Does Informant-Based Reporting of Cognitive Decline Correlate with Age-Adjusted Hippocampal Volume in Mild Cognitive Impairment and Alzheimer’s Disease?
Abstract
Background:
Informant-based measures are effective screening tools for cognitive impairment. The Alzheimer’s Questionnaire (AQ) is a subjective, informant-based measure that detects amnestic mild cognitive impairment (aMCI) and Alzheimer’s disease (AD) with high sensitivity and specificity and has been shown to predict amyloid burden.
Objective:
To determine whether informant-based report of cognitive decline correlates with hippocampal volume changes in MCI and AD.
Methods:
Retrospective chart review of 139 clinically referred patients with clinical diagnoses of aMCI or mild dementia due to AD was conducted. Diagnostic status (clinical diagnosis made by a neurologist), NeuroQuant measured MRI brain with percentile rank hippocampal volume, Montreal Cognitive Assessment (MoCA) total, AQ-Total score, and demographic variables were extracted from medical records. Spearman correlation was used to assess the relationship between hippocampal volume and AQ-Total. The AQ was used to assign diagnostic status. Thus, the relationship between the AQ and diagnostic status was excluded.
Results:
The sample include 88 female and 51 male participants. The mean age was 74.37±9.45, mean MOCA was 22.65±4.18, mean education was 14.80±3.35, and mean AQ score was 10.54±5.22. Hippocampal volume and the AQ correlation was r = –0.33 [95%CI –0.47 to –0.17], p < 0.0001.
Conclusion:
In a mixed-clinical sample of patients presenting to an outpatient memory disorders center, higher endorseme-nts of functional impairments by caregivers were significantly associated with smaller hippocampal volumes. When used in conjunction with other available measures, these findings further support the role of the AQ in clinical decision-making and demonstrate an additional relationship between clinical measures and volumetric MRI.
INTRODUCTION
Increasing evidence suggests that informant-based questionnaires that capture subjective memory complaints (SMC) are viable screening measures sensitive to objective cognitive impairment and that qualitative analysis of SMCs can predict prodromal clinical manifestations of dementia [1]. Examples of published questionnaires for SMC include the AD8 [2], the Informant Questionnaire on Cognitive Decline in the Elderly [3], and the Memory Functioning Questionnaire (MFQ) [4].
Evidence also suggests that SMC can predict amyloid positivity [5]. Some early studies with the MFQ have shown that poorer subjective ratings of memory, executive functioning, and overall cognition were significantly correlated with greater positron emission tomography (PET) tracer uptake, indicating greater amyloid burden [6, 7] and neurodegeneration [8]. Additionally, individuals with a higher number of SMCs were more likely to be amyloid positive even after controlling for neurodegeneration status (positive or negative), age, and education [6]. One study that explored the link between SMC and magnetic resonance imaging (MRI) reveals that SMC is associated with increased white matter hyperintensity volume and small vessel disease [9].
The Alzheimer’s Questionnaire (AQ) [10] is an informant-based SMC questionnaire that evaluates five domains associated with Alzheimer’s disease (AD), including memory, orientation, functional ability, visuospatial, and language. Prior research with the AQ has demonstrated its ability to differentiate patients with clinically diagnosed mild cognitive impairment (MCI) and AD from normal aging with high sensitivity (89.0%for MCI and 99.0%for AD) and specificity (91.0%for MCI and 96.0%for AD) [11].
The current primary aim is to determine the ability of subjective reports captured by the informant-based AQ to correlate to hippocampal (HC) volume in order to determine if an increase in subjective memory complaints is associated with neurodegeneration, as considered by HC atrophy in the brain. Given past research with the AQ showing that individuals with MCI and AD score higher than cognitively normal individuals, we hypothesized that individuals with lower HC volume will have higher AQ scores.
METHODS
Subjects
Archival records were queried from 139 patients who sought treatment at the Cleveland Clinic Lou Ruvo Center for Brain Health and who underwent a structural and volumetric MRI. Records from participants over the age of 50 with a diagnosis of mild to moderate AD or MCI, a completed an amyloid PET scan, data from the informant-based AQ, and with Mini-Mental State Examination [12] scores between 15–30 were included. Prospectively evaluated patients with mild to moderate AD or MCI also were enrolled. All participants with diagnoses of AD met the criteria established by the National Institute on Aging and the Alzheimer’s Association [13], and the diagnosis of MCI was based on the Petersen criteria [14]. Exclusion criteria included evidence of vascular, traumatic, or inflammatory causes of amnestic MCI (aMCI) evident by non-contrast MRI and history of major systemic diseases that could affect cognitive function, including other dementias (e.g., dementia with Lewy bodies, frontotemporal dementia, primary progressive aphasia, Parkinson’s disease dementia, vascular dementia), cardiopulmonary failure, hepatic or renal failure, diabetes mellitus, head injury, stroke, or other neurodegenerative diseases. Additional variables gathered from patients’ medical records include age, sex, education level, clinical diagnosis (established by a behavioral neurologist), and additional significant medical history. All study procedures were reviewed and approved through a minimal risk protocol by the Institutional Review Board at the Cleveland Clinic.
MRI procedure
MRI data acquisition and pre-processing
MR images were acquired on a 3T Siemens Trio scanner with a 12-channel head coil. Specifically, a 3D volumetric magnetization-prepared rapid acquisition (MPRAGE) T1-weighted scan was acquired with the following parameters: repetition time (TR) 2300 ms, echo time (TE) 3.31 ms, inversion time (TI) 900 ms, flip angle (FA) 9°, a field-of-view (FOV) of 256 mm, and a voxel resolution of 1.0 ×1.0×1.2 mm3. This T1-weighted structural image was used for the quantification of hippocampal volume by the NeuroQuant software package (CorTechs Laboratories, La Jolla, CA).
Other sequences in multiple planes were acquired for clinical qualitative evaluation, including a diff-usion weighted imaging sequence (TR 5100 ms, TE 105 ms, FA 180°, bandwidth 723 Hz/Px, FOV 220 mm, voxel resolution 1.2×1.2×4.0 mm3), a fluid attenuation inversion recovery (FLAIR) axial sequence (TR 9000 ms, TE 109 ms, FOV 240 mm, voxel resolution 0.8×0.8×5.0 mm3), a T2 fat supp-ressed axial sequence (TR 4560 ms, TE 104 ms, FA 160°, FOV 240 mm, voxel resolution 0.5×0.5 ×5.0 mm3), a T1-weighted axial sequence (TR 600 ms, TE 9 ms, FOV 240 mm, voxel resolution 0.9×0.9×5.0 mm3), an axial gradient echo seq-uence (TR 250 ms, TE 20 ms, voxel resolution 1.3×0.9×5.0 mm3), a T2 axial susceptibility-weighted images sequence (TR 27 ms, TE 20 ms, FA 17°, FOV 220 mm, voxel resolution 0.9×0.9×1.6 mm3), and a FLAIR sagittal sequence (TR 5000 ms, TE 388 ms, TI 2400 ms, bandwidth 751 Hz/Px, FOV 230 mm, voxel resolution 1.0×1.0×1.0 mm3).
NeuroQuant is a fully automated software package that was specifically developed for segmentation and volumetric analysis of brain structures. The software provides volumetric analysis of multiple brain structures for each hemisphere (right and left) as a percentage of the total intracranial volume; this value is based on the sum of all segmented brain structures, the brainstem, meninges, and cerebrospinal fluid (CSF) external to the brain surface, allowing for interindividual comparisons. The automatic segmentation process includes image filtering, artifact correction, segmentation, error measurement, and report generation. For the hippocampus, lateral ventricles, and the temporal horn of the lateral ventricles, a normative range based on previous segmentations of healthy individuals aged 50 to 95 years is provided. In this study we report age-adjusted percentile volumes for the hippocampus and the other areas of the brain included in the AD NeuroQuant evaluation, these being the forebrain parenchyma, cortical gray matter, cerebellum, amygdala, pallidum, putamen, caudate nucleus, thalamus, lateral ventricles, and inferior lateral ventricles.
Alzheimer’s Questionnaire
The AQ was administered to an informant accompanying the patient during clinical consultation as the standard of care. Each question is answered either “yes” or “no” and the sum of the positively endorsed items add up to a total score from 0 to 27. Six AQ items are weighted (i.e., worth two points) given their status as highly predictive of a clinical diagnosis of AD (e.g., repeating questions, disorientation to time) [10].
Analyses
All data were analyzed using Prizm GraphPad. Pearson bivariate correlations were conducted to measure the associations between demographic variables (age and education) and the AQ total score. Chi square analyses were conducted to measure potential differences in diagnostic status (aMCI versus dementia) by amyloid status (positive versus negative) and to measure differences in sex by diagnostic status and amyloid status. Independent samples t-tests were conducted to measure potential differences using age and education level as dependent variables, with amyloid status, diagnostic status, and sex as independent variables. Of note, education data was unavailable for 18 patients who were thus excluded from the t-test analysis evaluating differences in education level. An analysis of covariance (ANCOVA) was conducted to measure differences in the AQ total score with sex as the independent variable and education as a covariate. An independent samples t-test was conducted to measure differences in AQ total score using amyloid status (positive versus negative) as the independent variable. Differences in the AQ score by diagnostic status were excluded from analyses as the AQ was used, in part, to determine clinical diagnosis of MCI and AD. Finally, receiver operating characteristic (ROC) curves were plotted to assess overall classifica-tion accuracy of the AQ in predicting amyloid positivity.
RESULTS
The sample include 88 female and 51 male participants. Mean age was 74.37±9.45, mean MOCA was 22.65±4.18, mean education was 14.80±3.35, mean AQ score was 10.54±5.22
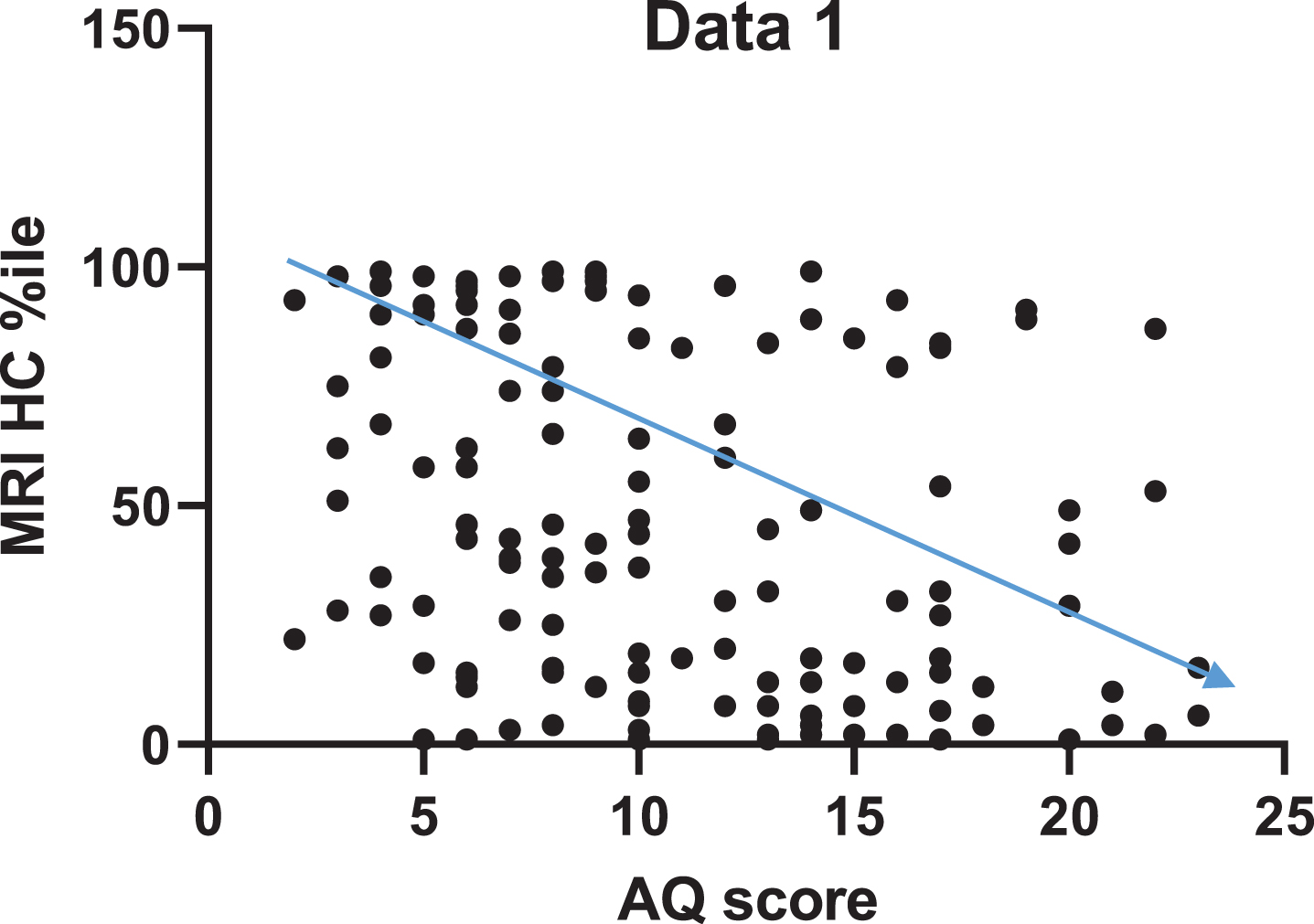
The correlation between HC volume and AQ are presented in Fig. 1. The AQ score was significantly correlated with HC volume (r = –0.33 [95%CI –0.47 to –0.17], p < 0.0001).
Fig. 1
Higher AQ scores are significantly correlated with a decrease in HC volume, as indicated by the inverse proportionality between AQ score and HC volume.
DISCUSSION
In a mixed-clinical sample of patients, higher endorsements of functional impairments by caregivers were significantly associated with smaller hippocampal volumes. When used in conjunction with other available measures, these findings further support the role of the AQ in clinical decision-making and demonstrate an additional relationship with established AD biomarker outcomes with volumetric MRI.
Subjective memory complaints and MRI have been investigated in other studies. Ryu et al. compared subjects with SMC and subjects without SMC and found micro- and macrostructural differences in HC and entorhinal cortex [15]. In another study of 28 patients from a memory clinic with isolated SCD, 35 community-recruited elders with similarly high levels of self-reported cognitive difficulties, and 35 community-recruited controls with low self-reported cognitive difficulties, high self-reported difficulties were associated with increased anxiety and amyloid-β deposition, whereas subclinical depression and (HC) atrophy were specifically associated with medical help seeking [16]. Other groups have detected diffusion tensor imaging changes associated with SMC [17] and cortical thinning [18]. The MEMENTO study is currently analyzing 2,300 participants with SMC and MCI to assess MRI and FDG PET. This study includes neuropsychological testing to determine the natural history of these condi-tions [19]
The findings from this study indicate that the AQ shows promise as a tool to improve diagnostic accuracy in individuals with potential AD (i.e., those with clinical diagnoses of amnestic MCI and AD). The data suggest that the stronger the subjective complaint, the more likely there is for HC atrophy. Thus, informant-based questionnaires may have a measure of predicting neurodegeneration. However, those with atypical presentations of AD (e.g., posterior cortical atrophy or logopenic primary progressive aphasia) may not be accurately classified by the AQ, as the types of cognitive complaints assessed by the measure as specific to typically presenting AD symptomology.
Overall, this study highlights the potential utility for informant-based subjective cognitive questionnaires in screening individuals with concerns of AD pathology. In this study, AD pathology (CSF, PET, or plasma) was not directly measured. The AQ is a cost-effective screening tool for inclusion in clinical trials and to improve diagnostic accuracy and appropriate referrals in settings where testing may be limited by resources and time demands (e.g., primary care). Further research is needed to determine the accuracy of informant-based measures in predicting neurodegeneration, but our findings indicate that the AQ has promise as an important diagnostic screening tool in clinical practice.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
This work is supported by NIH COBRE 5P20GM109025, NIH P20 AG068054, NIH R01AG059008, and the Keep Memory Alive Foundation.
REFERENCES
[1] | Tsutsumimoto K , Doi T , Makizako H , Hotta R , Nakakubo S , Makino K , Suzuki T , Shimada H ((2017) ) Association of social frailty with both cognitive and physical deficits among older people. J Am Med Dir Assoc 18: , 603–607. |
[2] | Galvin JE , Roe CM , Powlishta KK , Coats MA , Muich SJ , Grant E , Miller JP , Storandt M , Morris JC ((2005) ) The AD8: A brief informant interview to detect dementia. Neurology 65: , 559–564. |
[3] | Jorm AF (1996) Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly (Short IQCODE). Centre for Mental Health Research, The Australian National University Canberra, Australia. |
[4] | Gilewski MJ , Zelinski EM , Schaie KW ((1990) ) The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging 5: , 482–490. |
[5] | Brunet HE , Miller JB , Shi J , Chung B , Munter BT , Sabbagh MN ((2019) ) Does informant-based reporting of cognitive symptoms predict amyloid positivity on positron emission tomography? Alzheimers Dementia (Amst) 11: , 424–429. |
[6] | Amariglio R , Becker JA , Carmasin J , Wadsworth L , Lorius N , Sullivan C , Maye J , Gidicsin C , Pepin L , Sperling R , Johnson K , Rentz D ((2012) ) Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50: , 2880–2886. |
[7] | Snitz BE , Weissfeld LA , Cohen AD , Lopez OL , Nebes RD , Aizenstein HJ , McDade E , Price JC , Mathis CA , Klunk WE ((2015) ) Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry 23: , 985–993. |
[8] | Amariglio RE , Mormino EC , Pietras AC , Marshall GA , Vannini P , Johnson KA , Sperling RA , Rentz DM ((2015) ) Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology 85: , 56–62. |
[9] | van Rooden S , van den Berg-Huysmans AA , Croll PH , Labadie G , Hayes JM , Viviano R , van der Grond J , Rombouts SARB , Damoiseaux JS ((2018) ) Subjective cognitive decline is associated with greater white matter hyperintensity volume. J Alzheimers Dis 66: , 1283–1294. |
[10] | Sabbagh M , Malek-Ahmadi M , Kataria R , Belden C , Connor D , Pearson C , Jacobson S , Davis K , Yaari R , Upinder Singh U ((2010) ) The Alzheimer’s Questionnaire: Proof of concept study for a new informant-based dementia assessment. J Alzheimers Dis 22: , 1015–1021. |
[11] | Malek-Ahmadi M , Davis K , Belden CM , Laizure B , Jacobson SA , Yaari R , Singh U , Sabbagh MN ((2012) ) Validation and diagnostic accuracy of the Alzheimer’s Questionnaire (AQ). Age Ageing 41: , 396–399. |
[12] | Folstein MF , Folstein SE , McHugh PR ((1975) ) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: , 189–198. |
[13] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[14] | Petersen R , Caracciolo B , Brayne C , Gauthier S , Jelic V , Fratiglioni L ((2014) ) Mild cognitive impairment: A concept in evolution. J Intern Med 275: , 214–228. |
[15] | Ryu SY , Lim EY , Na S , Shim YS , Cho JH , Yoon B , Hong YJ , Yang DW ((2017) ) Hippocampal and entorhinal structures in subjective memory impairment: A combined MRI volumetric and DTI study. Int Psychogeriatr 29: , 785–792. |
[16] | Perrotin A , La Joie R , de La Sayette V , Barré L , Mézenge F , Mutlu J , Guilloteau D , Egret S , Eustache F , Chételat ((2017) ) Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimers Dement 13: , 550–560. |
[17] | Hsu YH , Huang CF , Huang WH , Deng JF , Tu MC ((2019) ) Microstructural correlates and laterality effect of prospective memory in non-demented adults with memory complaints. Dement Geriatr Cogn Disord 47: , 375–384. |
[18] | Schultz SA , Oh JM , Koscik RL , Dowling NM , Gallagher CL , Carlsson CM , Bendlin BB , LaRue A , Hermann BP , Rowley HA , Asthana S , Sager MA , Johnson SC , Okonkwo OC ((2015) ) Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement (Amst) 1: , 33–40. |
[19] | Dufouil C , Dubois B , Vellas B , Pasquier F , Blanc F , Hugon J , Hanon O , Dartigues JF , Harston S , Gabelle A , Ceccaldi M , Beauchet O , Krolak-Salmon P , David R , Rouaud O , Godefroy O , Belin C , Rouch I , Auguste N , Wallon D , Benetos A , Pariente J , Paccalin M , Moreaud O , Hommet C , Sellal F , Boutoleau-Bretonniére C , Jalenques I , Gentric A , Vandel P , Azouani C , Fillon L , Fischer C , Savarieau H , Operto G , Bertin H , Chupin M , Bouteloup V , Habert MO , Mangin JF , Chêne G ; MEMENTO cohort Study Group ((2017) ) Cognitive and imaging markers in non-demented subjects attending a memory clinic: Study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther 9: , 67. |




