Breast Cancer, Alzheimer’s Disease, and APOE4 Allele in the UK Biobank Cohort
Abstract
Background:
Cognitive problems are common in breast cancer patients. The apolipoprotein E4 (APOE4) gene, a risk factor for Alzheimer’s disease (AD), may be associated with cancer-related cognitive decline.
Objective:
To further evaluate the effects of the APOE4 allele, we studied a cohort of patients from the UK Biobank (UKB) who had breast cancer; some also had AD.
Methods:
Our analysis included all subjects with invasive breast cancer. Single nucleotide polymorphism (SNP) data for rs 429358 and rs 7412 was used to determine APOE genotypes. Cognitive function as numeric memory was assessed with an online test (UKB data field 20240).
Results:
We analyzed data from 2,876 women with breast cancer. Of the breast cancer subjects, 585 (20%) carried the APOE4 allele. Numeric memory scores were significantly lower in APOE4 carriers and APOE4 homozygotes than non-carriers (p = 0.046). 34 breast cancer subjects (1.1%) had AD. There was no significant difference in survival among genotypes ɛ3/ɛ3, ɛ3/ɛ4, and ɛ4/ɛ4.
Conclusion:
UKB data suggest that cognitive problems in women with breast cancer are, for the most part, mild, compared with other sequelae of the disease. AD, the worst cognitive problem, is relatively rare (1.1%) and, when it occurs, APOE genotype has little impact on survival.
INTRODUCTION
Cognitive problems are common in breast cancer patients [1]. Not all have these problems, and they can be subtle in the 75% older than 60. Aging, too, is related to cognitive decline, and it can be difficult to separate the effects of getting old from the effects of chemotherapy and hormonal treatment.
Chemotherapy can cause muddled thinking pat-ients call ‘chemobrain’ [2]. Tamoxifen can produce mental fogging that is difficult to distinguish from Alzheimer’s disease (AD) but which clears when tamoxifen is withdrawn [3].
The APOE genotype accounts for most of the AD risk. There are three common alleles of the APOE gene: APOE2, APOE3, and APOE4. In the general U.S. population, the ɛ4 allele prevalence is approximately 13%, about 2% being ɛ4 homozygous (2% of the U.S. population), and 11% being heterozygous (22% of the US population). Possession of one ɛ4 allele increases the risk of developing AD by 3 to 4-fold, and possession of two ɛ4 alleles increases risk by 15-fold, as compared with the ɛ3/ɛ3 genotype, with a large part of the variation being related to early age of onset. Over 60% of patients with non-familial AD carry the ɛ4 allele [4].
The ɛ4 allele may be associated with cancer-related cognitive decline. In one study, older women with breast cancer who were treated with chemotherapy and were carriers of the APOE4 allele were more likely to have cognitive problems than women without cancer who were also carriers of the APOE4 allele [1].
To further evaluate the effects of the APOE4 allele, we studied a cohort of patients from the UK Biobank (UKB) who had breast cancer; some also had AD.
METHODS
The UK Biobank is a large prospective observational study comprising approximately 500,000 men and women (N = 229,134 men, N = 273,402 women), more than 90% white, aged 40–69 years at enrollment. Participants were recruited from across 22 cen-ters located throughout England, Wales, and Scotland between 2006 and 2010 and continue to be longitudinally followed for capture of subsequent health events [5]. This methodology is like that of the Framingham Heart Study [6], with the exception that the UKB program collects postmortem samples, which Framingham did not.
Our UK Biobank application was approved as UKB project 57245 (S.L., P.H.R.).
Our analysis included all subjects with invasive breast cancer. Cancer diagnoses were ascertained through linkage to national cancer registries in England, Wales, and Scotland. Invasive breast cancer was coded using the 10th Revision of the International Classification of Diseases. Alzheimer’s disease was coded using the 10th Revision of the International Classification of Diseases. Death was ascertained via linkage to death registries. Complete follow-up was available through June 2020.
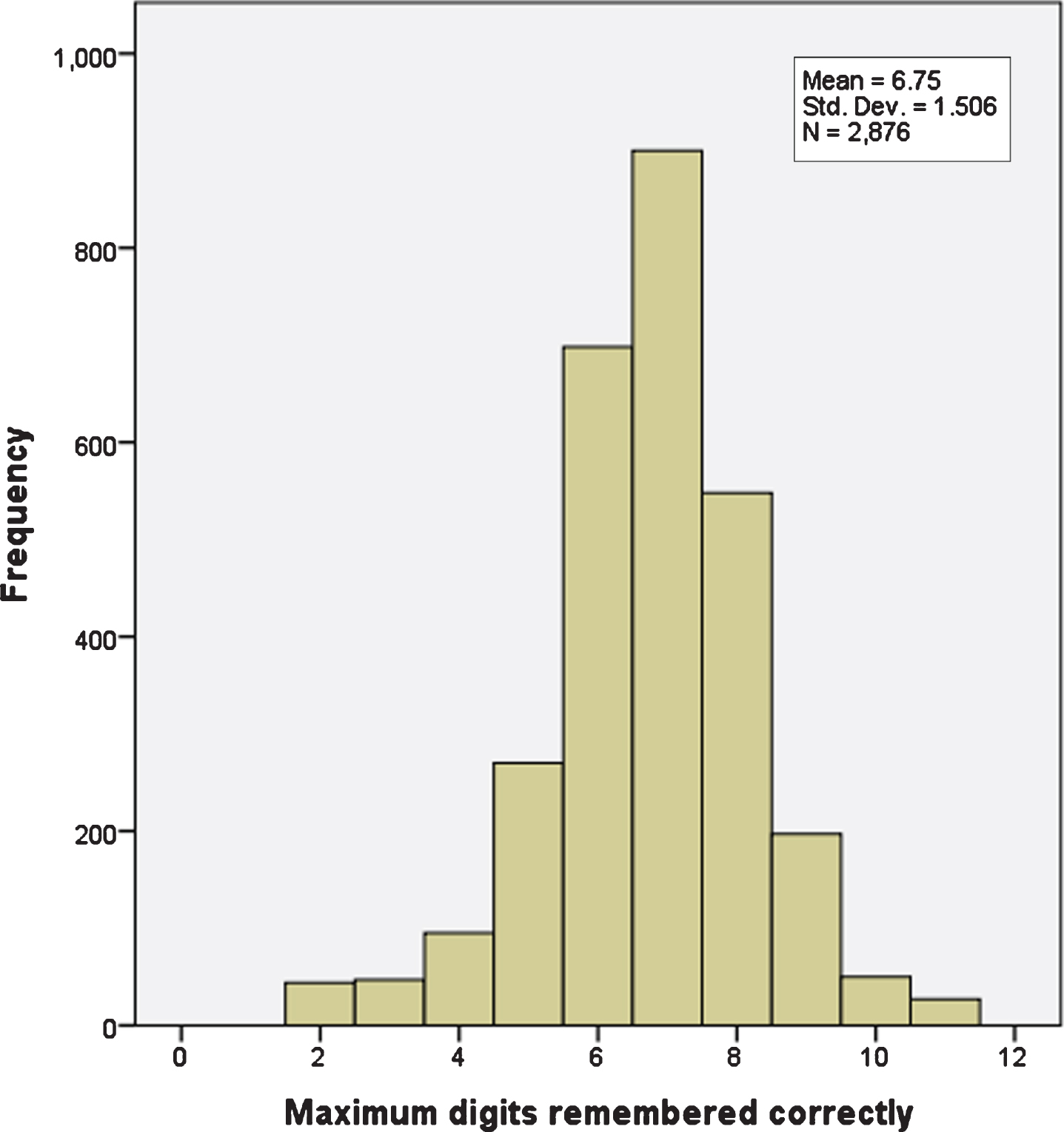
Single nucleotide polymorphism (SNP) data for rs429358 and rs7412 were used to determine APOE genotypes. Cognitive function as numeric memory was assessed with an online test (UKB data field 20240). Longest number correctly recalled during the numeric memory test was recorded, minimum score 2, maximum score 11. A value of –1 was assigned if the participant chose to abandon the test before completing the first round (Fig. 1).
Fig. 1
Numeric memory scores from 2,876 women with breast cancer. There are 10 distinct values, maximum 11, minimum 2.
We used the English Indices of Deprivation 2010 Index of multiple deprivation (UKB data field 26410) to correct for the effects of economic deprivation on numeric memory. The English Indices of Deprivation are measures of multiple deprivation at the small area level [7]. The model of multiple deprivation which underpins the Indices of Deprivation 2010 is based on the idea of distinct domains of deprivation which can be recognized and measured separately. These domains are experienced by individuals living in an area. People may be counted in one or more of the domains, depending on the number of types of deprivation that they experience. Each domain represents a specific form of deprivation experienced by people and each can be measured individually using indicators. Seven distinct domains have been identified in the English Indices of Deprivation: Income Deprivation, Employment Deprivation, Health Deprivation and Disability, Education Skills and Training Deprivation, Barriers to Housing and Services, Living Environment Deprivation, and Crime.
We used the English Indices of Deprivation Education score 2010 (UKB data field 26414) in a separate analysis to correct only for the effects of education on numeric memory. This score measures the extent of deprivation in terms of education, skills and training in an area. The indicators are structured into two sub-domains: one relating to children and young people and one relating to adult skills. The two sub-domains are designed to reflect the ‘flow’ and ‘stock’ of educational disadvantage within a region. Flow shows change during a period whereas stock indicates the quantity of education at a point in time. Seven indicators were used to calculate the score: 1) Average points score of pupils taking English, Math and Science Key Stage 2 exams; 2) Average points score of pupils taking English, Math and Science Key Stage 3 exams; 3) Average capped points score of pupils taking Key Stage 4 (General Certificate of Secondary Education or equivalent) exams; 4) Proportion of young people not staying on in school or non-advanced education above age 16; 5) Secondary school absence rate, the proportion of authorized and unauthorized absences from secondary school; 6) Proportion of those aged under 21 not entering Higher Education; 7) Proportion of adults aged 25–54 with no or low qualifications.
RESULTS
We analyzed data from 2,876 women with breast cancer. The age at enrollment was 57±7 (mean± SD). The women were 98% white and British.
Numeric memory scores from 2,876 women with breast cancer are shown in Table 1. Data were ana-lyzed by multivariate weighted least squares regression. Numeric memory score was dependent variable. APOE4 genotype (homozygous ɛ4/ɛ4, ɛ4 carrier, ɛ4 non-carrier), English Indices of Deprivation score were independent variables weighted by age. APOE4 carrier status had a significant effect on numeric memory score (p = 0.046). Women homozygous ɛ4/ɛ4 had the lowest score, ɛ4 carriers had an intermediate score, and ɛ4 non-carriers had the highest score. English Indices of Deprivation score significantly affected numeric memory score (p = 0.005) and was independent of the effect of APOE4 carrier status.
Table 1
Numeric memory scores from 2,876 women with breast cancer. Data were analyzed by multivariate weighted least squares regression. Numeric memory score was dependent variable. Genotype (homozygous ɛ4/ɛ4, ɛ4 carrier, ɛ4 non-carrier), English Indices of Deprivation 2010 Index of multiple deprivation score were independent variables weighted by age. APOE4 carrier status had a significant effect on numeric memory score (p = 0.046). English Indices of Deprivation score had a significant effect on numeric memory score (p = 0.005) that was independent of the effect of APOE4 carrier status. A second analysis was done, substituting the English Indices of Deprivation Education score for the English Indices of Deprivation 2010 Index of multiple deprivation score. APOE4 carrier status had a significant effect on numeric memory score (p = 0.05). Women homozygous ɛ4/ɛ4 had the lowest score, ɛ4 carriers had an intermediate score, and ɛ4 non-carriers had the highest score. English Indices of Deprivation Education score significantly affected numeric memory score (p < 0.001) and was independent of the effect of APOE4 carrier status
| Memory | Score | ||
| Genotype | N | Mean | SD |
| non-ɛ4 carrier | 2,291 | 6.77 | 1.488 |
| ɛ4 carrier | 541 | 6.67 | 1.555 |
| ɛ4/ɛ4 homozygote | 44 | 6.57 | 1.784 |
A second analysis was done, substituting the En-glish Indices of Deprivation Education score for the English Indices of Deprivation 2010 Index of multiple deprivation score. APOE4 carrier status had a significant effect on numeric memory score (p =0.05). Women homozygous ɛ4/ɛ4 had the lowest score, ɛ4 carriers had an intermediate score, ɛ4 non-carriers had the highest score. English Indices of Deprivation Education score significantly affected numeric memory score (p < 0.001) and was independent of the effect of APOE4 carrier status.
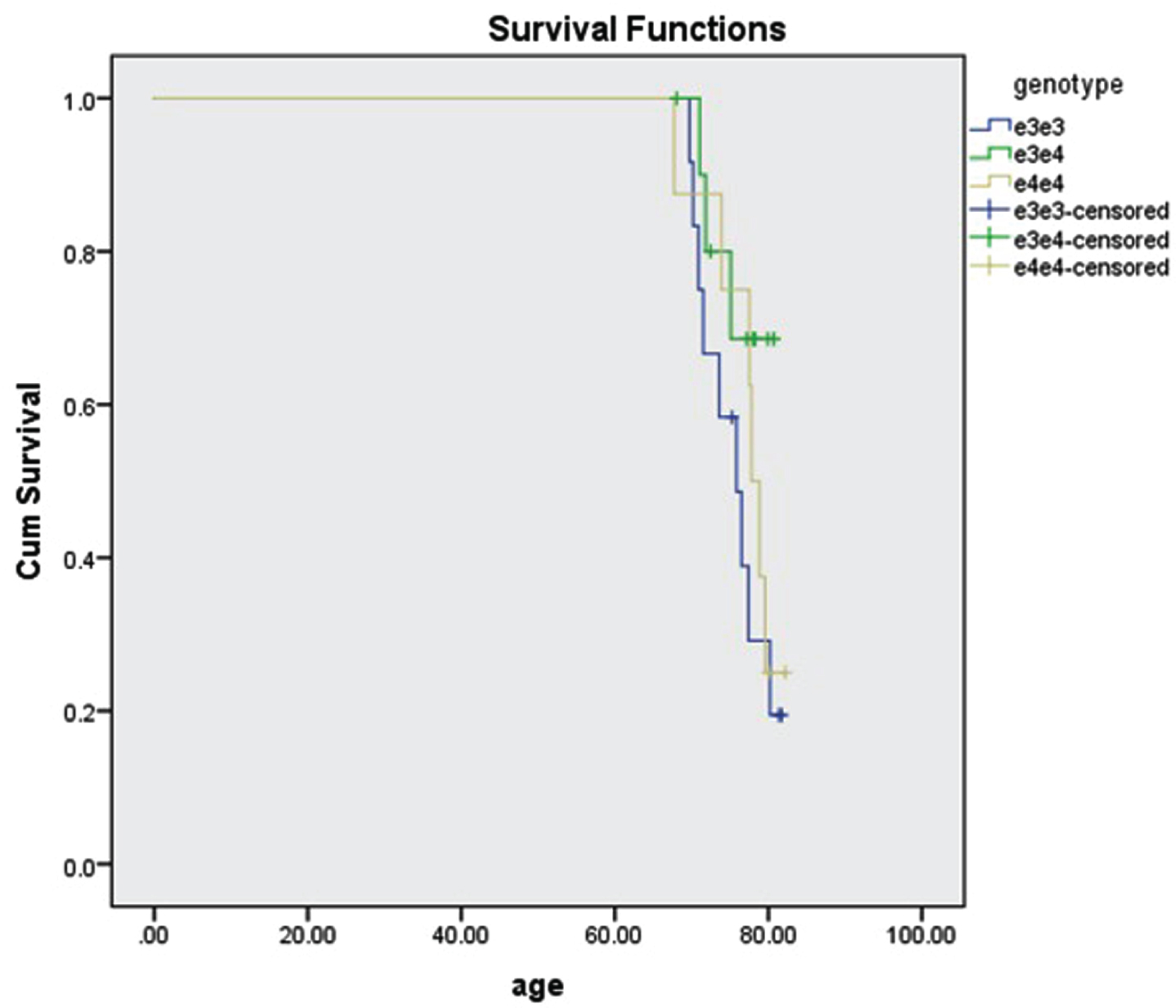
Of the 2,876 breast cancer subjects, 34 (1.1%) had AD. Their age was 76±4. We were able to determine APOE genotype in 31 of the subjects. 3 were missing rs7412 data. There was no significant difference in survival among genotypes ɛ3/ɛ3, ɛ3/ɛ4, and ɛ4/ɛ4 (p = 0.258, Fig. 2).
Fig. 2
Survival of 31 subjects with breast cancer and AD by APOE genotype. 12 subjects were ɛ3/ɛ3, 11 subjects were ɛ3/ɛ4, and 8 subjects were ɛ4/ɛ4. The survival difference was not significant (p = 0.258, log rank test).
DISCUSSION
Cancer survivors have difficulties with memory, attention, learning, and processing of information months or even years after completing treatment, and the APOE4 allele is a prime suspect. An APOE mouse model of cancer-related cognitive problems show-ed that the mice developed chemotherapy-related changes in spatial learning and memory as well as physical changes in brain regions involved in those functions [8].
Findings regarding the effects of APOE genotype on cognition in normal individuals have been inconsistent. It is difficult to substantiate whether cognitive deficits in APOE4 carriers are related to prodromal dementia, the prodromal hypothesis, or a direct con-tribution of APOE genotype to individual differences, the phenotype hypothesis. Multiple studies suggest that both prodromal and phenotype factors might be implicated [9]. Young ɛ4 carriers show a slight superiority in mental acuity as compared with ɛ3 homozygotes. At some point in middle age or late middle age, ɛ4 carriers start showing cognitive deficits as compared with ɛ3 homozygotes [10].
In our UKB cohort, the small significant decline in numeric memory scores of women with breast cancer and the APOE4 allele is consistent with a previous observation that older breast cancer patients with the ɛ4 allele who received chemotherapy, with or without hormonal therapy, experience minor cognitive declines over time [1].
Dementia prevalence in the UK is 6.6% in women age 75–79 [11], considerably greater than the 1.1% we found in the breast cancer patients with AD. The UK Biobank cohort is not representative of the general population regarding several sociodemographic, physical, lifestyle, and health-related characteristics. UK Biobank participants generally live in less socioeconomically deprived areas; are less likely to be obese, to smoke, and to drink alcohol daily; and have fewer self-reported health conditions. All-cau-se mortality is approximately half that of the UK population, and total cancer incidence rates are app-roximately 10% –20% lower. However, valid assessment of exposure-disease relationships may be widely generalizable and do not require participants to be representative of the population at large [12].
The much lower incidence rate of AD (1.1%) in the UKB cohort of white women of high education and socioeconomic status supports the current lifestyle recommendations for the prevention of AD. Because we analyzed UK white women of high education and socioeconomic status (SES), age at first birth and overall nulliparity rate in this high SES cohort would be higher than age at first birth and nulliparity rates in a lower SES ethnically matched group of women. On a relative basis, the UKB cohort has followed lifestyle recommendations for the prevention of AD: a heart-healthy lifestyle, good diet, exercise, control of blood pressure and blood sugar, prevention of obesity. These AD prevention recommendations like avoiding obesity (and its pro-estrogenic effects) would not be expected to prevent breast cancer when counteracted by late first birth and nulliparity effects. Stem cells in the breast are differentiated by pregnancy hormones, thereby removing them as candidate cells for malignant transformation, although the effect is only seen at younger ages of first birth [13].
Weaknesses in our analysis:
• UKB does not identify which subjects received chemotherapy and/or hormonal therapy. If it did, we might have been able to demonstrate a larger effect on numeric memory scores.
• UKB does not have information about pathology or stage. Our study cohort is well-educated and of high SES. We assume that most of the women follow the mammogram recommendations in the UK. Pathological stage at case presentation would almost certainly be at least somewhat earlier than in a cohort with lower education and SES. Impoverished patients often present at quite late stages of breast cancer. Despite the comparatively early diagnosis in UKB subjects, a significant fraction would still have required chemotherapy or, based on hormone receptor status of tumor, hormonal treatment.
• ɛ3/ɛ4 and ɛ3/ɛ2 are fundamentally different. We had too few ɛ2s to include statistical analysis of ɛ2 carriers and non-carriers. A larger sample would be required.
The UKB data we present above suggest that cognitive problems in women with breast cancer are, for the most part, mild, compared with other sequelae of the disease. AD, the worst cognitive problem, is relatively rare (1.1%) and, when it occurs, APOE genotype has little impact on survival.
Mild cognitive changes can be quite upsetting to patients experiencing them. Brain training, computer games, yoga, and physical activity may improve cognitive function in these patients [14].
It will be important to learn why some people are more vulnerable to cancer-related cognitive impairment. No doubt genetics plays a major role, probably greater than stress or treatment modality.
Further studies of the biology may allow prediction of which patients will be affected and how best to manage them.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Research reported in this paper was also supported by the Office of Research Infrastructure of the National Institutes of Health under award numbers S10OD018522 and S10OD026880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
[1] | Mandelblatt JS , Small BJ , Luta G , Hurria A , Jim H , McDonald BC , Graham D , Zhou X , Clapp J , Zhai W , Breen E , Carroll JE , Denduluri N , Dilawari A , Extermann M , Isaacs C , Jacobsen PB , Kobayashi LC , Holohan NK , Root J , Stern RA , Tometich D , Turner R , VanMeter JW , Saykin AJ , Ahles T ((2018) ) Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol 36: , JCO1800140. |
[2] | Moore HC ((2014) ) An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park) 28: , 797–804. |
[3] | Novick AM , Scott AT , Neill Epperson C , Schneck CD ((2020) ) Neuropsychiatric effects of tamoxifen: Challenges and opportunities. Front Neuroendocrinol 59: , 100869. |
[4] | Liu CC , Liu CC , Kanekiyo T , Xu H , Bu G ((2013) ) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9: , 106–118. |
[5] | Arthur RS , Wang T , Xue X , Kamensky V , Rohan TE ((2020) ) Genetic factors, adherence to healthy lifestyle behavior, and risk of invasive breast cancer among women in the UK Biobank. J Natl Cancer Inst 112: , 893–901. |
[6] | Mahmood SS , Levy D , Vasan RS , Wang TJ ((2014) ) The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 383: , 999–1008. |
[7] | Rezaeian M , Dunn G , St Leger S , Appleby L ((2005) ) The ecological association between suicide rates and indices of deprivation in English local authorities. Soc Psychiatry Psychiatr Epidemiol 40: , 785–791. |
[8] | Speidell AP , Demby T , Lee Y , Rodriguez O , Albanese C , Mandelblatt J , Rebeck GW ((2019) ) Development of a human APOE knock-in mouse model for study of cognitive function after cancer chemotherapy. Neurotox Res 35: , 291–303. |
[9] | O’Donoghue MC , Murphy SE , Zamboni G , Nobre AC , Mackay CE ((2018) ) APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 104: , 103–123. |
[10] | Smith CJ , Ashford JW , Perfetti TA ((2019) ) Putative survival advantages in young apolipoprotein ɛ4 carriers are associated with increased neural stress. J Alzheimers Dis 68: , 885–923. |
[11] | Prince M , Knapp M , Guerchet M , McCrone P , Prina M , Comas-Herrera A , Wittenberg R , Adelaja B , Hu B , King D , Rehill A , Salimkumar D (2014) Dementia UK: Update. Alzheimer’s Society. http://www.alzheimers.org.uk/dementiauk |
[12] | Fry A , Littlejohns TJ , Sudlow C , Doherty N , Adamska L , Sprosen T , Collins R , Allen NE ((2017) ) Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 186: , 1026–1034. |
[13] | Tiede B , Kang Y ((2011) ) From milk to malignancy: The role of mammary stem cells in development, pregnancy and breast cancer. Cell Res 21: , 245–257. |
[14] | Peterson BM , Johnson C , Case KR , Shackelford DYK , Brown JM , Lalonde TL , Hayward R ((2018) ) Feasibility of a combined aerobic and cognitive training intervention on cognitive function in cancer survivors: A pilot investigation. Pilot Feasibility Stud 4: , 50. |




