How Comorbidity Reflects on Cerebrospinal Fluid Biomarkers of Neurodegeneration in Aging
Abstract
Systemic comorbidity precipitates the risk for dementia. To comprehend the underlying mechanisms into a therapeutic perspective, we analyzed how comorbidity affects neurodegeneration-related cerebrospinal fluid (CSF) biomarkers of 55 cognitively intact subjects. The Charson Comorbidity Index (CCI) was correlated with CSF amyloid-β42 (Aβ42), amyloid-β40, total-tau, 181-phosphorylated-tau (p-tau), the Aβ42/p-tau ratio, neurogranin, and lactate. The age-related brain lesions at imaging were also considered. CCI had a raw association with Aβ42/p-tau and p-tau, and a stronger, age-independent correlation with lactate. These preliminary findings suggested that, in normal subjects, systemic comorbidity might increase CNS oxidative stress and, together with aging, contribute to develop an Alzheimer’s disease-like biochemical profile.
INTRODUCTION
With the worldwide increase of life expectancy and the parallel growing of neurodegenerative diseases cases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [1], the number of people living with dementia is destined to rise dramatically in next years.
Given the absence of disease-modifying treatments for dementia and idiopathic neurodegenerative disorders, prevention raises as a viable alternative to counteract such emerging issues. Indeed, several modifiable risk factors for dementia have been identified, including low educational level, blood hypertension, obesity, hearing loss, traumatic brain injury, alcohol misuse, smoking, depression, physical inactivity, social isolation, diabetes, and air pollution.
In addition, people with dementia suffer more frequently than general older population with comorbidities (e.g., cerebrovascular disease, stroke, diabetes), which in turn precipitate functional decline and quality of life [2]. Therefore, the chronic, systemic, age-related conditions [3–6] seem to impact on brain functions and contribute to clinic-pathological progression of dementia along its entire course, thus representing ideal targets for prevention strategies.
Because of its proximity to the central nervous system (CNS), cerebrospinal fluid (CSF) reflects neuropathological changes earlier than they become clinically manifest, thus providing an ideal source to track in vivo molecular events underlying neurodegenerative diseases [7].
Although still lacking, studies exploring relationships between medical comorbidity and CSF biomarkers of neurodegeneration in individuals without overt neurological impairment might help to elucidate mechanisms of neuronal vulnerability and provide hints to develop potential neuroprotective interventions.
Consequently, here we conducted a retrospective study in a group of cognitively intact subjects to define how systemic diseases could affect the biochemical profile of CSF. In particular, we investigated the association of the Charlson Comorbidity Index (CCI), a measure of comorbidities’ total burden [8], with a panel of CSF neurodegeneration-related biomarkers, including either the AD pathology’s markers or neurogranin and lactate levels, which assess respectively the development of a specific neuropathological process [9], or synaptopathy [10] and metabolic distress [11], two events profoundly intertwined with neurodegeneration.
METHODS
The study was performed at the Neurology Unit of Tor Vergata University Hospital (Rome, Italy) and involved 55 subjects without dementia receiving lumbar puncture for diagnostic purpose. None of them was suffering with neurodegenerative diseases (including parkinsonian syndromes, dementias, and motor neuron diseases). The prevalence of final neurological diagnoses were: headache/cerebrovascular condition = 27%; peripheral nervous system diseases = 21%; psychogenic disorders = 35%; confusional state 17%.
Dementia was ruled out by the Clinical Dementia Rating scale [12] and/or Mini-Mental State Examination (MMSE)>25 [13]. To avoid misdiagnosis, subjects with CSF amyloid-β42/181-phosphorylated-tau (Aβ42/p-tau) ratio suggestive of AD (<6.4) [14, 15] were excluded.
For all subjects, demographics and clinical data were collected and the CCI calculated. CCI is a global score that assesses (0 to 31 points) age and presence of 16 different comorbidities (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident/transient ischemic attacks, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, solid tumor, leukemia, lymphoma, AIDS) [8].
The burden of relevant brain lesions was considered by the age-related white matter changes (ARWMC) score, which evaluates the presence of both white matter and basal ganglia lesions on computer tomography or magnetic resonance imaging brain scans [16].
Lumbar puncture and CSF analysis were performed according with standard procedures, as previously described [10, 11]. The following biomarkers were assessed: amyloid-β42 (Aβ42), amyloid -β40 (Aβ40), total tau (t-tau), 181-phosphorylated-tau (p-tau), the Aβ42/p-tau ratio, neurogranin (NG), and lactate.
The study was conducted in agreement with local ethical standards and principles of Helsinki declaration. An informed consent was acquired for each participant.
Statistical analysis
The distribution of variables was evaluated with the Shapiro-Wilk test and the non-normally distributed were Log10-transformed to allow statistical calculations.
Correlations among clinical and biochemical variables were explored by the Pearson’s test. Then, a linear regression model adjusted for age was created to limit the effect of the main potential confounding factor.
Because of the possible gender difference of comorbidity [17], CCI was compared between in males and females by one-way ANOVA. A second linear regression analysis, including both age and sex as covariates, was thus performed.
A p value <0.05 was considered significant. Analysis was conducted in blind, by IBM-SPSS-22.
RESULTS
Demographic and clinical data are summarized in Table 1. Prevalence of main comorbidities in the population was: heart/vascular disorders = 38%; diabetes mellitus = 23%; solid localized tumor = 10%; other internal disorders = 13%; age >50 years = 90%.
Table 1
Clinical and demographic data of the population
| Variable | Descriptive statistics | ||
| Sex | (Female %) | 56% | |
| mean | st.dev. | ||
| Age | (y) | 66.4 | 13.0 |
| CCI | 4.0 | 2.1 | |
| t-tau | (pg/ml) | 228.1 | 121.4 |
| p-tau | (pg/ml) | 38.1 | 17.8 |
| Aβ42 | (pg/ml) | 1044.9 | 554.7 |
| Aβ40 | (pg/ml) | 8287.3 | 3711.6 |
| Aβ42/p-tau | 29.4 | 12.8 | |
| NG | (pg/ml) | 324.3 | 208.4 |
| Lactate | (mmol/l) | 1.8 | 1.0 |
| ARWMC-wm | 1.0 | 0.9 | |
| ARWMC-bg | 0.3 | 0.7 | |
CCI significantly correlated with p-tau (direct, R = 0.3, p = 0.02), Aβ42/p-tau ratio (inverse, R =–0.43, p = 0.001), lactate (direct, R = 0.37, p = 0.007) (Fig. 1A). No correlations resulted with ARWMC score.
Fig. 1
Association between CCI and CSF lactate. A) Graph representing direct correlation. B) Graph representing the linear model adjusted for age.
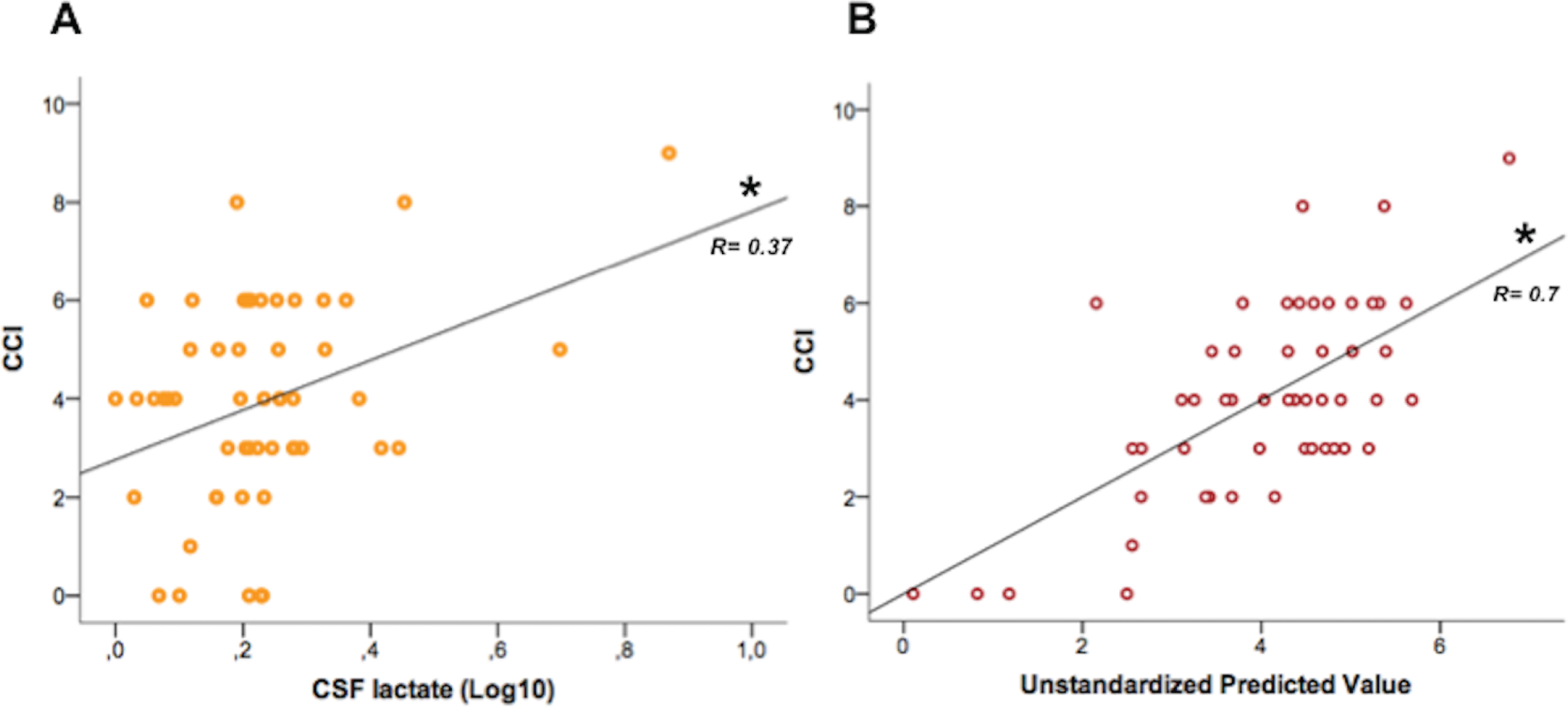
The linear model adjusted for age confirmed an independent association between CCI and lactate [F(2,49)=23.7, R2 = 0.49, p < 0.0001; T = 2.2, p = 0.03] (Fig. 1B) but not with p-tau and Aβ42/p-tau.
Gender effect
The burden of comorbidity was significantly higher in males (4.8±1.9, mean±st.dev.) than females (3.5±12.1, p = 0.02). The linear model adjusted for both sex and age did not show significant associations between CCI and biomarkers.
DISCUSSION
This study aimed to explore whether systemic comorbidities (evaluated by CCI) may affect the profile of neurodegeneration-related biomarkers in a group of subjects without dementia or neurodegenerative diseases. Given the overall scarcity of studies in this field, we provided initial evidence that the burden of systemic diseases could effectively influence the level of CSF biomarkers, probably as a consequence of brain changes.
CSF lactate is considered a readout of brain mitochondrial activity and redox balance; accordingly, a higher content has been found in several neurodegenerative disorders [11, 18]. Even in our population, lactate levels increased in parallel with CCI score, suggesting that bioenergetics impairment, mitochondrial dysfunction, and oxidative stress might mediate the neuronal injury induced by systemic conditions, independently from an age-related effect.
Although CCI evaluates up to 16 different pathologies, none of our subjects suffered with connective tissue and liver diseases, leukemia, lymphoma, or AIDS; conversely, they were mostly affected by heart and cerebrovascular diseases or diabetes, conditions typically associated to neurodegenerative diseases, which may precipitate neuronal vulnerability via oxidative stress and mitochondrial dysfunction [19–21]
Modification of AD core biomarkers (amyloid and tau proteins, and the synthetic ratio Aβ42/p-tau) may long precede clinical onset of dementia, mirroring an on-going pathological process with multifactorial origin [22]. Although within a range of normality [23–25], here the CSF levels of AD core biomarkers, namely p-tau and Aβ42/p-tau, showed raw associations with CCI, direct and inverse respectively. However, when the model was adjusted for age, the correlation was lost, suggesting that aging, more than systemic comorbidities, may favor an AD-like pathology as other authors similarly observed [26–28].
As well, CSF levels of NG were unrelated to systemic comorbidities burden. NG is a postsynaptic protein localized in dendritic spines, whose increased CSF content often reflects progression of cognitive impairment in patients with AD and PD as a consequence of spreading synaptopathy and neurodegeneration [10]. The absence of such correlation probably indicates its role as a specific marker of synaptic dysfunction rather than of global neuronal injury, consistently with previous findings [29, 30]. After all, none of the of patients here enrolled was diagnosed with neurodegenerative diseases, which typically underlie an overt process of synaptopathy with subsequent changes of related biomarkers [31–33].
Our study also disclosed relevance of gender in comorbidity’s occurrence. In fact, as also other authors demonstrated [17], CCI score was higher in males than females. Consequently, when sex was introduced as covariate in regression analysis, no significant results were obtained.
Finally, the lack of correlations between ARWMC scores and either CCI or CSF biomarkers suggests how the significant clinic-biochemical associations were not basically related to major brain lesions.
Definitely there are a number of limitations to be considered in this study, including the sample size, the cross-sectional design, the absence of biomarkers tracking other molecular events (e.g., vascular impairment, myelin disruption, neuronal loss), which indeed might have further outlined the impact of comorbidity on CNS. Then, we have also to acknowledge that underlying neurological conditions of enrolled subjects could affect to some extent CSF composition and biomarkers content [34].
Nevertheless, we found that systemic comorbidities (mostly vascular risk factors) reflect at CNS level with a subsequent modification of CSF biomarkers levels. The tendency is that concurrent medical conditions may increase oxidative stress, which is a well-known determinant of neurodegeneration. In addition, together with aging, they may predispose toward the development of an AD-like biochemical profile. Again, recent evidence demonstrated that frailty, intended as an accumulation of deficits weakening health outcomes, contributes to the pathophysiology and clinical manifestation of dementia, and, consequently, influences the expression of neuropathology and biomarkers [35–39].
Although further confirmatory studies, especially in prospective frames, are now necessary, these preliminary findings encourage strategies of neuroprotection based on prevention and cure of systemic diseases [32, 40].
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Dorsey ER , George BP , Leff B , Willis AW ((2013) ) The coming crisis: Obtaining care for the growing burden of neurodegenerative conditions. Neurology 80: , 1989–1996. |
[2] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: , 413–459. |
[3] | Chen TB , Yiao SY , Sun Y , Lee HJ , Yang SC , Chiu MJ , Chen TF , Lin KN , Tang LY , Lin CC , Wang PN ((2017) ) Comorbidity and dementia: A nationwide survey in Taiwan. PLoS One 12: , e0175475. |
[4] | Wang X , Zeng F , Jin WS , Zhu C , Wang QH , Bu Le X , Luo HB , Zou HQ , Pu J , Zhou ZH , Cui XP , Wang QS , Shi XQ , Han W , Wu Q , Chen HS , Lin H , Zhang LL , Zhang M , Lian Y , Xu ZQ , Zhou HD , Zhang T , Wang YJ ((2017) ) Comorbidity burden of patients with Parkinson’s disease and Parkinsonism between 2003 and 2012: A multicentre, nationwide, retrospective study in China. Sci Rep 7: , 1671. |
[5] | Wang QH , Wang X , Bu Le X , Lian Y , Xiang Y , Luo HB , Zou HQ , Pu J , Zhou ZH , Cui XP , Wang QS , Shi XQ , Han W , Wu Q , Chen HS , Lin H , Gao CY , Zhang LL , Xu ZQ , Zhang M , Zhou HD , Wang YJ ((2017) ) Comorbidity burden of dementia: A hospital-based retrospective study from 2003 to 2012 in seven cities in China. Neurosci Bull 33: , 703–710. |
[6] | Santos García D , Suárez Castro E , Expósito I , de Deus T , Tuñas C , Aneiros A , López Fernández M , Núñez Arias D , Bermúdez Torres M ((2017) ) Comorbid conditions associated with Parkinson’s disease: A longitudinal and comparative study with Alzheimer disease and control subjects. J Neurol Sci 373: , 210–215. |
[7] | Parnetti L , Gaetani L , Eusebi P , Paciotti S , Hansson O , El-Agnaf O , Mollenhauer B , Blennow K , Calabresi P ((2019) ) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18: , 573–586. |
[8] | Charlson ME , Pompei P , Ales KL , MacKenzie CR ((1987) ) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: , 373–383. |
[9] | Jack CR Jr , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R ; Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[10] | Sancesario GM , Di Lazzaro G , Alwardat M , Biticchi B , Basile V , Salimei C , Colona VL , Sinibaldi Salimei P , Bernardini S , Mercuri NB , Pisani A , Schirinzi T ((2020) ) Amyloid-β42/neurogranin ratio as a potential index for cognitive impairment in Parkinson’s disease. J Alzheimers Dis 76: , 1171–1178. |
[11] | Schirinzi T , Di Lazzaro G , Sancesario GM , Summa S , Petrucci S , Colona VL , Bernardini S , Pierantozzi M , Stefani A , Mercuri NB , Pisani A ((2020) ) Young-onset and late-onset Parkinson’s disease exhibit a different profile of fluid biomarkers and clinical features. Neurobiol Aging 90: , 119–124. |
[12] | Morris JC ((1997) ) Clinical Dementia Rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9: (Suppl 1), 173–176; discussion 177–178. |
[13] | Measso G , Cavarzeran F , Zappalà G , Lebowitz BD , Crook TH , Pirozzolo FJ , Amaducci LA , Massari D , Grigoletto F ((1993) ) The mini-mental state examination: Normative study of an Italian random sample. Dev Neuropsychol 9: , 77–85. |
[14] | Balasa M , Sánchez-Valle R , Antonell A , Bosch B , Olives J , Rami L , Castellví M , Molinuevo JL , Lladó A ((2014) ) Usefulness of biomarkers in the diagnosis and prognosis of early-onset cognitive impairment. J Alzheimers Dis 40: , 919–27. |
[15] | Schirinzi T , Sancesario GM , Di Lazzaro G , Scalise S , Colona VL , Imbriani P , Mercuri NB , Bernardini S , Lang AE , Pisani A ((2018) ) Clinical value of CSF amyloid-beta-42 and tau proteins in progressive supranuclear palsy. J Neural Transm 125: , 1373–1379. |
[16] | Wahlund LO , Barkhof F , Fazekas F , Bronge L , Augustin M , Sjögren M , Wallin A , Ader H , Leys D , Pantoni L , Pasquier F , Erkinjuntti T , Scheltens P ; European Task Force on Age-Related White Matter Changes ((2001) ) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32: , 1318–1322. |
[17] | Almagro P , Ponce A , Komal S , de la Asunción Villaverde M , Castrillo C , Grau G , Simon L , de la Sierra A ((2020) ) Multimorbidity gender patterns in hospitalized elderly patients. PLoS One 15: , e0227252. |
[18] | Gray E , Larkin JR , Claridge TD , Talbot K , Sibson NR , Turner MR ((2015) ) The longitudinal cerebrospinal fluid metabolomic profile of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 16: , 456–463. |
[19] | Santos CY , Snyder PJ , Wu WC , Zhang M , Echeverria A , Alber J ((2017) ) Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst) 7: , 69–87. |
[20] | Verdile G , Fuller SJ , Martins RN ((2015) ) The role of type 2 diabetes in neurodegeneration. Neurobiol Dis 84: , 22–38. |
[21] | Imbriani P , D’Angelo V , Platania P , Di Lazzaro G , Scalise S , Salimei C , El Atiallah I , Colona VL , Mercuri NB , Bonsi P , Pisani A , Schirinzi T , Martella G ((2020) ) Ischemic injury precipitates neuronal vulnerability in Parkinson’s disease: Insights from PINK1 mouse model study and clinical retrospective data. Parkinsonism Relat Disord 74: , 57–63. |
[22] | Sutphen CL , Jasielec MS , Shah AR , Macy EM , Xiong C , Vlassenko AG , Benzinger TL , Stoops EE , Vanderstichele HM , Brix B , Darby HD , Vandijck ML , Ladenson JH , Morris JC , Holtzman DM , Fagan AM ((2015) ) Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol 72: , 1029–1042. |
[23] | Forlenza OV , Radanovic M , Talib LL , Aprahamian I , Diniz BS , Zetterberg H , Gattaz WF ((2015) ) Cerebrospinal fluid biomarkers in Alzheimer’s disease: Diagnostic accuracy and prediction of dementia. Alzheimers Dement (Amst) 1: , 455–463. |
[24] | Schirinzi T , Di Lorenzo F , Sancesario GM , Di Lazzaro G , Ponzo V , Pisani A , Mercuri NB , Koch G , Martorana A ((2018) ) Amyloid-mediated cholinergic dysfunction in motor impairment related to Alzheimer’s disease. J Alzheimers Dis 64: , 525–532. |
[25] | Schirinzi T , Di Lazzaro G , Sancesario GM , Colona VL , Scaricamazza E , Mercuri NB , Martorana A , Sancesario G ((2017) ) Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer’s disease. J Neural Transm 124: , 1621–1625. |
[26] | Li G , Shofer JB , Petrie EC , Yu CE , Wilkinson CW , Figlewicz DP , Shutes-David A , Zhang J , Montine TJ , Raskind MA , Quinn JF , Galasko DR , Peskind ER ((2017) ) Cerebrospinal fluid biomarkers for Alzheimer’s and vascular disease vary by age, gender, and APOE genotype in cognitively normal adults. Alzheimers Res Ther 9: , 48. |
[27] | Mollenhauer B , Parnetti L , Rektorova I , Kramberger MG , Pikkarainen M , Schulz-Schaeffer WJ , Aarsland D , Svenningsson P , Farotti L , Verbeek MM , Schlossmacher MG ((2016) ) Biological confounders for the values of cerebrospinal fluid proteins in Parkinson’s disease and related disorders. J Neurochem 139: (Suppl 1), 290–317. |
[28] | Martorana A , Di Lorenzo F , Belli L , Sancesario G , Toniolo S , Sallustio F , Sancesario GM , Koch G ((2015) ) Cerebrospinal fluid Aβ42 levels: When physiological become pathological state. CNS Neurosci Ther 21: , 921–925. |
[29] | Hall S , Janelidze S , Zetterberg H , Brix B , Mattsson N , Surova Y , Blennow K , Hansson O ((2019) ) Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Mov Disord 35: , 513–518. |
[30] | Portelius E , Olsson B , Höglund K , Cullen NC , Kvartsberg H , Andreasson U , Zetterberg H , Sandelius Å , Shaw LM , Lee VMY , Irwin DJ , Grossman M , Weintraub D , Chen-Plotkin A , Wolk A , Mccluskey L , Elman L , Mcbride J , Toledo JB , Trojanowski JQ , Blennow K ((2018) ) Cerebrospinal fluid neurogranin concentration in neurodegeneration: Relation to clinical phenotypes and neuropathology. Acta Neuropathol 136: , 363–376. |
[31] | Di Lorenzo F , Motta C , Bonnì S , Mercuri NB , Caltagirone C , Martorana A , Koch G ((2019) ) LTP-like cortical plasticity is associated with verbal memory impairment in Alzheimer’s disease patients. Brain Stimul 12: , 148–151. |
[32] | Schirinzi T , Canevelli M , Suppa A , Bologna M , Marsili L ((2020) ) The continuum between neurodegeneration, brain plasticity, and movement: A critical appraisal. Rev Neurosci 31: , 723–742. |
[33] | Schirinzi T , Martella G , Imbriani P , Di Lazzaro G , Franco D , Colona VL , Alwardat M , Sinibaldi Salimei P , Mercuri NB , Pierantozzi M , Pisani A ((2019) ) Dietary vitamin E as a protective factor for Parkinson’s disease: Clinical and experimental evidence. Front Neurol 10: , 148. |
[34] | Baheerathan A , Pitceathly RD , Curtis C , Davies NW ((2020) ) CSF lactate. Pract Neurol 20: , 322–325. |
[35] | Wallace LMK , Theou O , Godin J , Andrew MK , Bennett DA , Rockwood K ((2019) ) Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: A cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol 18: , 177–184. |
[36] | Wallace LMK , Theou O , Darvesh S , Bennett DA , Buchman AS , Andrew MK , Kirkland SA , Fisk JD , Rockwood K ((2020) ) Neuropathological burden and the degree of frailty in relation to global cognition and dementia. Neurology 95: , e3269–e3279. |
[37] | Canevelli M , Arisi I , Bacigalupo I , Arighi A , Galimberti D , Vanacore N , D’Onofrio M , Cesari M , Bruno G ; Alzheimer’s Disease Neuroimaging Initiative (2020) Biomarkers and phenotypic expression in Alzheimer’s disease: Exploring the contribution of frailty in the Alzheimer’s Disease Neuroimaging Initiative. Geroscience, doi: 10.1007/s11357-020-00293-y. |
[38] | Fried LP , Tangen CM , Walston J , Newman AB , Hirsch C , Gottdiener J , Seeman T , Tracy R , Kop WJ , Burke G , McBurnie MA ; Cardiovascular Health Study Collaborative Research Group ((2001) ) Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: , 146–156. |
[39] | Koch G , Belli L , Giudice TL , Lorenzo FD , Sancesario GM , Sorge R , Bernardini S , Martorana A ((2013) ) Frailty among Alzheimer’s disease patients. CNS Neurol Disord Drug Targets 12: , 507–511. |
[40] | Alwardat M , Schirinzi T , Di Lazzaro G , Sancesario GM , Franco D , Imbriani P , Sinibaldi Salimei P , Bernardini S , Mercuri NB , Pisani A ((2019) ) Association between physical activity and dementia’s risk factors in patients with Parkinson’s disease. J Neural Transm 126: , 319–325. |




