adequacy of psychotropic prescriptions (in fact: the response to surroundings items of the ADRQL mainly reflect anxiety- and depression-related behaviors).
Very few studies of interventions aimed at reducing or optimizing psychotropic medications reported QoL outcomes. Using the Beer’s criteria [21], potentially harmful medications were identified by trained nurses in a randomized controlled trial including 227 elderly residents (93% dementia) from assisted living facilities. Psychotropic prescription was significantly reduced in the intervention group and there was a decline in QoL in both the intervention and control groups, although the decline was significantly lower in the intervention group. In addition, difference in hospitalization was observed in favor of the intervention group [47].
An educational deprescribing program focused on nonpharmacological prevention and management of behavioral and psychological symptoms of dementia (BPSD) was implemented and evaluated in 139 residents (98% dementia) from 23 nursing homes, taking regular antipsychotic medication for at least three months. Neuroleptic reduction of 82% was achieved, without change in BPSD or adverse outcomes [48].
In apparent contrast to our findings is a study that reports worsening of NPS after reducing antipsychotics which were compensated for in a psychosocial intervention group [43]. The worsening of symptoms could be due to better adequacy in neuroleptic prescription, since there was an 18% initial frequency of prescription, compared to 39% in our study. In another trial, the initial frequency of neuroleptic prescription (9%) remained stable throughout the follow-up, while QoL and NPS improved after psychosocial intervention [26].
In our sample, possible changing needs of patients due to drug reviews were spontaneously absorbed by “usual care”. It must be noted that the philosophy of the Albertia chain is to engage in non-pharmacological treatment methods and staff training on an ongoing basis. Several psychosocial programs had been implemented and integrated by the homes before implementing the CHROME criteria as “usual care”. At the time of the review of prescriptions, no special efforts were made by non-medical staff.
The present investigation had several limitations to be noted. The naturalistic, observational study design precluded attribution of QoL changes to CHROME intervention, although lack of overall negative effect could be reasonably demonstrated. The study’s primary and secondary outcome measures were recorded by personnel that routinely performed those evaluations for clinical follow-up of residents. Although blinded to the study’s goals and methodology, they did not strictly qualify as blind raters. In addition, overall prescribing was reduced, but prescription switch and medication dose, which could have influenced mood, behavior, and QoL, were not analyzed. As for effect measurement, future studies should include qualitative research methods to evaluate the subjective impact of CHROME implementation, since staff reports point in a better direction than the quantitative measures of QoL or NPS.
Implementing CHROME criteria without an external audit in view might produce unsystematic use (cherry-picking only certain components) and not deliver the results shown here. Integrating CHROME criteria in a home’s everyday practice requires some learning. Teaching materials have been simplified and schematized (see Table 1, Fig. 1, and Supplementary Material) since the implementation reported herein.
In conclusion, CHROME criteria offer practical solutions for issues faced by many non-specialized clinicians relative to psychopharmacological treatment of behavioral and psychological issues of people with dementia. Treatment is organized around six neuropsychiatric syndromes instead of BPSD. Focus is on treatment optimization (psychotropics, medical, environmental, and non-pharmacological), and not merely on drug elimination. Nonetheless, significant reductions occurred in most drug categories, not just antipsychotics. QoL was slightly improved, and no adverse events were identified. Diagnostic criteria, treatment guidelines, chemical restraint definition, legal issues, pharmaceutical best practices, and auditing methods are summarized. Implementation is easy and beneficial for people with dementia.
ACKNOWLEDGMENTS
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST
JO and JLA received honoraria from Albertia for their training services outside of this study; LP, SR and LG are employed at Albertia.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/ADR-210015.
REFERENCES
[1] | Schneider LS , Tariot PN , Dagerman KS , Davis SM , Hsiao JK , Ismail MS , Lebowitz BD , Lyketsos CG , Ryan JM , Stroup TS , Sultzer DL , Weintraub D , Lieberman JA ; CATIE-AD Study Group ((2006) ) Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 355: , 1525–1538. |
[2] | Ballard C , Hanney ML , Theodoulou M , Douglas S , McShane R , Kossakowski K , Gill R , Juszczak E , Yu LM , Jacoby R ; DART-AD investigators ((2009) ) The dementia antipsychotic withdrawal trial (DART-AD): Long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 8: , 151–157. |
[3] | Avila-Castells P , Garre-Olmo J , Calvó-Perxas L , Turró-Garriga O , Alsina E , Carmona O , Perkal H , Roig AM , Cuy JM , Lozano M , Molins A , Vallmajó N , López-Pousa S ; Registry Dementia of Girona Study Group ((2013) ) Drug use in patients with dementia: A register-based study in the health region of Girona (Catalonia/Spain). Eur J Clin Pharmacol 69: , 1047–1056. |
[4] | Olazarán J , Valle D , Serra JA , Cano P , Muñiz R ((2013) ) Psychotropic medications and falls in nursing homes: A cross-sectional study. J Am Med Dir Assoc 14: , 213–217. |
[5] | Maust DT , Kim HM , Seyfried LS , Chiang C , Kavanagh J , Schneider LS , Kales HC ((2015) ) Antipsychotics, other psychotropics, and the risk of death in patients with dementia: Number needed to harm. JAMA Psychiatry 72: , 438–445. |
[6] | McMaster M , Fielding E , Lim D , Moyle W , Beattie E ; AusQoL ((2018) ) A cross-sectional examination of the prevalence of psychotropic medications for people living with dementia in Australian long-term care facilities: Issues of concern. Int Psychogeriatr 30: , 1019–1026. |
[7] | Brimelow RE , Wollin JA , Byrne GJ , Dissanayaka NN ((2019) ) Prescribing of psychotropic drugs and indicators for use in residential aged care and residents with dementia. Int Psychogeriatr 31: , 837–847. |
[8] | García-Gollarte F , Baleriola-Júlvez J , Ferrero-López I , Cruz-Jentoft AJ ((2012) ) Inappropriate drug prescription at nursing home admission. J Am Med Dir Assoc 13: , 83.e9–83.e8.3E15. |
[9] | Resnick B , Kolanowski A , Van Haitsma K , Galik E , Boltz M , Ellis J , Behrens L , Eshraghi K , Zhu S ((2021) ) Current psychotropic medication use and contributing factors among nursing home residents with cognitive impairment. Clin Nurs Res 30: , 59–69. |
[10] | Majic T , Pluta JP , Mell T , Aichberger MC , Treusch Y , Gutzmann H , Heinz A , Rapp MA ((2010) ) The pharmacotherapy of neuropsychiatric symptoms of dementia: A cross-sectional study in 18 homes for the elderly in Berlin. Dtsch Arztebl Int 107: , 320–327. |
[11] | Muñiz R , Pérez-Wehbe AI , Couto F , Pérez M , Ramírez N , López A , Rodríguez J , Usieto T , Lavin L , Rigueira A , Agüera-Ortiz L , López-Alvarez J , Martín-Carrasco M , Olazarán J ((2020) ) The “CHROME criteria”: Tool to optimize and audit prescription quality of psychotropic medications in institutionalized people with dementia. Int Psychogeriatr 32: , 315–324. |
[12] | Curtin D , Gallagher PF , O’Mahony D ((2019) ) Explicit criteria as clinical tools to minimize inappropriate medication use and its consequences. Ther Adv Drug Saf 10: , 2042098619829431. |
[13] | National Institute for Health and Care Excellence, Dementia: Assessment, Management and Support for People Living with Dementia and their Carers, https://nice.org.uk/guidance/ng97, Last updated June 20, 2018, Accessed on March 7, 2021. |
[14] | Harrison SL , Cations M , Jessop T , Hilmer SN , Sawan M , Brodaty H ((2019) ) Approaches to deprescribing psychotropic medications for changed behaviours in long-term care residents living with dementia. Drugs Aging 36: , 125–136. |
[15] | Kristensen RU , Jensen-Dahm C , Gasse C , Waldemar G ((2021) ) Declining use of potentially inappropriate medication in people with dementia from 2000 to 2015: A repeated cross-sectional nationwide register-based study. J Alzheimers Dis 79: , 1459–1470. |
[16] | Reeve E , Bell JS , Hilmer SN ((2015) ) Barriers to optimising prescribing and deprescribing in older adults with dementia: A narrative review. Curr Clin Pharmacol 10: , 168–177. |
[17] | Turner JP , Edwards S , Stanners M , Shakib S , Bell JS ((2016) ) What factors are important for deprescribing in Australian long-term care facilities? Perspectives of residents and health professionals. BMJ Open 6: , e009781. |
[18] | Palagyi A , Keay L , Harper J , Potter J , Lindley RI ((2016) ) Barricades and brickwalls–a qualitative study exploring perceptions of medication use and deprescribing in long-term care. BMC Geriatr 16: , 15. |
[19] | National Institute for Health and Care Excellence ((2015) ) Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes, National Institute for Health and Care Excellence, London UK. |
[20] | O’Mahony D , O’Sullivan D , Byrne S , O’Connor MN , Ryan C , Gallagher P ((2015) ) STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2 [published correction appears in Age Ageing 2018;1:47:489]. Age Ageing 44: , 213–218. |
[21] | By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel ((2019) ) American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 67: , 674–694. |
[22] | UK National Prescribing Centre ((2002) ) Room for Review: A Guide to Medication Review: The Agenda for Patients, Practitioners and Managers, Medicines Partnership, London UK. |
[23] | Chenoweth L , Jessop T , Harrison F , Cations M , Cook J , Brodaty H ((2018) ) Critical contextual elements in facilitating and achieving success with a person-centred care intervention to support antipsychotic deprescribing for older people in long-term care. Biomed Res Int 2018: , 7148515. |
[24] | Jessop T , Harrison F , Cations M , Draper B , Chenoweth L , Hilmer S , Westbury J , Low LF , Heffernan M , Sachdev P , Close J , Blennerhassett J , Marinkovich M , Shell A , Brodaty H ((2017) ) Halting Antipsychotic Use in Long-Term care (HALT): A single-arm longitudinal study aiming to reduce inappropriate antipsychotic use in long-term care residents with behavioral and psychological symptoms of dementia. Int Psychogeriatr 29: , 1391–1403. |
[25] | Ballard C , Orrell M , Sun Y , Moniz-Cook E , Stafford J , Whitaker R , Woods B , Corbett A , Banerjee S , Testad I , Garrod L , Khan Z , Woodward-Carlton B , Wenborn J , Fossey J ((2017) ) Impact of antipsychotic review and non-pharmacological intervention on health-related quality of life in people with dementia living in care homes: WHELD-a factorial cluster randomised controlled trial. Int J Geriatr Psychiatry 32: , 1094–1103. |
[26] | Ballard C , Corbett A , Orrell M , Williams G , Moniz-Cook E , Romeo R , Woods B , Garrod L , Testad I , Woodward-Carlton B , Wenborn J , Knapp M , Fossey J ((2018) ) Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: A cluster-randomised controlled trial. PLoS Med 15: , e1002500. |
[27] | Olazarán-Rodríguez J , López-Alvarez J , Agüera-Ortiz L , López-Arrieta JM , Beltrán-Aguirre JL , García-García P , Rigueira-García A , Martín-Carrasco M , Quintana-Hernández D , Muñiz-Schwochert R ((2016) ) [The CHROME criteria for the accreditation of centers free of chemical restraints and for a quality prescription of psychotropic medications]. Psicogeriatría 6: , 91–98. [Article in Spanish] |
[28] | American Psychiatric Association ((2013) ) Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association, Arlington, VA. |
[29] | World Health Organization, International Classification of Diseases for Mortality and Morbidity Statistics, 11th Revision, https://icd.who.int/browse11/l-m/en, Last updated September 2020, Accessed on March 7, 2021. |
[30] | Jeste DV , Finkel SI ((2000) ) Psychosis of Alzheimer’s disease and related dementias: Diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry 8: , 29–34. |
[31] | Olin JT , Schneider LS , Katz IR , Meyers BS , Alexopoulos GS , Breitner JC , Bruce ML , Caine ED , Cummings JL , Devanand DP , Krishnan KR , Lyketsos CG , Lyness JM , Rabins PV , Reynolds CF 3rd , Rovner BW , Steffens DC , Tariot PN , Lebowitz BD ((2002) ) Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry 10: , 125–128. |
[32] | Cummings J , Mintzer J , Brodaty H , Sano M , Banerjee S , Devanand DP , Gauthier S , Howard R , Lanctôt K , Lyketsos CG , Peskind E , Porsteinsson AP , Reich E , Sampaio C , Steffens D , Wortmann M , Zhong K ; International Psychogeriatric Association ((2015) ) Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 27: , 7–17. |
[33] | Mendez MF ((2000) ) Mania in neurologic disorders. Curr Psychiatry Rep 2: , 440–445. |
[34] | Ng B , Camacho A , Lara DR , Brunstein MG , Pinto OC , Akiskal HS ((2008) ) A case series on the hypothesized connection between dementia and bipolar spectrum disorders: Bipolar type VI?. J Affect Disord 107: , 307–315. |
[35] | Muñiz R , López-Alvarez J , Agüera-Ortiz L , Perea L , Olazarán J ((2021) ) Syndrome-based prescription to optimize psychotropics: Are CHROME criteria a game changer?. Front Psychiatry 12: , 662228. |
[36] | Reisberg B , Ferris SH , de Leon MJ , Crook T ((1982) ) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139: , 1136–1139. |
[37] | Kasper JD , Black BS , Shore AD , Rabins PV ((2009) ) Evaluation of the validity and reliability of the Alzheimer Disease-related Quality of Life Assessment Instrument. Alzheimer Dis Assoc Disord 23: , 275–284. |
[38] | Reisberg B ((1988) ) Functional assessment staging (FAST). Psychopharmacol Bull 24: , 653–659. |
[39] | Cid-Ruzafa J , Damián-Moreno J ((1997) ) [Disability evaluation: Barthel’s index]. Rev Esp Salud Pública 71: , 127–137. [Article in Spanish] |
[40] | Lobo A , Ezquerra J , Gómez Burgada F , Sala JM , Seva Díaz A ((1979) ) [Cognocitive mini-test (a simple practical test to detect intellectual changes in medical patients)]. Actas Luso Esp Neurol Psiquiatr Cienc Afines 7: , 189–202. [Article in Spanish] |
[41] | Kaufer DI , Cummings JL , Ketchel P , Smith V , MacMillan A , Shelley T , Lopez OL , DeKosky ST ((2000) ) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12: , 233–239. |
[42] | Benjamini Y , Hochberg Y ((1995) ) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 57: , 289–300. |
[43] | Ballard C , Orrell M , YongZhong S , Moniz-Cook E , Stafford J , Whittaker R , Woods B , Corbett A , Garrod L , Khan Z , Woodward-Carlton B , Wenborn J , Fossey J ((2016) ) Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: A factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry 173: , 252–262. |
[44] | Bravo-José P , Sáez-Lleó CI , Peris-Martí JF ((2019) ) Deprescribing antipsychotics in long term care patients with dementia.140-145.45B. Farm Hosp 43: . |
[45] | Missotten P , Ylieff M , Di Notte D , Paquay L , De Lepeleire J , Buntinx F , Fontaine O ((2007) ) Quality of life in dementia: A 2-year follow-up study. Int J Geriatr Psychiatry 22: , 1201–1207. |
[46] | Agüera-Ortiz L , Hernandez-Tamames JA , Martinez-Martin P , Cruz-Orduña I , Pajares G , López-Alvarez J , Osorio RS , Sanz M , Olazarán J ((2017) ) Structural correlates of apathy in Alzheimer’s disease: A multimodal MRI study. Int J Geriatr Psychiatry 32: , 922–930. |
[47] | Pitkälä KH , Juola AL , Kautiainen H , Soini H , Finne-Soveri UH , Bell JS , Björkman M ((2014) ) Education to reduce potentially harmful medication use among residents of assisted living facilities: A randomized controlled trial. J Am Med Dir Assoc 15: , 892–898. |
[48] | Brodaty H , Aerts L , Harrison F , Jessop T , Cations M , Chenoweth L , Shell A , Popovic GC , Heffernan M , Hilmer S , Sachdev PS , Draper B ((2018) ) Antipsychotic deprescription for older adults in long-term care: The HALT Study. J Am Med Dir Assoc 19: , 592–600.e7. |




